Summary
Background:
Locked-in syndrome represents the most severe form of central pontine myelinolysis (CPM) and has been associated with a dismal outcome.
Case Report:
In this report we describe a case of severe locked-in syndrome after liver transplantation with spontaneous recovery with cessation of calcneurin inhibitor therapy and supportive treatment.
A 54-year old male received deceased-donor liver transplantation and developed decreased level of consciousness with spastic quadriplegia. A diagnosis of central pontine myelinolysis with extrapontine manifestations was confirmed by magnetic resonance imaging. His immunosuppresion was modified by switching from tacrolimus to sirolimus and addition of prednisone. The patient started to recover from symptoms fourth months after transplantation.
Conclusions:
Tacrolimus is known to have neurotoxic side effects and it may precipitate CPM in patients who have predisposing factors. Sirolimus and steroids should be considered as safe and an effective alternative for immunosuppression in the setting of CPM after liver transplantation.
Keywords: central pontine myelinolysis, calcineurin inhibitors, sirolimus
Background
Central pontine myelinolysis (CPM) is a demyelination disorder affecting mainly the pons; however it may affect other cerebral areas. It was first described in alcoholic and malnourished patients by Adams et al. [1]. It varies in severity with locked-in syndrome representing the most severe form of the disease [2]. It has a dismal prognosis. Although several therapies including steroids, plasmapharesis and intravenous immunoglobulins (IVIG) have been tried, there is no definitive treatment [3]. Here, we report a case of locked-in-syndrome after liver transplantation that recovered with cessation of calcineurin inhibitor (CNI) therapy and supportive care.
Case Report
A 54-year old patient presented with end-stage liver disease due to α1-antitrypsin deficiency, and non-alcoholic steato-hepatitis. Three months before transplant, he had marked deterioration of liver function with increasing bilirubin, profound coagulopathy, worsening encephalopathy and deterioration of mental status. In addition, he developed hyponatremia with serum sodium level ranging from 112 to 133 mmol/l; due to syndrome of inappropriate anti-diuretic hormone secretion (SIADH). Serum sodium was corrected gradually from 112 mmol/l, one week before transplant, to 133 mmol/L at the time of surgery. The patient received a whole liver graft from a young deceased donor, of identical blood group and size matched. Induction of immunosuppression was done using basiliximab and maintained with mycophenolate mofetil (MMF) and delayed tacrolimus due to hemodynamic instability.
After transplantation, the patient had intra-abdominal bleeding and required abdominal packing for hemostasis. On post-operative day (POD) 1, the patient recovered slowly, was confused but still could follow commands despite being on respiratory support. His serum sodium was 132 mmol/l. On POD 2, he was returned to the operating room for removal of abdominal pack and bleeding was brought under control. His serum sodium was 133 mmol/l. POD 3; a failed attempt at extubation was performed, complicated by excessive respiratory secretions and non drug-related drowsiness. He became febrile and peritoneal fluid analysis was positive for Staphyloccocci isolates, thus broad spectrum antibiotics were initiated.
On POD 4 the patient continued to be drowsy but oriented despite having normal sodium values (140 mmol/l). By POD 5, he started to have encephalopathy, along with increased serum sodium (151 mmol/l). Sodium was then restricted from intravenous and oral intake. Despite all these efforts sodium continued to rise on POD 6 and free water injections were needed, tacrolimus was started on that day. During the second week, the level of consciousness improved initially and the patient started to obey commands; serum Na was between 147 and 150 mmol/l However, by the end of the second week the patient’s LOC deteriorated again with arm flexing to stimuli and spontaneous eye opening but no response to commands. MRI of the brain did not show any signs of myelinolysis at this point. Next the patient developed palatal tremors, GCS was 7, and the condition was diagnosed clinically as locked-in-syndrome, he was switched from Tacrolimus to sirolimus on POD 14. POD 21, the patient started to improve gradually by biting a mouth swab purposefully. POD 22, he started to follow commands by squeezing hands bilaterally and moving his tongue, but was unable to move his feet at that time. MMF was stopped due to related pancytopenia, prednisone was started at 7.5 mg orally daily. There was a continued and gradual improvement and by POD 33, the patient was weaned from the ventilator and transferred to the ward, Graft function remained normal and stable throughout the entire process. An MR of the brain finally confirmed the diagnosis of central pontine myelinolysis on POD 39. At this point, the patient was able to open his eyes and follow objects with his head.
On POD 47 the patient developed aspiration pneumonia, which was medically treated and a gastrostomy tube was inserted to improve nutrition and prevent future aspirations.
One week later, he started to blink his eyes and was able to nod to verbal commands. On POD 60 the patient was fully alert and responsive, able to move his limbs and speak with a slow and slurred speech, his swallowing improved by POD 75. Four months after transplant, he is able to speak clearly, has started to feed himself and can walk with support with ongoing physiotherapy to achieve further mobility.
Nine months after transplant, he still has a degree of difficulty with daily living activities and with mobility, cognitive impairment and behavioural disturbance.
Discussion
Central pontine myelinolysis (CPM) is characterized by symmetrical loss of myelin in the basis pontis, with relative preservation of axons and neuronal cell bodies [4]. It affects mainly the pons however, extrapontine myelinolysis occurs in about 10% of cases. The Pons is believed more vulnerable to demyelination than the cerebral hemispheres due to the anatomical structural rigidity of the protuberance which makes fibers more susceptible to vasogenic edema and to the leaking of myelinotoxic substances from the vessels [2].
Physiologically, glial cells play a critical role in regulation of extracellular osmolarity and electrolyte balance to protect the neurons they envelop. In case of cerebral fluid imbalance, these cells activate the Na+-K+ ATPase pump system to counteract any derangements. However, in patients with chronic liver disease, glial cells have lower adaptive capacity due to the chronic metabolic derangements. So, when rapid correction of sodium occurs, the cells are unable to prevent cerebral edema, an osmotic injury of the endothelium results with subsequent release of myelinotoxic factors, stripping of the myelin sheath from the axon and damage of oligodendrocytes [2,4]. Interestingly, the extensive myelinolysis of glial cells is not associated with marked immunological response as indicated by the marked absence of scavenger cells, suggesting an important contribution of apoptosis to this process [4].
Liver transplant patients represent the third largest group of CPM cases [2]. The incidence of CPM after liver transplantation varies between centers; in two large studies [5,6], CPM was diagnosed in less than 1% of cases, however in another report [7], the authors described 5 CPM cases out of 27 patients transplanted (18.5%). Presentation of CPM varies from asymptomatic to a fully developed locked-in-syndrome which is characterized by the combination of quadriplegia, loss of ability of the patient to communicate except by the use of the eyes, and an inability to follow commands [8].
Early reports of CPM in liver transplant patients frequently described fatal outcomes, and diagnosis was often only established postmortem, Boon et al. [9], reported that 5 cases were diagnosed by examination of 50 necropsy specimens, none of these cases were diagnosed before death. While recent advances in imaging modalities have allowed much earlier diagnosis [10], imaging findings often lag clinical presentation. Radiological evidence often takes 1–2 weeks to appear and a similar lag is present between recovery of the neurological symptoms and disappearance of the radiological findings [2].
The exact etiology of CPM is unclear; it seems that rapid correction of hyponatremia is a frequent and important triggering factor [2]. Interestingly, CPM has been described in eunatremic [11] and hypernatremic patients [4] suggesting the multifactorial nature of this disease.
Several risk factors have been associated with development of CPM in liver transplant recipients: these include metabolic derangements (severe hyponatremia and hypokalemia), hypocholesterolemia and chronic encephalopathy before transplant, bleeding or vascular complication during and after surgery, use of calcineurin inhibitors (CNIs) including cyclosporine and tacrolimus, and infection after the transplant [8]. Moreover, patients with chronic encephalopathy are more susceptible to the neurotoxic effect of tacrolimus and have higher risk of developing serious neurological complications after liver transplantation [12].
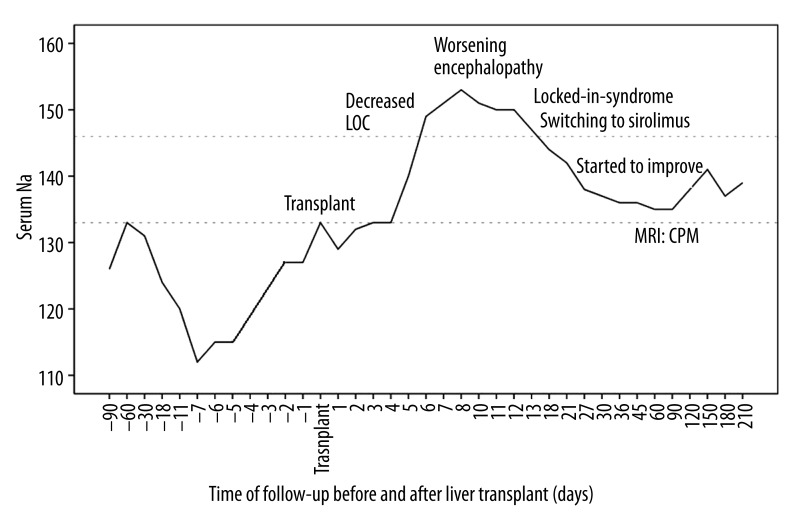
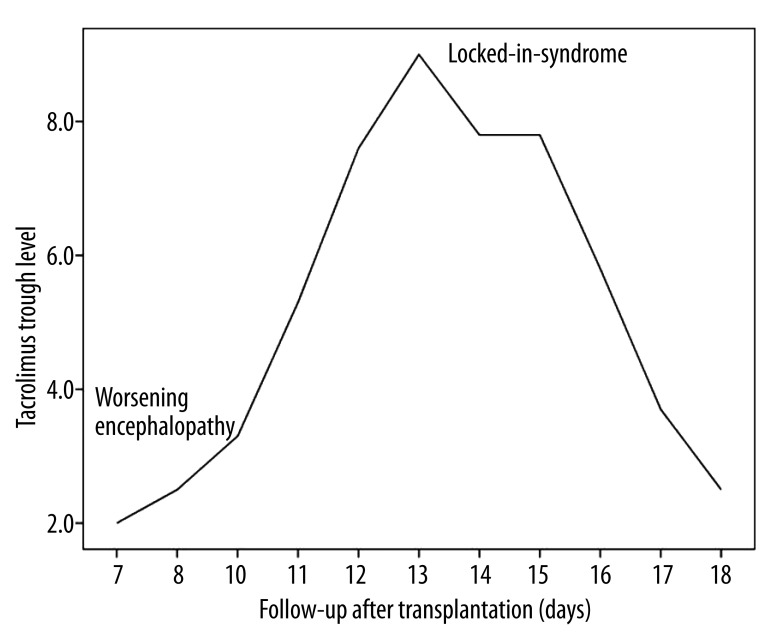
Our patient had chronic hyponatremia due to SIADH, chronic encephalopathy, had intra-abdominal bleeding and infection after transplant, and received tacrolimus for immunosuppression. Although the correction of hyponatremia was not rapid (from 112 to 133 mmol/l over 7 days), there was a further relatively sharp increase of almost 20 mmol/l over 2 days (days 3–5 postop) coinciding with some of the most florid symptoms. Neurological symptoms correlated with increasing serum sodium (Figure 1), and there was coincidence of locked-in-syndrome and the peak tacrolimus trough level (Figure 2).
Figure 1.
Serum sodium before and after liver transplant in relation to neurological symptoms.
Figure 2.
Trough tacrolimus levels after liver transplantation in relation to neurological symptoms.
In the literature, strategies for treatment of CPM vary between centers and include conversion of CNIs to sirolimus, use of steroids, plasmapharesis and intravenous immunoglobulins (IVIG). CNIs are known for their neurotoxic side effects and they may trigger CPM in liver transplant patients [13]. The exact mechanism is not fully understood however they may decrease expression of P-glycoprotein in the brain endothelial cells which results in disruption of the blood-brain barrier and subsequent vasogenic edema [14]. The newer class of immunosuppressants, m-TOR inhibitors, including sirolimus and everolimus, does not appear to have associated neurological complications in liver transplant patients [15]. Several reports described dramatic clinical and radiological improvement of CPM after switching immunosuppression from tacrolimus to sirolimus without negative impact on the liver graft functions [8,13,14].
Our patient was managed by conversion of tacrolimus to sirolimus, adding prednisone to immunosuppression, and correction of fluid and electrolyte imbalance, which resulted in gradual but important recovery.
Steroids were described to have a protective effect in a rat model of CPM, probably by preventing disruption of the blood-brain barrier through inhibition of mediators like tumor necrosis factor and prostaglandins [16], and recovery of neurological symptoms related to CPM after use of steroids has also been reported in patients [17].
Plasmapharesis and IVIG have been used either alone [18,19] or in combination [20] for treatment of CPM, with varying responses. One paper reported recovery of three patients using plasmapheresis after 2 months in the first 2 subjects and 12 months in the third individual. They hypothesized that plasmapharesis improved the outcome by clearing the myelinotoxic substances, formation of antimyelin antibodies and enhancing remyelination [2,18]. We considered this treatment protocol but it was not used as our patient started to improve spontaneously.
This case report describes a liver transplant subject who developed a clinical picture of the most severe form of CPM, the locked-in-syndrome, with spontaneous, near complete, and continuing recovery. Several factors may have contributed to this outcome, including the gradual development of the neurological disorder, the gradual correction of sodium and the immunosuppression adjustment including discontinuation of CNI, and substitution of mTOR inhibitor and steroids.
Conclusions
This report emphasizes several important facts of this complex and often devastating clinical problem. Rapid electrolyte fluxes (especially Na+ rise) may be critical inciting factors in the later postoperative period as well as the more frequently described perioperative period and underline the importance of ongoing attention to controlling electrolyte flux for many days after liver transplant. While controlled trials to confirm efficacy would be exceedingly difficult, conversion to non-CNI based immunosuppression remains a reasonable and important part of the treatment of CPM after liver transplant. The dramatic improvement in the patient described, despite the severity of the symptoms, suggests a dismal prognosis is certainly not inevitable, and supports persistence with supportive measures in these very difficult cases.
References:
- 1.Adams RD, Victor M, Mancall EL. Central pontine myelinolysis: a hitherto undescribed disease occurring in alcoholic and malnourished patients. AMA Arch Neurol Psychiatry. 1959;81(2):154–72. [PubMed] [Google Scholar]
- 2.Lampl C, Yazdi K. Central pontine myelinolysis. Eur Neurol. 2002;47(1):3–10. doi: 10.1159/000047939. [DOI] [PubMed] [Google Scholar]
- 3.Zhang ZW, Kang Y, Deng LJ, et al. Therapy of central pontine myelinolysis following living donor liver transplantation: Report of three cases. World J Gastroenterol. 2009;15(31):3960–63. doi: 10.3748/wjg.15.3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ashrafian H, Davey P. A review of the causes of central pontine myelinosis: yet another apoptotic illness? Eur J Neurol. 2001;8(2):103–9. doi: 10.1046/j.1468-1331.2001.00176.x. [DOI] [PubMed] [Google Scholar]
- 5.Lee EM, Kang JK, Yun SC, et al. Risk factors for central pontine and extrapontine myelinolysis following orthotopic liver transplantation. Eur Neurol. 2009;62(6):362–68. doi: 10.1159/000242426. [DOI] [PubMed] [Google Scholar]
- 6.Bonham CA, Dominguez EA, Fukui MB, et al. Central nervous system lesions in liver transplant recipients: prospective assessment of indications for biopsy and implications for management. Transplantation. 1998;66(12):1596–604. doi: 10.1097/00007890-199812270-00005. [DOI] [PubMed] [Google Scholar]
- 7.Cartier RL, Armijo MJ, Quiroz ZG, Matamala CJ. [Central pontine myelinolysis after liver transplantation. Report of five cases] Rev Med Chil. 2010;138(10):1264–71. [PubMed] [Google Scholar]
- 8.Cui R, Fayek S, Rand EB, et al. Central pontine myelinolysis: A case report and clinical-pathological review. Pediatr Transplant. 2012;16(6):E251–56. doi: 10.1111/j.1399-3046.2011.01591.x. [DOI] [PubMed] [Google Scholar]
- 9.Boon AP, Carey MP, Adams DH, et al. Central pontine myelinolysis in liver transplantation. J Clin Pathol. 1991;44(11):909–14. doi: 10.1136/jcp.44.11.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruzek KA, Campeau NG, Miller GM. Early diagnosis of central pontine myelinolysis with diffusion-weighted imaging. AJNR Am J Neuroradiol. 2004;25(2):210–13. [PMC free article] [PubMed] [Google Scholar]
- 11.Huq S, Wong M, Chan H, Crimmins D. Osmotic demyelination syndromes: central and extrapontine myelinolysis. J Clin Neurosci. 2007;14(7):684–88. doi: 10.1016/j.jocn.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 12.Balderramo D, Prieto J, Cardenas A, Navasa M. Hepatic encephalopathy and post-transplant hyponatremia predict early calcineurin inhibitor-induced neurotoxicity after liver transplantation. Transpl Int. 2008;24(8):812–19. doi: 10.1111/j.1432-2277.2011.01280.x. [DOI] [PubMed] [Google Scholar]
- 13.Fukazawa K, Nishida S, Aguina L, Pretto E., Jr Central pontine myelinolysis (CPM) associated with tacrolimus (FK506) after liver transplantation. Ann Transplant. 2011;16(3):139–42. doi: 10.12659/aot.882008. [DOI] [PubMed] [Google Scholar]
- 14.Forgacs B, Merhav HJ, Lappin J, Mieles L. Successful Conversion to Rapamycin for Calcineurin Inhibitor-Related Neurotoxicity Following Liver Transplantation. Transplant Proc. 2005;37(4):1912–14. doi: 10.1016/j.transproceed.2005.02.101. [DOI] [PubMed] [Google Scholar]
- 15.Kawahara T, Asthana S, Kneteman NM. m-TOR inhibitors: what role in liver transplantation? J Hepatol. 2011;55(6):1441–51. doi: 10.1016/j.jhep.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 16.Sugimura Y, Murase T, Takefuji S, et al. Protective effect of dexamethasone on osmotic-induced demyelination in rats. Exp Neurol. 2005;192(1):178–83. doi: 10.1016/j.expneurol.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 17.Hagiwara K, Okada Y, Shida N, Yamashita Y. Extensive central and extrapontine myelinolysis in a case of chronic alcoholism without hyponatremia: a case report with analysis of serial MR findings. Intern Med. 2008;47(5):431–35. doi: 10.2169/internalmedicine.47.0634. [DOI] [PubMed] [Google Scholar]
- 18.Finsterer J, Engelmayer E, Trnka E, Stiskal M. Immunoglobulins are effective in pontine myelinolysis. Clin Neuropharmacol. 2000;23(2):110–13. doi: 10.1097/00002826-200003000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Bibl D, Lampl C, Gabriel C, et al. Treatment of central pontine myelinolysis with therapeutic plasmapheresis. Lancet. 1999;353(9159):1155. doi: 10.1016/S0140-6736(99)01145-9. [DOI] [PubMed] [Google Scholar]
- 20.Saner FH, Koeppen S, Meyer M, et al. Treatment of central pontine myelinolysis with plasmapheresis and immunoglobulins in liver transplant patient. Transpl Int. 2008;21(4):390–91. doi: 10.1111/j.1432-2277.2007.00608.x. [DOI] [PubMed] [Google Scholar]