Abstract
Objective
Continuous drug delivery to the ocular surface remains difficult due to the rapid tear clearance of topically applied agents. The purpose of this study was to evaluate biodegradable and biocompatible drug delivery systems on the ocular surface using poly-lactic-co-glycolic acid (PLGA) based polymers.
Methods
Fluorescein-labeled albumin and doxycycline were individually encapsulated into a PLGA-based matrix using a water-oil-water double emulsion method. The drug elution rates for various microspheres were evaluated spectrofluorometrically. Particle size was measured using image analysis software. Subconjunctival injections of PLGA microspheres were used to evaluate safety and inflammatory response to the polymer in the murine model. Efficacy of the drug delivery system was evaluated by a single subconjunctival injection of PLGA-doxycycline (a broad metalloproteinase inhibitor) prior to induction of desiccating stress (DS) model in C57BL/6 mice for 5 days.
Results
PLGA-based microspheres successfully elute encapsulated drugs of interest continuously over controlled periods of time. Mean PLGA-based microparticle diameter was 4.6 μm±1.54 μm. Drug elution rates and delivery times were easily modifiable by altering polymers and synthesis parameters. In vitro studies demonstrate successful continuous elution of encapsulated drugs for at least 2 weeks. In vivo testing of PLGA-doxycycline was efficacious in preventing DS-induced corneal barrier disruption with desiccating stress, similarly to topically applied doxycycline.
Conclusions
PLGA-based drug delivery systems are safe and non-inflammatory. They can be successfully used to treat ocular surface and corneal diseases by continuously delivering biopharmaceuticals of interest.
Keywords: PLGA, Doxycycline, Desiccating stress
Introduction
Drug delivery systems in both research and clinical applications have been gaining interest over the past decade due to the significant developments in novel biopharmaceuticals that necessitate sustained therapeutic concentration levels to achieve desired effects. Polymeric-based delivery systems have been utilized to help attain time-controlled drug delivery. Achieving sustained therapeutic levels on the ocular surface remains a challenge due to the continuous basal production of tears and tear clearance through the lacrimal drainage system. These physiologic mechanisms limit the efficacy topical treatments to the ocular surface and may limit compliance due to difficult dosing schedules.
Microparticles fabricated from biodegradable poly (DL-lactic-co-glycolic acid) (PLGA) copolymers have been widely utilized as carriers for bioactive molecules [1]. PLGA has been demonstrated to be both biocompatible and biodegradable, and is approved by the FDA for specific human clinical applications [2]. The advantage of polymeric-based scaffold systems is the variability in drug delivery rates achievable by altering fundamental synthesis parameters. Degradation rates can be altered to be from days to years simply by varying the molecular weight of the polymeric chains, ratio of lactic to glycolic acid, or the structure and size of the microparticle. Release rates of encapsulated molecules are also controlled by the structure and solubility of the drugs themselves, as well as the microenvironment of degradation once the microspheres are delivered. For example, an inflammatory response following implantation can decrease the pH microenvironment and effect the structure, solubility, diffusivity, and activity of the drugs being delivered [3]. Prior studies have successfully demonstrated the use of biodegradable polymeric systems in non-ocular environments [4], and evaluated the parameters that affect drug release kinetics in vitro [5,6].
PLGA microspheres have been used in dentistry to deliver doxycycline as both a matrix metalloproteinase inhibitor and as an antibiotic for treatment of periodontal disease [7]. Likewise they have been used in a limited number of ophthalmic applications [8–11] Doxycycline-loaded particles have been created and characterized with the intention of treating ocular surface disease [8]. However, none have specifically used PLGA to treat ocular surface disease or more specifically, an experimentally induced dry eye disease.
Dry eye is a common disease, with a reported prevalence of up to 15% of the population. The eye irritation and blurred vision associated with this condition can cause significant morbidity [12,13]. The corneal epithelial surface provides a critical barrier function to maintain corneal integrity by preventing intrusion by microbes, inflammatory mediators, and inflammatory cells. The intercellular tight-junctional complexes in the cornea and conjunctiva not only provide a defensive barrier, but also restrict the penetration of therapeutic drugs in ocular diseases. Keratoconjunctivitis sicca (KCS) can also lead to corneal ulceration, which causes a permanent decrease in vision and potentially even blindness due to chronic elevated pro-inflammatory cytokines and chemokines [14,15]. The desiccating and hyperosmolar stress in KCS have been found to activate an array of signaling pathways, including mitogen-activated protein kinase, which ultimately regulates various matrix metalloproteinases (MMP-9, -1, -3, and -13) as well as epithelial differentiation [16]. The upregulation of these proteins and enzymes results in tight junction protein degradation and increased epithelial desquamation.
The purpose of our investigation was to determine the potential use of PLGA microspheres as a drug delivery system to modulate the ocular surface in an experimental dry eye murine model. This study is the first attempt to use PLGA to treat ocular surface disease, and, more specifically, an experimentally induced dry eye disease.
Methods
Preparation of PLGA microspheres
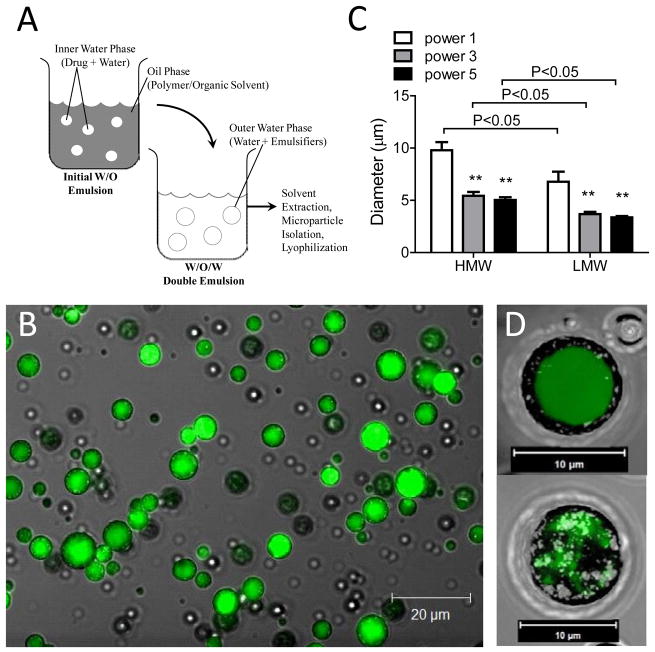
The PLGA microspheres were prepared by a double-emulsion solvent extraction method (W1/O/W2, water-in-oil-in-water), shown in Figure 1A [5,6]. The initial water phase (W1) consists of the drug of interest suspended in aqueous solution. The oil phase (O) consists of the biodegradable polymer dissolved in organic solvent, which will form the polymeric mesh. The final water phase (W2) formulates the microspheres. Fluorescein isothiocyanate-labeled human albumin (FITC-albumin) (Sigma-Aldrich, A7016) and doxycycline hyclate (Sigma-Aldrich, D9891) were separately encapsulated into this PLGA-based matrix.
Figure 1.
(A) Schematic of water/oil/water(W/O/W) double-emulsion microparticle synthesis technique. (B) Confocal image of FITC-albumin loaded high molecular weight PLGA microspheres at ultrasonication power setting #1 with 40x objective. Scale bar = 20μm. (C) Microparticle size when synthesized with low and high molecular weight (LMW and HMW, respectively) PLGA at ultrasonication power settings #1, 3, and 5. (** = p<0.05 compared to power #1). Decreases in polymer size and ultrasonication power decreases microparticle size. (D) 3-D Confocal projection of FITC-albumin loaded PLGA microsphere at day 0 (top) and day 14 (bottom). Note PLGA degradation and loss of drug at day 14. Image obtained at 63x under oil. Scale bar = 10μm.
Two PLGA polymers were evaluated for microsphere synthesis, low molecular weight [(LMW-PLGA) average MW 5000–15000 lactide:glycolide (50:50)] and high molecular weight [(HMW-PLGA) average MW 40000–75000 lactide:glycolide (50:50)] (Sigma-Aldrich, St. Louis, MO). Briefly, 500 mg of polymer was dissolved in 1.5 mL of methylene chloride (DCM). Unused polymer was stored under argon gas. Aqueous drug solution (250μL) or aqueous FITC-albumin solution (750 μL) [W1] was added to the organic phase solution, containing the polymer. This solution was emulsified using an ultrasonicator (Virsonic 60 Ultrasonic Cell Disrupter, Virtis Co., Gardiner, NY) for 15–20 seconds to form the initial water-oil suspension. FITC-BSA containing particles were ultrasonicated at power settings #1, 3, or 5, and drug-containing particles were ultrasonicated at power setting #3. Next, 5 mL of ice cold 2% (w/v) polyvinyl alcohol [average MW 13,000–23,000 87–89% hydrolyzed] (Sigma-Aldrich, St. Louis, MO) was added to the emulsion and ultrasonicated at the previous power setting for an additional 15–20 seconds to complete the double-emulsion process. The resulting W1/O/W2 mixture was then poured into 150 mL of rapidly stirred, ice cold 2% polyvinyl alcohol. 150 mL of ice cold 2% isopropanol was then added to the mixture for 15–30 minutes to allow for solvent extraction and microsphere hardening. A 37 micron polyester mesh (Spectra Mesh Polyester Mesh 37 micron opening, Lot #3243525, Spectrum Labs, Rancho Dominguez, CA) was used to sieve and isolate monodispersed microspheres. The microspheres were then centrifuged, washed at 600g for 5 minutes at 4°C (Eppendorf Centrifuge 5810R, Westbury, NY), flash frozen with liquid nitrogen, and lyophilized at −20°C for 72 hours before storage at −20°C.
Characterization of PLGA microspheres
Microspheres were fixed in 4% paraformaldehyde phosphate-buffered saline solution and mounted on a microscope slide for visualization. All images of the microspheres were acquired using a Zeiss 510 laser scanning confocal system mounted on an Axio Imager microscope and Plan-Apochromat 40x and 63x oil objective lens (Carl Zeiss Microimaging, Thornwood, NY). FITC-albumin loaded PLGA microspheres were excited with a 488 nm argon laser source. Particle size and distribution of microspheres were then analyzed using NIS-Elements software (Nikon, Melville, NY).
In vitro release of microspheres
The drug elution rates for FITC-albumin loaded LMW-PLGA and HMW-PLGA microspheres, created at ultrasonication power settings #1, 3, and 5, in balanced salt saline solution were measured daily in vitro, at 37°C, over 20 days by spectrofluorometry (SpectraMax M2, Molecular Devices, Sunnyvale, CA). The eluent was separated from the microspheres and measured in a 384 well low volume fluorescence microplate. Initial synthesis parameters were 30 mg FITC-Albumin (BSA) with 500 mg of PLGA polymer. Concentrations of FITC-albumin were extrapolated from a standardized FITC-albumin fluorescent calibration curve.
In vivo study
This research protocol was approved by the Baylor College of Medicine Center for Comparative Medicine, and it conformed to the standards in the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
To evaluate the distribution, safety, and inflammatory response to the microspheres, nonstressed C57BL/6 mice (n=3) received bilateral subconjunctival injections of FITC-albumin loaded HMW-PLGA microspheres (10uL, 1mg) using a 33-gauge needle (Hamilton Inc., Reno, NV). Injection was chosen over topical administration to avoid particle loss with tear turnover. Mice were euthanized after 14 days.
As a proof of concept, the drug delivery system was evaluated by examining the effects of doxycycline, in the experimental dry eye mouse model that has been reported to have increased MMP-9 levels [17]. Doxycycline is a semisynthetic tetracycline that is often used to treat matrix metalloproteinase mediated diseases such as rosacea, recurrent epithelial corneal erosions, and sterile corneal ulcerations [18–20] It has been shown to suppress inflammatory cytokine expression on the ocular surface, and to significantly improve desiccating stress-induced corneal barrier disruption[16,21,22].
Initial synthesis parameters of doxycycline-loaded PLGA were 10 mg of doxycycline hyclate and 500 mg of HMW-PLGA. A single subconjunctival (10 μL, 1 mg) injection of doxycycline-loaded or sham (BSS)-loaded PLGA microspheres was given using a 33 gauge needle prior to induction of 5 days of desiccating stress (DS) in C57BL/6 mice. DS was induced in the mice, aged 6 to 8 weeks, by subcutaneous injection of 0.5mg/0.2mL scopolamine hydrobromide (Sigma-Aldrich, St. Louis, MO) in alternating hindquarters, four times a day, in addition to exposure to an air draft and < 40% ambient humidity for 18 hours per day, as previously reported [23].
Twenty four C57BL/6 mice were divided into four experimental groups (n=6/group): non-stressed controls (NS, no dry eye treatment and no PLGA injection); five days of desiccating stress (DS5) without injection; DS5 with sham balanced salt solution loaded PLGA injection (DS5+S-PLGA); DS5 with doxycycline-PLGA injection (DS5+D-PLGA).
Corneal permeability
Corneal epithelial permeability was assessed with Oregon green dextran (OGD, MW 70,000, Molecular Probes, Eugene, OR) based on a previously published method [16]. OGD was instilled onto the ocular surface 1 min prior to euthanization (0.5 ul of 50 mg/mL). The corneas were then rinsed with 2 ml of PBS and photographed with a SMZ 1500 stereoscopic zoom microscope (Nikon, Melville, NY), under a fluorescence excitation at 470 nm. Images were processed using Metavue 6.24r software (Molecular Devices, Sunnyvale, CA). The severity of corneal OGD staining was digitally graded as previously reported [24]. Mean fluorescence intensity measured inside the 2mm central zone by the image analysis software (Metavue 6.2r; Molecular Devices, Sunnyvale, CA) was averaged within each group. Results are presented as mean ± standard deviation of fluorescence gray levels.
Statistical analysis
Analysis of variance (ANOVA) with Tukey’s post hoc testing was used for statistical comparisons, and p < 0.05 was considered statistically significant. These tests were performed using GraphPad Prism 5.0 software (GraphPad Software, San Diego, CA).
Results
Microsphere morphology
To evaluate microsphere morphology, FITC-albumin loaded PLGA particles were synthesized and photographed using laser scanning confocal microscopy. Microspheres prepared with a W/O/W technique (Figure 1A) demonstrated uniformity in drug encapsulation and sphericity as well as monodispersity when suspended in solution (Figure 1C), permitting a reproducible technique for biological applications. Microsphere size decreased at higher ultrasonication powers for both HMW-PLGA (p<0.001) and LMW-PLGA (p<0.01) (Figure 1B). Likewise, the use of LMW-PLGA resulted in the formation of smaller particles. The protocol used produced a synthesis yield of 50–60 %, and greater than 99% of microspheres synthesized demonstrated uniform drug encapsulation. Larger particles were naturally able to encapsulate more of the target compound. However, when particle volume was taken into account, low and high molecular weight microspheres showed similar levels of encapsulation efficiency. Figure 1C (inset) demonstrates the loss of FITC-albumin out of an individual PLGA microsphere as well as the degradation of the polymeric scaffold after two weeks in vitro.
In vitro drug release rate
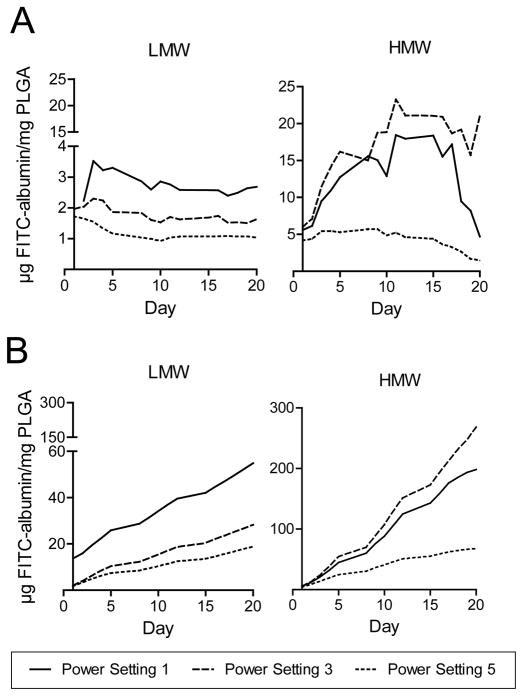
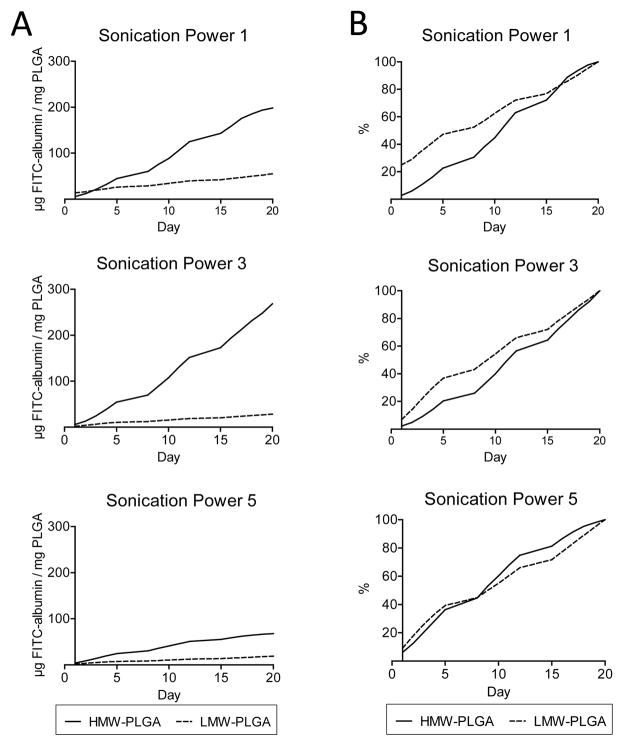
To assess drug release, an in vitro elution assay was performed. PLGA microparticles loaded with FITC-albumin were kept at 37°C for 20 days and each day, at the same time, was sampled. At the end the third week, samples were measured spectrofluorometrically. Our results demonstrated continuous elution of encapsulated drugs over 20 days. Figure 1D shows a three dimensional confocal projection of FITC-albumin elution and PLGA degradation from day zero to fourteen. Characteristics of the sustained release of target compound from the microparticles are exhibited in Figures 2 and 3. Lower drug elution rates and a larger initial elutional burst were observed at higher ultrasonication powers (Figure 2). These trends were likewise seen with the smaller LMW-PLGA particles when compared to the larger HMW-PLGA ones (Figure 3).
Figure 2.
(A) Daily elution of FITC-albumin from microparticles synthesized with low and high molecular weight (LMW and HMW, respectively) PLGA. Increase in ultrasonication power resulted in lower drug elution rates for LMW particles. (B) Cumulative elution of FITC-albumin from microparticles synthesized with low and high (LMW and HMW, respectively) molecular weight PLGA.
Figure 3.
(A) Comparison of cumulative elution from low and high molecular weight PLGA (LMW and HMW, respectively) at ultrasonication power settings #1, 3, and 5. HMW-PLGA exhibits greater cumulative elution. (B) Comparison of percent total elution from low and high molecular weight (LMW and HMW, respectively) PLGA at ultrasonication power settings #1, 3, and 5. Note initial burst phenomenon is more prevalent at lower ultrasonication powers and with LMW-PLGA.
In vivo evaluation in dry eye experimental murine model
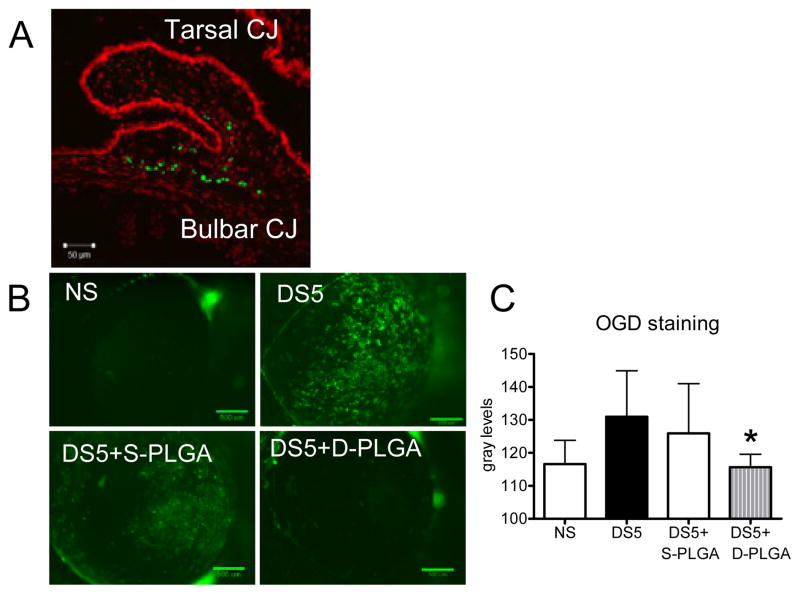
To gauge the safety of in vivo use of PLGA microshperes, a single subconjunctival injection of FITC-loaded particles was performed. Mice were then followed for 14 days for signs of inflammation. Subjects were then euthanized and eyes were sectioned and photographed with laser confocal microscopy. Injection resulted in localization to the subconjunctival and sub-Tenon space. No significant inflammation was evoked two weeks post-injection (Figure 4A).
Figure 4.
(A) Representative image of subconjunctival injections of fluorescent PLGA microparticles two weeks post-injection. Note dispersion of particles along the subconjunctival space. (B) Representative digital images of corneas used to score Oregon Green Dextran-488 (OGD) permeability in nonstressed control mice (NS) and mice subjected to desiccating for 5 days (DS5) and DS5 treated with either Sham-PLGA (DS5+Sham PLGA) or Doxycycline-PLGA (DS5+Doxycycline-PLGA). Sham-PLGA microparticles treatment demonstrates similar results to nonstressed controls, while doxycycline-loaded PLGA microparticles demonstrate efficacy in mitigating desiccating stress. (C) OGD corneal staining score of mice before (NS) and after 5 days of desiccating stress (DS5) and DS5 treated with either Sham-PLGA (DS5+S-PLGA) or Doxycycline-PLGA (DS5+D-PLGA). * = P < 0.05 vs. DS5+S-PLGA.
We have previously shown that our DS model disrupts corneal barrier function in mice and mimics the corneal epithelial disease seen in humans [25]. Using this model, we showed that daily, topical doxycycline was efficacious in decreasing DS-induced corneal barrier disruption.
To evaluate PLGA as a drug delivery system, we prepared doxycycline-loaded PLGA particles, and injected them subconjunctivally in both eyes, once, immediately prior to the induction of DS. No topical treatment was used. Control mice received subconjunctival injections of BSS-loaded PLGA. Following five days of desiccating stress, the intensity of corneal OGD staining was measured.
It was found that DS induced a significant corneal barrier disruption, which was prevented by treatment with PLGA-doxycycline, while Sham-PLGA had no effect (Figures 4B & C). These results parallel our previously tested reported findings showing efficacy of treatment with daily topical doxycycline in an experimental dry eye [22].
Discussion
In this study, we modified an established microsphere fabrication technique for clinical applications in treatment of ocular surface disease. We also demonstrated biocompatibility of the microparticles as well as their ability to deliver a drug of interest and thus attain a desirable biological response.
Drug elution rates and delivery time were easily modifiable by altering the synthesis parameters of microparticle size via ultrasonication power and polymer molecular weight. It was found that higher ultrasonication powers and lower molecular weight PLGA resulted in a smaller microparticle. However, to say that drug elution is only determined by microparticle size, and that the two aforementioned variables only affect microparticle size, is an oversimplification as many variables appear to contribute to the elution profile.
The elution profile can be broken down into two main parts: 1) the initial burst, and 2) the ensuing steady elution rate. The initial burst is created by the drug which leaches out during the final steps of the synthesis and the drug which is able to escape from the microparticles immediately after resuspension but prior to microparticle degradation. Encapsulation efficiency is one of the greatest factors contributing to the initial burst. Immediately after the solvent extraction, microparticles begin degrading until they are flash-frozen and lyophilized. Although excess liquid is removed prior to lyophilization, a portion of the drug that escapes the microspheres during this time is present immediately when the particles are resuspended in an aqueous solution. Porosity is another important aspect of the initial drug burst; a high ratio of the starting pore size to the size of the molecules encapsulated will allow for a greater degree of drug elution irrespective of microparticle degradation rate.
In our data, the burst phenomenon was greater in the LMW-PLGA particles, at lower ultrasonication powers due to the two aforementioned reasons. LMW-PLGA particles had lower encapsulation efficiency, exhibited by their lower cumulative elution than HMW-PGLA particles. The increased rate seen between days one and five also suggests that LMW-PLGA tends to form more porous spheres, and that this porosity is increased by the use of lower sonication powers.
The behavior of LMW-PLGA can be understood by considering how microparticles are formed with the W/O/W technique. When dissolved in an organic solvent and then agitated in an aqueous solution, polymers form spheres similar to the way fat forms micelles in the digestive system. When cooled, these polymers spontaneously form bonds between them. Proximity of one polymer molecule to another and degree of overlap is what determines how strong a bond is created between them. Longer polymers have the potential of forming very strong bonds with numerous other polymers, leading to a more dense mesh of polymer, and thus a less porous microparticle. This characteristic of larger polymers forming a more dense mesh also makes them less susceptible to disruption, and likewise they create larger particles even when higher ultrasonication powers are used.
Drug elution rates following the initial burst are largely determined by the degradation of the microparticle. Smaller particles typically release drugs at a faster rate because of the increased ratio of surface area to volume of the microsphere. Particle size can largely be controlled by the length of the polymer and ultrasonication power used in synthesis; however, one is relatively limited in how small particles can be made using this synthesis protocol. Smaller particles require centrifugation at extremely high speeds to isolate them from solution in the last step of synthesis. This results in greater difficulty of resuspension after lyophilization and the potential formation of aggregates in solution. Another parameter that needs to be adjusted in synthesizing smaller microspheres is the time needed for solvent extraction and microsphere hardening. Smaller microparticles degrade and release drugs more rapidly, decreasing the encapsulation yield and increasing formation of aggregates during lyophilization. Thus, drug elution rates can be increased by decreasing the size of the microparticles synthesized but practically to a certain extent.
Irrespective of the molecular weight of the polymer used, higher ultrasonication powers resulted in lower drug elution rates. This is contrary to the presumption that smaller particles will have a higher elution rate due to their higher cumulative surface area, and thus exposure to aqueous environment. A potential reason for this is because higher ultrasonication powers also lead to decreased porosity and lower encapsulation efficiencies. Thus, it will take longer for drug to be able to escape from the particles, and once it is able to, there will be a smaller concentration gradient to drive the diffusion.
As illustrated by our results, the advantage of PLGA-based scaffolds for drug delivery is the ability to alter drug release rates simply by changing the molecular weight of the polymer, the ratio of lactic acid to glycolic acid groups on the polymeric chain, or the size of the particles via the amount of ultrasonication power used.
The use of PLGA as drug delivery system on the ocular surface carries unique challenges as opposed to other regions of the eye. Even though these particles provide the continuous release of a drug, delivery of any therapeutic substance to the ocular surface will always be hindered by tear clearance. Promising attempts have been made to overcome this hurdle with the development of mucoadhesive microparticles [26]. However, the use of such a particle is beyond the aims of this study, which was to prove that PLGA could be used to enhance the current treatment of DED.
Previous studies with doxycycline in an experimental dry eye mouse model, and in humans, have demonstrated success in improving corneal epithelial irregularity and providing symptomatic improvement in patients [19,22]. Frequent daily administration (4x/ day) was required to mitigate the effects of desiccating stress. We chose this established model to demonstrate efficacy and the advantages of a sustained drug-release system. A single subconjunctival injection of doxycycline-loaded PLGA microspheres given to the mice prior to induction of desiccating stress yielded significant improvement in corneal barrier function. By simplifying dosing schedules and providing continuous drug release, the use of microparticles allow increased flexibility in designing experimental projects. It also offers a new tool to evaluate the effects of novel growth factors and signaling peptides, which require a constantly high signaling concentration that might be difficult to sustain with topical administration.
Ocular drug delivery to achieve specific therapeutic response can be challenging for patients since it requires multiple dosing regimens throughout the day to achieve specific desired concentrations. For example, treatment of herpetic keratitis requires nine drops a day of trifluridine or intraocular pressure lowering regimens to treat advanced glaucoma require multiple complex drug dosing [27,28]. In areas of experimental eye research, modulating the ocular surface with growth factors may also require multiple dosing regimens. The difficulty in attaining therapeutic threshold levels is due to multiple factors resulting in increased drug clearance rates. By designing a convenient biologically compatible, continuous drug delivery system, many of these obstacles may be overcome.
Summary
PLGA-based drug delivery systems have been studied extensively and are capable polymers for clinical applications because of their biocompatibility and established synthesis protocols. We successfully demonstrated an in vitro and in vivo application of PLGA-based microspheres for continuous drug release. PLGA-doxycycline was efficacious in preventing desiccating stress-induced corneal barrier disruption in experimental dry eye, similar to daily administered doxycycline. Our study validates PLGA-based drug delivery systems as a promising tool to treat ocular diseases by continuously delivering biopharmaceuticals of interest. Current investigations are underway to evaluate the efficacy in delivering growth factors in vivo to modulate ocular surface diseases.
Acknowledgments
The authors of this paper would like to gratefully acknowledge the support from the NIH Grant EY11915 (SCP), Fight for Sight 2430063503 (EC) and Lions Foundation for Sight (CSDP). We also greatly appreciate Dr. Antonios Mikos and Dr. Rebekah Drezek at Rice University for their support and technical services.
Funding
This work was supported by National Institutes of Health Grants EY11915 to S.C.P.; Fight for Sight Fellowship to E.C.; Research to Prevent Blindness, Oshman Foundation; William Stamps Farish Fund; Hamill Foundation, Lions Foundation for Sight to C.S.D.P.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Cohen S, Yoshioka T, Lucarelli M, Hwang LH, Langer R. Controlled delivery systems for proteins based on poly(lactic/glycolic acid) microspheres. Pharm Res. 1991;8:713–720. doi: 10.1023/a:1015841715384. [DOI] [PubMed] [Google Scholar]
- 2.Suggs L, Mikos A. Synthetic biodegradable polymers for medical applications. In: Mark J, editor. Physical Properties of Polymers Handbook. Woodbury, NY: American Institute of Physics; 1996. pp. 615–624. [Google Scholar]
- 3.Shive MS, Anderson JM. Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv Drug Deliv Rev. 1997;28:5–24. doi: 10.1016/s0169-409x(97)00048-3. [DOI] [PubMed] [Google Scholar]
- 4.Cleek RL, Rege AA, Denner LA, Eskin SG, Mikos AG. Inhibition of smooth muscle cell growth in vitro by an antisense oligodeoxynucleotide released from poly(DL-lactic-co-glycolic acid) microparticles. J Biomed Mater Res. 1997;35:525–530. doi: 10.1002/(sici)1097-4636(19970615)35:4<525::aid-jbm12>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 5.Mao S, Xu J, Cai C, Germershaus O, Schaper A, Kissel T, et al. Effect of WOW process parameters on morphology and burst release of FITC-dextran loaded PLGA microspheres. Int J Pharm. 2007;334:137–148. doi: 10.1016/j.ijpharm.2006.10.036. [DOI] [PubMed] [Google Scholar]
- 6.McGinity JW, O’Donnell PB. Preparation of microspheres by the solvent evaporation technique. Adv Drug Deliv Rev. 1997;28:25–42. doi: 10.1016/s0169-409x(97)00049-5. [DOI] [PubMed] [Google Scholar]
- 7.Mundargi RC, Srirangarajan S, Agnihotri SA, Patil SA, Ravindra S, et al. Development and evaluation of novel biodegradable microspheres based on poly(d,l-lactide-co-glycolide) and poly(epsilon-caprolactone) for controlled delivery of doxycycline in the treatment of human periodontal pocket: in vitro and in vivo studies. J Control Release. 2007;119:59–68. doi: 10.1016/j.jconrel.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 8.Aksungur P, Demirbilek M, Denkbas EB, Vandervoort J, Ludwig A, et al. Development and characterization of Cyclosporine A loaded nanoparticles for ocular drug delivery: Cellular toxicity, uptake, and kinetic studies. J Control Release. 2011;151:286–294. doi: 10.1016/j.jconrel.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 9.Vega E, Egea MA, Valls O, Espina M, Garcia ML. Flurbiprofen loaded biodegradable nanoparticles for ophtalmic administration. J Pharm Sci. 2006;95:2393–2405. doi: 10.1002/jps.20685. [DOI] [PubMed] [Google Scholar]
- 10.Bertram JP, Saluja SS, McKain J, Lavik EB. Sustained delivery of timolol maleate from poly(lactic-co-glycolic acid)/poly(lactic acid) microspheres for over 3 months. J Microencapsul. 2009;26:18–26. doi: 10.1080/02652040802095250. [DOI] [PubMed] [Google Scholar]
- 11.Park K, Chen Y, Hu Y, Mayo AS, Kompella UB, et al. Nanoparticle-mediated expression of an angiogenic inhibitor ameliorates ischemia-induced retinal neovascularization and diabetes-induced retinal vascular leakage. Diabetes. 2009;58:1902–1913. doi: 10.2337/db08-1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCarty CA, Bansal AK, Livingston PM, Stanislavsky YL, Taylor HR. The epidemiology of dry eye in Melbourne, Australia. Ophthalmology. 1998;105:1114–1119. doi: 10.1016/S0161-6420(98)96016-X. [DOI] [PubMed] [Google Scholar]
- 13.Pflugfelder SC, Maskin SL, Anderson B, Chodosh J, Holland EJ, et al. A randomized, double-masked, placebo-controlled, multicenter comparison of loteprednol etabonate ophthalmic suspension, 0.5%, and placebo for treatment of keratoconjunctivitis sicca in patients with delayed tear clearance. Am J Ophthalmol. 2004;138:444–457. doi: 10.1016/j.ajo.2004.04.052. [DOI] [PubMed] [Google Scholar]
- 14.Jehangir S. Dry eye syndrome in Pakistani community. J Pak Med Assoc. 1990;40:66–67. [PubMed] [Google Scholar]
- 15.Jones DT, Monroy D, Ji Z, Atherton SS, Pflugfelder SC. Sjogren’s syndrome: cytokine and Epstein-Barr viral gene expression within the conjunctival epithelium. Invest Ophthalmol Vis Sci. 1994;35:3493–3504. [PubMed] [Google Scholar]
- 16.Li DQ, Chen Z, Song XJ, Luo L, Pflugfelder SC. Stimulation of matrix metalloproteinases by hyperosmolarity via a JNK pathway in human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2004;45:4302–4311. doi: 10.1167/iovs.04-0299. [DOI] [PubMed] [Google Scholar]
- 17.Chotikavanich S, de Paiva CS, Li dQ, Chen JJ, Bian F, et al. Production and activity of matrix metalloproteinase-9 on the ocular surface increase in dysfunctional tear syndrome. Invest Ophthalmol Vis Sci. 2009;50:3203–3209. doi: 10.1167/iovs.08-2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akpek EK, Merchant A, Pinar V, Foster CS. Ocular rosacea: patient characteristics and follow-up. Ophthalmology. 1997;104:1863–1867. [PubMed] [Google Scholar]
- 19.Dursun D, Kim MC, Solomon A, Pflugfelder SC. Treatment of recalcitrant recurrent corneal erosions with inhibitors of matrix metalloproteinase-9, doxycycline and corticosteroids. Am J Ophthalmol. 2001;132:8–13. doi: 10.1016/s0002-9394(01)00913-8. [DOI] [PubMed] [Google Scholar]
- 20.Seedor JA, Perry HD, McNamara TF, Golub LM, Buxton DF, et al. Systemic tetracycline treatment of alkali-induced corneal ulceration in rabbits. Arch Ophthalmol. 1987;105:268–271. doi: 10.1001/archopht.1987.01060020122043. [DOI] [PubMed] [Google Scholar]
- 21.de Paiva CS, Corrales RM, Villarreal AL, Farley W, Li DQ, et al. Apical corneal barrier disruption in experimental murine dry eye is abrogated by methylprednisolone and doxycycline. Invest Ophthalmol Vis Sci. 2006;47:2847–2856. doi: 10.1167/iovs.05-1281. [DOI] [PubMed] [Google Scholar]
- 22.de Paiva CS, Corrales RM, Villarreal AL, Farley WJ, Li DQ, et al. Corticosteroid and doxycycline suppress MMP-9 and inflammatory cytokine expression, MAPK activation in the corneal epithelium in experimental dry eye. Exp Eye Res. 2006;83:526–535. doi: 10.1016/j.exer.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 23.Pflugfelder SC, Farley W, Luo L, Chen LZ, de Paiva CS, et al. Matrix metalloproteinase-9 knockout confers resistance to corneal epithelial barrier disruption in experimental dry eye. Am J Pathol1. 2005;66:61–71. doi: 10.1016/S0002-9440(10)62232-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Paiva CS, Chotikavanich S, Pangelinan SB, Pitcher JD, 3rd, Fang B, et al. IL-17 disrupts corneal barrier following desiccating stress. Mucosal Immunol. 2009;2:243–253. doi: 10.1038/mi.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dursun D, Wang M, Monroy D, Li DQ, Lokeshwar BL, et al. A mouse model of keratoconjunctivitis sicca. Invest Ophthalmol Vis Sci. 2002;43:632–638. [PubMed] [Google Scholar]
- 26.Yoncheva K, Vandervoort J, Ludwig A. Development of mucoadhesive poly(lactide-co-glycolide) nanoparticles for ocular application. Pharm Dev Technol. 2011;16:29–35. doi: 10.3109/10837450903479954. [DOI] [PubMed] [Google Scholar]
- 27.Wilhelmus KR. Therapeutic interventions for herpes simplex virus epithelial keratitis. Cochrane Database Syst Rev. 2008:CD002898. doi: 10.1002/14651858.CD002898.pub2. [DOI] [PubMed] [Google Scholar]
- 28.Gurwitz JH, Glynn RJ, Monane M, Everitt DE, Gilden D, et al. Treatment for glaucoma: adherence by the elderly. Am J Public Health. 1993;83:711–716. doi: 10.2105/ajph.83.5.711. [DOI] [PMC free article] [PubMed] [Google Scholar]