Abstract
Cell migration and morphogenesis are key events in tissue development and organogenesis. In Caenorhabditis elegans, the migratory path of the distal tip cells determines the morphology of the hermaphroditic gonad. The distal tip cells undergo a series of migratory phases interspersed with turns to form the gonad. A wide variety of genes have been identified as crucial to this process, from genes that encode components and modifiers of the extracellular matrix to signaling proteins and transcriptional regulators. The connections between extracellular and transmembrane protein functions and intracellular pathways are essential for distal tip cell migration, and the integration of this information governs gonad morphogenesis and determines gonad size and shape.
INTRODUCTION
The Caenorhabditis elegans hermaphroditic gonad consists of two symmetric U-shaped arms. The development and morphogenesis of this structure occurs postembryonically. At hatching, the gonad primordium is made up of four cells, Z1, Z2, Z3, and Z4.1 Z1 and Z4 give rise to the somatic structures of the gonad and are located in the anterior- and posterior-most regions of the primordium, respectively. Z2 and Z3 give rise to the germ cell lineage.1 The morphology of the mature hermaphroditic gonad relies on the migration of two distal tip cells (DTCs), which are descended from Z1 and Z4.2,3 The DTCs are born during the first larval stage (L1) and a single DTC lies at each end of the gonad primordium, which, at the end of gonad development, will become the distal tip of each gonad arm.1 DTCs form caps around the distal-most mitotic germ cells and extend long cytonemes at least 10 cell lengths along the proximal-distal axis4 (Figure1(a)).
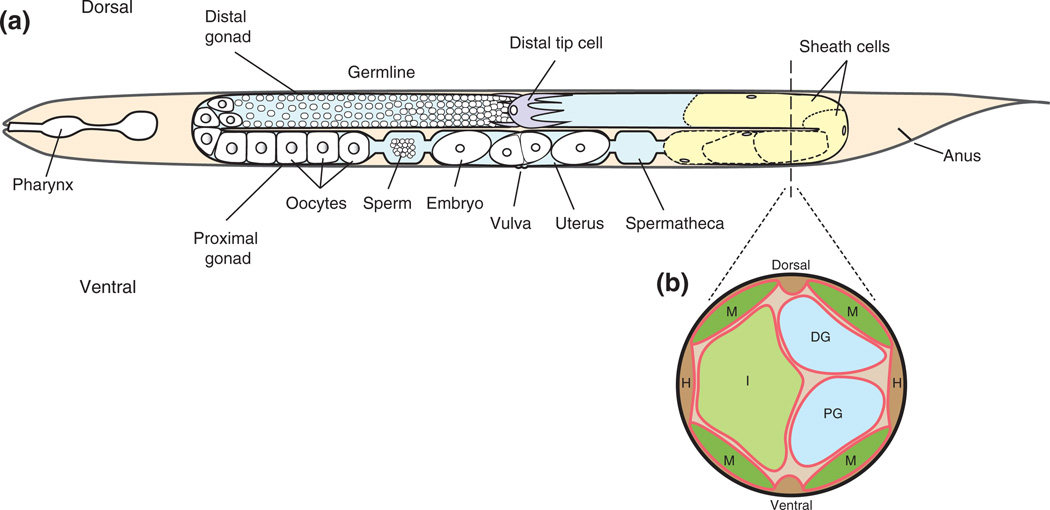
FIGURE 1.
Schematic representation of the adult hermaphrodite. Gonadal structures are featured in a lateral view4 (a) and a cross-sectional view (b). (a) The anterior gonad arm (left) depicts germ cells in the distal gonad; proximal gonad structures are labeled. The posterior arm (right) depicts the somatic structures of the gonad including a distal tip cell (purple) and sheath cells (yellow). The dotted line running through the posterior gonad arm represents the approximate location of the schematic cross-sectional slice. (b) The cross-sectional view depicts the basement membranes (red) that surround the body wall muscles (M), hypodermis (H), intestine (I), distal gonad (DG), and proximal gonad (PG). This schematic is based on an electron micrograph.
The gonad is surrounded by its own basement membrane5 and this basement membrane contacts basement membranes along the adjacent body wall muscles and hypodermis6 (Figure 1(b)). The molecules that make up the gonadal basement membrane can either be produced and secreted by gonadal cells or by nearby tissues, such as the body wall muscles, and assembled in the gonadal basement membrane.7,8 Different tissues have varying basement membrane thicknesses,5 and tissue- and stage-specific expression and assembly of basement membrane components suggest that basement membranes can differ molecularly.6 Directly under the gonadal basement membrane, sheath cells lie in a single layer and, by adulthood, cover the proximal germ cells and all oocytes. Sheath cells are important for gonad function and maintenance and, to achieve their proper placement, are thought to migrate along germ cells as the DTCs migrate and elongate the gonad arms9 (Figure 1(a)).
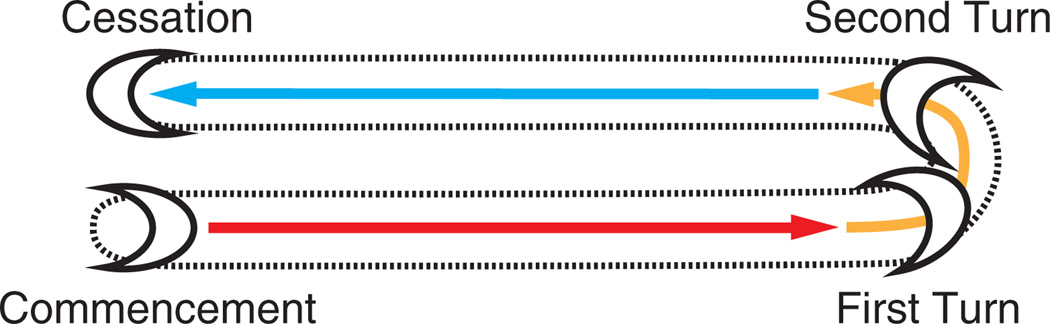
DTC migration occurs in a stereotypic manner and commences during the second larval (L2) stage (Box 1). DTC migration can be divided into three phases10–12 (Figure 2). DTCs first migrate away from the midbody on the ventral basement membrane, which overlies the ventral muscles (phase 1). During the third larval (L3) stage, the DTCs make two turns (phase 2). They first turn away from the ventral basement membrane and migrate toward the dorsal side of the animal along the basement membrane that lies on the lateral hypodermis. Upon reaching the dorsal basement membrane, the cells turn again to migrate back toward the midbody of the animal. Phase 3 of migration takes place during the fourth larval (L4) stage, and the DTCs migrate on the basement membrane that overlies the dorsal muscles. Cessation of migration occurs at the onset of young adulthood, when the DTCs reach the midbody, placing them approximately opposite the vulva.1,2 The final morphologies of the gonad arms reflect the migratory paths that their two corresponding DTCs have undertaken (Figures 1(a) and 2).
BOX 1.
WHY STUDY DISTAL TIP CELL MIGRATION?
Cell migration is an essential process for development, immunity, and tissue repair. Its misregulation leads to birth defects and many pathological conditions. Significant advances in understanding the molecular and cellular events required for directed cell migration have been made using a variety of elegant cell culture systems. However, using that information to understand migration within vertebrate tissues has been a challenge because of the inability to visualize cell behaviors in situ. In this regard, the transparent body and the stereotypic cell migrations during C. elegans development have provided new insights into the mechanisms that regulate cell movements in vivo. DTCs are particularly amenable to cell migration analyses.3 The hermaphroditic gonad develops postembryonically and is easily visualized. The C. elegans genome is conserved, which allows the translation of functions to other organisms, including humans. Genetics and RNAi-based screens11,12 are readily applied and have identified genes linked to cell migration in other systems, validating the DTC model of migration. These screens have also revealed the genes that are novel to the study of migration, providing new insights into the mechanisms of cell motility and directionality. For example, significant roles for extracellular matrix proteins14 and matrix metalloproteases15,16 in DTC migration suggest new ideas about how cells navigate in vivo. Analyses of DTC turning and stopping have identified a novel mechanism for regulating gonad shape and size through the coordinated expression of integrin adhesion receptors.17 Future studies will illuminate additional molecules and mechanisms that govern the dynamics of DTC migration.
FIGURE 2.
The U-shaped migratory path of the distal tip cell shapes the hermaphrodite gonad arm during larval development. Schematic representation of a posterior gonad arm shows the distal tip cell (crescent shapes) at different stages of migration. Arrows depict the direction of distal tip cell migration and colored arrows show the extent of distal tip cell migration during each phase and larval stage: phase 1/larval stage 2 (red), phase 2/larval stage 3 (yellow) and phase 3/larval stage 4 (blue). The key genes associated with the indicated distal tip cell activities are listed in Table 1.
In general, migration relies on the response of cells to either long-range or short-range cues, such as secreted guidance factors or the extracellular matrix (ECM), respectively. Furthermore, the cells must then correctly receive and interpret these signals to respond and migrate accordingly. Reception and coordination of these cues are crucial for the modification of the cytoskeleton to produce motility.13 To better understand these mechanisms that govern DTC migration, both forward and reverse genetics have been used to identify genes required for this process.11,12 Loss of function of specific genes through either mutant alleles or RNA interference (RNAi) has revealed a broad spectrum of gene functions involved in DTC migration, such as genes for cell fate specification, cell signaling, cell adhesion, and gene expression, as well as genes that encode ECM components and the enzymes that modify and remodel the ECM (Table 1). Furthermore, recent work has identified a subset of these genes as required cell autonomously in the DTC during migration. Many of the genes that have been linked to DTC migration affect pathways related to cytoskeletal organization, but our understanding of actin rearrangements in the DTC during migration remains quite limited.
TABLE 1.
Key Regulators of DTC Migration by Stage
| General regulators | ina-1, pat-2 |
| lam-1, epi-1 | |
| emb-9, let-2 | |
| Commencement | gon-1 |
| mig-6 | |
| First turn | unc-6, unc-5, unc-40 |
| src-1 | |
| pat-2 | |
| Second turn | talin |
| Pathfinding | mig-17 |
| pat-3 | |
| fbl-1 | |
| ced-10, mig-2 | |
| vab-10 | |
| Cessation | vab-3, ina-1 |
| cacn-1 |
Genes that function in each of the three migration phases have been identified. Those that function in phases 1 and 2 tend to give the most dramatic defects, including shortened gonad arms, ventralized migration paths, or abnormal turns. While following the correct path is essential throughout all phases of DTC migration, phenotypes that affect pathfinding along the dorsal basement membrane (during phase 3) are particularly easy to recognize in L4 and young adult hermaphrodites. In fact, dorsal pathfinding defects were the most common DTC migration defects observed in a genome-wide RNAi screen.11,12 We have organized this review according to the major activities of the DTCs during the three migration phases: commencement; turning, both away from the ventral basement membrane and onto the dorsal basement membrane; pathfinding; and the cessation of migration11,12 (Figure 2; Table 1).
COMMENCEMENT OF MIGRATION ALONG THE VENTRAL BASEMENT MEMBRANE
In the gonad primordium, the expression of genes that encode components of the cell migration machinery is initiated in Z1 and Z4 cells. Basement membrane proteins, the cell-adhesion receptor subunits ina-1/α integrin18 and pat-3/β integrin,19,20 cytoskeletal components, and proteins that regulate these components are expressed early in the process. At mid-stage L2, the DTCs begin migration away from the midbody and the primordial vulva. They follow a linear path along the ventral basement membrane, one DTC going toward the head and the other toward the tail. Surprisingly, genetic and RNAi screens for DTC migration genes have identified only two genes, gon-1 and mig-6, that are essential for initiation of this phase of migration, and both are associated with the ECM.
ECM Proteins are Required to Initiate DTC Migration
Reduction or loss of function of either gon-1 or mig-6 yields similar phenotypes in which the DTCs fail to initiate migration or show severely limited gonadal extension on the ventral side (Figure 3(b)). GON-1 is a secreted protein in the ADAMTS (a disintegrin and metalloprotease with thrombospondin type 1 repeats) family and possesses a matrix metalloprotease domain and multiple thrombospondin type-1-like repeats.16 The ADAMTS proteins have important roles in tissue development and remodeling and in diseases such as cancer.21 MIG-6 is orthologous to Drosophila and vertebrate papilin, a multidomain protein in the ADAMTS-like (ADAMTSL) family of proteins.14,22 ADAMTS and ADAMTSL proteins share a ‘papilin cassette’ composed of thrombospondin type 1 repeats and a cysteine-rich region but, unlike ADAMTS proteases, ADAMTSL proteins lack a catalytic domain.22,23
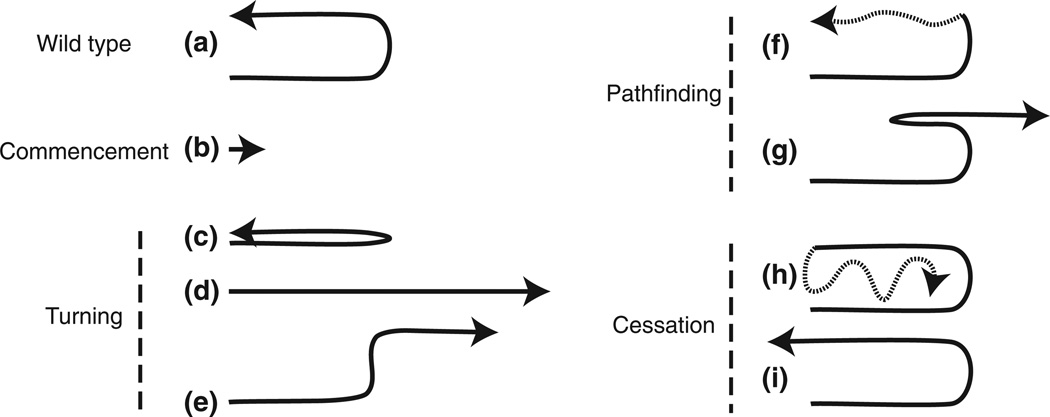
FIGURE 3.
Distal tip cell migration defects. Arrows show the direction of migration. Sections represented by dotted lines indicate potential variability in migration paths. A wild-type U-shaped migratory path is shown in (a). Defects are grouped according to the migration stages: commencement (b, no migration), turning (c, ventralized; d, no turn; e, wrong turn), pathfinding (f, meandering on dorsal; g, change in direction), and cessation (h, perpetual migration; i, overshoot). Only posterior gonads are represented, although these defects also occur in the anterior gonad arms.
Expression of gon-1 is turned on in the DTCs in L2 at the start of migration and continues through L4, suggesting that GON-1’s role extends beyond the commencement of migration.16 It is also expressed in the body wall muscles where it appears to shape the gonad arms. Normally, gonad arms exhibit a tubular structure, whereas animals that lack muscle expression of GON-1 exhibit misshapen, nontubular gonad arms, suggesting that muscle GON-1 aids in the expansion and morphogenesis of the arm.24
GON-1 requires its metalloprotease domain to rescue gonadogenesis,16 which suggests that GON-1 might promote DTC migration and affect gonad morphology by remodeling the ECM. Interestingly, human ADAMTS4 or ADAMTS9 was able to rescue gonadogenesis in hermaphrodites carrying the gon-1(0) null allele.25 Both ADAMTS4 and ADAMTS9 can degrade aggrecan, a proteoglycan found in cartilage ECM.26,27 No homolog of aggrecan has been identified in C. elegans and whether GON-1 cleaves proteoglycans, such as UNC-52/perlecan, in the basement membrane remains to be determined.
Gonad expression of mig-6/papilin (also called ppn-1) is first detected in Z1 and Z4 cells of the primordium, and the expression is maintained throughout the entire DTC migration process.11 A unique aspect of mig-6 is the expression of a DTC-specific isoform. The mig-6 transcript is alternatively spliced into a long (MIG-6L) and a short (MIG-6S) form. Both forms contain the papilin cassette and six Kunitz-type (KU) domains, but the MIG-6L form includes a unique C-terminal region of five more KU domains, an Ig motif and a protease and lacunin (PLAC) domain.14 Mutant alleles and RNAi treatments specific to one or both forms of MIG-6 revealed that each isoform has a distinct role in DTC migration. MIG-6L is important for the onset of migration, and MIG-6S is required for DTC pathfinding and is discussed in that section.
MIG-6L is expressed solely in the DTC, and MIG-6 is detected in the basement membranes of the DTC, gonad, pharynx, and intestine.14 The start of DTC migration relies on the function of the MIG-6L isoform, and the loss of MIG-6L results in DTCs exhibiting decreased rates of migration and gonad arms that appear similar to those found in gon-1 mutants.11,14 Drosophila papilin has been shown to bind to and regulate an ADAMTS collagenase in vitro,22 suggesting the possibility that MIG-6L may regulate GON-1 localization or activity in the gonadal basement membrane (Figure 4). This idea remains to be tested.
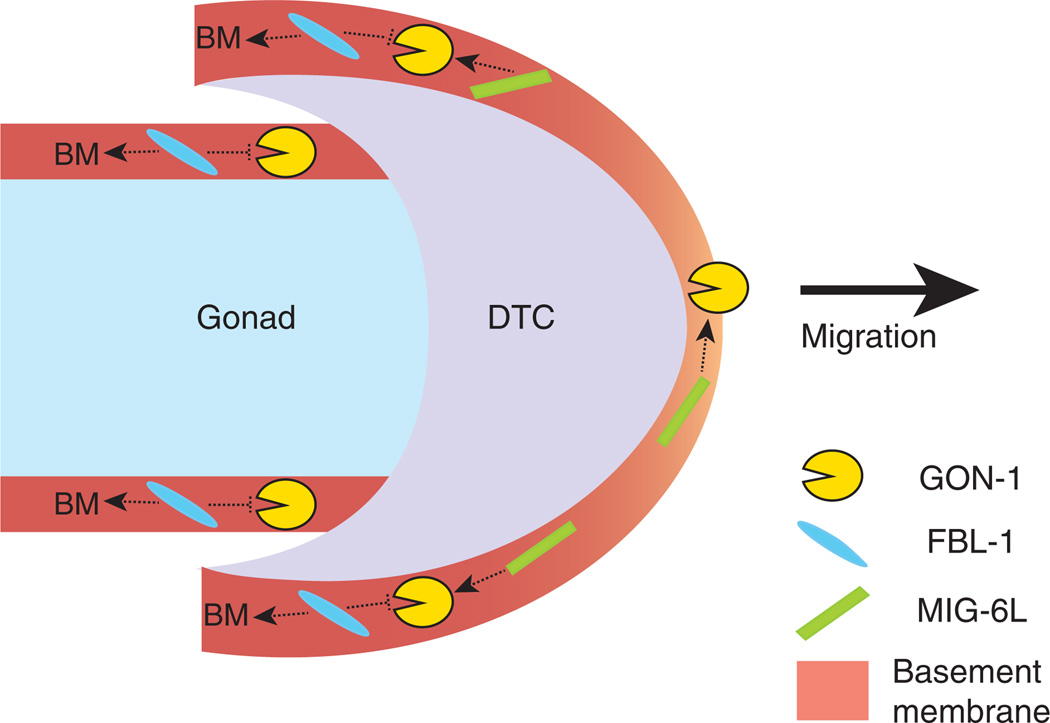
FIGURE 4.
A molecular model for the commencement of distal tip cell (DTC) migration. Both GON-1 and MIG-6L are crucial for DTC migration, and we speculate that MIG-6L positively regulates GON-1 activity or localization (dotted arrows). Mutant phenotypes suggest that FBL-1 may act to maintain the integrity of the basement membrane (BM, dotted arrows) and this effect of FBL-1 might limit GON-1 activity (dotted repression). Because GON-1 and MIG-6L are expressed by the DTC, their expression levels are potentially higher than that of FBL-1 in the DTC basement membrane. We propose that this imbalance in expression results in an optimum level of GON-1 activation and matrix remodeling, perhaps at the front of the DTC (light orange BM), to allow DTC migration. By contrast, higher levels of FBL-1 in the gonadal basement membrane limit the ability of GON-1 to remodel the matrix or to support migration.
Knockdowns of integral components of the basement membrane, such as laminin (epi-1/laminin αB and lam-1/laminin β; our unpublished observations)5,28 or type IV collagen (emb-9/collagen IV α1 and let-2/collagen IV α2),14,29 give similar DTC phenotypes to gon-1 and mig-6(l) mutants. It seems likely, however, that these migration defects are due to the reduction or loss of an essential structural component of the basement membrane, which would impact the cell’s ability to incorporate newly synthesized basement membrane proteins as the ECM is remodeled during DTC migration.
fbl-1 Mutants Suppress the gon-1 Phenotype
A candidate screen for ECM genes that modify the gon-1 phenotype identified a suppressor, fbl-1, that encodes a member of the conserved fibulin family of ECM proteins.25 FBL-1 is expressed by the intestinal cells in two isoforms, FBL-1C and FBL-1D. FBL-1C, but not the FBL-1D isoform, localizes to the basement membrane surrounding the gonad during gonadogenesis.8,30 fbl-1 mutants exhibited premature cessation of DTC migration and gonad arms that were double the width of wild-type arms.8,25 Furthermore, germline cells were observed to leak out of the gonad in fbl-1 mutant adult animals,8 which is often a consequence of gonadal basement membrane defects.28 These phenotypes and the previously described gon-1 phenotypes are suppressed in gon-1;fbl-1 double mutants.25
Although the double mutant data strongly suggest that FBL-1 may serve as a substrate for GON-1’s metalloprotease activity, cleaved FBL-1 fragments have not been detected.25,31 In mammalian cells, fibulin-1 binds to ADAMTS-1 and enhances the cleavage of the proteoglycan aggrecan, but is not itself cleaved by the protease.31 This finding suggests that FBL-1 could act to regulate GON-1 activity. However, the enlarged gonad arms and leakage of germ cells observed in fbl-1 single mutants suggest that FBL-1 provides structural integrity to the basement membrane, which may affect GON-1 function.
The possible regulation of GON-1 by FBL-1 and MIG-6L, coupled with their different expression patterns, gives rise to a potential model of how DTC migration starts. fbl-1 is expressed by the intestine and assembles on the gonadal basement membrane, while gon-1 and mig-6(l) are expressed by the DTCs,14,25 which could result in levels of GON-1 and MIG-6L that are higher than FBL-1 in the DTC basement membrane. This imbalance could provide the optimal environment for GON-1 activity such that DTC migration occurs (Figure 4). In the absence of FBL-1, GON-1 may excessively remodel a defective basement membrane to create the fbl-1 mutant phenotype. In the case of gon-1;fbl-1 double mutants, a permissive environment for DTC migration is created because of the combination of impaired basement membrane remodeling (no GON-1) and compromised basement membrane structure (no FBL-1).
DTC TURNING
The Turn from the Ventral Basement Membrane Toward the Dorsal Side
The first turn away from the ventral side separates the first and second phases of DTC migration and is one of the most studied features of the DTC migratory pathway. This reorientation of the DTC also causes the DTC to switch from the ventral muscle basement membrane to the lateral hypodermal basement membrane, two migration substrates that are proposed to differ in molecular composition.6 The UNC-6/netrin pathway is key for this turn, and a myriad of genes that modulate netrin signaling has been identified.
Netrin-Mediated Repulsion of the DTC
The netrin receptors, UNC-5 and UNC-40, and their laminin-related ligand, UNC-6/netrin, are required for the first DTC turn and initiate the second phase of migration.32 Mutations in these netrin genes result in a phenotype that is characterized by the absence of phase 2 of migration such that migration phase 3 is completed on the ventral, rather than dorsal, basement membrane (Figure 3(c)).
Temporal and spatial expression patterns of UNC-6 in ventral epidermoblasts and neurons have led to the proposal that this protein is distributed in a gradient that peaks near the ventral midline.33,34 The expression of unc-40 appears in DTCs from stage L2 until L4,35 whereas unc-5 expression in the DTC is activated at the time of reorientation away from the ventral basement membrane.36 UNC-5 and UNC-40 both are required for the first turn and, thus, the upregulation of unc-5 provides a switch to mediate repulsion from ventral toward the dorsal side.32,36,37 The expression of unc-5 requires DAF-12, a heterochronic nuclear receptor that is important for developmental timing.36,38
UNC-129, a predicted transforming growth factor (TGF)-β homolog, was first identified as a suppressor of ectopic UNC-5 signaling and is expressed in the dorsal body wall muscles, suggesting that it acted as an attractant.39–41 Recent work on UNC-129, however, has revealed a nuanced mechanism of netrin regulation. UNC-129 affects UNC-5 and UNC-40 signaling as these receptors function to interpret the UNC-6 signal and may allow the DTCs to remain sensitive to UNC-6 as they travel further away from the ventral side.42
Repulsion depends on intracellular signaling downstream of the UNC-5 cytoplasmic domain.43 SRC-1, one of two C. elegans Src non-receptor tyrosine kinases, binds and phosphorylates the UNC-5 receptor.44,45 Localization of SRC-1 to the cytoplasmic domain of UNC-5 rescues unc-5 mutants, but not unc-6 or unc-40 mutant phenotypes,45 which suggests that this phosphorylation event is necessary for downstream signaling of UNC-5 during DTC migration. In mammalian cells, Src and focal adhesion kinase (FAK) function together to phosphorylate UNC-5.46 FAK is an essential component of integrin signaling in vertebrates. However, in C. elegans, a large deletion in the FAK homolog kin-32 did not affect DTC migration,47 indicating that differences exist between species in the netrin signaling pathway.
In neurons, UNC-5 signals through MAX-2, a p21-activated kinase (PAK).48 PAKs function upstream of actin polymerization and microtubule disassembly in migrating vertebrate cells. Therefore, MAX-2 may potentially link UNC-5 signaling to cytoskeletal regulators in the DTC. Information about the localization of UNC-5 and its effectors within the DTC would help to elucidate how cytoskeletal dynamics are locally regulated to elicit cell reorientation and turning.
Integrin-Mediated Cell Adhesion Changes at the Ventral Turn
Integrins function as cell-adhesion receptors and consist of an α subunit (INA-1 and PAT-2) and a β subunit (PAT-3). Upon stimulation by a ligand, integrins signal from the ‘outside-in’ to activate intracellular pathways that include FAK, Src, and other kinase cascades. Evidence from mammalian systems shows cooperative roles for integrins with netrin receptors in regulating developmental cell movements.49 While it seems likely that these receptors work together in the DTC, coordinated signaling through SRC-1 or other conserved molecules has not been demonstrated.
The INA-1/PAT-3 integrin is important for cell migrations (neural cells as well as DTCs),18 while the other C. elegans integrin PAT-2/PAT-3 is prevalent in contractile, nonmotile cells, such as body wall muscles.6 It is now clear that both receptors play distinct roles during DTC migration and that the regulation of their expression is crucial to gonad morphogenesis. ina-1 expression is induced prior to the start of migration and is required for the DTC to complete migration.17,18 ina-1 mutant gonad arms are occasionally misdirected, exhibit defective morphologies, and leak germ cells.18 During migration on the ventral basement membrane, the expression of VAB-3, a Pax6 transcription factor, is initiated in the DTCs and directly activates pat-2 expression at the first turn in L3. In the absence of vab-3, DTCs have a meandering migratory path associated with a lack of pat-217 (Figure 3(h)). In wild type animals, the upregulation of pat-2 expression provides a second adhesion receptor (PAT-2/PAT-3), which may allow the DTC to adjust to the different basement membrane substrates upon which the cell migrates, for example, the body wall muscle basement membrane versus the hypodermal basement membrane. Moreover, this shift in integrin receptor expression in combination with netrin-mediated repulsion of the DTC may provide an essential receptor repertoire for adhesion along the new migratory path (Figure 5).
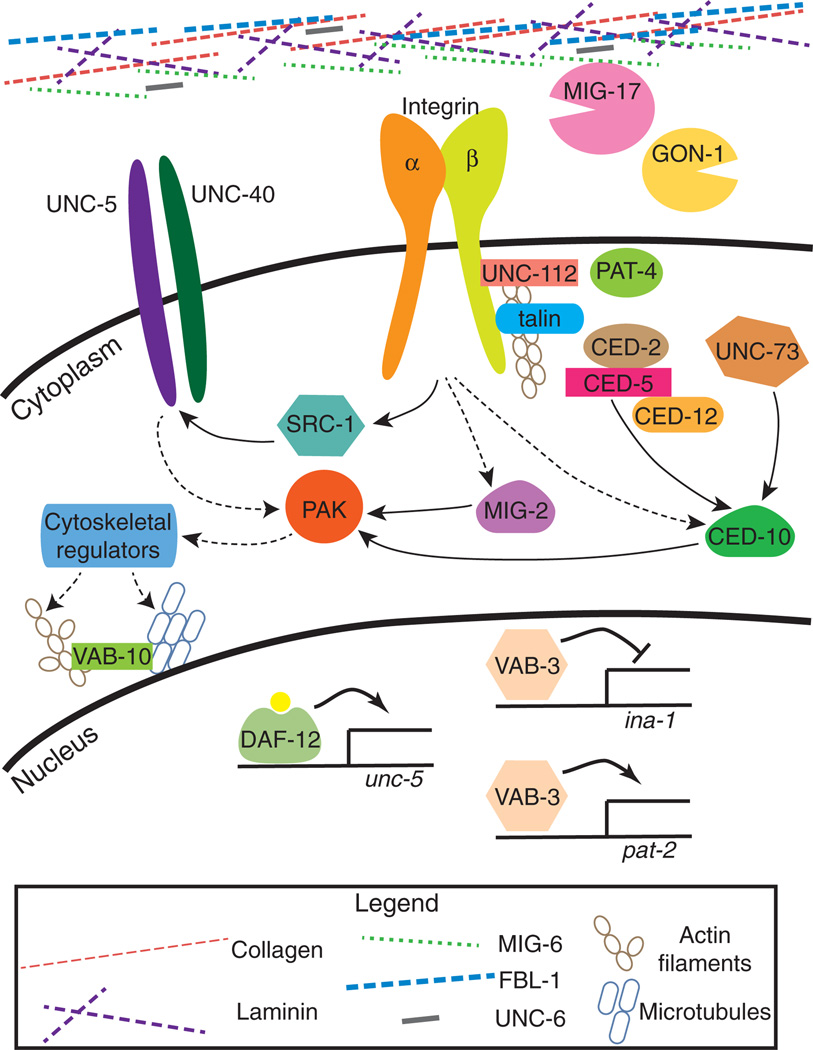
FIGURE 5.
A summary of genes crucial for distal tip cell (DTC) migration. Solid line arrows represent direct interactions and dotted line arrows represent indirect or speculative interactions. PAT-3 is represented by integrin β and INA-1 or PAT-2 is represented by integrin α. UNC-6/netrin; CED-2/CrkII; CED-5/Dock180; CED-12/ELMO; UNC-73/Trio GEF (guanine nucleotide exchange factor); UNC-112/kindlin; PAT-4/ILK; MIG-2/Rac; CED-10/Rac; VAB-10/spectraplakin; VAB-3/Pax6; MIG-6/papilin; FBL-1/fibulin; PAK/PAK-1; or MAX-2.
Turning Toward the Midbody
After turning away from the ventral basement membrane, the DTCs must maneuver another turn back toward the midbody. Unlike UNC-6/netrin, which is a major cue for the ventral to dorsal turn, a protein with a similarly significant role has not been identified for the turn onto the dorsal basement membrane. However, several genes have been linked to DTC reorientation back toward the midbody.
Adhesion and Signaling Proteins Mediate the Dorsal Turn Back to the Midbody
Two integrin-associated molecules, SRC-1 and talin, have significant roles in turning toward the midbody. Postembryonic RNAi knockdown of src-1 or talin caused DTCs to turn away from the midbody on the dorsal basement membrane50,51 (Figure 3(e)) or, in some cases, reduced src-1 expression can give DTCs that do not complete any turns and stay on the ventral basement membrane51 (Figure 3(d)). Talin binds to the PAT-3 cytoplasmic tail and regulates integrin activity,52 so its reduction may affect the ability of integrins to bind to appropriate dorsal ECM ligands. Because SRC-1 is a tyrosine kinase, its phenotypes are likely from lack of phosphorylation of signaling targets that are required for turning. These targets could include the cytoplasmic tails of integrins or UNC-5.43,53 Therefore, SRC-1 potentially links and coordinates the netrin and integrin signaling pathways (Figure 5).
In addition to turning defects, talin knock down can block migration and causes misshapen gonad arms, possibly because of its effects on sheath cell structure.50 Talin is thought to affect cell migration through PAT-3/β integrin,54 and while there are similarities between the pat-3 and talin knockdown phenotypes, the differences between these two phenotypes suggest that there are distinct aspects to their function in DTC migration.
The involvement of integrins and integrin-related proteins during the second turn also suggests that DTC adhesion is key in mediating the changes in cell polarity necessary for turning. Like the first turn, the DTC switches from migrating on the hypodermal to dorsal muscle basement membrane, which can affect the nature of DTC adhesion. Talin and SRC-1 together may function to modulate one branch of integrin signaling that is specific to turning. The dual role of SRC-1 in netrin and integrin signaling may be required to coordinate netrin repulsion with the changes in adhesion pathways.
DTC PATHFINDING ALONG THE DORSAL BASEMENT MEMBRANE
Subsequent to reorientation, the DTC migrates on the dorsal basement membrane toward the midbody. Genes related to ECM protein production and remodeling and genes that mediate and regulate cell adhesion have been implicated in pathfinding in this phase of migration. Pathfinding is required during all migration phases as the DTCs interpret extracellular cues. Because defects in pathfinding are particularly visible during phase 3, many genes have been associated with migration on the dorsal basement membrane, as described in this section.
MIG-17 is a Matrix Metalloprotease Required for DTC Pathfinding
The mig-17 gene encodes an ADAM (a disintegrin and metalloprotease) family member that is expressed by the body wall muscles, but, once secreted, localizes to the surfaces of muscles and the gonad. Localization to the gonadal surface is important for DTC pathfinding, as the loss of mig-17 causes the DTC to meander during phase 3 ofmigration and results in a malformed gonad arm15 (Figure 3(f)).
MIG-17 tissue targeting relies on the presence of its glycosylated prodomain.55 However, MIG-17’s protease activity depends on the cleavage of its prodomain. This cleavage is regulated spatially and temporally during development; it occurs at L3 and is reduced in the gonadal basement membrane of older adults, when DTC migration is completed.56 Basement membrane composition can also affect MIG-17 targeting. For example, mutations affecting the short form of mig-6 (mig-6S) exhibit similar phenotypes to mig-17 mutants,14 suggesting that the reduction of MIG-6S may affect the composition or organization of the basement membrane and thereby impact MIG-17 function. Genetic analysis has revealed potential interactions between MIG-17 and the basement membrane components FBL-1, LET-2, a type IV collagen chain, and NID-1, the C. elegans nidogen ortholog.8,29 These genetic interactions suggest that MIG-17 may directly impact the assembly and remodeling of the basement membrane.
Proteolytic targets for MIG-17 have not been identified, but proper posttranslational modifications are important for its intracellular trafficking and localization. For example, mig-23, encoding a nucleoside diphosphatase (NDPase), is required for MIG-17 glycosylation. A mutant form of MIG-17 that cannot be N-glycosylated is unable to rescue mig-17 mutants. Supporting this finding, mig-23 is expressed by body wall muscle cells and must be expressed in the same cell as mig-17 for the proper secretion and localization of MIG-17.57 Similarly, genes that encode the conserved oligomeric Golgi (COG) complex for intracellular vesicle trafficking, the cogc genes, are required in body wall muscles for MIG-17 glycosylation and secretion.58
Attachment of glycosaminoglycans onto proteoglycans has also been implicated in DTC migration. Mutations in SQV-5 and MIG-22, encoding a chondroitin synthase and a chondroitin polymerizing factor, respectively, cause pathfinding defects.59 Furthermore, hst-2, a heparan 2-o-sulfotransferase, is needed for the DTC to stay in contact with the dorsal basement membrane.60 The role of HST-2 in the DTC is particularly interesting because the basement membrane proteoglycan UNC-52/perlecan and the transmembrane proteoglycan SDN-1/syndecan are both modified with heparan sulfate side chains and have been linked to DTC turning and migration.61,62 Other ECM and transmembrane proteins that have been linked to phase 3 migration and basement membrane integrity include TEN-1,63,64 DGN-1,65 DIG-1,36 and laminin.5,28 These studies underscore the importance of basement membrane composition in DTC migration and pathfinding.
Integrins Regulate DTC Pathfinding
PAT-3 is required for embryogenesis, therefore a dominant-negative approach was used to bypass this early requirement for pat-3 and test its role in DTC migration. Transgenic animals carrying dominant-negative pat-3 exhibit DTCs that undergo supernumerary turns and dorsal pathfinding defects20 (Figure 3(h)), implicating integrin–receptor interactions in directing the DTCs back to the midbody. Analyses of the PAT-3 cytoplasmic tail have revealed that the conserved Asn-Pro-X-Tyr (NPXY) and Thr-Thr (TT) motifs, both of which are in the talin binding region of PAT-3, are required for proper pathfinding along the dorsal basement membrane.20,66 This region is also important for ‘inside out’ signaling which shifts integrins between low and high affinity binding to ligands and is linked to talin binding to the cytoplasmic tail of the β subunit.52,67 Interestingly, pat-3 phenotypes generated by mutant NPXY or TT motifs often share similarities to ina-1 hypomorphicmutants, suggesting that the talin-dependent PAT-3 activity is due to the INA-1/PAT-3 heterodimeric form in the DTC.17,18,20,66
Protein Turnover Regulates PAT-3 Expression
The level of PAT-3 expression in the DTC is crucial for its migration and is regulated by protein turnover. RNF-121, a conserved E3 ligase RING finger protein that is expressed in the DTCs, functions as an endoplasmic reticulum (ER)-anchored ubiquitin ligase that targets PAT-3 for degradation in an ER-associated manner. When RNF-121 was overexpressed, the level of PAT-3 was reduced, and animals exhibited pathfinding defects similar to those that carry the dominant-negative form of pat-3. Therefore, an optimal level of DTC PAT-3 expression must be maintained for DTC pathfinding.68 Whether a particular heterodimeric form of integrin is targeted by RNF-121 or whether this turnover is crucial for the switch from INA-1 to PAT-2 heterodimers in DTCs is not yet known.
Effectors Linking Adhesive Signals to the Cytoskeleton
Integrin-mediated adhesion to the basement membrane is ultimately transduced to the cytoskeleton. A number of genes that are required for downstream integrin signaling are important for DTC migration. For example, pat-4 and unc-112 encode the integrin-linked kinase (ILK) ortholog69 and the band 4.1 and ERM (FERM) domain protein, Mig2/kindlin, respectively.70 UNC-112/kindlin and PAT-3 recruit PAT-4/ILK to dense bodies in muscles.69,71 Loss of either pat-4 or unc-112 through RNAi treatments resulted in similar DTC phenotypes observed in pat-3 dominant-negative animals. Moreover, the gonadal sheath cell actin cytoskeleton appears disrupted in pat-4 and unc-112 knockdown animals, suggesting that these proteins are necessary to link integrins to the actin cytoskeleton.72
Small GTPases in the Rho/Rac family of conserved proteins are critical for actin cytoskeleton dynamics. In C. elegans, Rac GTPases are encoded by three genes, mig-2, ced-10, and rac-2.73 The loss of either CED-10 or MIG-2 causes the DTC to either prematurely halt migration or make an extra turn that completely reverses its direction while migrating on the dorsal basement membrane17,74,75 (Figure 3(g)). This phenotype is also observed in animals mutant for genes that encode positive regulators of CED-10/Rac, such as unc-73, a guanine nucleotide exchange factor (GEF) Trio,75 ced-2/CrkII, ced-5/Dock180, and ced-12/ELMO. CED-5 localizes to focal adhesions and interacts with CED-2 and CED-12.76–79 These three CED proteins interact and function as a GEF to regulate CED-10 activity.80 Not surprisingly given their localization to focal adhesions, knock down of pat-3/β-integrin in a gain of function mig-2/Rac allelic background shows a reduced pat-3 pathfinding phenotype.81 In addition, pat-3 dominant-negative DTC phenotypes are exacerbated when coupled with mutants in the Rac GEF gene unc-73 or in ced-5.81 These findings indicate that CED-10 and MIG-2 are activated downstream of integrins in the DTC.
One pathway that has been shown to be downstream of the Rac GTPases in DTC migration consists of PAK, PAK interacting exchange factor (PIX), and G-protein-coupled receptor-kinase interactor (GIT). Active Rac stimulates this pathway, which then controls cytoskeletal dynamics to promote cell migration.82–84 The PAK genes in C. elegans, pak-1 and max-2, are required for DTC migration and normal DTC morphology. Mutations in pak-1 or max-2 gave misshapen DTCs and relatively mild pathfinding defects during the third phase of migration.85 Double mutant pak-1;max-2 animals exhibited more severe DTC pathfinding and turning defects. Genetic analysis revealed a role for PAK-1 functioning with PIX-1 and GIT-1 in controlling DTC migration and morphology downstream of integrins.85
Several other genes have been shown to genetically interact with Rac GTPase pathways. The abl-1/Abl kinase, which interacts with ABI-1, a cytoskeletal regulatory protein of the WASP-family verprolin-homologous (WAVE) protein complex, can suppress ced mutant phenotypes in the DTC.86 Mutations in the C. elegans homolog of the Drosophila Cactus binding protein Cactin/CACN-1 enhanced the ced-10 mutant phenotypes.87 Although the molecular mechanisms governing the interactions of CACN-1 or ABL-1 with CED-10 or MIG-2 have not been deciphered, the genetic data support potential roles for these molecules in modulating Rac signaling between adhesion and cytoskeletal dynamics. Evidence for cytoskeletal reorganization during DTC migration and turning has recently been provided by the comparison of DTCs with wild type or mutant vab-10, which encodes a spectraplakin family member that has binding domains for both actin filaments and microtubules (Figure 5).88 This study shows not only the rearrangements of the cytoskeleton during migration, but also that mutation of this regulatory molecule perturbs the cytoskeleton and causes DTC migration and nuclear translocation defects.
In addition to linking adhesion molecules to the cytoskeleton, Rac signaling may also have a role in DTC polarity. One clue comes from the mom-5/Frizzled receptor gene that mediates Wnt polarity signals. Mutations in mom-5 result in a DTC migration phenotype that is very similar to the ced-10 defect (Figure 3(g)) and mutations in other Wnt-related genes, such as gsk-3, apr-1/APC, and egl-20/Wnt also cause DTC migration defects.89 The mom-5 defect could be suppressed by the overexpression of either CED-5 or CED-10,89 suggesting that Rac GTPase acts downstream of cell polarity signaling in addition to its role in adhesion signaling during DTC migration.
CESSATION OF DTC MIGRATION
Once the three phases of migration are complete, the DTCs must cease migrating at the appropriate site on the dorsal basement membrane. The homeodomain-paired domain transcription factor VAB-3 plays a significant role in cessation. Certain mutations in vab-3 cause perpetual migration by hermaphrodite DTCs resulting in excessively long, coiled gonad arms12,17 (Figure 3(h)). This phenotype is due to prolonged expression of the INA-1 integrin receptor in the DTC; reduction of ina-1 expression relieves the perpetual migration defect. Strikingly, the simultaneous reduction of two Rac GTPases, ced-10 and mig-2, in a vab-3 mutant background also alleviates the perpetual migration defect. These findings establish the importance of INA-1 and the downstream effectors CED-10 and MIG-2 in stopping migration and defining organ size.17
Rac GTPases have been implicated in another extended migration phenotype. Reduction of CACN-1 causes an ‘overshoot’ phenotype in which the DTCs do not stop opposite the vulva but continue migrating along the dorsal basement membrane past the midbody87 (Figure 3(i)). This phenotype is suppressed by a ced-10/Rac loss of function allele. These two studies, therefore, support the idea that migration cessation depends on the regulation of Rac signaling by integrins and CACN-1.
CONCLUSION
DTC migration relies on the coordination of multiple steps: commencement, two turns, pathfinding, and cessation. Genes integral to each of these steps have been identified and analyses of their functions have defined pathways that are critical to DTC migration and gonadogenesis. The integration of this work has served to identify gaps in our knowledge and outstanding questions of the field. Overall, the composition and organization of the ECM have proven to be vital determinants of DTC migration, from initiation of migration through DTC pathfinding and cessation. There is evidence that the basement membrane that surrounds the gonad differs in composition from the basement membrane along the body wall muscles, as well as the basement membrane that covers the hypodermis. What roles do differences in assembly or remodeling of these basement membranes play in controlling DTC migration?
Another area that demands attention is the potential cross-talk between the major signaling pathways that are required for DTC migration. For example, the Src kinase, SRC-1, is critical for both the occurrence and the directionality of the two turns that the DTC performs. SRC-1 is required for transmission of the UNC-6/netrin signal through UNC-5, and Src kinases are intimately involved in the transduction of integrin signals. Information about how regulation of shared components in distinct signaling pathways is coordinated is instrumental to understanding the roles of intracellular signaling in DTC migration.
Finally, how is the DTC cytoskeleton involved in its migration? The regulation of cell morphology is closely linked to cytoskeletal regulation and organization, both crucial for cell migration as shown in other systems.90 The RNAi screen for DTC migration defects has revealed that genes encoding cytoskeletal components (actin and tubulin) and cytoskeletal regulators (e.g., gex-3, zen-4, erm-1) are necessary.11 Clarification as to how the DTC cytoskeleton is organized and regulated will further our understanding of the mechanisms that dictate DTC migration and gonad morphogenesis.
ACKNOWLEDGMENTS
The authors acknowledge support from the NIH (R01GM059383) and the NIGMS Cell Migration Consortium (NIH U54 GM064346). MCW is a postdoctoral fellow of the New Jersey Commission on Cancer Research.
REFERENCES
- 1.Kimble J, Hirsh D. The postembryonic cell lineages of the hermaphrodite and male gonads in Caenorhabditis elegans. Dev Biol. 1979;70:396–417. doi: 10.1016/0012-1606(79)90035-6. [DOI] [PubMed] [Google Scholar]
- 2.Kimble JE, White JG. On the control of germ cell development in Caenorhabditis elegans. Dev Biol. 1981;81:208–219. doi: 10.1016/0012-1606(81)90284-0. [DOI] [PubMed] [Google Scholar]
- 3.Hubbard EJ, Greenstein D. The Caenorhabditis elegans gonad: a test tube for cell and developmental biology. Dev Dyn. 2000;218:2–22. doi: 10.1002/(SICI)1097-0177(200005)218:1<2::AID-DVDY2>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 4.Hall DH, Winfrey VP, Blaeuer G, Hoffman LH, Furuta T, Rose KL, Hobert O, Greenstein D. Ultra-structural features of the adult hermaphrodite gonad of Caenorhabditis elegans: relations between the germ line and soma. Dev Biol. 1999;212:101–123. doi: 10.1006/dbio.1999.9356. [DOI] [PubMed] [Google Scholar]
- 5.Huang CC, Hall DH, Hedgecock EM, Kao G, Karantza V, Vogel BE, Hutter H, Chisholm AD, Yurchenco PD, Wadsworth WG. Laminin α subunits and their role in C. elegans development. Development. 2003;130:3343–3358. doi: 10.1242/dev.00481. [DOI] [PubMed] [Google Scholar]
- 6.Kramer JM. Basement Membranes. WormBook; 2005. The C. elegans Research Community. Available at: http://www.wormbook.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Graham PL, Johnson JJ, Wang S, Sibley MH, Gupta MC, Kramer JM. Type IV collagen is detectable in most, but not all, basement membranes of Caenorhabditis elegans and assembles on tissues that do not express it. J Cell Biol. 1997;137:1171–1183. doi: 10.1083/jcb.137.5.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kubota Y, Kuroki R, Nishiwaki K. A fibulin-1 homolog interacts with an ADAM protease that controls cell migration in C. elegans. Curr Biol. 2004;14:2011–2018. doi: 10.1016/j.cub.2004.10.047. [DOI] [PubMed] [Google Scholar]
- 9.Lints R, Hall DH. Reproductive System, Somatic Gonad. WormAtlas; 2009. [Google Scholar]
- 10.Hedgecock EM, Culotti JG, Hall DH, Stern BD. Genetics of cell and axon migrations in Caenorhabditis elegans. Development. 1987;100:365–382. doi: 10.1242/dev.100.3.365. [DOI] [PubMed] [Google Scholar]
- 11.Cram EJ, Shang H, Schwarzbauer JE. A systematic RNA interference screen reveals a cell migration gene network in C. elegans. J Cell Sci. 2006;119:4811–4818. doi: 10.1242/jcs.03274. [DOI] [PubMed] [Google Scholar]
- 12.Nishiwaki K. Mutations affecting symmetrical migration of distal tip cells in Caenorhabditis elegans. Genetics. 1999;152:985–997. doi: 10.1093/genetics/152.3.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vicente-Manzanares M, Horwitz AR. Cell migration: an overview. Methods Mol Biol. 2011;769:1–24. doi: 10.1007/978-1-61779-207-6_1. [DOI] [PubMed] [Google Scholar]
- 14.Kawano T, Zheng H, Merz DC, Kohara Y, Tamai KK, Nishiwaki K, Culotti JG. C. elegans mig-6 encodes papilin isoforms that affect distinct aspects of DTC migration, and interacts genetically with mig-17 and collagen IV. Development. 2009;136:1433–1442. doi: 10.1242/dev.028472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nishiwaki K, Hisamoto N, Matsumoto K. A metalloprotease disintegrin that controls cell migration in Caenorhabditis elegans. Science. 2000;288:2205–2208. doi: 10.1126/science.288.5474.2205. [DOI] [PubMed] [Google Scholar]
- 16.Blelloch R, Kimble J. Control of organ shape by a secreted metalloprotease in the nematode Caenorhabditis elegans. Nature. 1999;399:586–590. doi: 10.1038/21196. [DOI] [PubMed] [Google Scholar]
- 17.Meighan CM, Schwarzbauer JE. Control of C. elegans hermaphrodite gonad size and shape by vab-3/Pax6-mediated regulation of integrin receptors. Genes Dev. 2007;21:1615–1620. doi: 10.1101/gad.1534807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baum PD, Garriga G. Neuronal migrations and axon fasciculation are disrupted in ina-1 integrin mutants. Neuron. 1997;19:51–62. doi: 10.1016/s0896-6273(00)80347-5. [DOI] [PubMed] [Google Scholar]
- 19.Gettner SN, Kenyon C, Reichardt LF. Characterization of β pat-3 heterodimers, a family of essential integrin receptors in C. elegans. J Cell Biol. 1995;129:1127–1141. doi: 10.1083/jcb.129.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee M, Cram EJ, Shen B, Schwarzbauer JE. Roles for β(pat-3) integrins in development and function of Caenorhabditis elegans muscles and gonads. J Biol Chem. 2001;276:36404–36410. doi: 10.1074/jbc.M105795200. [DOI] [PubMed] [Google Scholar]
- 21.Porter S, Clark IM, Kevorkian L, Edwards DR. The ADAMTS metalloproteinases. Biochem J. 2005;386:15–27. doi: 10.1042/BJ20040424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kramerova IA, Kawaguchi N, Fessler LI, Nelson RE, Chen Y, Kramerov AA, Kusche-Gullberg M, Kramer JM, Ackley BD, Sieron AL, et al. Papilin in development; a pericellular protein with a homology to the ADAMTS metalloproteinases. Development. 2000;127:5475–5485. doi: 10.1242/dev.127.24.5475. [DOI] [PubMed] [Google Scholar]
- 23.Apte SS. A disintegrin-like and metalloprotease (reprolysin-type) with thrombospondin type 1 motif (ADAMTS) superfamily: functions and mechanisms. J Biol Chem. 2009;284:31493–31497. doi: 10.1074/jbc.R109.052340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blelloch R, Anna-Arriola SS, Gao D, Li Y, Hodgkin J, Kimble J. The gon-1 gene is required for gonadal morphogenesis in Caenorhabditis elegans. Dev Biol. 1999;216:382–393. doi: 10.1006/dbio.1999.9491. [DOI] [PubMed] [Google Scholar]
- 25.Hesselson D, Newman C, Kim KW, Kimble J. GON-1 and fibulin have antagonistic roles in control of organ shape. Curr Biol. 2004;14:2005–2010. doi: 10.1016/j.cub.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 26.Somerville RP, Longpre JM, Jungers KA, Engle JM, Ross M, Evanko S, Wight TN, Leduc R, Apte SS. Characterization of ADAMTS-9 and ADAMTS-20 as a distinct ADAMTS subfamily related to Caenorhabditis elegans GON-1. J Biol Chem. 2003;278:9503–9513. doi: 10.1074/jbc.M211009200. [DOI] [PubMed] [Google Scholar]
- 27.Tortorella MD, Burn TC, Pratta MA, Abbaszade I, Hollis JM, Liu R, Rosenfeld SA, Copeland RA, Decicco CP, Wynn R, et al. Purification and cloning of aggrecanase-1: a member of the ADAMTS family of proteins. Science. 1999;284:1664–1666. doi: 10.1126/science.284.5420.1664. [DOI] [PubMed] [Google Scholar]
- 28.Kao G, Huang CC, Hedgecock EM, Hall DH, Wadsworth WG. The role of the laminin β subunit in laminin heterotrimer assembly and basement membrane function and development in C. elegans. Dev Biol. 2006;290:211–219. doi: 10.1016/j.ydbio.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 29.Kubota Y, Ohkura K, Tamai KK, Nagata K, Nishiwaki K. MIG-17/ADAMTS controls cell migration by recruiting nidogen to the basement membrane in C. elegans. Proc Natl Acad Sci U S A. 2008;105:20804–20809. doi: 10.1073/pnas.0804055106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muriel JM, Dong C, Hutter H, Vogel BE. Fibulin-1C and Fibulin-1D splice variants have distinct functions and assemble in a hemicentin-dependent manner. Development. 2005;132:4223–4234. doi: 10.1242/dev.02007. [DOI] [PubMed] [Google Scholar]
- 31.Lee NV, Rodriguez-Manzaneque JC, Thai SN, Twal WO, Luque A, Lyons KM, Argraves WS, Iruela-Arispe ML. Fibulin-1 acts as a cofactor for the matrix metalloprotease ADAMTS-1. J Biol Chem. 2005;280:34796–34804. doi: 10.1074/jbc.M506980200. [DOI] [PubMed] [Google Scholar]
- 32.Ziel JW, Sherwood DR. Roles for netrin signaling outside of axon guidance: a view from the worm. Dev Dyn. 2010;239:1296–1305. doi: 10.1002/dvdy.22225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wadsworth WG. Moving around in a worm: netrin UNC-6 and circumferential axon guidance in C. elegans. Trends Neurosci. 2002;25:423–429. doi: 10.1016/s0166-2236(02)02206-3. [DOI] [PubMed] [Google Scholar]
- 34.Wadsworth WG, Bhatt H, Hedgecock EM. Neuroglia and pioneer neurons express UNC-6 to provide global and local netrin cues for guiding migrations in C. elegans. Neuron. 1996;16:35–46. doi: 10.1016/s0896-6273(00)80021-5. [DOI] [PubMed] [Google Scholar]
- 35.Chan SS, Zheng H, Su MW, Wilk R, Killeen MT, Hedgecock EM, Culotti JG. UNC-40, a C. elegans homolog of DCC (deleted in colorectal cancer), is required in motile cells responding to UNC-6 netrin cues. Cell. 1996;87:187–195. doi: 10.1016/s0092-8674(00)81337-9. [DOI] [PubMed] [Google Scholar]
- 36.Su M, Merz DC, Killeen MT, Zhou Y, Zheng H, Kramer JM, Hedgecock EM, Culotti JG. Regulation of the UNC-5 netrin receptor initiates the first reorientation of migrating distal tip cells in Caenorhabditis elegans. Development. 2000;127:585–594. doi: 10.1242/dev.127.3.585. [DOI] [PubMed] [Google Scholar]
- 37.Merz DC, Zheng H, Killeen MT, Krizus A, Culotti JG. Multiple signaling mechanisms of the UNC-6/netrin receptors UNC-5 and UNC-40/DCC in vivo. Genetics. 2001;158:1071–1080. doi: 10.1093/genetics/158.3.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Motola DL, Cummins CL, Rottiers V, Sharma KK, Li T, Li Y, Suino-Powell K, Xu HE, Auchus RJ, Antebi A, et al. Identification of ligands for DAF-12 that govern dauer formation and reproduction in C. elegans. Cell. 2006;124:1209–1223. doi: 10.1016/j.cell.2006.01.037. [DOI] [PubMed] [Google Scholar]
- 39.Colavita A, Culotti JG. Suppressors of ectopic UNC-5 growth cone steering identify eight genes involved in axon guidance in Caenorhabditis elegans. Dev Biol. 1998;194:72–85. doi: 10.1006/dbio.1997.8790. [DOI] [PubMed] [Google Scholar]
- 40.Colavita A, Krishna S, Zheng H, Padgett RW, Culotti JG. Pioneer axon guidance by UNC-129, a C. elegans TGF-β. Science. 1998;281:706–709. doi: 10.1126/science.281.5377.706. [DOI] [PubMed] [Google Scholar]
- 41.Nash B, Colavita A, Zheng H, Roy PJ, Culotti JG. The forkhead transcription factor UNC-130 is required for the graded spatial expression of the UNC-129 TGF-β guidance factor in C. elegans. Genes Dev. 2000;14:2486–2500. doi: 10.1101/gad.831500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.MacNeil LT, Hardy WR, Pawson T, Wrana JL, Culotti JG. UNC-129 regulates the balance between UNC-40 dependent and independent UNC-5 signaling pathways. Nat Neurosci. 2009;12:150–155. doi: 10.1038/nn.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lai Wing Sun K, Correia JP, Kennedy TE. Netrins: versatile extracellular cues with diverse functions. Development. 2011;138:2153–2169. doi: 10.1242/dev.044529. [DOI] [PubMed] [Google Scholar]
- 44.Killeen M, Tong J, Krizus A, Steven R, Scott I, Pawson T, Culotti J. UNC-5 function requires phosphorylation of cytoplasmic tyrosine 482, but its UNC-40-independent functions also require a region between the ZU-5 and death domains. Dev Biol. 2002;251:348–366. doi: 10.1006/dbio.2002.0825. [DOI] [PubMed] [Google Scholar]
- 45.Lee J, Li W, Guan KL. SRC-1 mediates UNC-5 signaling in Caenorhabditis elegans. Mol Cell Biol. 2005;25:6485–6495. doi: 10.1128/MCB.25.15.6485-6495.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li W, Aurandt J, Jurgensen C, Rao Y, Guan KL. FAK and Src kinases are required for netrin-induced tyrosine phosphorylation of UNC5. J Cell Sci. 2006;119:47–55. doi: 10.1242/jcs.02697. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 47.Cram EJ, Fontanez KM, Schwarzbauer JE. Functional characterization of KIN-32, the Caenorhabditis elegans homolog of focal adhesion kinase. Dev Dyn. 2008;237:837–846. doi: 10.1002/dvdy.21457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lucanic M, Kiley M, Ashcroft N, L’Etoile N, Cheng HJ. The Caenorhabditis elegans P21-activated kinases are differentially required for UNC-6/netrin-mediated commissural motor axon guidance. Development. 2006;133:4549–4559. doi: 10.1242/dev.02648. [DOI] [PubMed] [Google Scholar]
- 49.Nikolopoulos SN, Giancotti FG. Netrin-integrin signaling in epithelial morphogenesis, axon guidance and vascular patterning. Cell Cycle. 2005;4:e131–e135. [PubMed] [Google Scholar]
- 50.Cram EJ, Clark SG, Schwarzbauer JE. Talin loss-of-function uncovers roles in cell contractility and migration in C. elegans. J Cell Sci. 2003;116:3871–3878. doi: 10.1242/jcs.00705. [DOI] [PubMed] [Google Scholar]
- 51.Itoh B, Hirose T, Takata N, Nishiwaki K, Koga M, Ohshima Y, Okada M. SRC-1, a non-receptor type of protein tyrosine kinase, controls the direction of cell and growth cone migration in C. elegans. Development. 2005;132:5161–5172. doi: 10.1242/dev.02103. [DOI] [PubMed] [Google Scholar]
- 52.Kim C, Ye F, Ginsberg MH. Regulation of Integrin Activation. Annu Rev Cell Dev Biol. 2011 doi: 10.1146/annurev-cellbio-100109-104104. [DOI] [PubMed] [Google Scholar]
- 53.Anthis NJ, Campbell ID. The tail of integrin activation. Trends Biochem Sci. 2011;36:191–198. doi: 10.1016/j.tibs.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moulder GL, Huang MM, Waterston RH, Barstead RJ. Talin requires β-integrin, but not vinculin, for its assembly into focal adhesion-like structures in the nematode Caenorhabditis elegans. Mol Biol Cell. 1996;7:1181–1193. doi: 10.1091/mbc.7.8.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ihara S, Nishiwaki K. Prodomain-dependent tissue targeting of an ADAMTS protease controls cell migration in Caenorhabditis elegans. EMBO J. 2007;26:2607–2620. doi: 10.1038/sj.emboj.7601718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ihara S, Nishiwaki K. Stage-specific activation of MIG-17/ADAMTS controls cell migration in Caenorhabditis elegans. FEBS J. 2008;275:4296–4305. doi: 10.1111/j.1742-4658.2008.06573.x. [DOI] [PubMed] [Google Scholar]
- 57.Nishiwaki K, Kubota Y, Chigira Y, Roy SK, Suzuki M, Schvarzstein M, Jigami Y, Hisamoto N, Matsumoto K. An NDPase links ADAM protease glycosylation with organ morphogenesis in C. elegans. Nat Cell Biol. 2004;6:31–37. doi: 10.1038/ncb1079. [DOI] [PubMed] [Google Scholar]
- 58.Kubota Y, Sano M, Goda S, Suzuki N, Nishiwaki K. The conserved oligomeric Golgi complex acts in organ morphogenesis via glycosylation of an ADAM protease in C. elegans. Development. 2006;133:263–273. doi: 10.1242/dev.02195. [DOI] [PubMed] [Google Scholar]
- 59.Suzuki N, Toyoda H, Sano M, Nishiwaki K. Chondroitin acts in the guidance of gonadal distal tip cells in C. elegans. Dev Biol. 2006;300:635–646. doi: 10.1016/j.ydbio.2006.08.037. [DOI] [PubMed] [Google Scholar]
- 60.Kinnunen T, Huang Z, Townsend J, Gatdula MM, Brown JR, Esko JD, Turnbull JE. Heparan 2-O-sulfotransferase, hst-2, is essential for normal cell migration in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2005;102:1507–1512. doi: 10.1073/pnas.0401591102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Merz DC, Alves G, Kawano T, Zheng H, Culotti JG. UNC-52/perlecan affects gonadal leader cell migrations in C. elegans hermaphrodites through alterations in growth factor signaling. Dev Biol. 2003;256:173–186. doi: 10.1016/s0012-1606(03)00014-9. [DOI] [PubMed] [Google Scholar]
- 62.Schwabiuk M, Coudiere L, Merz DC. SDN-1/syndecan regulates growth factor signaling in distal tip cell migrations in C. elegans. Dev Biol. 2009;334:235–242. doi: 10.1016/j.ydbio.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 63.Trzebiatowska A, Topf U, Sauder U, Drabikowski K, Chiquet-Ehrismann R. Caenorhabditis elegans teneurin, ten-1, is required for gonadal and pharyngeal basement membrane integrity and acts redundantly with integrin ina-1 and dystroglycan dgn-1. Mol Biol Cell. 2008;19:3898–3908. doi: 10.1091/mbc.E08-01-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Drabikowski K, Trzebiatowska A, Chiquet-Ehrismann R. ten-1, an essential gene for germ cell development, epidermal morphogenesis, gonad migration, and neuronal pathfinding in Caenorhabditis elegans. Dev Biol. 2005;282:27–38. doi: 10.1016/j.ydbio.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 65.Johnson RP, Kang SH, Kramer JM. C. elegans dystroglycan DGN-1 functions in epithelia and neurons, but not muscle, and independently of dystrophin. Development. 2006;133:1911–1921. doi: 10.1242/dev.02363. [DOI] [PubMed] [Google Scholar]
- 66.Xu X, Ahn JH, Tam P, Yu EJ, Batra S, Cram EJ, Lee M. Analysis of conserved residues in the β pat-3 cytoplasmic tail reveals important functions of integrin in multiple tissues. Dev Dyn. 2010;239:763–772. doi: 10.1002/dvdy.22205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Moser M, Legate KR, Zent R, Fassler R. The tail of integrins, talin, and kindlins. Science. 2009;324:895–899. doi: 10.1126/science.1163865. [DOI] [PubMed] [Google Scholar]
- 68.Darom A, Bening-Abu-Shach U, Broday L. RNF-121 is an endoplasmic reticulum-membrane E3 ubiquitin ligase involved in the regulation of β-integrin. Mol Biol Cell. 2010;21:1788–1798. doi: 10.1091/mbc.E09-09-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mackinnon AC, Qadota H, Norman KR, Moerman DG, Williams BD. C. elegans PAT-4/ILK functions as an adaptor protein within integrin adhesion complexes. Curr Biol. 2002;12:787–797. doi: 10.1016/s0960-9822(02)00810-2. [DOI] [PubMed] [Google Scholar]
- 70.Rogalski TM, Mullen GP, Gilbert MM, Williams BD, Moerman DG. The UNC-112 gene in Caenorhabditis elegans encodes a novel component of cell-matrix adhesion structures required for integrin localization in the muscle cell membrane. J Cell Biol. 2000;150:253–264. doi: 10.1083/jcb.150.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lin X, Qadota H, Moerman DG, Williams BD. C. elegans PAT-6/actopaxin plays a critical role in the assembly of integrin adhesion complexes in vivo. Curr Biol. 2003;13:922–932. doi: 10.1016/s0960-9822(03)00372-5. [DOI] [PubMed] [Google Scholar]
- 72.Xu X, Rongali SC, Miles JP, Lee KD, Lee M. pat-4/ILK and unc-112/Mig-2 are required for gonad function in Caenorhabditis elegans. Exp Cell Res. 2006;312:1475–1483. doi: 10.1016/j.yexcr.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 73.Lundquist EA. Small GTPases. WormBook; 2006. The C. elegans Research Community. Available at: http://www.wormbook.org. [Google Scholar]
- 74.Reddien PW, Horvitz HR. CED-2/CrkII and CED-10/Rac control phagocytosis and cell migration in Caenorhabditis elegans. Nat Cell Biol. 2000;2:131–136. doi: 10.1038/35004000. [DOI] [PubMed] [Google Scholar]
- 75.Lundquist EA, Reddien PW, Hartwieg E, Horvitz HR, Bargmann CI. Three C. elegans Rac proteins and several alternative Rac regulators control axon guidance, cell migration and apoptotic cell phagocytosis. Development. 2001;128:4475–4488. doi: 10.1242/dev.128.22.4475. [DOI] [PubMed] [Google Scholar]
- 76.Gumienny TL, Brugnera E, Tosello-Trampont AC, Kinchen JM, Haney LB, Nishiwaki K, Walk SF, Nemergut ME, Macara IG, Francis R, et al. CED-12/ELMO, a novel member of the CrkII/Dock180/Rac pathway, is required for phagocytosis and cell migration. Cell. 2001;107:27–41. doi: 10.1016/s0092-8674(01)00520-7. [DOI] [PubMed] [Google Scholar]
- 77.Wu YC, Horvitz HR. C. elegans phagocytosis and cell-migration protein CED-5 is similar to human DOCK180. Nature. 1998;392:501–504. doi: 10.1038/33163. [DOI] [PubMed] [Google Scholar]
- 78.Wu YC, Tsai MC, Cheng LC, Chou CJ, Weng NY. C. elegans CED-12 acts in the conserved crkII/DOCK180/Rac pathway to control cell migration and cell corpse engulfment. Dev Cell. 2001;1:491–502. doi: 10.1016/s1534-5807(01)00056-9. [DOI] [PubMed] [Google Scholar]
- 79.Zhou Z, Caron E, Hartwieg E, Hall A, Horvitz HR. The C. elegans PH domain protein CED-12 regulates cytoskeletal reorganization via a Rho/Rac GTPase signaling pathway. Dev Cell. 2001;1:477–489. doi: 10.1016/s1534-5807(01)00058-2. [DOI] [PubMed] [Google Scholar]
- 80.Reddien PW, Horvitz HR. The engulfment process of programmed cell death in caenorhabditis elegans. Annu Rev Cell Dev Biol. 2004;20:193–221. doi: 10.1146/annurev.cellbio.20.022003.114619. [DOI] [PubMed] [Google Scholar]
- 81.Lee M, Shen B, Schwarzbauer JE, Ahn J, Kwon J. Connections between integrins and Rac GTPase pathways control gonad formation and function in C. elegans. Biochim Biophys Acta. 2005;1723:248–255. doi: 10.1016/j.bbagen.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 82.Hoefen RJ, Berk BC. The multifunctional GIT family of proteins. J Cell Sci. 2006;119:1469–1475. doi: 10.1242/jcs.02925. [DOI] [PubMed] [Google Scholar]
- 83.Frank SR, Hansen SH. The PIX-GIT complex: a G protein signaling cassette in control of cell shape. Semin Cell Dev Biol. 2008;19:234–244. doi: 10.1016/j.semcdb.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ong CC, Jubb AM, Zhou W, Haverty PM, Harris AL, Belvin M, Friedman LS, Koeppen H, Hoeflich KP. p21-activated kinase 1: PAK’ed with potential. Oncotarget. 2011;2:491–496. doi: 10.18632/oncotarget.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lucanic M, Cheng HJ. A RAC/CDC-42-independent GIT/PIX/PAK signaling pathway mediates cell migration in C. elegans. PLoS Genet. 2008;4:e1000269. doi: 10.1371/journal.pgen.1000269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hurwitz ME, Vanderzalm PJ, Bloom L, Goldman J, Garriga G, Horvitz HR. Abl kinase inhibits the engulfment of apoptotic [corrected] cells in Caenorhabditis elegans. PLoS Biol. 2009;7:e99. doi: 10.1371/journal.pbio.1000099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tannoury H, Rodriguez V, Kovacevic I, Ibourk M, Lee M, Cram EJ. CACN-1/Cactin interacts genetically with MIG-2 GTPase signaling to control distal tip cell migration in C. elegans. Dev Biol. 2010;341:176–185. doi: 10.1016/j.ydbio.2010.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim HS, Murakami R, Quintin S, Mori M, Ohkura K, Tamai KK, Labouesse M, Sakamoto H, Nishiwaki K. VAB-10 spectraplakin acts in cell and nuclear migration in Caenorhabditis elegans. Development. 2011;138:4013–4023. doi: 10.1242/dev.059568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cabello J, Neukomm LJ, Gunesdogan U, Burkart K, Charette SJ, Lochnit G, Hengartner MO, Schnabel R. The Wnt pathway controls cell death engulfment, spindle orientation, and migration through CED-10/Rac. PLoS Biol. 2010;8:e1000297. doi: 10.1371/journal.pbio.1000297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ridley AJ. Life at the leading edge. Cell. 2011;145:1012–1022. doi: 10.1016/j.cell.2011.06.010. [DOI] [PubMed] [Google Scholar]
WEB RESOURCES
- WormAtlas. Altun ZF, Hall DH, editors. 2002–2006 Available at: http://www.wormatlas.org.
- Chalfie M, editor. WormBook, the Online Review of C. elegans Biology. doi: 10.1895/wormbook.1.177.1. ISSN:1551-8507. Available at: http://www.wormbook.org. [DOI] [PMC free article] [PubMed]