Abstract
The “Bystander” and “Good Samaritan” effects involve the transfer of toxic or beneficial compounds from one cell to a generally adjacent other through gap junction channels and through extracellular routes. The variety of injuries in which bystander cell killing or protection occurs has greatly expanded in the last decade to include infectious agents and therapeutic compounds, radiation injury, chaperones in cell therapy and apoptosis in development. This has been accompanied by the appreciation that both gap junction mediated and paracrine routes are used for the signaling of the “kiss of life” and the “kiss of death” and that manipulations of these pathways and the molecules that use them may find therapeutic utility in treatment of a variety of pathological conditions.
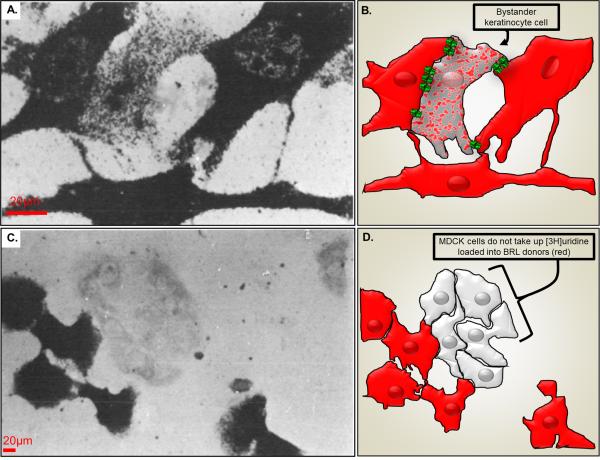
More than thirty years ago, John Pitts and his colleagues published several papers describing the outcome of experiments in which mutant cell lines deficient in the enzyme necessary to incorporate hypoxanthine into nuclear DNA were co-cultured with normal cells. When radio-labeled hypoxanthine was added to the culture and autoradiography used to detect probe distribution, wildtype cells displayed intense labeling, but enzyme deficient cells adjacent to the wildtype cells also showed incorporation of label into DNA, although labeling was less intense (Fig. 1). The interpretation was that radiolabeled hypoxanthine was being transferred from cell to cell and that the enzyme deficient cells were being rescued by their wildtype neighbors (the “Good Samaritans” providing the “kiss of life”). The phenomenon was shown to be specific for pairing of certain cell types (Fig. 1C) and was termed “metabolic cooperation” (for detailed description of these early studies see1). Subsequently, cell killing as a result of transfer of toxic nucleotides was described and termed the “kiss of death”2 (see Fig 2).
Figure 1.
Pioneering metabolic labeling experiments on coupling competent and coupling deficient cells. A.) Uridine nucleotide transfer between dermal fibroblast donors and epidermal keratinocyte recipients. Donor cell cultures were labeled with [3H]uridine and the autoradiograph was exposed for 3 weeks prior to developing. B.) Illustration showing keratinocyte (spotted cell) that has received [3H]uridine from the pre-loaded donor dermal fibroblast (red) likely through gap junctions (green). C.) Lack of uridine nucleotide transfer between BRL cells and MDCK cells. D.) Illustration of cell location where a lack of gap junction coupling leads to no [3H]uridine transfer from pre-loaded donor BRL cells (red) and MDCK cells (light gray). Modified from Hunter and Pitts97.
Figure 2.
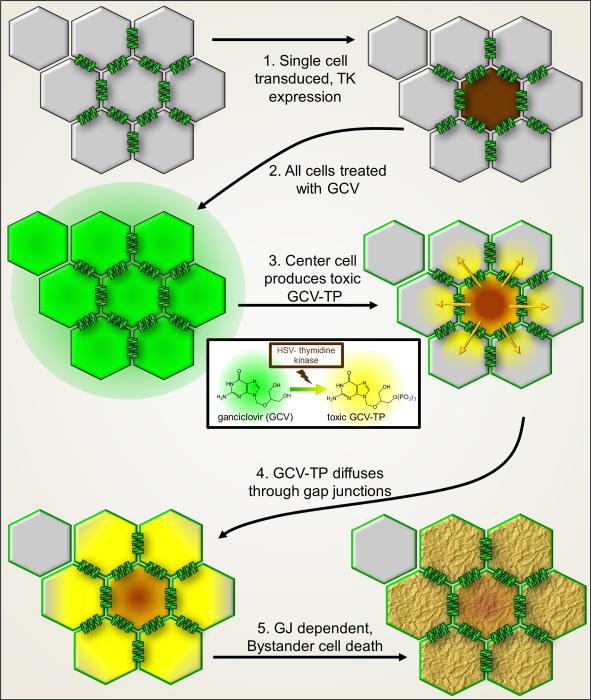
Schematic diagram of Bystander cell killing as therapy. Cells interconnected by gap junction channels (one cell is not coupled by gap junctions). 1. Only one of eight cells is transduced by a virus with genetic code for the Herpes simplex virus thymidine kinase (HSV-tk), which induces expression in only that single cell (brown). 2. Treatment of the cell population with membrane permeant ganciclovir (GCV). 3. HSV-tk in the center cell converts GCV to the toxic phosphorylated and membrane impermeant GCV-triphosphate (GCV-TP, yellow color). 4. GCV-TP, with Mr 495 Da, is gap junction permeant and diffuses to neighboring cells. 5. Bystanders cells coupled to the center cell receive GCV-TP which intercalates into DNA, terminating synthesis and resulting in Bystander cell death.
Both “Good Samaritan” and “kiss of death” functions are two outcomes of Bystander phenomena, where cells in contact or nearby are protected or sensitized as a consequence of proximity. Intercellular communication of Bystander effects can be mediated by two major mechanisms that are quite distinct: Paracrine signaling through secreted molecules or exosomes and direct diffusion of relatively small molecules through gap junction channels. [While other types of direct cytoplasmic contact have been claimed in certain cell types, such as cell fusion and tunneling nanotubes3, they are not considered in detail here]. Numerous types of Bystander responses have now been reported, including radiation effects, where non-irradiated cells receive signals from neighboring or distant cells following exposure to ionizing radiation4, wound healing5, spread of dsDNA6, protective or nurturing effects in stem cell therapy, extension of range of tissue invasion by infectious agents, neuro- and cardio- protection and spread of apoptosis, and exploitation of Bystander killing in tumor therapy. In addition to roles in cancerous and infected cells, the Bystander Effect may also play important roles in certain tissues under normal conditions7. The goal of this review is to summarize the somewhat controversial evidence emerging from numerous studies indicating that gap junctions may either have a protective effect in cell survival or may provide a conduit by which death may spread.
The players in intercellular signaling
Gap junction channels are distinct from other channel types in at least three major ways: Their proteins are encoded by unique gene families in vertebrates and invertebrates, they form intercellular channels between pairs of adjacent cells and the channel pore is large, allowing flux of small molecules as well as ions. Each of these properties has important implications for the spread of signals from cell to cell and each is considered in more detail below.
Proteins encoding vertebrate gap junction channels are the connexins, named according to the molecular weight (Mr) of the protein (in kDa) predicted by cDNAs encoding them; the major gap junction protein of neurons, encoded by a cDNA predicting a 36kDa protein, is thus Cx36. There are about twenty connexin genes in mammalian genomes, with high homologies among orthologues connecting the same cell types (for example, Cx35 and Cx34.7, found between neurons in fish brain, are highly homologous to Cx36 expressed in mammalian neurons). Invertebrate gap junction proteins are encoded by proteins termed innexins, where proteins generally follow a different nomenclature (InxX, where X is an integer denoting the order of discovery); highest homologies are within Classes, so that all Caenorhabditis innexins are more similar to each other than to any Drosophila innexins. A search of mammalian gene sequence databases with innexin sequences revealed a group of weakly homologous cDNAs encoding proteins termed pannexins8; there are three pannexins (Panx1-3) in genomes of most vertebrates, and alternate splicing may generate even more proteins. Connexins, innexins and pannexins are all four transmembrane proteins; however, there is no sequence homology between connexins and the other two gene families, which are only distant relatives of each other. Innexin sequences incorporated into DNA viruses of parasitic wasps (termed vinnexins9) represent a subgroup of innexins.
Gap junction channels extend from the cytoplasmic domains of the gap junction proteins (both N- and C-termini as well as the domain linking transmembrane domains 2 and 3) across extracellular space and then into the cytoplasm of the adjacent cell. Cysteine residues in the extracellular loops of individual connexins are believed to provide β barrel structure for the hexameric connexon contributed by each cell, the tight electrostatic bonds between residues aligned in part by the paired cysteine residues seal the connexons to each other so tightly that there is no ionic leakage. It is widely believed that unpaired connexons may be functional under either physiological or pathological conditions and for some connexins, notably those of the lens, there is clear evidence for nonjunctional channel formation as well as gap junction channels. However, for other connexins such as Cx43, this remains the subject of debate, since other molecules such as pannexin1 (Panx1) can perform this function10. Moreover, pharmacological dissection of underlying pathways are problematic, because most drugs that block connexin channels inhibit pannexin channels at even lower concentrations11. A few reports indicate than pannexins may form intercellular channels at very low incidence under conditions of overexpression12,13, but the possible presence of connexin channels in these studies was not rigorously excluded, and most studies have not detected gap junction channels formed by Panx114. By contrast, it is clear that Panx1 forms nonjunctional channels in many cell types; these channels are responsible for numerous cell functions, principally those requiring flux of moderately large molecules, such as ATP, into or out of the cell.
The pore diameter of the gap junction channel is large enough to allow intercellular diffusion of current carrying ions, many metabolites, and second messenger molecules. Most relevant for the current carrying capacity necessary for electrotonic coupling are potassium and, to some extent, chloride; in some cases, electrically driven sodium flux may occur, with generally pathological consequences15. Second messenger molecules that are gap junction permeant include cyclic adenosine monophosphate (cAMP; see Fig. 3), cyclic guanoside monophosphate (GMP), calcium, inositol trisphosphate (IP3; see Fig. 4), diacylglycerol (DAG), and other molecules with Mr less than 1000 Da. Metabolites that are gap junction permeant include all amino acids and essential compounds in metabolic pathways including most pentose and hexose sugars and their phosphates. A period of controversy rattled the field in the 70's, when some authors suggested that large molecules (including enzymes, RNA and even organelles) could be transferred through gap junctions while others maintained that gap junction channels had a strict permeability limit of about 1 kDa16,17. This smaller size limit prevailed in studies with fluorescent tracers of various diameters17,18. Reports of exchange of much larger molecules are likely the result of fixation artifact, or could potentially occur as a consequence of pinocytosis or of long term junctional turnover. Since coupled cells sip each other's cytoplasm through incorporation of double walled vesicles (termed the “connexosome” by Dale Laird19), the possibility for exchange of large molecules by this route needs to be inspected carefully. Nevertheless, the possibility that miRNA may cross gap junctions was introduced from studies showing transfer (albeit very slow) of siRNA between transfected cells20. Junctional transfer of miRNA is becoming widely accepted21, even though in most cases the alternative routes of connexosome or tunneling nanotube transfer or the release and uptake of vesicles containing the miRNA (exosomes) have not been conclusively ruled out.
Figure 3.
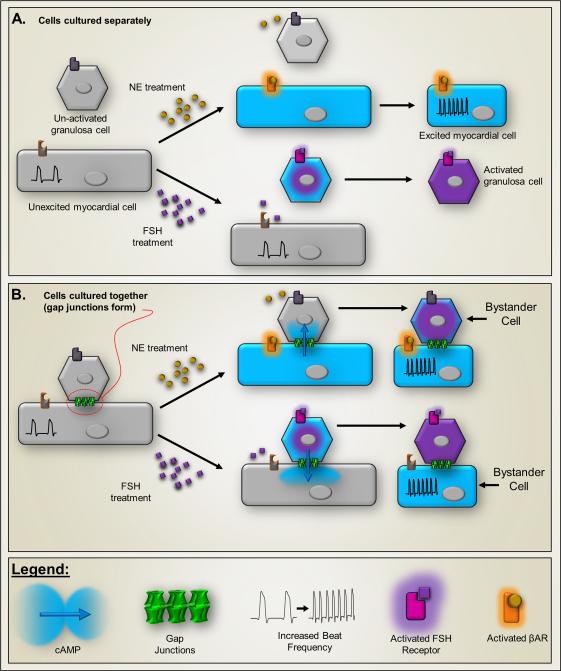
Intercellular second messenger signaling evidenced in co-culture of mouse myocardial cells with rat ovarian granulosa cells. Myocardial cells respond to adrenergic agonists (βAR) with increased beat frequency (insets), whereas (B) granulosa cells produce plasminogen activator when stimulated by follicle stimulating hormone (FSH). In both cases, the second messenger is likely cAMP, which is elevated in the stimulated cell (illustrated by blue color). A.) Because the cell types express different receptors, when cultured alone neither responds to the other's agonist. B.) When cultured together myocardial and granulosa cells form gap junctions (green structures). Co-culture produces a Bystander effect in which addition of either norepinephrine or FSH to the co-cultures results in cross-stimulation, evoking changes in both cell types. This presumably results from cell-to-cell transfer of a second messenger, which is likely to be cyclic AMP (MW 329). Illustration represents experiments described in 22.
Fig. 4.
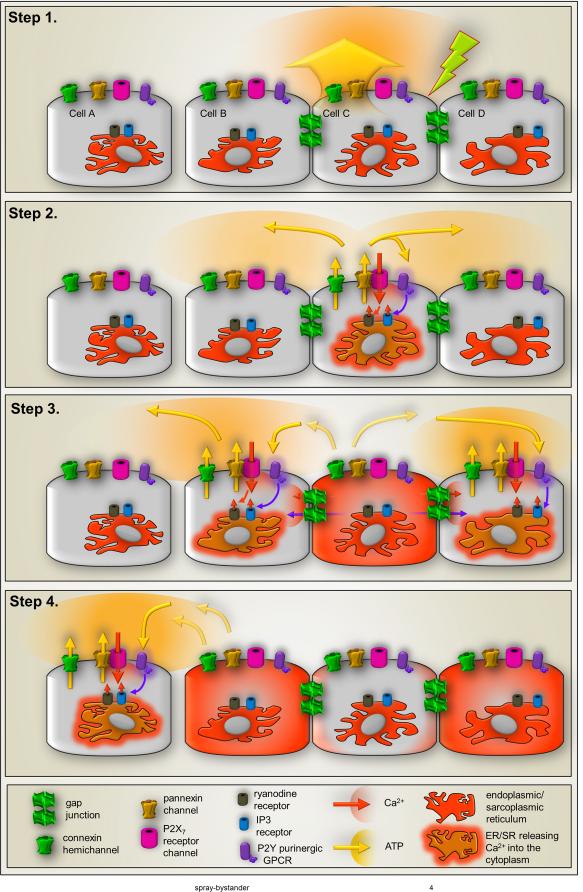
Components of intercellular calcium waves. Schematic representation of steps involved in transmission of intercellular Ca2+ waves through gap junctions and through ATP release. Step 1: Mechanical, electrical or pharmacological stimulation (green arrow) of one cell leads to Ca2+ entry through ion channels and ATP release (yellow arrow) that can occur through vesicular and non-vesicular mechanisms that involve transporters and channels such as pannexin1 channels and connexin hemichannels. Step 2: Autocrine activation of cell surface metabotropic P2Y (purple) and ionotropic P2X (magenta) receptors by released ATP induces increase in intracellular Ca2+ levels of the stimulated cell by Ca2+ influx (orange arrow) through P2XRs and Ca2+ mobilization from intracellular stores triggered by activation of IP3 receptors (blue cylinder) on endoplasmic/sarcoplasmic reticulum mediated by P2YR-generated IP3 (purple arrow) and Ca2+-induced Ca2+ release (small orange arrow) mediated by activation of ryanodine receptors (brown cylinder). Besides contributing to ATP-mediated Ca2+ influx, activation of the P2X7R subtype (magenta) also provide a mechanism for ATP-induced ATP release through its interaction and activation of pannexin1 channels (yellow. Opening of connexin hemichannels (green on cell surface) have also been proposed to contribute a pathway for ATP release from stimulated cells. Step 3: The Ca2+ signal generated in the stimulated cell spreads to adjacent cells when Ca2+ itself and the second messenger IP3 pass though gap junctions (green channels between cells), thereby inducing Ca2+ release from Ca2+ and IP3 sensitive intracellular stores (see Step 2), and when ATP activates P2 receptors in the adjacent cells (as in Step 2). Besides contributing to increase in intracellular Ca2+ levels of neighboring cells, paracrine activation of P2 receptors can induce further ATP release in the path of the Ca2+ wave spread through P2X7R-mediated activation of pannexin1 channels (as in Step 2). Step 4: Cells that are not coupled by gap junctions can be recruited into the Ca2+ wave spread and the Ca2+ signal can “jump” cell free spaces when released ATP diffuses and activates P2 receptors in these cells, triggering the events described in steps 2-3. Similar mechanisms contribute to long range signal spread.
Coupling is good
The earliest evidence that second messenger signaling could occur through gap junctions came from studies in which the laboratories of Bernie Gilula and Bill Beers cocultured cardiac myocytes with rat ovarian cumulus cells and then treated cells with agonists specifically elevating cAMP in each cell type22. Both cell types responded to each treatment, indicating that a second messenger, likely cAMP, diffused between the cells (Fig. 3; 22). Subsequent studies have confirmed gap junction mediated cAMP exchange between cell pairs, as evaluated by a variety of methods, most recently using as readout the transcellular activation of cAMP sensitive currents23.
The most abundant literature dealing with gap junction mediated second messenger exchange comes from studies of Ca2+ wave spread in many types of cells in culture, in tissue slice and in vivo. After the initial demonstration that both Ca2+ and IP3 could diffuse between coupled pairs of hepatocytes24, numerous authors have used Ca2+ sensitive fluorophores to show that mechanical, electrical or pharmacological stimulation of one cell in culture, slice or even in tissue would lead to spread of Ca2+ elevations in adjacent cells. These intercellular Ca2+ elevations spread at a velocity of about 10-20 µm/sec, with the extent of spread being modeled as a threshold for evocation of Ca2+- or IP3-induced Ca2+ release initiated and maintained by Ca2+ and the IP3 elevation caused by the original stimulus (see Fig. 4; 25). It has been interesting to observe that while Ca2+ wave spread is generally sensitive to gap junction blockers, there is usually a prominent extracellular component of transmission that involves ATP release from stimulated cells and activation of purinergic (P2) receptors on adjacent cells (Fig. 4). This ATP release pathway has been attributed by some to Cx43 hemichannels26, but Panx1 has generally not been excluded and is favored for such a role by a growing number of investigators27.
The laboratories of Gerry Dienel and Christian Giaume have both reported that delivery of metabolic compounds through gap junctions can be regulated by need 28,29; see 30 for commentary; astrocytes recently being termed “good scouts” in this regard31. A surprising finding from these studies, with unexplored significance, is that certain sugars are more gap junction permeant than others.
Bystander innocence
Suicidal genes are those whose exogenous expression kills the transfected/transduced cell; a widely used suicide gene is herpes virus thymidine kinase. Interest in the original findings of metabolic cooperation1 was re-ignited with the discovery that suicide gene therapy could be used to kill not only the cells expressing the suicide gene, but also spread to those in direct contact; thus, transduction of a few as 10-50% of tumor cells could result in virtually total tumor cell killing32. This was an exciting finding of possible translational relevance, since it offered the potential to dramatically increase the efficacy of therapy within a tumor. Clinical trials for treating brain tumors involve the use of recombinant retroviruses bearing a herpes simplex virus thymidine kinase (HSV tk) gene. The strategy is to infect tumor cells and thereby sensitize them to the drug ganciclovir, which was developed to treat herpes viral infection (Fig. 2). Ganciclovir is a guanosine analog that is metabolized to a toxic product by HSV thymidine kinase but not by the thymidine kinase of the mammalian host. The phosphorylated 3’ dehydroxy product is incorporated into nascent DNA chains of proliferating cells and causes cell death by chain termination33.
Ganciclovir is cell permeable but its phosphorylated product is not. However, phosphorylated ganciclovir has a molecular weight below 300 Da and thus passes readily between cells though gap junctions. Evidence for contact dependence comes from studies mixing tagged cell types34 and from use of transfected cells, where gap junction expression was shown to correlate precisely with the transfer of cell death 35-37, see 38 for review. It should be noted that Bystander killing by other thymidine constructs may be gap junction independent; the mechanism of action for an alternative suicide gene therapy model involving thymidine phosphorylase-5’-deoxy-5-fluorouridine has been reported to involve secretion of the toxic molecule and uptake by neighbors39.
Recent adaptations of the method include introduction of HSV tk under the control of a stem cell reporter, so that the potentially tumor-producing cells can be destroyed by activation of a controllable suicide gene40 and chemical modifications of HSV tk to enhance both its direct potency on transduced cells and probability of successfully killing all untransfected Bystanders (see 41).
There is evidence that a naturally occurring Bystander Effect may occur in liver, where gap junction blockers may act as hepatoprotectants, minimizing liver damage from such drugs as thioacetamide (TAA) and acetaminophen6. In this study, a single TAA dose that led to marked liver damage and mortality in wildtype mice caused substantially less liver damage and death in Cx32 null mice. Using a high throughput screen for small molecules conferring protection from Bystander cell killing, the authors found 2-APB (2-Aminoethyl diphenylborinate), which was shown to be more effective when applied to Cx32 than Cx43 containing gap junctions. This drug was also found to be highly hepatoprotective, even when given six hours following acetaminophen. The drug 2-APB was initially identified42 as a gap junction channel blocker and subsequently demonstrated43 to display a high degree of selectivity for gap junctions formed by some connexins over those formed by others.
Contagious Bystanders
In cardiac myocytes and in mouse hearts infected with T. cruzi, the protozoan parasite that is the causative agent in Chagas disease, cardiac function is globally compromised; however, the altered expression of Cx43 and its gene are limited in both extent and location (for review, see44). This finding suggests that the general deterioration in cardiac function seen in this disease may result in part from spread of damage signals from more seriously compromised cells to healthier ones.
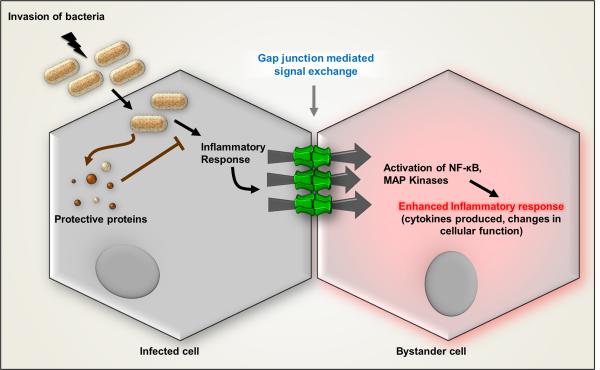
An excellent example of one type of Bystander transfer of injurious material has come from cellular level studies of infection by the enterobacteria Shigella, Lysteria, and Salmonella (Fig. 5; 45). In these studies, primarily focused on Shigella, the authors examined monolayer cultures of an intestinal epithelial cell line. Shigella invades intestinal endothelial cells after crossing the intestinal epithelium and enters the basolateral surface. Shigella is recognized by a pattern recognition receptor activated by a bacterial peptide glycan. This triggers a series of signaling events ultimately leading to massive interleukin (IL-8) secretion. What is most interesting about this signaling pathway is that its activation in secondarily stimulated cells is more severe than in the infected one, as a result of modulation of response in the invaded cell. The authors term the spread “propagation” although the extent to which it is regenerative is not explored.
Fig. 5.
Contagious Bystander. Bacterial infection generates proinflammatory signals in the infected cells that spread to the uninfected Bystander cells through gap junction channels to amplify the innate immune response. NF-kB and MAP kinases are activated in uninfected Bystanders to produce IL-8 following infection of adjacent cells. Modified from schematic diagram in Kasper et al.45.
Demonstration that gap junctions are involved in this “propagation” phenomenon included the absence of paracrine signaling and quantification of images showing IL-8 secretion only in cells directly in contact with the infected ones. Moreover, IL-8 secretion by Bystanders was inhibited by the gap junction channel blocker 18-βGA (found effective at 2 and 5 μM, concentrations that are disturbingly much lower than known to block gap junctions) and depended upon the expression of Cx43. A role of Cx43 hemichannels was ruled out by the requirement that both adjacent cells must express Cx43. Thus, it was concluded that Shigella infection leads to pro-inflammatory pathway activation (including NF-kb, JNK, ERK, and p38) that spreads through gap junctions from infected to uninfected cells leading to IL-8 expression in Bystanders. This mechanism amplifies IL-8 response of the monolayer by increasing the number of IL-8 producing cells per infection site, attracting neutrophils to the infected area and thereby contributing to innate immunity.
A different set of experiments also implicated gap junction proteins in Shigella invasion. Here, authors showed that expression of Cx26 in individual cells greatly facilitated Shigella penetration46, and the enhancement was attributed to hemichannel activity. Whether this might be the result of leakage of a chemoattractive molecule from Cx26 expressing cells or whether hemichannels may provide co-receptors for invasion (as occurs with Herpes virus and tight junction proteins47) is unexplored.
Another example of contagious Bystander Effect is observed in HIV infection. In an in vitro model for neuroAIDS, a small percentage of HIV-infected astrocytes (less than 5%) that showed Cx43 upregulation was able to amplify and spread toxic signals to uninfected cells via gap junctions48. The astrocytic kiss of death was later demonstrated to spread to endothelial cells in an in vitro model of the blood brain barrier (BBB) that consisted of a co-culture of endothelial cells and astrocytes grown on opposite sides of a porous membrane49. A small percentage of HIV-infected astrocytes was able to induce endothelial apoptosis and disrupt barrier function as shown by increased albumin permeability. This effect was significantly attenuated by the gap junction blockers 18-αGA and carbenoxolone. The alternative hypothesis, that the spread of toxic signals occurred via soluble factors, was discarded after incubating the BBB model with cell-free virus and supernatants of HIV-infected astrocyte cultures, without detecting changes in BBB permeability. In addition, signaling pathways concentrated at the astrocyte endfeet that are involved in controlling vascular tone appeared relevant to barrier disruption, because blocking the activation of lipoxygenase and cyclooxygenase pathways as well as the calcium activated potassium channels was protective against the BBB disruption caused by the few HIV-infected astrocytes. However, nitric oxide signaling did not contribute to BBB disruption in this model.
Calcium dysregulation may be involved in increased BBB permeability which is a hallmark of neuroAIDS and other neurodegenerative diseases. It has been established that endothelial permeability is regulated by extracellular calcium via calcium dependent VE-cadherin interactions with the junctional complexes50 as well as by changes in intracellular calcium and intercellular calcium waves51. The Bystander effect in endothelium was manifested in observations where hyperpermeability induced by extracellular Ca2+-free solution was significantly counteracted by buffering intracellular calcium changes with BAPTA-AM and by the connexin mimetic peptide Gap27. Low extracellular calcium triggers the release of ATP that diffuses and activates P2Y receptors on neighbor cells and generates IP3 that triggers calcium release from the endoplasmic reticulum. IP3 passing via gap junctions contributes synergistically to the Ca2+ wave propagation52. A future target to counteract BBB disruption therefore might be based in regulating calcium signaling and cell-cell communication.
Radiation-induced injury
Ionizing irradiation exerts genetic and biochemical effects to induce apoptosis or trigger DNA repair not only in the irradiated cells but in non-irradiated cells nearby, a potentially serious consequence of radiation exposure during diagnostic procedures such as X ray and CT and cancer radiotherapy. It was originally thought that the major health concerns were limited to DNA damage, potentially leading to cancer and other disturbances of cell function and homeostasis. However, this dogma was challenged by observations that similar effects could be seen in adjacent normal non-irradiated cells, another instance of the Bystander Effect. Two possible routes have been explored: Secreted water soluble factors and direct intercellular communication via gap junctions53-55. Studies of mechanisms responsible for radiation-induced Bystander cell killing have generally used two distinct paradigms: a) direct observation of cell cultures after spatially localized exposure to high or low radiation levels, and b) application of medium from exposed cultures to non-irradiated cells. In the latter scenario, water soluble molecules are believed to either act locally as paracrine signals or even exert effects at very long distances, as by hormones.
Changes occurring both in the irradiated cells and in the Bystanders can include induced chromosomal abnormalities, genomic instability, changes in protein expression and mutations, all of which may lead to malignant transformation55,56. Tissue culture medium from human keratinocytes that had been exposed to 0.5 or 5 Gy irradiation was found to increase Ca2+ levels, decrease mitochondrial potential and increase reactive oxygen species in non-irradiated cells57. Even lower doses of 60Co gamma irradiation increased apoptotic cell death in non-irradiated cells58,59.
While Bystander spread of cell death is a major concern following irradiation, increased proliferation and the potential uncontrolled growth that may ultimately result are also of note. In the case of the loss of growth control, it is noteworthy that cell contact has been proposed to be more important than either gap junction mediated signal spread or release of soluble factors60.
Soluble factors that have been implicated in radiation-induced Bystander effects include protein kinase C (PKC) isoforms61, and transforming growth factor beta62 which are generally believed to arise from the generation of reactive oxygen species (ROS) such as superoxide radicals. Such clastogenic factors appear in blood plasma from patients exposed to ionizing radiation causing chromosomal alterations in non-irradiated patient blood in coculture63. Additionally, irradiated cells have been shown to release ATP, providing an extracellular route for this phenomenon64, and Cx43 hemichannels have been proposed for this function65.
Chaperones and network integration in cell therapy
The importance of gap junction coupling in achieving stem cell integration into cardiac tissue is summarized in a recent review66. One critical lesson from the earliest stem cell transplantation studies was that skeletal muscle is a poor choice for cardiac engraftment; because skeletal muscle lacks gap junctions, these cells establish an electrical barrier that is highly arrhythmogenic. Ideally, stem cells should incorporate within the tissue, establishing gap junction contact and seamless current flow, thereby creating an anti-arrhythmogenic substrate. With regard to the nervous system, evidence has been provided that gap junctions play a critical role in the integration of neural stem cells into both slice cultures and host tissue and that gap junctions also are critical for the neuroprotection resulting from cell stem implantation in neurodegenerative disease67. In these cases, the relevant gap junction protein appeared to be Cx43, whose expression was initially high in implanted cells, then declined over time, offering a window of optimal incorporation of the implanted cells. Among the noteworthy aspects of this study are that the P2 receptor antagonist suramin did not affect Ca2+ wave transmission between neural stem cells and host cells, implying that the major route was gap junction mediated, and that the protective action in two different types of Purkinje cell neurodegeneration models implies gap junction involvement at late stages in these diseases.
The unfairness of sharing: Gap junctions may extend apoptosis beyond the initial lesion
The role of gap junctions in cellular response to tissue injury has been studied extensively, with authors adopting either of two opposing viewpoints 68,69. The first is that a cell accumulating toxic metabolites in response to a pathological alteration could spread cytotoxicity to adjacent cells through open gap junction channels. On the other hand, the enormous effective volume provided by a network of coupled cells would be expected to optimize buffering of the toxic molecules, reducing concentrations to nontoxic levels. Evidence for changes in gap junction communication includes dephosphorylation of Cx43 in response to hypoxia in both brain tissue and astrocyte cultures, whereas expression of Cx32 and Cx36 were reported to be increased while Cx43 was decreased in ischemic brain injury70. In inflamed brain, Cx43 expression and dye coupling were reduced, and expression of certain proteins that are components of tight junctions was induced71. Traumatic nerve injury upregulates Cx43 in the facial nucleus72 and in both dorsal root and trigeminal ganglia, where coupling is also increased73,74. Changes in both connexin expression and coupling have been reported following ischemic or impact injury to organotypic hippocampal cultures, where initial increases in coupling reverse, declining during the re-oxygeneration75.
A number of studies report that gap junctions are neuroprotective. For example, Cx43 heterozygotes (in which Cx43 expression is reduced by half) show larger ischemic infarcts, gap junction blockade increased excitotoxicity in dissociated culture76 and Cx32 knockouts showed enhanced neuronal vulnerability in response to global brain ischemia70. Kozoriz et al77 reported that it is the carboxyl terminal domain of Cx43 that mediates this neuroprotection during stroke. Middle cerebral artery occlusion in mice in which Cx43 was truncated at residue 258 (M258 mutants) showed increased infarct volume, astrogliosis and inflammatory cell invasion at 4 days after injury. Cx43 hemichannel activity was implicated in this phenomenon as inferred from carbenoxolone-inhibitable propidium iodide uptake and Ca2+ wave studies in astrocytes cultured from the M258 mice. A similar protective effect of the Cx43 carboxyl terminus was demonstrated in cardiac ischemia using M258 and Cx43 heterozygotes78.
Studies showing that gap junctions are neurodestructive include the finding that the gap junction blocker octanol decreased neuronal death in a global cerebral ischemia model79, and that decreased coupling reduces vulnerability to hypoxic and traumatic injury in slices. Many other studies have indicated that cell killing can be spread through gap junctions. For example, octanol and heptanol were shown to reduce infarct volume and myocardial fibrosis in ischemia80,81 and halothane reduced injury from focal brain ischemia82. Frantseva et al83 showed that carbenoxolone, heptanol, and antisense knockdown of Cx26, Cx32, Cx43, all reduced hypoxia-induced cell death in slice cultures. In response to weight drop, Lin and Takemoto84 showed that expression of mutants in a PKC isoform responsible for spinocerebellar ataxia type 14 (SCA14) exerted a dominant effect on native PKC in a neuronal cell line, thereby opening gap junction channels and extending cell death. This affect was rescued by a decoy peptide.
Garcia Dorado et al85 showed in cardiac ischemia reperfusion that gap junction coupling synchronized ischemia-induced contractions and spread ischemic cell death. However, Ayer et al86 found no attenuation of neurological deficits with octanol or Cx43 delivered subcutaneously in a subarachnoid hemorrhage model. De Pina Benabou et al87 found that oxygen/glucose deprivation (OGD) increased gap junction coupling and caspase 3 activation in hippocampal slice cultures, and treatment with the gap junction blocker carbenoxolone before, during or 60 minutes after OGD reduced delayed neuronal death. Here, carbenoxolone was shown to be neuroprotective when administered to ischemic rat pups immediately after intrauterine hypoxia, preventing caspase 3 activation and decreasing long term neuronal death.
Evidence that gap junctions may be involved in transmitting apoptotic cell death in vivo was demonstrated in studies showing that during retinal development, dying cells in ganglion and inner nuclear layers occurred in clusters and that this clustering was reduced by treatment of animals with carbenoxolone (88see Fig. 6). Moreover, scrape loading of retinas with cytochrome c led to cleavage of caspase 3 and spread of damage to coupled cells that was blocked by carbenoxolone and heptanol. We later showed that gap junctions remain open during the spread of death signal from one cell to another (Fig. 7; 89), a finding that was also demonstrated between retinal photoreceptors in retinitis pigmentosis90 and first shown in dying astrocytes91. Spread of apoptotic signals was also demonstrated in Cx32 transfected BHK cells92.
Fig. 6.
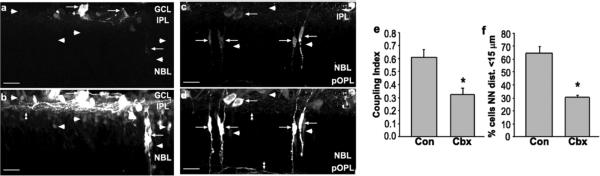
Bystander cell death in the developing retina. a, b, Z-series projection (20 μm) of retinal sections from P5 mouse injected with vehicle and scrape-loaded with RD (rhodamine dextran) and NB (neurobiotin). a, RD labels cells in ganglion cell layer (GCL) and neuroblast layer (NBL, arrows). b, NB (reacted with Cy2-streptavidin) is localized in cells that were scrape-loaded with RD (arrows) and other cells not loaded with RD (arrowheads). Many processes in the inner plexiform layer (IPL) were labeled with NB (double arrowhead). Arrowheads in a indicate position of coupled cells in b, c, d, Carbenoxolone treatment decreases spread of NB. c, RD labeling (arrows) of scrape-loaded retina from animal treated with the gap junction blocker carbenoxolone (20 mg/kg, s.c.). d, NB labeling of same field shows only a few NB-labeled cells that do not colocalize with RD (arrowheads). Processes in IPL and pOPL are indicated by double arrowheads. Scale bars in a-d, 20 μm. e, Coupling Index for control (Con) and carbenoxolone (CBX) indicates a significant decrease in dye coupling by carbenoxolone treatment (asterisks indicate groups significantly different from controls; Student's t test, p < 0.01). f, carbenoxolone reduces the percentage of dying cells with a NN distance shorter than 15 μm in a manner that closely parallels the decrease in the Coupling Index. From Cusato et al,88.
Fig. 7.
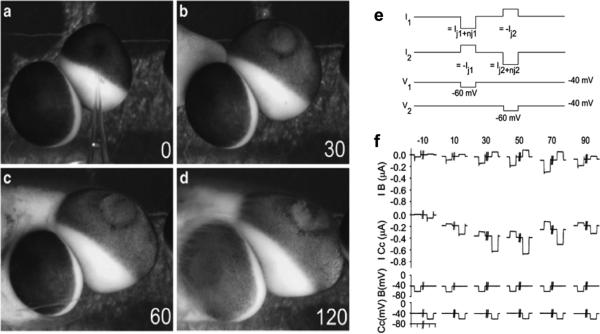
Cytochrome c (cyC) injection causes primary and Bystander cell death in Xeonpus oocytes. (a–d) Time lapse photographs of electrically coupled oocytes following injection of cyC into the cell at the upper right. (a) At the time of injection, both cells have normal pigmentation and morphology. (b) At 30 min after introducing cyC, the injected cell shows a loss of pigmentation at the animal pole and has begun to swell. (c) At 60 min post-cyC, the injected cell continues to swell and becomes somewhat distorted. (d) At 120 min post-cyC, the injected cell has lost structural integrity and the uninjected ‘Bystander’cell has begun to lose pigmentation and swell. Gap junctional coupling measurements. (e) Protocol for dual cell two-electrode voltage clamp studies. Cells were held at -40 mV and stepped to -60 mV for 1 s. The downward deflection of the stepped cell is the total current (Gtotal), which is the sum of the junctional current (Gj) and nonjunctional current (Gnj). The upward deflection in the follower cell is the current that has passed from one cell to the other via gap junctions (Gj). (f) Current recordings from a pair of cells in which one cell was injected with cyC at t=0 min. The Gj and Gnj in the cyC-injected cell (I cyC) increase as the cell dies. The Bystander cell also undergoes an increase in Gj equal to that of the cyC-injected cell (I B), but the increase in Gnj is less pronounced. Bottom two traces show the timing of the voltage pulses to each cell, which were 1 s in duration and 10 s apart. From Cusato et al.89.
Cx43 expression in GFAP positive glia within the mouse spinal cord is a major determinant of the degree of injury spread and functional recovery after crush injury as demonstrated by Huang and colleagues93. This work also showed that ATP release after spinal injury was greatly reduced when Cx43 expression was eliminated from most glia near the injury site. Huang et al, proposed several possible, non-mutually exclusive bystander pathways through which glial Cx43 could lead to spreading injury and reduced neuronal function around the spinal cord injury site. These include the avenues for direct ATP release illustrated in Figure 4 such as secondary activation of Panx1 channels and/or through Cx43 hemichannels. Glial Cx43 might also mediate these effects over longer timescales through bystander activities such as second messenger transfer and altered cytokine release. Further research in this area is needed to reveal which of these bystander pathways is dominant and the order and time scales over which the processes act. In these examples, improvements to treatment of human disease or injuries might be realized by preventing the bystander “kiss of death” though blockade of Cx43 activities.
Conclusions: Therapeutic possibilities
In this review, we have summarized a number of tissue types and injuries where gap junction dependent or independent Bystander cell killing or survival has been proposed. Ultimately, the assignment of mechanism of action depends on the final active molecule, and that appears to differ in various tissues and injuries. For example, ATP is likely a major player in Bystander cell death in brain, where release through Panx1 or Cx43 hemichannels provides the stimulus for excitotoxic neuronal loss94. And whether such a stimulus is destructive depends in part on whether cells are adequately coupled to provide volume buffering of toxic molecules. Good things such as second messengers and necessary metabolites also pass through gap junctions, and this supportive function may provide a role for delivery of supporting and chaperone molecules. Metabolic cooperation can save cells in culture but whether gap junctions may function as drug delivery devices has not been adequately explored.
With regard to well tolerated drugs with which to examine whether gap junctions are neurodestructive or neuroprotective, the licorice derivative, carbenoxolone in particular has been a favorite for in vivo experiments in which gap junctions are chronically blocked because it is well tolerated at even high doses. However, as reported by Leshchenko et al,95, carbenoxolone does not cross the blood brain barrier and therefore its action on the nervous system must be confined to the periphery. It should be noted that studies intending to use carbenoxolone as a mechanism to inhibit hydroxysteroid dehydrogenase have reported worsened outcome of cerebral ischemia, which is attributed to remodeling of the middle cerebral artery96.
Acknowledgments
Research in the authors’ laboratories is currently supported by RO1 grants HL094889 (DCS), AR057139 (DCS, MMT), T32 HL007675 (SVL-Q), 5T32NS007439 (RJS), DK081435 (SOS, MMT) and DK091466 (MMT, SOS)
References
- 1.Pitts JD. The discovery of metabolic co-operation. Bioessays. 1998;20:1047–51. doi: 10.1002/(SICI)1521-1878(199812)20:12<1047::AID-BIES11>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 2.Fujimoto WY, Subak-Sharpe JH, Seegmiller JE. Hypoxanthine-guanine phosphoribosyltransferase deficiency: chemical agents selective for mutant or normal cultured fibroblasts in mixed and heterozygote cultures. Proc Natl Acad Sci U S A. 1971;68:1516–9. doi: 10.1073/pnas.68.7.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kimura S, Hase K, Ohno H. Tunneling nanotubes: emerging view of their molecular components and formation mechanisms. Experimental cell research. 2012;318:1699–706. doi: 10.1016/j.yexcr.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 4.Hamada N, Maeda M, Otsuka K, Tomita M. Signaling pathways underpinning the manifestations of ionizing radiation-induced bystander effects. Curr Mol Pharmacol. 2011;4:79–95. doi: 10.2174/1874467211104020079. [DOI] [PubMed] [Google Scholar]
- 5.Yang L, Cranson D, Trinkaus-Randall V. Cellular injury induces activation of MAPK via P2Y receptors. J Cell Biochem. 2004;91:938–50. doi: 10.1002/jcb.10774. [DOI] [PubMed] [Google Scholar]
- 6.Patel SJ, King KR, Casali M, Yarmush ML. DNA-triggered innate immune responses are propagated by gap junction communication. Proc Natl Acad Sci U S A. 2009;106:12867–72. doi: 10.1073/pnas.0809292106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klee P, Allagnat F, Pontes H, Cederroth M, Charollais A, Caille D, Britan A, Haefliger JA, Meda P. Connexins protect mouse pancreatic beta cells against apoptosis. The Journal of clinical investigation. 2011;121:4870–9. doi: 10.1172/JCI40509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Panchin YV. Evolution of gap junction proteins--the pannexin alternative. J Exp Biol. 2005;208:1415–9. doi: 10.1242/jeb.01547. [DOI] [PubMed] [Google Scholar]
- 9.Marziano NK, Hasegawa DK, Phelan P, Turnbull MW. Functional interactions between polydnavirus and host cellular innexins. Journal of virology. 2011;85:10222–9. doi: 10.1128/JVI.00691-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spray DC, Ye ZC, Ransom BR. Functional connexin “hemichannels”: a critical appraisal. Glia. 2006;54:758–73. doi: 10.1002/glia.20429. [DOI] [PubMed] [Google Scholar]
- 11.Scemes E, Spray DC, Meda P. Connexins, pannexins, innexins: novel roles of “hemi-channels”. Pflugers Arch. 2009;457:1207–26. doi: 10.1007/s00424-008-0591-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bruzzone R, Hormuzdi SG, Barbe MT, Herb A, Monyer H. Pannexins, a family of gap junction proteins expressed in brain. Proc Natl Acad Sci U S A. 2003;100:13644–9. doi: 10.1073/pnas.2233464100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lai CP, Bechberger JF, Thompson RJ, MacVicar BA, Bruzzone R, Naus CC. Tumor-suppressive effects of pannexin 1 in C6 glioma cells. Cancer Res. 2007;67:1545–54. doi: 10.1158/0008-5472.CAN-06-1396. [DOI] [PubMed] [Google Scholar]
- 14.Sosinsky GE, Boassa D, Dermietzel R, Duffy HS, Laird DW, MacVicar B, Naus CC, Penuela S, Scemes E, Spray DC, Thompson RJ, Zhao HB, Dahl G. Pannexin channels are not gap junction hemichannels. Channels (Austin) 2011;5:193–7. doi: 10.4161/chan.5.3.15765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruiz-Meana M, Garcia-Dorado D, Hofstaetter B, Piper HM, Soler-Soler J. Propagation of cardiomyocyte hypercontracture by passage of Na(+) through gap junctions. Circ Res. 1999;85:280–7. doi: 10.1161/01.res.85.3.280. [DOI] [PubMed] [Google Scholar]
- 16.Kolodny GM. Transfer of cytoplasmic and nuclear proteins between cells in culture. J Mol Biol. 1973;78:197–210. doi: 10.1016/0022-2836(73)90438-5. [DOI] [PubMed] [Google Scholar]
- 17.Pitts JD, Simms JW. Permeability of junctions between animal cells. Intercellular transfer of nucleotides but not of macromolecules. Experimental cell research. 1977;104:153–63. doi: 10.1016/0014-4827(77)90078-7. [DOI] [PubMed] [Google Scholar]
- 18.Flagg-Newton J, Simpson I, Loewenstein WR. Permeability of the cell-to-cell membrane channels in mammalian cell juncton. Science. 1979;205:404–7. doi: 10.1126/science.377490. [DOI] [PubMed] [Google Scholar]
- 19.Laird DW. Life cycle of connexins in health and disease. Biochem J. 2006;394:527–43. doi: 10.1042/BJ20051922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Valiunas V, Polosina YY, Miller H, Potapova IA, Valiuniene L, Doronin S, Mathias RT, Robinson RB, Rosen MR, Cohen IS, Brink PR. Connexin-specific cell-to-cell transfer of short interfering RNA by gap junctions. J Physiol. 2005;568:459–68. doi: 10.1113/jphysiol.2005.090985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hosoda T, Zheng H, Cabral-da-Silva M, Sanada F, Ide-Iwata N, Ogorek B, Ferreira-Martins J, Arranto C, D'Amario D, del Monte F, Urbanek K, D'Alessandro DA, Michler RE, Anversa P, Rota M, Kajstura J, Leri A. Human cardiac stem cell differentiation is regulated by a mircrine mechanism. Circulation. 2011;123:1287–96. doi: 10.1161/CIRCULATIONAHA.110.982918. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Lawrence TS, Beers WH, Gilula NB. Transmission of hormonal stimulation by cell-to-cell communication. Nature. 1978;272:501–6. doi: 10.1038/272501a0. [DOI] [PubMed] [Google Scholar]
- 23.Qu Y, Dahl G. Function of the voltage gate of gap junction channels: selective exclusion of molecules. Proc Natl Acad Sci U S A. 2002;99:697–702. doi: 10.1073/pnas.022324499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saez JC, Connor JA, Spray DC, Bennett MV. Hepatocyte gap junctions are permeable to the second messenger, inositol 1,4,5-trisphosphate, and to calcium ions. Proc Natl Acad Sci U S A. 1989;86:2708–12. doi: 10.1073/pnas.86.8.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iacobas DA, Suadicani SO, Spray DC, Scemes E. A stochastic two-dimensional model of intercellular Ca2+ wave spread in glia. Biophys J. 2006;90:24–41. doi: 10.1529/biophysj.105.064378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kang J, Kang N, Lovatt D, Torres A, Zhao Z, Lin J, Nedergaard M. Connexin 43 hemichannels are permeable to ATP. J Neurosci. 2008;28:4702–11. doi: 10.1523/JNEUROSCI.5048-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iglesias R, Dahl G, Qiu F, Spray DC, Scemes E. Pannexin 1: the molecular substrate of astrocyte “hemichannels”. J Neurosci. 2009;29:7092–7. doi: 10.1523/JNEUROSCI.6062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gandhi GK, Cruz NF, Ball KK, Theus SA, Dienel GA. Selective astrocytic gap junctional trafficking of molecules involved in the glycolytic pathway: impact on cellular brain imaging. J Neurochem. 2009;110:857–69. doi: 10.1111/j.1471-4159.2009.06173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rouach N, Koulakoff A, Abudara V, Willecke K, Giaume C. Astroglial metabolic networks sustain hippocampal synaptic transmission. Science. 2008;322:1551–5. doi: 10.1126/science.1164022. [DOI] [PubMed] [Google Scholar]
- 30.Stout RF, Jr., Spray DC, Parpura V. Astrocytic 'power-grid': Delivery upon neuronal demand. Cellscience. 2009;5:34–43. [PMC free article] [PubMed] [Google Scholar]
- 31.Dienel GA. Astrocytes are ‘good scouts’: being prepared also helps neighboring neurons. J Cereb Blood Flow Metab. 2010;30:1893–4. doi: 10.1038/jcbfm.2010.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ram Z, Walbridge S, Shawker T, Culver KW, Blaese RM, Oldfield EH. The effect of thymidine kinase transduction and ganciclovir therapy on tumor vasculature and growth of 9L gliomas in rats. J Neurosurg. 1994;81:256–60. doi: 10.3171/jns.1994.81.2.0256. [DOI] [PubMed] [Google Scholar]
- 33.Cheng YC, Grill SP, Dutschman GE, Nakayama K, Bastow KF. Metabolism of 9-(1,3-dihydroxy-2-propoxymethyl)guanine, a new anti-herpes virus compound, in herpes simplex virus-infected cells. J Biol Chem. 1983;258:12460–4. [PubMed] [Google Scholar]
- 34.Bi WL, Parysek LM, Warnick R, Stambrook PJ. In vitro evidence that metabolic cooperation is responsible for the bystander effect observed with HSV tk retroviral gene therapy. Hum Gene Ther. 1993;4:725–31. doi: 10.1089/hum.1993.4.6-725. [DOI] [PubMed] [Google Scholar]
- 35.Vrionis FD, Wu JK, Qi P, Waltzman M, Cherington V, Spray DC. The bystander effect exerted by tumor cells expressing the herpes simplex virus thymidine kinase (HSVtk) gene is dependent on connexin expression and cell communication via gap junctions. Gene Ther. 1997;4:577–85. doi: 10.1038/sj.gt.3300438. [DOI] [PubMed] [Google Scholar]
- 36.Estin D, Li M, Spray D, Wu JK. Connexins are expressed in primary brain tumors and enhance the bystander effect in gene therapy. Neurosurgery. 1999;44:361–8. doi: 10.1097/00006123-199902000-00068. discussion 368-9. [DOI] [PubMed] [Google Scholar]
- 37.Elshami AA, Saavedra A, Zhang H, Kucharczuk JC, Spray DC, Fishman GI, Amin KM, Kaiser LR, Albelda SM. Gap junctions play a role in the ‘bystander effect’ of the herpes simplex virus thymidine kinase/ganciclovir system in vitro. Gene Ther. 1996;3:85–92. [PubMed] [Google Scholar]
- 38.Andrade-Rozental AF, Rozental R, Hopperstad MG, Wu JK, Vrionis FD, Spray DC. Gap junctions: the “kiss of death” and the “kiss of life”. Brain Res Brain Res Rev. 2000;32:308–15. doi: 10.1016/s0165-0173(99)00099-5. [DOI] [PubMed] [Google Scholar]
- 39.Denning C, Pitts JD. Bystander effects of different enzyme-prodrug systems for cancer gene therapy depend on different pathways for intercellular transfer of toxic metabolites, a factor that will govern clinical choice of appropriate regimes. Hum Gene Ther. 1997;8:1825–35. doi: 10.1089/hum.1997.8.15-1825. [DOI] [PubMed] [Google Scholar]
- 40.Cheng F, Ke Q, Chen F, Cai B, Gao Y, Ye C, Wang D, Zhang L, Lahn BT, Li W, Xiang AP. Protecting against wayward human induced pluripotent stem cells with a suicide gene. Biomaterials. 2012;33:3195–204. doi: 10.1016/j.biomaterials.2012.01.023. [DOI] [PubMed] [Google Scholar]
- 41.Preuss E, Muik A, Weber K, Otte J, von Laer D, Fehse B. Cancer suicide gene therapy with TK.007: superior killing efficiency and bystander effect. J Mol Med (Berl) 2011;89:1113–24. doi: 10.1007/s00109-011-0777-8. [DOI] [PubMed] [Google Scholar]
- 42.Harks EG, Camina JP, Peters PH, Ypey DL, Scheenen WJ, van Zoelen EJ. Theuvenet AP: Besides affecting intracellular calcium signaling, 2-APB reversibly blocks gap junctional coupling in confluent monolayers, thereby allowing measurement of single-cell membrane currents in undissociated cells. FASEB J. 2003;17:941–3. doi: 10.1096/fj.02-0786fje. [DOI] [PubMed] [Google Scholar]
- 43.Bai D, del Corsso C, Srinivas M, Spray DC. Block of specific gap junction channel subtypes by 2-aminoethoxydiphenyl borate (2-APB). J Pharmacol Exp Ther. 2006;319:1452–8. doi: 10.1124/jpet.106.112045. [DOI] [PubMed] [Google Scholar]
- 44.Adesse D, Goldenberg RC, Fortes FS, Jasmin, Iacobas DA, Iacobas S, Campos de Carvalho AC, de Narareth Meirelles M, Huang H, Soares MB, Tanowitz HB, Garzoni LR, Spray DC. Gap junctions and chagas disease. Adv Parasitol. 2011;76:63–81. doi: 10.1016/B978-0-12-385895-5.00003-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kasper CA, Sorg I, Schmutz C, Tschon T, Wischnewski H, Kim ML, Arrieumerlou C. Cell-cell propagation of NF-kappaB transcription factor and MAP kinase activation amplifies innate immunity against bacterial infection. Immunity. 2010;33:804–16. doi: 10.1016/j.immuni.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 46.Tran Van Nhieu G, Clair C, Bruzzone R, Mesnil M, Sansonetti P, Combettes L. Connexin-dependent inter-cellular communication increases invasion and dissemination of Shigella in epithelial cells. Nat Cell Biol. 2003;5:720–6. doi: 10.1038/ncb1021. [DOI] [PubMed] [Google Scholar]
- 47.Derangeon M, Spray DC, Bourmeyster N, Sarrouilhe D, Herve JC. Reciprocal influence of connexins and apical junction proteins on their expressions and functions. Biochim Biophys Acta. 2009;1788:768–78. doi: 10.1016/j.bbamem.2008.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eugenin EA, Berman JW. Gap junctions mediate human immunodeficiency virus-bystander killing in astrocytes. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:12844–50. doi: 10.1523/JNEUROSCI.4154-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eugenin EA, Clements JE, Zink MC, Berman JW. Human immunodeficiency virus infection of human astrocytes disrupts blood-brain barrier integrity by a gap junction-dependent mechanism. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:9456–65. doi: 10.1523/JNEUROSCI.1460-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baumgartner W, Hinterdorfer P, Ness W, Raab A, Vestweber D, Schindler H, Drenckhahn D. Cadherin interaction probed by atomic force microscopy. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:4005–10. doi: 10.1073/pnas.070052697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.De Bock M, Culot M, Wang N, da Costa A, Decrock E, Bol M, Bultynck G, Cecchelli R, Leybaert L. Low extracellular Ca(2+) conditions induce an increase in brain endothelial permeability that involves intercellular Ca(2+) waves. Brain Res. 2012 doi: 10.1016/j.brainres.2012.06.046. [DOI] [PubMed] [Google Scholar]
- 52.Scemes E, Suadicani SO, Dahl G, Spray DC. Connexin and pannexin mediated cell-cell communication. Neuron Glia Biol. 2007;3:199–208. doi: 10.1017/S1740925X08000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barcellos-Hoff MH, Brooks AL. Extracellular signaling through the microenvironment: a hypothesis relating carcinogenesis, bystander effects, and genomic instability. Radiat Res. 2001;156:618–27. doi: 10.1667/0033-7587(2001)156[0618:esttma]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 54.Mothersill C, Seymour CB. Cell-cell contact during gamma irradiation is not required to induce a bystander effect in normal human keratinocytes: evidence for release during irradiation of a signal controlling survival into the medium. Radiat Res. 1998;149:256–62. [PubMed] [Google Scholar]
- 55.Azzam EI, de Toledo SM, Gooding T, Little JB. Intercellular communication is involved in the bystander regulation of gene expression in human cells exposed to very low fluences of alpha particles. Radiat Res. 1998;150:497–504. [PubMed] [Google Scholar]
- 56.Azzam EI, de Toledo SM, Little JB. Direct evidence for the participation of gap junction-mediated intercellular communication in the transmission of damage signals from alpha -particle irradiated to nonirradiated cells. Proc Natl Acad Sci U S A. 2001;98:473–8. doi: 10.1073/pnas.011417098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lyng FM, Seymour CB, Mothersill C. Early events in the apoptotic cascade initiated in cells treated with medium from the progeny of irradiated cells. Radiat Prot Dosimetry. 2002;99:169–72. doi: 10.1093/oxfordjournals.rpd.a006753. [DOI] [PubMed] [Google Scholar]
- 58.Mothersill C, Seymour CB. Bystander and delayed effects after fractionated radiation exposure. Radiat Res. 2002;158:626–33. doi: 10.1667/0033-7587(2002)158[0626:badeaf]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 59.Mothersill C, Stamato TD, Perez ML, Cummins R, Mooney R, Seymour CB. Involvement of energy metabolism in the production of ‘bystander effects’ by radiation. Br J Cancer. 2000;82:1740–6. doi: 10.1054/bjoc.2000.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gerashchenko BI, Howell RW. Cell proximity is a prerequisite for the proliferative response of bystander cells co-cultured with cells irradiated with gamma-rays. Cytometry A. 2003;56:71–80. doi: 10.1002/cyto.a.10092. [DOI] [PubMed] [Google Scholar]
- 61.Baskar R, Balajee AS, Geard CR, Hande MP. Isoform-specific activation of protein kinase c in irradiated human fibroblasts and their bystander cells. Int J Biochem Cell Biol. 2008;40:125–34. doi: 10.1016/j.biocel.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 62.Gow MD, Seymour CB, Ryan LA, Mothersill CE. Induction of bystander response in human glioma cells using high-energy electrons: a role for TGF-beta1. Radiat Res. 2010;173:769–78. doi: 10.1667/RR1895.1. [DOI] [PubMed] [Google Scholar]
- 63.Morgan WF. Is there a common mechanism underlying genomic instability, bystander effects and other nontargeted effects of exposure to ionizing radiation? Oncogene. 2003;22:7094–9. doi: 10.1038/sj.onc.1206992. [DOI] [PubMed] [Google Scholar]
- 64.Ohshima Y, Tsukimoto M, Takenouchi T, Harada H, Suzuki A, Sato M, Kitani H, Kojima S. gamma-Irradiation induces P2X(7) receptor-dependent ATP release from B16 melanoma cells. Biochimica et biophysica acta. 2010;1800:40–6. doi: 10.1016/j.bbagen.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 65.Ohshima Y, Tsukimoto M, Harada H, Kojima S. Involvement of connexin43 hemichannel in ATP release after gamma-irradiation. J Radiat Res. 2012;53:551–7. doi: 10.1093/jrr/rrs014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Maizels L, Gepstein L. Gap junctions, stem cells, and cell therapy: Rhythmic/arrhythmic implications. Heart Rhythm. 2011 doi: 10.1016/j.hrthm.2011.12.018. [DOI] [PubMed] [Google Scholar]
- 67.Jaderstad J, Jaderstad LM, Li J, Chintawar S, Salto C, Pandolfo M, Ourednik V, Teng YD, Sidman RL, Arenas E, Snyder EY, Herlenius E. Communication via gap junctions underlies early functional and beneficial interactions between grafted neural stem cells and the host. Proc Natl Acad Sci U S A. 2010;107:5184–9. doi: 10.1073/pnas.0915134107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Perez Velazquez JL, Frantseva MV, Naus CC. Gap junctions and neuronal injury: protectants or executioners? Neuroscientist. 2003;9:5–9. doi: 10.1177/1073858402239586. [DOI] [PubMed] [Google Scholar]
- 69.Takano T, Oberheim N, Cotrina ML, Nedergaard M. Astrocytes and ischemic injury. Stroke. 2009;40:S8–12. doi: 10.1161/STROKEAHA.108.533166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Oguro K, Jover T, Tanaka H, Lin Y, Kojima T, Oguro N, Grooms SY, Bennett MV, Zukin RS. Global ischemia-induced increases in the gap junctional proteins connexin 32 (Cx32) and Cx36 in hippocampus and enhanced vulnerability of Cx32 knock-out mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21:7534–42. doi: 10.1523/JNEUROSCI.21-19-07534.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Duffy HS, John GR, Lee SC, Brosnan CF, Spray DC. Reciprocal regulation of the junctional proteins claudin-1 and connexin43 by interleukin-1beta in primary human fetal astrocytes. J Neurosci. 2000;20:RC114. doi: 10.1523/JNEUROSCI.20-23-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rohlmann A, Laskawi R, Hofer A, Dobo E, Dermietzel R, Wolff JR. Facial nerve lesions lead to increased immunostaining of the astrocytic gap junction protein (connexin 43) in the corresponding facial nucleus of rats. Neurosci Lett. 1993;154:206–8. doi: 10.1016/0304-3940(93)90208-3. [DOI] [PubMed] [Google Scholar]
- 73.Cherkas PS, Huang TY, Pannicke T, Tal M, Reichenbach A, Hanani M. The effects of axotomy on neurons and satellite glial cells in mouse trigeminal ganglion. Pain. 2004;110:290–8. doi: 10.1016/j.pain.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 74.Hanani M, Huang TY, Cherkas PS, Ledda M, Pannese E. Glial cell plasticity in sensory ganglia induced by nerve damage. Neuroscience. 2002;114:279–83. doi: 10.1016/s0306-4522(02)00279-8. [DOI] [PubMed] [Google Scholar]
- 75.Frantseva MV, Kokarovtseva L, Perez Velazquez JL. Ischemia-induced brain damage depends on specific gap-junctional coupling. J Cereb Blood Flow Metab. 2002;22:453–62. doi: 10.1097/00004647-200204000-00009. [DOI] [PubMed] [Google Scholar]
- 76.Ozog MA, Siushansian R, Naus CC. Blocked gap junctional coupling increases glutamate-induced neurotoxicity in neuron-astrocyte co-cultures. J Neuropathol Exp Neurol. 2002;61:132–41. doi: 10.1093/jnen/61.2.132. [DOI] [PubMed] [Google Scholar]
- 77.Kozoriz MG, Bechberger JF, Bechberger GR, Suen MW, Moreno AP, Maass K, Willecke K, Naus CC. The connexin43 C-terminal region mediates neuroprotection during stroke. J Neuropathol Exp Neurol. 2010;69:196–206. doi: 10.1097/NEN.0b013e3181cd44df. [DOI] [PubMed] [Google Scholar]
- 78.Maass K, Chase SE, Lin X, Delmar M. Cx43 CT domain influences infarct size and susceptibility to ventricular tachyarrhythmias in acute myocardial infarction. Cardiovascular research. 2009;84:361–7. doi: 10.1093/cvr/cvp250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rami A, Volkmann T, Winckler J. Effective reduction of neuronal death by inhibiting gap junctional intercellular communication in a rodent model of global transient cerebral ischemia. Exp Neurol. 2001;170:297–304. doi: 10.1006/exnr.2001.7712. [DOI] [PubMed] [Google Scholar]
- 80.Rawanduzy A, Hansen A, Hansen TW, Nedergaard M. Effective reduction of infarct volume by gap junction blockade in a rodent model of stroke. J Neurosurg. 1997;87:916–20. doi: 10.3171/jns.1997.87.6.0916. [DOI] [PubMed] [Google Scholar]
- 81.Garcia-Dorado D, Inserte J, Ruiz-Meana M, Gonzalez MA, Solares J, Julia M, Barrabes JA, Soler-Soler J. Gap junction uncoupler heptanol prevents cell-to-cell progression of hypercontracture and limits necrosis during myocardial reperfusion. Circulation. 1997;96:3579–86. doi: 10.1161/01.cir.96.10.3579. [DOI] [PubMed] [Google Scholar]
- 82.Warner DS, Ludwig PS, Pearlstein R, Brinkhous AD. Halothane reduces focal ischemic injury in the rat when brain temperature is controlled. Anesthesiology. 1995;82:1237–45. doi: 10.1097/00000542-199505000-00019. discussion 27A. [DOI] [PubMed] [Google Scholar]
- 83.Frantseva MV, Kokarovtseva L, Naus CG, Carlen PL, MacFabe D, Perez Velazquez JL. Specific gap junctions enhance the neuronal vulnerability to brain traumatic injury. J Neurosci. 2002;22:644–53. doi: 10.1523/JNEUROSCI.22-03-00644.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lin D, Takemoto DJ. Protection from ataxia-linked apoptosis by gap junction inhibitors. Biochem Biophys Res Commun. 2007;362:982–7. doi: 10.1016/j.bbrc.2007.08.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Garcia-Dorado D, Rodriguez-Sinovas A, Ruiz-Meana M. Gap junction-mediated spread of cell injury and death during myocardial ischemia-reperfusion. Cardiovasc Res. 2004;61:386–401. doi: 10.1016/j.cardiores.2003.11.039. [DOI] [PubMed] [Google Scholar]
- 86.Ayer R, Chen W, Sugawara T, Suzuki H, Zhang JH. Role of gap junctions in early brain injury following subarachnoid hemorrhage. Brain Res. 2010;1315:150–8. doi: 10.1016/j.brainres.2009.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.de Pina-Benabou MH, Szostak V, Kyrozis A, Rempe D, Uziel D, Urban-Maldonado M, Benabou S, Spray DC, Federoff HJ, Stanton PK, Rozental R. Blockade of gap junctions in vivo provides neuroprotection after perinatal global ischemia. Stroke. 2005;36:2232–7. doi: 10.1161/01.STR.0000182239.75969.d8. [DOI] [PubMed] [Google Scholar]
- 88.Cusato K, Bosco A, Rozental R, Guimaraes CA, Reese BE, Linden R, Spray DC. Gap junctions mediate bystander cell death in developing retina. J Neurosci. 2003;23:6413–22. doi: 10.1523/JNEUROSCI.23-16-06413.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cusato K, Ripps H, Zakevicius J, Spray DC. Gap junctions remain open during cytochrome c-induced cell death: relationship of conductance to ‘bystander’ cell killing. Cell Death Differ. 2006;13:1707–14. doi: 10.1038/sj.cdd.4401876. [DOI] [PubMed] [Google Scholar]
- 90.Ripps H. Cell death in retinitis pigmentosa: gap junctions and the ‘bystander’ effect. Exp Eye Res. 2002;74:327–36. doi: 10.1006/exer.2002.1155. [DOI] [PubMed] [Google Scholar]
- 91.Lin JH, Weigel H, Cotrina ML, Liu S, Bueno E, Hansen AJ, Hansen TW, Goldman S, Nedergaard M. Gap-junction-mediated propagation and amplification of cell injury. Nat Neurosci. 1998;1:494–500. doi: 10.1038/2210. [DOI] [PubMed] [Google Scholar]
- 92.Udawatte C, Ripps H. The spread of apoptosis through gap-junctional channels in BHK cells transfected with Cx32. Apoptosis. 2005;10:1019–29. doi: 10.1007/s10495-005-0776-8. [DOI] [PubMed] [Google Scholar]
- 93.Huang C, Han X, Li X, Lam E, Peng W, Lou N, Torres A, Yang M, Garre JM, Tian GF, Bennett MV, Nedergaard M, Takano T. Critical role of connexin 43 in secondary expansion of traumatic spinal cord injury. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:3333–8. doi: 10.1523/JNEUROSCI.1216-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Thompson RJ, Zhou N, MacVicar BA. Ischemia opens neuronal gap junction hemichannels. Science. 2006;312:924–7. doi: 10.1126/science.1126241. [DOI] [PubMed] [Google Scholar]
- 95.Leshchenko Y, Likhodii S, Yue W, Burnham WM, Perez Velazquez JL. Carbenoxolone does not cross the blood brain barrier: an HPLC study. BMC Neurosci. 2006;7:3. doi: 10.1186/1471-2202-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Osmond JM, Dorrance AM. 11beta-hydroxysteroid dehydrogenase type II inhibition causes cerebrovascular remodeling and increases infarct size after cerebral ischemia. Endocrinology. 2009;150:713–9. doi: 10.1210/en.2008-0808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hunter GK, Pitts JD. Non-selective junctional communication between some different mammalian cell types in primary culture. J Cell Sci. 1981;49:163–75. doi: 10.1242/jcs.49.1.163. [DOI] [PubMed] [Google Scholar]