Abstract
Sphingolipids serve an important role as effector molecules in signaling pathways bearing on apoptosis and cell survival. The balance between proapoptotic ceramide and prosurvival sphingosine-1-phosphate, sometimes termed the “sphingolipid rheostat,” has received particular attention. Less well studied is the role of the follicular lymphoma variant translocation 1 (FVT1) gene, which was identified through its involvement in an atypical follicular lymphoma translocation and which encodes an enzyme in the synthetic pathway of ceramide. We investigated the expression of FVT1 in a variety of B-cell non-Hodgkin lymphomas and found that FVT1 is significantly underexpressed by germinal center–type diffuse large B-cell lymphoma (DLBCL) when compared with non–germinal center–type DLBCL, follicular lymphoma, and normal tonsil control samples. Increased expression of FVT1 correlated with decreased survival, suggesting that changes in the expression of FVT1 and in the concentrations of bioactive sphingolipids may be important in the pathogenesis and treatment of some types of DLBCL.
Keywords: Follicular lymphoma variant translocation 1 gene, FVT1, Lymphoma, Sphingolipids, Ceramide
While sphingolipids have long been recognized as cellular structural elements with important roles in the integrity and substructure of lipid bilayer membranes, recent work has revealed a surprising function for these molecules at the center of fundamental biochemical pathways regulating cell survival, apoptosis, senescence, migration, and inflammatory response.1 Ceramide, in particular, has emerged as a key player in the promotion of apoptosis2 and is generated in response to a wide variety of stressors, including tumor necrosis factor-α exposure,3 corticosteroid treatment,4 irradiation,4,5 FAS-FAS ligand interaction,6 and DNA damage.6 In contrast, ceramide’s downstream metabolite sphingosine-1-phosphate (S1P) has potent antiapoptotic and growth-promoting functions, including the activation of nuclear factor (NF)-κB and the induction of cyclooxygenase-2.7,8
The relationship between proapoptotic ceramide and prosurvival S1P has been termed the “sphingolipid rheostat,” with the implication that the relative abundance of these 2 molecules drives the cell toward death or survival.9 The enzyme sphingosine kinase, which in concert with ceramidases converts ceramide to S1P, would thus control the set point of this rheostat; indeed, sphingosine kinase 1 (SPHK1) messenger RNA (mRNA) and protein levels of sphingosine kinase are increased in a variety of primary cancers, including those of lung, colon, breast, ovary, stomach, uterus, and kidney,1 indicating a propensity for malignant cells to detoxify ceramide to S1P and thereby attain a growth advantage. Recently, SPHK1 mRNA levels have been shown to be increased relative to normal control samples in B-cell non-Hodgkin lymphoma (B-NHL),10 refractory anemia with excess blasts in transformation,11 and acute leukemia.11
The follicular lymphoma variant translocation 1 gene (FVT1), which was first identified through its involvement in a variant t(2;18) translocation in follicular lymphoma, codes for 3-ketodihydrosphingosine reductase, a key enzyme in the synthetic pathway of ceramide.12 We have previously found FVT1 to be differentially expressed in Epstein-Barr virus (EBV)-positive and EBV-negative primary effusion lymphoma (PEL).13 Expression of FVT1 is also up-regulated in T-cell malignancies and in stimulated lymphocytes in vitro.14 An unpublished Bayesian analysis of gene expression microarray data for a series of diffuse large B-cell lymphoma (DLBCL) cases identified FVT1 expression as among the strongest predictors of overall survival.15
Given these several lines of evidence suggesting that alteration in expression of FVT1 may characterize certain types of lymphomas, and in light of the general importance of the sphingolipid metabolic pathway in the cell cycle, we assessed levels of FVT1 mRNA in a variety of B-NHLs. We found that germinal center–type (GC) DLBCL is characterized by reduced expression of FVT1, a feature that sets it apart from non-GC-type DLBCL (non-GC DLBCL) and other types of B-NHL. Furthermore, in DLBCL, the level of FVT1 expression correlates with survival. These findings may hold pathophysiologic and therapeutic implications.
Materials and Methods
Selection of Primary Cases
The registry of snap-frozen and cryopreserved tissue in the Immunopathology Laboratory, Department of Pathology and Laboratory Medicine, Weill Cornell Medical College, New York, NY, was searched for samples of cases of B-NHL at initial diagnosis, with the prior approval of the institutional review board. We selected 38 cases for analysis, including 17 DLBCLs, 6 chronic lymphocytic leukemias, 8 follicular lymphomas, 2 Burkitt lymphomas, 2 splenic marginal zone lymphomas, and 3 normal tonsils. The DLBCL cases comprised 7 GC DLBCLs and 10 non-GC DLBCLs, as described subsequently. Survival data were available for 15 of the 17 DLBCL cases, with an estimated average follow-up of 59 months (range, 1–131 months). For patients with available data, all were treated with initial CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone) chemotherapy; only 1 patient received rituximab in addition to CHOP.
RNA Isolation, Reverse Transcription, and Polymerase Chain Reaction (PCR)
Total RNA was isolated from serial microtome sections using the RNeasy Mini kit (Qiagen, Valencia, CA) according to the manufacturer’s specifications. In addition, total RNA was isolated from 4 PEL cell lines (2 Kaposi sarcoma herpes-virus (KSHV+/EBV+ and 2 KSHV+/EBV−) and 3 DLBCL cell lines of known immunophenotype (non-GC DLBCL, Ly3 and Ly10; GC DLBCL, Ly7). Following spectrophotometric RNA quantification, reverse transcription was carried out using the SuperScript III First Strand Synthesis System (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. PCR was performed using an Applied Biosystems 7500 Real-Time PCR System with SYBR Green PCR Mastermix (Applied Biosystems, Foster City, CA) and the following primer sequences: FVT1 forward, 5′-GGGCGCATGTGGT-GGTTA-3′; FVT1 reverse, 5′-ATAGCACTCGATAGCAAT-GCACTT-3′; glyceraldehyde-3-phosphate dehydrogenase (GAPDH) forward, 5′-TTGCCATCAATGACCCCT TCA-3′; GAPDH reverse, 5′-CGCCCCACT TGATTTTGGA-3′. PCR products were subjected to melting curve analysis, and selected products were sized using polyacrylamide or agarose gel electrophoresis, as appropriate. Cycle threshold (Ct) values were used to calculate the ΔCt for FVT1 vs GAPDH and statistically analyzed by the Mann-Whitney U test using Microsoft Excel (Microsoft, Redmond, WA) and WinSTAT (A-Prompt, Lehigh, PA). All P values are 2-tailed.
Protein Isolation and Western Blot Analysis
Total protein was extracted from 3 DLBCL cell lines (Ly2, Ly3, and Ly8) maintained in 20% fetal bovine serum and Iscove modified Dulbecco medium. Similarly, total protein was extracted from selected patient samples (5 GC DLBCL and 4 non-GC DLBCL) via serial microtome sections. After total protein quantification via the Bradford assay method, 40 μg of protein for each sample was electrophoresed via 6% to 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis, transferred to a nitrocellulose membrane, blocked with 5% milk in tris(hydroxymethyl)aminomethane-buffered saline, and probed using purified rabbit monoclonal antibody to actin (dilution 1:2,000; Sigma-Aldrich, St Louis, MO), a polyclonal rabbit antibody to FVT1 (dilution 1:2,000), and an antirab-bit secondary antibody (dilution 1:2,000 for FVT1, dilution 1:3,000 for actin; Invitrogen) according to a standard Western blotting protocol. The anti-FVT1 was a polyclonal rabbit antibody to the following peptide: CMMQREKSENADKTA (custom made by Covance, Princeton, NJ). Labeled protein was detected using ECL Western Blotting Reagents (GE Healthcare, Piscataway, NJ) and, in the case of the patient samples, densitometrically quantified using Iquant software (Molecular Dynamics, Sunnyvale, CA).
Immunohistochemical Analysis
Immunostaining of the DLBCL cases for CD10 (clone NCL-CD10-270, Vision BioSystems, Norwell, MA), BCL6 (clone PG-B6p, DakoCytomation, Glostrup, Denmark), and MUM1 (clone MUM1p, DakoCytomation) for classification into the GC and non-GC categories was performed using the Bond Max Autostainer (Vision BioSystems, Norwell, MA). Immunohistochemical staining was also performed using the FVT1 rabbit antisera. Formalin-fixed, paraffin-embedded tissue sections were deparaffinized, and endogenous peroxidase was inactivated, after which antigen retrieval was performed using the Bond Epitope Retrieval Solution 1 or the Bond Epitope Retrieval Solution 2 (Vision BioSystems) at 99°C to 100°C for 20 to 30 minutes. For FVT1, antigen retrieval was performed using 0.1% trypsin at 37°C for 10 to 15 minutes. Following antigen retrieval, the sections were incubated sequentially with the primary antibody for 25 minutes, postprimary for 15 minutes, and then polymer for 25 minutes (Bond Polymer Define HRP System, Vision BioSystems) followed by colorimetric development with diaminobenzidine (Vision BioSystems). The H&E-stained and immunohistochemically stained slides counterstained with hematoxylin were subsequently reviewed by 2 hematopathologists (D.R.C. and A.C.), and each case was classified as GC type or non-GC type by the method previously described.16
Additional Gene Expression Data Analysis and Survival Analysis
Additional gene expression and survival data were obtained from the Lymphoma/Leukemia Molecular Profiling Project (http://llmpp.nih.gov), corresponding to the DLBCL cases reported by Alizadeh et al17 and by Rosenwald et al.18 These results and the survival data for our primary DLBCL cases were analyzed by Kaplan-Meier survival analysis and Cox proportional hazards regression using Microsoft Excel and WinSTAT. All P values are 2-tailed.
Results
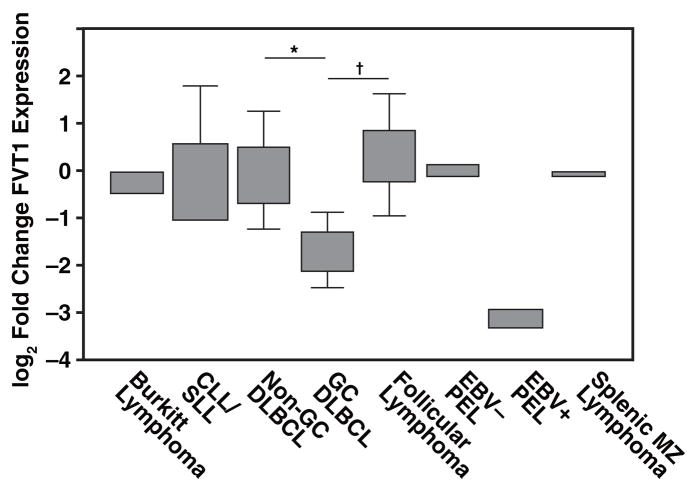
FVT1 expression is significantly decreased in GC DLBCL Figure 1 relative to non-GC DLBCL (P < .001), follicular lymphoma (P < .01), and normal tonsil controls (P < .05). There was a 9-fold difference in FVT1 expression in EBV+ and EBV− PEL (Figure 1), consistent with our previous observations.13 There were no significant differences in FVT1 expression among the remaining subgroups of B-NHL. Although immunohistochemical expression of BCL-6, CD10, and MUM1 was used in the assignment of DLBCL cases to the GC or non-GC type, FVT1 expression correlated significantly only with MUM1 expression (P < .04).
Figure 1.
Expression of follicular lymphoma variant translocation 1 (FVT1) in B-cell non-Hodgkin lymphomas by reverse transcription–polymerase chain reaction. FVT1 expression is significantly decreased in germinal center–type diffuse large B-cell lymphoma (GC DLBCL) relative to the normal tonsil control samples (P < .05), non-GC DLBCL (* P < .001), and follicular lymphoma († P < .01). There is also a 9-fold difference in expression in Epstein-Barr virus (EBV)+ and EBV− primary effusion lymphoma (PEL). Boxes represent the second and third quartiles, while whiskers represent the full range of values. CLL/SLL, chronic lymphocytic leukemia/ small lymphocytic lymphoma; MZ, marginal zone.
We also evaluated FVT1 expression in previously reported DLBCL gene expression microarray studies17,18 and confirmed that in these independent data sets, FVT1 is significantly underexpressed in GC DLBCL relative to non-GC DLBCL Table 1.
Table 1.
Expression of FVT1 in GC-Type DLBCL Is Significantly Decreased in Comparison With Non-GC-Type DLBCL*
| Study | Groups Compared | Statistical Test | P |
|---|---|---|---|
| Present | GC, non-GC | Mann-Whitney U | <.001 |
| Alizadeh et al17 | GC, ABC | Mann-Whitney U | <.01 |
| Rosenwald et al18 | GC, ABC, type III | ANOVA | <.05 |
ABC, activated B-cell; ANOVA, analysis of variance; DLBCL, diffuse large B-cell lymphoma; FVT1, follicular lymphoma variant translocation 1; GC, germinal center.
Each study listed shows similar findings in independent data sets.
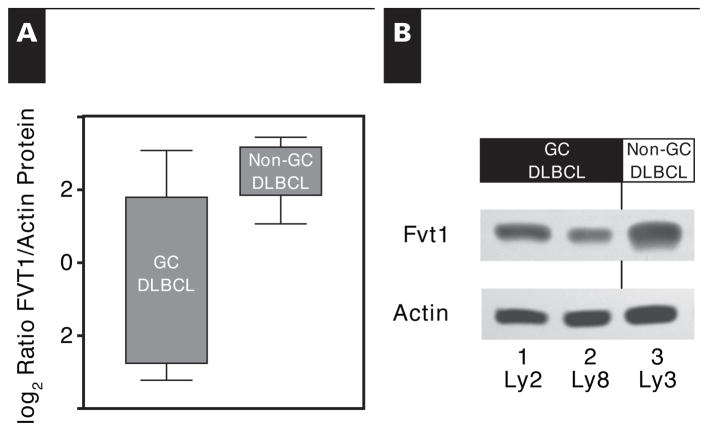
To assess protein expression, we raised an antibody to FVT1. While immunohistochemical analysis with this antibody demonstrated widespread expression in lymphocytes, it did not delineate specific reactive or neoplastic populations. However, we successfully used the antibody in immunoblot analysis, detecting a predominant band with the expected size and relative intensity in EBV+ and EBV− PEL cell lines (not shown). Protein levels of FVT1, assessed via this manner in selected GC DLBCL and non-GC DLBCL patient samples and cell lines, parallel these mRNA levels Figure 2.
Figure 2.
Amount of follicular lymphoma variant translocation 1 (FVT1) protein as detected by Western blot. A, Amounts of FVT1 protein and actin in 12 selected patient samples were detected by Western blot and quantified by densitometry. Although not statistically significant (P = .12), the trend for FVT1 protein parallels the gene expression findings, with decreased amounts in the germinal center–type cases relative to those of non–germinal center–type cases. B, Amounts of FVT1 protein and actin were detected by Western blot for 3 representative diffuse large B-cell lymphoma cell lines. Those of the germinal center type (Ly2 and Ly8) contain a decreased amount of FVT1 relative to one of the non–germinal center type (Ly3). Boxes represent the second and third quartiles, while whiskers represent the full range of values.
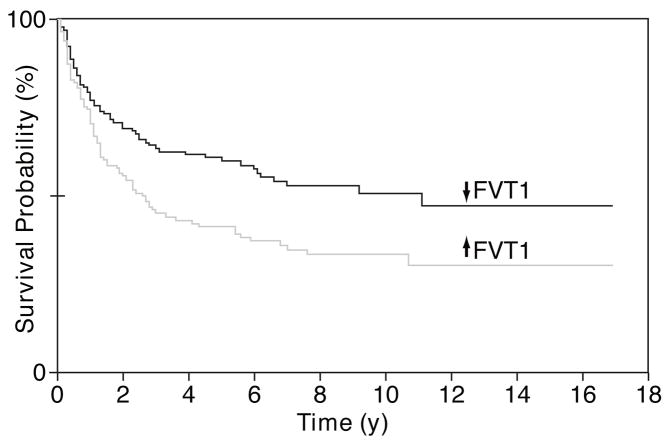
The previously reported DLBCL cases17,18 were separated into categories comprising cases with increased FVT1 expression and cases with decreased FVT1 expression. By Kaplan-Meier survival probability analysis Figure 3, patients with increased expression of FVT1 survived for a significantly shorter period (P < .01). By univariate Cox proportional hazards regression, FVT1 expression is a significant predictor of survival (P = .05); however, multivariate Cox proportional hazards regression, including terms for FVT1 expression and the subclass (GC or non-GC) of the DLBCL, shows that the predictive effect of FVT1 expression on survival is not independent of GC/non-GC subclassification (P = .55). Similar analysis of our small set of primary DLBCL cases did not yield significant associations between survival and FVT1 expression or GC/non-GC subclass.
Figure 3.
Kaplan-Meier survival probability analysis for patients with diffuse large B-cell lymphoma (DLBCL). The previously published DLBCL cases were separated into 2 categories: cases with increased follicular lymphoma variant translocation 1 (FVT1) expression and cases with decreased FVT1 expression. Patients with increased expression of FVT1 survived for a significantly shorter time (P < .01).
Discussion
Although FVT1 bears in its name a designation as a “follicular lymphoma variant” translocation gene, this is something of a misnomer, as in fact this gene is only rarely rearranged in follicular lymphoma. Nevertheless, some investigators14 have proposed that this locus may be deregulated due to the t(14;18) translocation that affects its neighbor, BCL2, some 10 kb downstream.12 Our data indicate that, in fact, FVT1 expression by follicular lymphoma does not differ significantly when compared with control tonsil and other types of low-grade B-cell lymphoma.
While we did not find evidence for altered expression of FVT1 in follicular lymphoma, the GC type of DLBCL demonstrated decreased expression of FVT1 when compared with normal control samples, follicular lymphoma, and non-GC DLBCL. We confirmed this finding by examining publicly available gene expression data from 2 previously published landmark studies of DLBCL. In addition, when these previously reported cases are segregated into groups with increased or decreased expression of FVT1, survival is significantly shorter in the group with increased expression. Of note, similar analysis of our primary DLBCL cases did not show a significant association between survival and FVT1 expression, a finding likely attributable to the small number of cases (n = 15); indeed, we also could not demonstrate in this group a significant difference in survival between GC and non-GC DLBCL cases, an association well-established in the literature for such prerituximab era cases.16
The relationship between FVT1 expression and survival in DLBCL raises the possibility that the relatively decreased expression of FVT1 in EBV+ PEL might carry similar implications. However, there are few longitudinal studies in the literature of survival or prognostic factors in this rare, extremely aggressive entity.19 While 1 recent study using a small sample of patients with nonuniform treatment protocols found no association between EBV status and survival in PEL,20 further study seems necessary before definitive conclusions regarding survival in PEL, EBV status, and FVT1 expression can be reached.
Our results also lead us to consider the possible biochemical role that FVT1 may have in the cell. First, although we found that the gene expression is paralleled on the protein level, in a complex synthetic pathway there may not be a one-to-one correspondence between enzyme abundance and activity. Nevertheless, mRNA levels of enzymes of ceramide metabolism have previously been used as surrogates in the analysis of the kinetics of this pathway.11 As the 3-ketodihydrosphingosine reductase encoded by FVT1 catalyzes the conversion of 3-ketosphinganine to sphinganine, which is further modified to form ceramide, decreased levels of this enzyme in GC DLBCL and EBV+ PEL might be expected to reduce levels of intracellular ceramide. Decreased ceramide would, in turn, lessen the potential for apoptosis, perhaps contributing to lymphomagenesis in GC DLBCL and EBV+ PEL. Such an interpretation is supported by the finding that alterations in the activity of enzymes before and after 3-ketodihydrosphingosine reductase in the metabolic pathway directly affect intracellular ceramide concentration.21–24 Of note, however, 3-ketodihydrosphingosine reductase is not the rate-limiting enzyme in sphingolipid synthesis,21 suggesting that FVT1 expression may also reflect larger patterns of regulation within this pathway.
In support of this, we have identified a putative palindromic NF-κB binding site in the 5′ flanking region of FVT1 (421 base pairs 5′ to transcription start), potentially underscoring the connection between FVT1 expression and fundamental signaling pathways relating to lymphocyte differentiation, activity, and cell cycle control. However, further work is necessary to clarify the role in such processes of FVT1 and the bioactive sphingolipids in general.
Our findings indicate that decreased expression of FVT1 is a consistent finding in a subset of NHLs. Furthermore, the specific association of this finding with GC DLBCL and the inverse relationship between FVT1 expression and survival in DLBCL suggests that alterations in the expression of FVT1 may have an especially important role in the pathogenesis of GC DLBCL. Finally, as many chemotherapeutic agents target the metabolic pathways pertaining to ceramide23,25,26 and as new agents designed to modulate the signaling of S1P are advancing toward widespread clinical use,27,28 further investigation into the role of FVT1 in lymphomagenesis may yield insights of therapeutic importance.
Acknowledgments
Supported in part by grant NIH-R01-CA68939 from the National Institutes of Health, Bethesda, MD (to E.C.).
References
- 1.Ogretmen B, Hannun YA. Biologically active sphingolipids in cancer pathogenesis and treatment. Nat Rev Cancer. 2004;4:604–616. doi: 10.1038/nrc1411. [DOI] [PubMed] [Google Scholar]
- 2.Ruvolo PP. Ceramide regulates cellular homeostasis via diverse stress signaling pathways. Leukemia. 2001;15:1153–1160. doi: 10.1038/sj.leu.2402197. [DOI] [PubMed] [Google Scholar]
- 3.Osawa Y, Uchinami H, Bielawski J, et al. Roles for C16-ceramide and sphingosine 1-phosphate in regulating hepatocyte apoptosis in response to tumor necrosis factor-alpha. J Biol Chem. 2005;280:27879–27887. doi: 10.1074/jbc.M503002200. [DOI] [PubMed] [Google Scholar]
- 4.Quintans J, Kilkus J, McShan CL, et al. Ceramide mediates the apoptotic response of WEHI 231 cells to anti-immunoglobulin, corticosteroids and irradiation. Biochem Biophys Res Commun. 1994;202:710–714. doi: 10.1006/bbrc.1994.1988. [DOI] [PubMed] [Google Scholar]
- 5.Vit JP, Rosselli F. Role of the ceramide-signaling pathways in ionizing radiation–induced apoptosis. Oncogene. 2003;22:8645–8652. doi: 10.1038/sj.onc.1207087. [DOI] [PubMed] [Google Scholar]
- 6.Pettus BJ, Chalfant CE, Hannun YA. Ceramide in apoptosis: an overview and current perspectives. Biochim Biophys Acta. 2002;1585:114–125. doi: 10.1016/s1388-1981(02)00331-1. [DOI] [PubMed] [Google Scholar]
- 7.Maceyka M, Payne SG, Milstien S, et al. Sphingosine kinase, sphingosine-1-phosphate, and apoptosis. Biochem Biophys Acta. 2002;1585:193–201. doi: 10.1016/s1388-1981(02)00341-4. [DOI] [PubMed] [Google Scholar]
- 8.Pettus BJ, Bielawski J, Porcelli AM, et al. The sphingosine kinase 1/ sphingosine-1-phosphate pathway mediates COX-2 induction and PGE2 production in response to TNF-alpha. FASEB J. 2003;17:1411–1121. doi: 10.1096/fj.02-1038com. [DOI] [PubMed] [Google Scholar]
- 9.Taha TA, Hannun YA, Obeid LM. Sphingosine kinase: biochemical and cellular regulation and role in disease. J Biochem Mol Biol. 2006;39:113–131. doi: 10.5483/bmbrep.2006.39.2.113. [DOI] [PubMed] [Google Scholar]
- 10.Bayerl MG, Abou-Elella A, Bruggeman RD, et al. Sphingosine kinase 1 protein and mRNA is highly expressed in B-cell NHL and is a potential target for pharmacological inhibition. Mod Pathol. 2007;20:232A–267A. [Google Scholar]
- 11.Sobue S, Iwasaki T, Sugisaki C, et al. Quantitative RT-PCR analysis of sphingolipid metabolic enzymes in acute leukemia and myelodysplastic syndromes. Leukemia. 2006;20:2042–2046. doi: 10.1038/sj.leu.2404386. [DOI] [PubMed] [Google Scholar]
- 12.Kihara A, Igarashi Y. FVT-1 is a mammalian 3-ketodihydrosphingosine reductase with an active site that faces the cytosolic side of the endoplasmic reticulum membrane. J Biol Chem. 2004;279:49243–49250. doi: 10.1074/jbc.M405915200. [DOI] [PubMed] [Google Scholar]
- 13.Fan W, Bubman D, Chadburn A, et al. Distinct subsets of primary effusion lymphoma can be identified based on their cellular gene expression profile and viral association. J Virol. 2005;79:1244–1251. doi: 10.1128/JVI.79.2.1244-1251.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rimokh R, Gadoux M, Bertheas MF, et al. FVT-1, a novel human transcription unit affected byvariant translocation t(2;18)(p11;q21) of follicular lymphoma. Blood. 1993;81:136–142. [PubMed] [Google Scholar]
- 15.Lee KE. dissertation. College Station: Texas A&M University; 2004. [Accessed October 1, 2008]. Bayesian Models for DNA Microarray Data Analysis; pp. 41–50. http://txspace.tamu.edu/handle/1969.1/2465. [Google Scholar]
- 16.Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103:275–282. doi: 10.1182/blood-2003-05-1545. [DOI] [PubMed] [Google Scholar]
- 17.Alizadeh AA, Eisen MB, Davis RE, et al. Distinct types of diffuse large B-cell lymphoma identified bygene expression profiling. Nature. 2000;403:503–511. doi: 10.1038/35000501. [DOI] [PubMed] [Google Scholar]
- 18.Rosenwald A, Wright G, Chan WC, et al. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:1937–1947. doi: 10.1056/NEJMoa012914. [DOI] [PubMed] [Google Scholar]
- 19.Chen YB, Rahemtullah A, Hochberg E. Primary effusion lymphoma. Oncologist. 2007;12:569–576. doi: 10.1634/theoncologist.12-5-569. [DOI] [PubMed] [Google Scholar]
- 20.Boulanger E, Gerard L, Gabarre J, et al. Prognostic factors and outcome of human herpesvirus 8–associated primary effusion lymphoma in patients with AIDS. J Clin Oncol. 2005;19:4372–4380. doi: 10.1200/JCO.2005.07.084. [DOI] [PubMed] [Google Scholar]
- 21.Perry DK. Serine palmitoyltransferase: role in apoptotic de novo ceramide synthesis and other stress responses. Biochim Biophys Acta. 2002;1585:146–152. doi: 10.1016/s1388-1981(02)00335-9. [DOI] [PubMed] [Google Scholar]
- 22.Kalen A, Borchardt RA, Bell RM. Elevated ceramide levels in GH4C1 cells treated with retinoic acid. Biochim Biophys Acta. 1992;1125:90–96. doi: 10.1016/0005-2760(92)90160-w. [DOI] [PubMed] [Google Scholar]
- 23.Bose R, Verheij M, Haimovitz-Friedman A, et al. Ceramide synthase mediates daunorubicin-induced apoptosis: an alternative mechanism for generating death signals. Cell. 1995;82:405–414. doi: 10.1016/0092-8674(95)90429-8. [DOI] [PubMed] [Google Scholar]
- 24.Garzotto M, White-Jones M, Jiang Y, et al. 12-O-tetradecanoylphorbol-13-acetate–induced apoptosis in LNCaP cells is mediated through ceramide synthase. Cancer Res. 1998;58:2260–2264. [PubMed] [Google Scholar]
- 25.Reynolds CP, Maurer BJ, Kolesnick RN. Ceramide synthesis and metabolism as a target for cancer therapy. Cancer Lett. 2004;206:169–180. doi: 10.1016/j.canlet.2003.08.034. [DOI] [PubMed] [Google Scholar]
- 26.Radin NS. Killing tumors byceramide-induced apoptosis: a critique of available drugs. Biochem J. 2003;371:243–256. doi: 10.1042/BJ20021878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mansoor M, Melendez AJ. Recent trials for FTY720 (fingolimod): a new generation of immunomodulators structurally similar to sphingosine. Rev Recent Clin Trials. 2008;3:62–69. doi: 10.2174/157488708783330486. [DOI] [PubMed] [Google Scholar]
- 28.Liu Q, Zhao X, Frissora F, et al. FTY720 demonstrates promising preclinical activityfor chronic lymphocytic leukemia and lymphoblastic leukemia/lymphoma. Blood. 2008;111:275–284. doi: 10.1182/blood-2006-10-053884. [DOI] [PMC free article] [PubMed] [Google Scholar]