Abstract
Background
Keratinocyte carcinomas (KCs) are the most common malignancies of the skin. As lesions have a low mortality rate, understanding quality-of-life (QoL) factors is necessary in their management.
Objective
To assess QoL and associated patient characteristics in those with a history of keratinocyte carcinomas.
Methods
We conducted a cross-sectional study of veterans with a history of KCs enrolled in a randomized controlled trial for chemoprevention of keratinocyte carcinomas. Study dermatologists counted actinic keratoses (AKs) and assessed for skin photodamage. QoL was assessed using Skindex-29 and KC-specific questions. Demographics were self-reported.
Results
Participants (n = 931) enrolled at 5 clinical sites had worse QoL on all subscales (emotions, functioning, and symptoms) compared to a reference group of patients without skin disease. Univariate analysis demonstrated worse QoL associated with higher AK count, past 5-fluorouracil (5-FU) use, and greater sun sensitivity. Multivariate analysis demonstrated that higher AK count and past 5-FU use were independently related to diminished QoL. Higher comorbidities showed modest associations on the symptoms and functioning subscales. Number of previous KCs was not independently associated with any QoL differences.
Limitations
Study population may not be generalizable to the general population. Counting of AKs is of limited reliability. Previous 5-FU use is self reported.
Conclusions
A history of ever use of 5-FU and present AKs was strongly associated with worse QoL. We find it more useful to consider these patients as having the chronic condition “actinic neoplasia syndrome,” whose burden may be best measured by factors other than their history of KCs.
INTRODUCTION
Keratinocyte carcinomas (KCs) are the most common malignancies, consisting of basal cell and squamous cell carcinomas. Over one million new cases of KCs are diagnosed annually in the United States, similar to the incidence of all non-cutaneous cancers combined.1–3 Mortality rates from a population-based follow-back study for nonmelanoma skin cancer was 0.91 per 100,000 per year.4 However, unlike other cancers in which end points focus on mortality, morbidity is critical to understanding the burden of disease for KCs, as these skin cancers are generally not fatal, but may lead to severe disfigurement and related complications.
This study sought to describe quality of life (QoL) and associated patient characteristics in those with a history of KCs. On the basis of previous studies and our clinical experience, we hypothesized that skin-related quality of life would be better in patients who were male, married, and more educated, and in patients with fewer comorbidities, less photodamage, or fewer KCs in the past. We studied a large sample of patients (n = 931), who were evaluated at the time of entry into a chemoprevention trial for KC across multiple centers in the United States.
METHODS
Study population
The Veterans Affairs Topical Tretinoin Chemoprevention Trial (VATTC) was a multicenter randomized trial of topical tretinoin for chemoprevention of KCs (key personnel from the VATTC are listed in the Appendix). Eligibility for the VATTC trial required two or more primary KCs (basal cell and/or invasive squamous cell carcinoma) in the prior 5 years. All patients were free of KC at enrollment as verified by dermatologist examination and did not report use of 5-fluorouracil (5-FU), calcipotriene, or retinoid products in the prior 60 days. All data in this report were gathered at the baseline interview and initial (pre-intervention) dermatological examination. All participants gave signed informed consent. This study was overseen by multiple independent committees, including 8 institutional review boards.5
Measures
In this study, quality of life refers to skin-related quality of life, as measured by Skindex-29 and supplementary items.6 Skindex is a self-administered questionnaire to measure the effects of skin conditions on QoL. It consists of 29 items reported as 3 scales—emotions, symptoms, and functioning. Scales range from 0 to 100, with higher numbers indicative of worse QoL. Questions refer to the past week and are scored on a 5-point scale, from “never” to “all the time”.
In addition to Skindex, patients responded to six KC-specific items. The purpose of these items was to measure specific effects of KC on QoL that may not be fully captured by the Skindex instrument. These items were composed based on input from experienced clinicians and patients with KCs, and they reflect the effects of actinic damage and KCs. These KC-specific questions inquired about bother from scars, bother about appearance, bother about persistence of skin condition, worry about treatment, and worry that the skin condition will spread. The items were scored in a similar manner to the Skindex items, which were averaged to generate a KC-specific worry/bother score. Therefore, both skin-and disease-specific instruments were used as measurement tools for a comprehensive, specific assessment.7
Education level, marital status, previous 5-FU and other medication use, and sun-sensitivity indicators were collected using self-response surveys. An index to measure sensitivity to sun was developed by using patient responses about hair and skin color, freckling, susceptibility to sunburn, reaction to first sun exposure, ability to tan, and tan-line duration.8
Data collection
VATTC patients were asked to complete the Skindex questionnaire at entry into the study. Actinic keratoses (AKs) on the face and ears were counted by a study dermatologist at the baseline visit. Distribution of AK counts was highly skewed; hence for descriptive purposes and for multivariate analyses, the counts were grouped into 5 categories. From the medical record, information was obtained on the number of primary basal cell carcinomas and/or primary invasive squamous cell carcinomas located anywhere on the body in the past 5 years. Data on KCs were also grouped into a 5-point scale. Visible photodamage was assessed by the examiners’ comparison with a standard set of photographs and rated on a 0 to 8 scale, 8 being the most photodamaged skin.9 Data on comorbidities were obtained from a nationwide VA database of patient records as described elsewhere.5
Statistical analysis
We calculated Skindex-29 subscale scores and compared them with scores of patients with other skin diseases, and of persons without skin disease, as reported previously.10 Charlson index was calculated on the basis of a weighted sum of 19 dichotomous (0–1) indicator variables, with weights varying from 1–6. A sun-sensitivity index was calculated ranging from 0 to 1, with 1 being the most sensitive and indicating lighter skin, light hair, and tendency to burn.11,12
We next determined which patient characteristics were associated with worse QoL, using univariate linear regression models in Stata statistical software (release 8.2, StataCorp, College Station, TX). We included characteristics that we thought likely related to QoL, including age, gender, education level, marital status, AK count category, history of previous 5-FU use, degree of sun-sensitivity, past KC number category, and Charlson index. All P values are two tailed. Additionally, we conducted a backwards stepwise regression analysis, which retained items associated with the P value being less than .10, to reveal independent associations of QoL with aforementioned patient characteristics. Dependent variables consisted of the emotion, functioning, and symptoms subscales, as well as the KC-specific worry/bother score.
RESULTS
Study population
The VATTC study recruited 1131 patients from 6 VA medical centers across the nation. For this report, 5 centers (937 enrolled patients) with adequate information and QoL assessments were included. Six patients had incomplete Skindex forms and were excluded from the final analysis. The remaining 931 patients were predominately elderly, Caucasian, and male, with substantial evidence of photodamage. For example, 31% had more than 5 AKs on the face and ears, 64% had 3 or more KCs in the prior 5 years, and 16% had 7 or more KCs in the previous 5 years (Table I).
Table I.
Characteristics of 931 patients with history of two or more keratinocyte carcinomas*
| Mean (SD) | |
|---|---|
| Age (y) | 71.0 (9.1) |
| Education level (0 to 3) | 1.67 (0.64) |
| AK group (1 to 5) | 1.20 (1.06) |
| KC group (1 to 5) | 2.53 (1.50) |
| Photodamage (0 to 8 scale) | 5.48 (1.53) |
| Sun sensitivity index (0 to 1)† | 0.53 (0.21) |
| Gender | 97% men |
| Marital status | 64% married |
| Race | 99.5% Caucasian |
| Ever used 5-FU | 19% |
| Education level 0 to 3 | |
| 0 = No schooling | 0% |
| 1 = High school or less | 43% |
| 2 = College or equivalent | 48% |
| 3 = Postgraduate | 9% |
| AK group (1 to 5)* | |
| 0 = None | 27% |
| 1 = 1–5 | 42% |
| 2 = 6–15 | 20% |
| 3 = 16–25 | 7% |
| 4 = ≥26 | 4% |
| KC group (1 to 5)* | |
| 1 = History of 2 KCs | 36% |
| 2 = History of 3 KCs | 22% |
| 3 = History of 4 KCs | 11% |
| 4 = History of 5–6 KCs | 15% |
| 5 = History of ≥7 KCs | 16% |
AK, Actinic keratoses; KC, keratinocyte carcinomas; SD, standard deviation.
AK and KC groups categorized into quintiles for descriptive purposes and multivariate analysis.
Sun sensitivity index: 0 = least sensitive; 1 = most sensitive.
Skindex scores
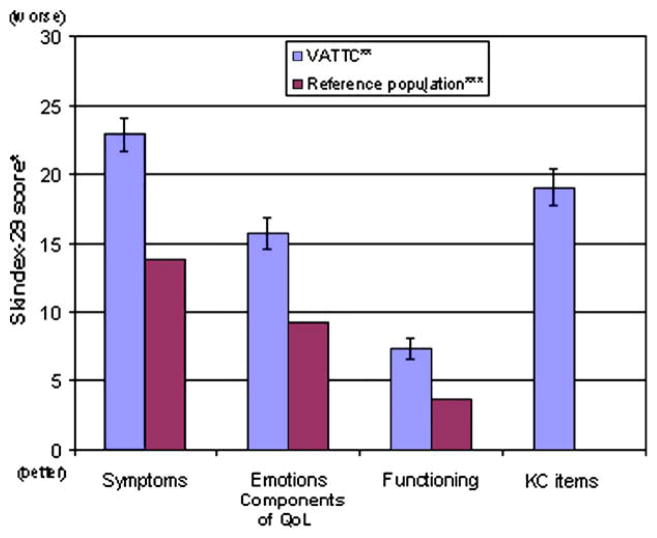
Overall, the participants had worse skin-related QoL in all domains compared to a historical reference sample of persons without skin disease (Fig 1). Two items in the emotions scale stood out as making the largest contribution to the elevated mean score. Both “I worry that my skin condition may get worse” and “I worry that my skin condition may be serious” had scores that were double the overall emotions scale score. The KC-specific worry/bother questions also demonstrated items that scored significantly higher than others within the same group, namely “I am worried that my skin condition will spread” and “I am bothered by the persistence/reoccurrence of my skin condition” (Fig 2).
Fig 1.
Baseline QoL in VATTC trial participants and a reference population. Skindex-29 scores of patients with the actinic neoplasia syndrome (ANS) compared to a historical reference group without skin disease. ANS is a chronic syndrome consisting of a history of KCs with persistent AKs. *, Chren MM, Lasek RJ, Flocke SA, Zyzanski SJ. Improved discriminative and evaluative capability of a refined version of Skindex, a QoL instrument for patients with skin diseases. Arch Dermatol 1997;11:1433–40. **, VA Topical TretinoinChemoprevention Trial. ***, Lasek RJ, Chren MM. Acne vulgaris and the QoL of adult dermatology patients. Arch Dermatol 1998:134:454–8.
Fig 2.
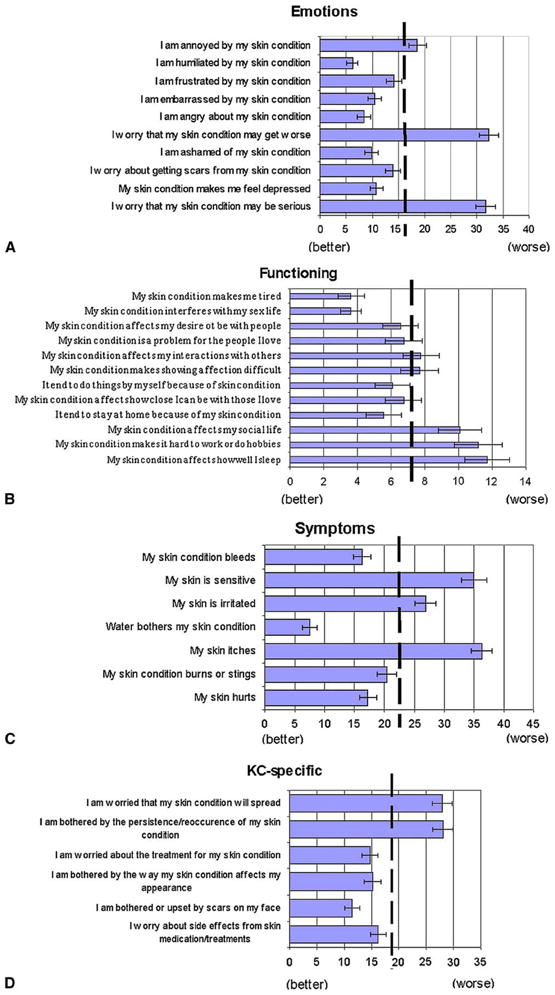
Baseline QoL means for each item of the Skindex-29 with standard errors shown. These figures depict means for each item, as well as KC-specific questions. Dashed vertical line represents the mean of each specific subscale. A, B, C, and D show the emotions, functioning, and symptoms subscales, and KC-specific items, respectively.
Univariate analysis
Worse QoL on all 3 subscales and the KC-specific items was significantly associated with higher AK count, past 5-FU use, and greater sun sensitivity (Table II). Weaker but still significant associations with worse QoL were found with higher number of past KCs on the symptoms and KC-specific items. Younger age was strongly associated with worse QoL on all domains except the symptoms scale. Women and unmarried patients had worse symptoms than other patients, and more educated patients had worse KC-specific effects. No other patient characteristic, including visible photodamage and comorbidities, was related to QoL in univariate analysis.
Table II.
Univariate associations with worse quality of life
| Variable | Regression coefficient (P value)
|
|||
|---|---|---|---|---|
| Emotions | Symptoms | Functioning | KC-specific | |
| Greater AK count | 1.69 (.002) | 2.9 (<.001) | 1.19 (.002) | 1.79 (.003) |
| Ever 5-FU use | 5.24 (<.001) | 6.25 (<.001) | 3.08 (.004) | −6.12 (<.001) |
| Greater sun sensitivity | 9.24 (.001) | 14.6 (<.001) | 4.37 (.03) | 10.57 (.001) |
| Greater No. of KCs | 0.73 (.06) | 0.83 (.038) | 0.36 (.2) | 0.85 (.049) |
| Greater photodamage | −0.12 (.8) | 0.36 (.4) | −0.05 (.9) | −0.12 (.8) |
| Intervention (yes/no) | 0.62 (.6) | 0.12 (.9) | 0.18 (.8) | 0.5 (.7) |
| Age (per 10 years) | −1.96 (.002) | −0.60 (.4) | −1.13 (.01) | −2.58 (<.001) |
| Women (vs men) | 0.77 (.8) | 7.76 (.02) | 0.27 (.9) | 3.31 (.4) |
| More education | 1.47 (.10) | 1.54 (.10) | 0.58 (.4) | 2.79 (.006) |
| Married (vs not) | −1.29 (.3) | −3.54 (.004) | −1.02 (.2) | −1.05 (.4) |
| Higher Charlson score | 0.33 (.3) | 0.6 (.07) | 0.29 (.2) | 0.08 (.8) |
AK, Actinic keratosis; 5-FU, 5-fluorouracil; KC, keratinocytic carcinoma.
Multivariate analysis
In multivariate analysis (Table III), higher AK count and past 5-FU use were independently related to worse QoL in all domains, including the KC-specific worry/bother group. Younger age was independently related to worse QoL in all domains except symptoms. In addition, greater sun sensitivity was marginally associated with emotions, but strongly associated with symptoms. Higher comorbidities showed modest, yet significant, associations with the symptoms and functioning subscales. Female gender, higher education level, and being unmarried were all independently associated with worse symptoms. Greater photodamage was not independently related to worse QoL. Number of previous KCs was not independently associated with QoL.
Table III.
Multivariate association with worse quality of life
| Variable | Regression coefficient (P value)
|
|||
|---|---|---|---|---|
| Emotions | Symptoms | Functioning | KC-specific | |
| Greater AK count | 1.43 (.01) | 2.33 (<.0001) | 1.15 (.004) | 1.49 (.02) |
| Ever 5-FU use | 4.17 (.006) | 3.99 (.01) | 2.58 (.02) | 4.35 (.01) |
| Greater sun sensitivity | 5.43 (.054) | 11.11 (<.001) | — | 5.83 (.07) |
| Greater No. of KCs | — | — | — | 0.84 (.052) |
| Greater photodamage | — | — | — | — |
| Intervention (yes/no) | — | — | — | — |
| Age (per 10 years) | −2.27 (.001) | — | −1.54 (.001) | −2.45 (.001) |
| Women (vs men) | — | 9.36 (.005) | — | — |
| More education | — | 1.83 (.049) | — | 2.25 (.03) |
| Married (vs not) | — | −3.88 (.002) | — | — |
| Higher Charlson score* | 0.57 (.08) | 0.71 (.03) | 0.46 (.049) | — |
For abbreviations, see Table II legend.
Dash signifies exclusion from multivariate model because P >.10.
A higher Charlson score correlates with higher morbidity; value shown here is coefficient per unit of Charlson. Standard error equals 0.06.
DISCUSSION
We conducted a cross-sectional analysis of correlates of skin-related QoL in patients with actinic neoplasia syndrome (ANS) and found that AK count, ever-use of 5-FU, and younger age were the most prominent factors predictive of worse QoL in this population; the number of KCs in the past 5 years was not an independent correlate of QoL. As expected, younger age, gender, education, comorbidities, and sun sensitivity were associated with various domains of QoL.
Evaluations of the impact of KCs generally view them as discrete events (cancer diagnoses), which have definable morbidity risks. It is also recognized that once a person has one KC, the risk of developing a second is markedly increased and that KCs are frequently associated with other manifestations of chronic sun damage, such as AKs and solar elastosis. We suggest an alternate view of persons who have had multiple KCs: that they have a chronic illness consequent to ultraviolet irradiation, associated with various manifestations including neoplasms such as basal cell carcinomas, squamous cell carcinomas, and AKs, as well as other signs of photodamage. We call this collection of manifestations of chronic ultraviolet damage ANS. This syndrome is associated with high risk of additional actinically induced neoplasms and hence a frequent concern about these. It should be noted that important aspects of ANS might include cutaneous changes other than neoplasia.
For the purpose of this study we operationally defined ANS as a history of at least 2 KCs in the past 5 years. We noted that the number of KCs was not actually an important predictor of diminished QoL, but rather a history of treatment with 5-FU and numbers of AKs on examination were the most important predictors. This suggests that it is not the KCs that are primarily responsible for the QoL impact, but other aspects of this syndrome.
Previous work using a generic skin-related QoL instrument, the Dermatology Life Quality Index (DLQI) has suggested that nonmelanoma skin cancer itself has little effect on overall QoL.13–16 This skin-specific instrument may lack sensitivity in measuring disease-specific features of KCs, as characteristics of this disease are unique compared with those of chronic benign skin conditions. Similar results showing skin cancer’s minimal impact on QoL were obtained using standard gamble health utility measures in assessing nonmelanoma skin cancer.17 One research group initially found decreased utilities in a small pilot study; these results were not replicated with a larger cohort. Researchers noted that a lack of sensitivity of the measurement method may have contributed to these results.18
A recent study using the Skindex instrument showed independent association of higher education levels, being married, and fewer comorbidities with better QoL; age and previous history of nonmelanoma skin cancer had no effect.19 In contrast, our results suggest that higher education levels are independently associated with worse symptoms and KC-specific worry/bother items. Our results concur in that being married showed strong association with improved QoL in the symptoms domain, and previous history of basal cell or squamous cell carcinoma had no independent effect in any domain. Higher numbers of KCs had a borderline association with the KC-specific items.
Our population also demonstrated slight, but significant association of higher comorbidities with worse QoL in the symptoms and functioning domains, a finding that agrees with previous literature.19,20 The Charlson score for comorbidities also showed a correlation with age, a variable whose association with QoL also strengthened when Charlson score was included in the model (Table IV).
Table IV.
Correlations among quality-of-life subscales and selected risk factors
| Emotions | Symptoms | Functioning | KC items | Age | Gender | Marital | Educational level | Photodamage | AK group | KC group | SS score | 5-FU use | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Emotions | 1 | ||||||||||||
| Symptoms | 0.64 | 1 | |||||||||||
| Functioning | 0.80 | 0.65 | 1 | ||||||||||
| KC items | 0.86 | 0.62 | 0.69 | 1 | |||||||||
| Age | −0.10 | −0.03 | −0.08 | −0.12 | 1 | ||||||||
| Gender | 0.02 | 0.08 | <0.01 | 0.03 | −0.12 | 1 | |||||||
| Marital | −0.04 | −0.09 | −0.04 | −0.03 | −0.09 | 0.09 | 1 | ||||||
| Educational level | 0.05 | 0.05 | 0.03 | 0.09 | −0.18 | 0.09 | 0.13 | 1 | |||||
| Photodamage | −0.02 | 0.03 | −0.01 | −0.01 | 0.27 | −0.04 | −0.02 | −0.07 | 1 | ||||
| AK group | 0.10 | 0.16 | 0.10 | 0.10 | 0.12 | −0.09 | −0.01 | −0.01 | 0.34 | 1 | |||
| KC group | 0.06 | 0.07 | 0.04 | 0.07 | 0.11 | −0.11 | −0.01 | −0.1 | 0.12 | 0.06 | 1 | ||
| SS score | 0.11 | 0.17 | 0.07 | 0.11 | −0.16 | 0.06 | −0.01 | 0.02 | 0.06 | 0.15 | 0.07 | 1 | |
| 5-FU use | −0.12 | −0.13 | −0.10 | −0.12 | −0.02 | 0.04 | 0.03 | −0.05 | −0.08 | −0.19 | −0.15 | −0.16 | 1 |
| Charlson | 0.03 | 0.06 | 0.04 | <0.01 | 0.25 | −0.06 | −0.04 | −0.16 | 0.04 | 0.02 | 0.12 | −0.02 | −0.01 |
SS, Sun sensitivity; for other abbreviations, see legend to Table II.
An instrument specific for skin cancer–related QoL has been developed by Rhee et al21 (Skin Cancer Index [SCI]). This group found that age younger than 50 years was associated with worse QoL on both the SCI and DLQI.21 Our study also showed younger age to be modestly, but significantly, associated with worse QoL. This result is difficult to compare with those of Rhee et al, as the mean age of our population was 71. However, given the results of both studies, skin-related factors likely become less important to QoL with increasing age. The same study by Rhee et al showed that female sex was predictive of lower QoL on the SCI total score, SCI appearance, and DLQI scales. Our study supports the finding that female gender was associated with the symptoms subscale; however, the power of this finding is low, as our study only enrolled 30 women.
Greater visible photodamage was not related to QoL by our own measures in either univariate or multivariate associations. Additionally, the means of specific Skindex items referring to appearance also scored lower than their respective overall scale means (see Fig 2). On the emotions scale, these were “I am ashamed of my skin condition”, “I am embarrassed by my skin condition”, and “I am humiliated by my skin condition.” KC supplemental items referring to appearance were “I am bothered or upset by scars on my face” and “I am bothered by the way my skin condition affects my appearance.” These findings suggest that this population of elderly male patients is not particularly concerned about physical appearance aspects of ANS.
One study examining QoL in nonmelanoma cervicofacial skin cancer found that all subscales of the Functional Assessment of Cancer Therapy–General (FACT-G) instrument were high compared to historical norms. FACT-G is a validated instrument designed for cancer patients, measuring physical, social/family, emotional, and functional well-being.22 In light of the results of our study, ANS has a demonstrable effect on QoL, although the effects are modest in contrast to inflammatory skin conditions such as acne vulgaris and psoriasis.6
Our study has significant limitations. In particular, the population studied consisted of volunteers for a chemoprevention trial who had at least 2 KCs in the 5 years prior to enrollment. They were all veterans and predominantly Caucasian, male, and elderly. Hence the results may not generalize to other contexts. Furthermore, the counting of AKs is known to be subject to considerable error, despite being counted by experienced, board-certified dermatologists. The history of 5-FU use was obtained by self-report only, without verification by medical records. The assessment of visible photodamage was done with a validated scale, but the raters were of unknown reliability. The strengths of this study include a validated instrument for the assessment of QoL, validation of history of past KCs by examination of medical records, a geographically diverse population, a large sample size, and access to previous medical records used for measurement of comorbidities.
We presume that a history of ever-use of 5-FU served as a proxy for a history of many AKs in the past. Hence from these data we may suggest that AKs, although associated with low or at most modest risk of subsequent squamous cell carcinoma, are at the same time associated with a significant impact on QoL and may be particularly important for the QoL impact of the ANS. Hence, AKs may be deserving of therapeutic intervention on that basis, although the type of therapeutic intervention that will be most helpful in this regard remains to be determined.
We verified that ANS has an impact on QoL. In this group of heavily sun-damaged patients, aspects of ANS other than the skin cancers themselves appear to be the most important determinants of that QoL impact. This finding supports viewing ANS as a chronic illness as opposed to a series of specific cancer episodes and underscores the need to better address concerns about risks of recurrence and spread of actinic neoplasms in this population.
CAPSULE SUMMARY.
Keratinocyte carcinomas (KCs) have high incidence and low mortality.
Quality-of-life (QoL) impacts are critical for KCs.
We studied QoL predictors in 931 high risk patients (97% men).
Actinic keratosis counts and 5-FU history were strongest predictors.
Number of prior KCs was not an independent QoL predictor.
Actinic neoplasia syndrome may best describe these patients.
Acknowledgments
This trial was supported by the VA Cooperative Studies Program (CSP#402), Office of Research and Development, Department of Veterans Affairs. Additional support was received from the American Cancer Society. The study medication was donated by the OrthoNeutrogena division of Ortho-McNeil Pharmaceutical, Inc. Dr Weinstock is also supported by grants R01CA106592, R01CA106807, R25CA087972, and R01AR49342 from the National Institutes of Health. Dr Chren’s work is supported by a Midcareer Investigator Award (K24-AR052667) from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health.
Abbreviations used
- AK
actinic keratoses
- ANS
actinic neoplasia syndrome
- DLQI
Dermatology Life Quality Index
- FACT-G
Functional Assessment of Cancer Therapy–General
- 5-FU
5-fluorouracil
- KC
keratinocyte carcinoma
- QoL
quality of life
- SCI
Skin Cancer Index
- VATTC
Veterans Affairs Topical Tretinoin Chemoprevention (Trial)
Appendix. Key personnel of the VATTC Trial
Study Chairman’s Office: Martin Weinstock, MD (Chair), Kimberly Marcolivio, Providence VAMC
Executive Committee: Martin Weinstock, Providence, RI; Stephen Bingham, Perry Point, MD; John J. DiGiovanna, Providence, RI; Russell Hall, Durham, NC; Mark Naylor, Oklahoma City, OK; J. Richard Taylor, Miami, FL; Julia Vertrees, Albuquerque, NM; Clifton White, Portland, OR
Clinical Centers: Durham VAMC: Russell Hall (primary investigator), Navjeet Sidhu-Malik, Deborah Hannah; Hines VAMC: David Eilers (primary investigator), Tehming Liang, Nadia Sakla, Ann Kreuger; Long Beach VAMC: Gary Cole (primary investigator), Edward Jeffes, Terri Labrador; Miami VAMC: J. Richard Taylor (primary investigator), Robert Kirsner (primary investigator), Jonette E. Kerri, Anna G. Falabella; Margarita Givens; Oklahoma City VAMC: Mark Naylor (primary investigator), Mary Beth Benson, Lisa Perry; Phoenix VAMC: James Kalivas (primary investigator), Catherine Yanni, Selma Targovnik, Janet Austin, Susan Collier
Cooperative Studies Program Coordinating Center, Perry Point, MD: Joseph F. Collins, ScD; Stephen Bingham; Beverly Calvert; Philip Connor; Colleen Crigler; Dawn Davis; Pat Grubb, Judy Kelly; Gail Kirk, Karen Lawson; Linda Linzy, Lorrine Palmer, Maxine Rhoads
Cooperative Studies Program Clinical Research Pharmacy Coordinating Center, Albuquerque, NM: Mike Sather, PhD, FASHP; Erica Copeland; Carol Fye; William Gagne; Patricia Grimes de Naranjo; Chad Messick; Julia Vertrees
Dermatopathologists: Michael Piepkorn, Bellevue, WA; Clifton White, Portland, OR
Data and Safety Monitoring Board: Robert Lew, Boston, MA; Irwin Braverman, New Haven, CT; Bernard Cole, Lebanon, NH; Richard Kalish, Stony Brook, NY; David McLean, Vancouver, BC; Bruce Harris Thiers, Charleston, SC
Footnotes
Conflicts of interest: None declared.
References
- 1.Albert MR, Weinstock MA. Keratinocyte carcinoma. CA Cancer J Clin. 2003;53:292–302. doi: 10.3322/canjclin.53.5.292. [DOI] [PubMed] [Google Scholar]
- 2.Miller DL, Weinstock MA. Nonmelanoma skin cancer in the United States: incidence. J Am Acad Dermatol. 1994;30:774–8. doi: 10.1016/s0190-9622(08)81509-5. [DOI] [PubMed] [Google Scholar]
- 3.Geller AC, Annas GD. Epidemiology of melanoma and non-melanoma skin cancer. Semin Oncol Nurs. 2003;19:2–11. doi: 10.1053/sonu.2003.50000. [DOI] [PubMed] [Google Scholar]
- 4.Lewis KG, Weinstock MA. Nonmelanoma skin cancer mortality (1988–2000): the Rhode Island follow-back study. Arch Dermatol. 2004;140:837–42. doi: 10.1001/archderm.140.7.837. [DOI] [PubMed] [Google Scholar]
- 5.Weinstock MA, Bingham SF, Lew RA, Hall R, Eilers D, Kirsner R, et al. Topical tretinoin therapy and all-cause mortality. Arch Dermatol. 2009;145:18–24. doi: 10.1001/archdermatol.2008.542. [DOI] [PubMed] [Google Scholar]
- 6.Chren MM, Lasek RJ, Flocke SA, Zyzanski SJ. Improved discriminative and evaluative capability of a refined version of Skindex, a quality-of-life instrument for patients with skin diseases. Arch Dermatol. 1997;133:1433–40. [PubMed] [Google Scholar]
- 7.Patrick DL, Deyo RA. Generic and disease-specific measures in assessing health status and quality of life. Med Care. 1989;27:S217–32. doi: 10.1097/00005650-198903001-00018. [DOI] [PubMed] [Google Scholar]
- 8.Clouser MC, Harris RB, Roe DJ, Saboda K, Ranger-Moore J, Duckett L, et al. Risk group, skin lesion history, and sun sensitivity reliability in squamous cell skin cancer progression. Cancer Epidemiol Biomarkers Prev. 2006;15:2292–7. doi: 10.1158/1055-9965.EPI-06-0405. [DOI] [PubMed] [Google Scholar]
- 9.Griffiths CE, Wang TS, Hamilton TA, Voorhees JJ, Ellis CN. A photonumeric scale for the assessment of cutaneous photo-damage. Arch Dermatol. 1992;128:347–51. [PubMed] [Google Scholar]
- 10.Lasek RJ, Chren MM. Acne vulgaris and the quality of life of adult dermatology patients. Arch Dermatol. 1998;134:454–8. doi: 10.1001/archderm.134.4.454. [DOI] [PubMed] [Google Scholar]
- 11.Christian JB, Lapane KL, Hume AL, Eaton CB, Weinstock MA. Association of ACE inhibitors and angiotensin receptor blockers with keratinocyte cancer prevention in the randomized VATTC trial. J Natl Cancer Inst. 2008;100:1223–32. doi: 10.1093/jnci/djn262. [DOI] [PubMed] [Google Scholar]
- 12.Weinstock MA. Assessment of sun sensitivity by questionnaire: validity of items and formulation of a prediction rule. J Clin Epidemiol. 1992;45:547–52. doi: 10.1016/0895-4356(92)90104-u. [DOI] [PubMed] [Google Scholar]
- 13.Blackford S, Roberts D, Salek MS, Finlay A. Basal cell carcinomas cause little handicap. Qual Life Res. 1996;5:191–4. doi: 10.1007/BF00434740. [DOI] [PubMed] [Google Scholar]
- 14.Rhee JS, Loberiza FR, Matthews BA, Neuburg M, Smith TL, Burzynski M. Quality of life assessment in nonmelanoma cervicofacial skin cancer. Laryngoscope. 2003;113:215–20. doi: 10.1097/00005537-200302000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Rhee JS, Matthews BA, Neuburg M, Smith TL, Burzynski M, Nattinger AB. Skin cancer and quality of life: assessment with the Dermatology Life Quality Index. Dermatol Surg. 2004;30:525–9. doi: 10.1111/j.1524-4725.2004.30169.x. [DOI] [PubMed] [Google Scholar]
- 16.Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)–a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19:210–6. doi: 10.1111/j.1365-2230.1994.tb01167.x. [DOI] [PubMed] [Google Scholar]
- 17.Chen SC, Bayoumi AM, Soon SL, Aftergut K, Cruz P, Sexton SA, et al. A catalog of dermatology utilities: a measure of the burden of skin diseases. J Investig Dermatol Symp Proc. 2004;9:160–8. doi: 10.1046/j.1087-0024.2003.09112.x. [DOI] [PubMed] [Google Scholar]
- 18.Lear W, Akeroyd JE, Mittmann N, Murray C. Measurement of utility in nonmelanoma skin cancer. J Cutan Med Surg. 2008;12:102–6. doi: 10.2310/7750.2008.07034. [DOI] [PubMed] [Google Scholar]
- 19.Chen T, Bertenthal D, Sahay A, Sen S, Chren MM. Predictors of skin-related quality of life after treatment of cutaneous basal cell carcinoma and squamous cell carcinoma. Arch Dermatol. 2007;143:1386–92. doi: 10.1001/archderm.143.11.1386. [DOI] [PubMed] [Google Scholar]
- 20.Rhee JS, Matthews BA, Neuburg M, Smith TL, Burzynski M, Nattinger AB. Quality of life and sun-protective behavior in patients with skin cancer. Arch Otolaryngol Head Neck Surg. 2004;130:141–6. doi: 10.1001/archotol.130.2.141. [DOI] [PubMed] [Google Scholar]
- 21.Rhee JS, Matthews BA, Neuburg M, Logan BR, Burzynski M, Nattinger AB. The skin cancer index: clinical responsiveness and predictors of quality of life. Laryngoscope. 2007;117:399–405. doi: 10.1097/MLG.0b013e31802e2d88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11:570–9. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]