Abstract
INTRODUCTION
We examine whether regular cigarette smokers were more likely to be exposed to and use cannabis at an earlier age, and further, upon initiation, whether their initial experiences with cannabis varied from those reported by never/non-regular cigarette smokers.
METHOD
A sample of 3797 Australian twins and siblings aged 21–46 years was used. Survival analyses examined whether cigarette smokers were at increased likelihood of early opportunity to use cannabis and early onset of cannabis use. Logistic regression examined whether cigarette smokers reported greater enjoyment of their cannabis experience, inhaling on the first try, differing positive and negative initial subjective reactions, smoked cigarettes with cannabis the first time and were more likely to try cannabis again within a week.
RESULTS
Regular cigarette smokers were more likely to report an earlier opportunity to use cannabis and early onset of cannabis use. Regular cigarette smokers were also considerably more likely to have enjoyed their first experience with cannabis and reported higher rates of positive initial reactions. They were more likely to report inhaling on the first try and smoking cigarettes with cannabis. Potentially negative subjective reactions were also elevated in regular cigarette smokers. Importantly, cigarette smokers were at 1.87 increased odds of smoking cannabis within a week of their initial use.
CONCLUSION
These findings indicate that the well-known overlap in cannabis and cigarette smoking behaviors may evolve as early as opportunity to use and extend through the course of the substance use trajectory.
Keywords: cannabis, cigarette, initial reaction, onset, opportunity
1. INTRODUCTION
Cannabis is the most commonly used illicit drug in developed nations (Degenhardt et al., 2008). Over 90% of those reporting lifetime cannabis use also report having smoked cigarettes at least once in their lifetime (Agrawal et al., 2012). Tobacco smoking typically precedes onset of cannabis use (Degenhardt et al., 2010) and has been posited to increase the likelihood of cannabis involvement (Kandel and Yamaguchi, 1993). Additionally, cigarette smokers are at increased likelihood of using cannabis (Kandel, 2002). One study also found that cannabis exposure opportunity occurred more frequently, and at earlier ages, in tobacco users than non-users (Wagner and Anthony, 2002).
Cigarette smoking may also influence some of the earliest experiences that an individual has with cannabis. Cigarette smokers may smoke cigarettes with cannabis when using it for the first time or relate their experiences with cannabis to their prior experiences with cigarettes. Early subjective reactions to cannabis smoking, such as feeling relaxed, predict later cannabis use disorders (Fergusson et al., 2003). However, the extent to which cigarette smoking modifies these early experiences remains unexplored.
In this study, we utilize data from Australian men and women to examine two important research questions. First, we investigate whether cigarette smoking was associated with an earlier age at first opportunity to use and onset of cannabis use. Second, we test whether early experiences with cannabis, including characteristics of first use (self-reported inhalation, smoking cigarettes with cannabis, positive (e.g., pleasurable buzz) and negative (e.g., coughing) subjective experiences and enjoyment), as well as using cannabis again within a week of first use, were differentially reported by cigarette smokers.
2. METHODS
2.1 Sample
Data on 3,824 young adult (mean age 32.1, age range 21–46 years, 3348 twins and 476 of their non-twin siblings) Australian male and female twin and siblings were drawn from the Cannabis Twin Study. Participants were recruited from 4,131 twin pairs in the Australian Twin Registry (ATR) born between 1972 and 1979. Details regarding sample recruitment are available in a related publication (Lynskey et al., 2012). The final sample consisted of 3,797 participants (63.9% female, representing 75.9% of participants contacted; 27 individuals missing data on cannabis use were excluded).
2.2 Assessment
Computer-assisted telephone interviews (CATI) were used to gather self-report information from all participants. A modified version of the Semi-Structured Assessment of the Genetics of Alcoholism (SSAGA-OZ; Bucholz et al., 1994) was used, which included questions on early experiences with cannabis and a detailed assessment of cigarette smoking behavior derived from the Composite International Diagnostic Interview (CIDI; Cottler et al., 1991). In addition, the SSAGA-OZ interview collected full DSM-IV (American Psychiatric Association, 1994) diagnostic information on alcohol, nicotine, cannabis and other illicit drug abuse/dependence as well as other psychopathology, including conduct disorder.
2.3 Measures
2.3.1 Cigarette smoking
Defined as having smoked 100 or more cigarettes during the lifetime (Centers for Disease Control, 2007).
2.3.2 Age at cannabis exposure and onset
Age at which participants first had access to cannabis, irrespective of whether they used it or not (Wagner and Anthony, 2002) and age at first cannabis use.
2.3.3 Early experiences with cannabis
An extensive series of items was used to elicit the respondent’s earliest experiences with smoking cannabis. These included:
How soon after initial experimentation the respondent had tried cannabis again: responses, across 9 categories, ranged from ‘never’ to ‘the same day’. For these analyses, using cannabis within a week of initial experimentation was coded as 1.
How deeply the respondent inhaled the first time they tried cannabis: responses, in 4 categories, ranged from ‘in my lungs, and deeply’ to ‘just in my mouth.’ For these analyses, inhaling into the lungs (deeply or not deeply) was coded as 1.
Whether they smoked cigarettes when they first smoked cannabis (yes/no).
How much they enjoyed smoking cannabis the first time they tried it: responses, in 4 categories, ranged from ‘not at all’ to ‘a lot’. For these analyses, enjoying cannabis a lot was coded as 1.
Initial positive and negative subjective reactions to cannabis: based on yes/no responses to items described by Fergusson and colleagues (Fergusson et al., 2003) supplemented with subjective initial reactions developed for cigarette smokers, dealing with physical responses to smoking (Pomerleau et al., 1998).
2.3.4 Covariates
Only covariates that could have exerted an impact during the developmental period during which cannabis onset occurs were included. In addition to age and sex, lifetime measures of history of regular alcohol use (used once a month for six months or longer, but only if regular alcohol use had been initiated prior to the onset of cannabis use) and DSM-IV diagnosis of a lifetime history of conduct disorder were included.
2.4 Statistical Analysis
For age at first opportunity to use cannabis and age at onset of cannabis use, discrete-time proportional hazards models were fitted to the data using STATA (Stata Corp, 2003). Ages at which individuals reported first opportunity and first use tended to cluster around 12–28 years of age (i.e., not continuously distributed) and hence, we elected to use a piecewise constant for the baseline hazard (δ14, 15, 16, 17, 18, 19–20 and δ21 years) and a logit model. Analyses were adjusted for clustered data using a robust variance estimator. In addition, covariates representing sex, zygosity (dizygotic twin or not and membership in a same or opposite sex dizygotic pair) and interactions between sex and the zygosity terms were introduced into the models to account for the twin data.
Initial comparisons of early experiences with cannabis across smokers and non-smokers were conducted in SAS v9.2 (SAS Institute, 2012). Subsequent analyses examining the association between these early experiences and cigarette smoking were conducted in STATA. Logistic regression was used with robust standard errors for adjustment of non-independence of twin and sibling observations. Additionally, the covariates described above (sex, zygosity and their interactions) were added to the model. All analyses were also repeated by randomly selecting one member of the twin pair.
3. RESULTS
In the full sample, 2,601 individuals (69%) reported lifetime cannabis use. Of these cannabis users, 1,393 (55%) were regular smokers and the remaining 1,208 (46%) had never smoked 100 or more cigarettes (never regular smokers) or were lifetime non-smokers. Rates of regular cigarette smoking were markedly lower in those who had never used cannabis (12%).
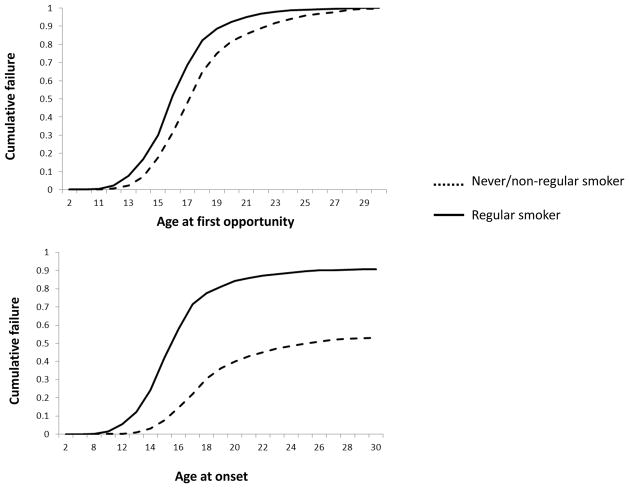
Likelihood of exposure to and onset of cannabis use in smokers and non-smokers is shown in Figure 1. Regular cigarette smoking was associated with both an earlier opportunity to use cannabis and with earlier onset of first use. The hazard ratios for opportunity to use cannabis and for onset of use were 2.35 (95% C.I. 2.16–2.56) and 3.49 (95% C.I. 3.18–3.83) respectively. As early age at first opportunity was correlated with early age at first cannabis use, we controlled for the effects of age at opportunity in the model for onset of cannabis use, the hazard ratio remained significantly elevated (2.72, 95% C.I. [2.45–3.02]). Thus, regular cigarette smokers were more likely to report both earlier opportunity to use and earlier onset of cannabis use.
Figure 1.
Likelihood of opportunity to use cannabis and its onset of use at various ages of onset in regular cigarette smokers and those who are never smokers or did not smoke regularly in a sample of 2601 cannabis users.
3.1 Early experiences with cannabis in cigarette smokers
Differences in early experiences with cannabis between the never/non-regular and regular cigarette smokers are shown in Table 1. Results are presented from individual models with cannabis-related measures as outcomes, adjustment of covariates described above and application of robust standard errors for familial clustering. Regular smokers reported more rapid escalation in cannabis involvement and more positive initial subjective reactions. For instance, compared with never/non-regular smokers, regular cigarette smokers were 87% more likely to try cannabis again within the first week. Similarly, they were at 2.41 increased likelihood of reporting that they liked the taste of cannabis the first time they used it. Regular cigarette smokers were also more likely to report some of the putatively negative reactions, such as confusion, getting the jitters and experiencing nausea/vomiting. In contrast, no differences were noted for coughing, burning in the throat or headaches across the groups. Results remained unchanged when analyses were replicated by randomly selecting one member of the twin pair (not shown).
Table 1.
Opportunity to use cannabis, onset of cannabis use and initial cannabis experiences among 2601 Australian male and female twins reporting a lifetime history of cannabis use, across regular and non-regular/never cigarette smokers.
| Never smoked regularly/never smoked (N=1208) | Regular smoker (N=1393) | Odds-ratio [95% C.I.] | p-value | |
|---|---|---|---|---|
| Mean [SD] age at first opportunity to use | 18.0 [3.4] | 16.7 [2.7] | - | <.0001 |
| Mean [SD] age at first use | 18.8 [3.7] | 17.1 [2.9] | - | <.0001 |
| Tried again within a week | 11.9 | 21.5 | 1.87 [1.49–2.34] | <.0001 |
| Inhaled into lungs the first time | 51.7 | 72.6 | 2.52 [2.11–3.00] | <.0001 |
| Smoked cigarettes with cannabis the first time | 5.6 | 47.1 | 16.4 [11.7–20.21] | <.0001 |
| Enjoyed the first time a great deal | 7.9 | 18.8 | 2.29 [1.75–2.98] | <.0001 |
| Initial subjective reactions | ||||
| Like the taste | 8.7 | 19.3 | 2.41 [1.88–3.09] | <.0001 |
| Coughed | 86.7 | 85.1 | 0.84 [0.66–1.05] | 0.24 |
| Felt dizzy | 60.7 | 77.0 | 2.08 [1.74–2.49] | <.0001 |
| Felt relaxed | 47.4 | 54.4 | 1.29 [1.10–1.52] | 0.0003 |
| Giggled a lot | 57.0 | 71.0 | 1.79 [1.43–2.01] | <.0001 |
| Felt pleasurable rush or buzz | 30.2 | 46.3 | 1.82 [1.53–2.16] | <.0001 |
| Felt nauseous/vomited | 15.1 | 25.6 | 2.01 [1.63–2.49] | <.0001 |
| Had a headache | 15.5 | 15.7 | 1.05 [0.84–1.31] | 0.9053 |
| Got the jitters | 8.0 | 16.1 | 2.23 [1.70–2.92] | <.0001 |
| Felt a burning in the throat | 43.5 | 42.4 | 0.94 [0.80–1.11] | 0.57 |
| Felt confused | 18.2 | 33.5 | 2.18 [1.80–2.63] | <.0001 |
4. DISCUSSION
Consistent with prior work by Wagner and Anthony (2002), the first key finding of this study was that both opportunity to use cannabis and its first use occurred earlier in cigarette smokers. This earlier onset can be attributed to a general predisposition to precocious or non-normative behaviors, of which cigarette smoking and early-onset cannabis use are components (Huizink et al., 2010; Korhonen et al., 2010; Storr et al., 2011). This may also include the role of deviant peers who promote cigarette smoking and also facilitate opportunity to use cannabis at younger ages (Creemers et al., 2010; Kobus, 2003; Lynskey et al., 1998). Other shared predisposing factors might include genetic vulnerability to drug use as well as genes that influence general disinhibition and impulsive behaviors (Huizink et al., 2010; Korhonen et al., 2012; Neale et al., 2006).
Regular cigarette smokers in this study reported that they inhaled cannabis on that first occasion. This may indicate respiratory adaptation, perhaps attributable to expertise with cigarette smoking. One study comparing cannabis use topographies in cigarette smokers failed to note differences in inhalation depth or other topographical measures (Simmons and Tashkin, 1995) but further exploration of the role of cigarette smoking on early cannabis smoking topography is needed.
Cigarette smokers also consistently reported greater positive initial subjective experiences with cannabis. However, no significant reduction in coughing or burning in the throat was noted. Increased reports of positive reactions may indicate respiratory adaptation and the possibility that individuals who may be primed to respond favorably to smoking-related cues may anticipate, experience or, at least subjectively, recall and report more positive experiences with all smoked substances.
Cigarette smoking was also associated with similar subjective responses (Pomerleau et al., 1998). Therefore, we cannot rule out the possibility that either tandem cigarette smoking elicited more positive subjective reactions to cannabis or that individuals attributed their subjective response to cigarette smoking to the use of cannabis. In addition, some individuals may report similar subjective reactions across multiple substances. Typical subjective reactions to tobacco and cannabis are correlated (Zeiger et al., 2012) with greater correlations within the positive and negative domains of reactions. Thus, it is possible that the effect of cigarette smoking on initial reactions to cannabis may reflect similar initial or general subjective reactions to cigarettes as well. This is consistent with another study that reported that a common heritable drug sensitivity factor linked general (not initial) subjective reactions to alcohol, tobacco and cannabis (Haberstick et al., 2011).
It is worth noting that cigarette smokers also reported experiencing greater dizziness, nausea, confusion and feeling the jitters or trembling muscles than non-smokers. While these may be typically considered negative experiences, their subjective experience is likely to vary by the individual’s expectations of the psychoactive effects of cannabis. However, while positive subjective reactions have been consistently implicated in the subsequent emergence of cannabis use disorders (Fergusson et al., 2003a; Grant et al., 2005; Le et al., 2009; Lyons et al., 1997; Scherrer et al., 2009; Zeiger et al., 2010), fewer studies have noted the predictive utility of negative reactions. Additionally, increased positive and negative reactions may be attributed to a higher initial dose of 9-tetrahydrocannabinol from cannabis smoke, which regular smokers were more likely to inhale. Finally, the possibility of interactions between nicotine and 9-tetrahydrocannabinol should also be explored. Animal studies indicate that nicotine may enhance the rewarding effects of cannabis (Valjent et al., 2002).
There were some limitations to this study. First, all reports of initial experiences were based on retrospective recall. Some bias in recall may be attributed to respondents reporting their first cannabis use about 15 years prior to the interview. Furthermore, there is the possibility that recall of early experiences varies by current levels of cannabis and tobacco involvement. Those who had used cannabis 11 or more times during their lifetime were asked about recency of use – only 9% reported cannabis use in the 30 days prior to the interview. However, 44% of lifetime regular cigarette smokers were current cigarette smokers at the time of interview. Second, this is a study of adult Australian twins and siblings and may not generalize to other populations. However, there is strong support for the generalizability of twin samples (Kendler and Prescott, 2006).
In conclusion, we find that cannabis-related behaviors, even those as early as first opportunity to use and reactions surrounding initial experimentation, are altered in cigarette smokers. This suggests that cannabis use trajectories should be viewed in the context of co-occurring cigarette smoking. Furthermore, as rates of cigarette smoking decrease in youth (Johnston et al., 2011), there may be corresponding reductions in cannabis involvement, even though these changes are not currently apparent.
Acknowledgments
Funding: This research is supported by R01DA23668 and K02DA32573 to AA. Data collection was funded by National Institute on Drug Abuse (NIDA) grant: DA18267 (ML) and facilitated through access to the Australian Twin Registry, a national resource supported by an Enabling Grant (ID 628911) from the National Health & Medical Research Council. Funding agencies played no role in data analysis, manuscript preparation or any other aspect of the study.
We thank Anjali Henders, Dixie Statham, Richard Parker, Soad Hancock, Judith Moir, Sally Rodda, Pieta-Maree Shertock, Heather Park, Jill Wood, Pam Barton, Fran Husband and Adele Somerville.
Footnotes
Conflict of Interest: None.
Author contributions
AA and MTL conceived of hypotheses. AA conducted all analyses and AA and MTL prepared the first draft and all revised drafts. MTL collected all data. PAFM provided expertise on assessment and phenotypic coding. NGM supervised data collection and provided expertise on data analysis. All authors approved submitted versions of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agrawal A, Budney AJ, Lynskey MT. The co-occurring use and misuse of cannabis and tobacco: a review. Addiction. 2012;107:1221–1233. doi: 10.1111/j.1360-0443.2012.03837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- Bucholz KK, Cadoret RJ, Cloninger RC, Dinwiddie SH, Hesselbrock V, Nurnberger JI, Reich T, Schmidt I, Schuckit MA. A new, semi-structured psychiatric interview for use in genetic linkage studies. J Stud Alcohol. 1994;55:149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control (CDC) Cigarette smoking among adults: United States, 2006. MMWR. 2007;56:1157–1161. [PubMed] [Google Scholar]
- Cottler LB, Robins LN, Grant BF, Blaine J, Towle LH, Wittchen HU, Sartorius N. The CIDI-core substance abuse and dependence questions: cross-cultural and nosological issues. The WHO/ADAMHA Field Trial. Br J Psychiatry. 1991;159:653–8. 653–658. doi: 10.1192/bjp.159.5.653. [DOI] [PubMed] [Google Scholar]
- Creemers HE, Dijkstra JK, Vollebergh WA, Ormel J, Verhulst FC, Huizink AC. Predicting life-time and regular cannabis use during adolescence; the roles of temperament and peer substance use: the TRAILS study. Addiction. 2010;105:699–708. doi: 10.1111/j.1360-0443.2009.02819.x. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Chiu WT, Sampson N, Kessler RC, Anthony JC, Angermeyer M, Bruffaerts R, de GG, Gureje O, Huang Y, Karam A, Kostyuchenko S, Lepine JP, Mora ME, Neumark Y, Ormel JH, Pinto-Meza A, Posada-Villa J, Stein DJ, Takeshima T, Wells JE. Toward a global view of alcohol, tobacco, cannabis, and cocaine use: findings from the WHO World Mental Health Surveys. PLoS Med. 2008;5:e141. doi: 10.1371/journal.pmed.0050141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt L, Dierker L, Chiu WT, Medina-Mora ME, Neumark Y, Sampson N, Alonso J, Angermeyer M, Anthony JC, Bruffaerts R, de GG, de GR, Gureje O, Karam AN, Kostyuchenko S, Lee S, Lepine JP, Levinson D, Nakamura Y, Posada-Villa J, Stein D, Wells JE, Kessler RC. Evaluating the drug use “gateway” theory using cross-national data: consistency and associations of the order of initiation of drug use among participants in the WHO World Mental Health Surveys. Drug Alcohol Depend. 2010;108:84–97. doi: 10.1016/j.drugalcdep.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fergusson DM, Horwood LJ, Lynskey MT, Madden PA. Early reactions to cannabis predict later dependence. Arch Gen Psychiatry. 2003b;60:1033–1039. doi: 10.1001/archpsyc.60.10.1033. [DOI] [PubMed] [Google Scholar]
- Grant JD, Scherrer JF, Lyons MJ, Tsuang M, True WR, Bucholz KK. Subjective reactions to cocaine and marijuana are associated with abuse and dependence. Addict Behav. 2005;30:1574–1586. doi: 10.1016/j.addbeh.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Haberstick BC, Zeiger JS, Corley RP, Hopfer CJ, Stallings MC, Rhee SH, Hewitt JK. Common and drug-specific genetic influences on subjective effects to alcohol, tobacco and marijuana use. Addiction. 2011;106:215–224. doi: 10.1111/j.1360-0443.2010.03129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizink AC, Levalahti E, Korhonen T, Dick DM, Pulkkinen L, Rose RJ, Kaprio J. Tobacco, cannabis, and other illicit drug use among finnish adolescent twins: causal relationship or correlated liabilities? J Stud Alcohol Drugs. 2010;71:5–14. doi: 10.15288/jsad.2010.71.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LD, O’Malley P, Bachman JG, Schulenberg J. Marijuana use is rising; ecstasy use is beginning to rise; and alcohol use is declining among US teens. University of Michigan News Service; Ann Arbor: 2011. [Google Scholar]
- Kandel D. Stages and Pathways of Drug Involvement. Cambridge University Press; New York, NY: 2002. [Google Scholar]
- Kandel D, Yamaguchi K. From beer to crack: developmental patterns of drug involvement. Am J Public Health. 1993;83:851–855. doi: 10.2105/ajph.83.6.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA. Genes, Environment and Psychopathology: Understanding the Causes of Psychiatric and Substance Use Disorders. Guilford Press; New York: 2006. Twinning and twin models; pp. 34–50. [Google Scholar]
- Kobus K. Peers and adolescent smoking. Addiction. 2003;98(Suppl 1):37–55. doi: 10.1046/j.1360-0443.98.s1.4.x. [DOI] [PubMed] [Google Scholar]
- Korhonen T, Latvala A, Dick DM, Pulkkinen L, Rose RJ, Kaprio J, Huizink AC. Genetic and environmental influences underlying externalizing behaviors, cigarette smoking and illicit drug use across adolescence. Behav Genet. 2012;42:614–625. doi: 10.1007/s10519-012-9528-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen T, Levalahti E, Dick DM, Pulkkinen L, Rose RJ, Kaprio J, Huizink AC. Externalizing behaviors and cigarette smoking as predictors for use of illicit drugs: a longitudinal study among Finnish adolescent twins. Twin Res Hum Genet. 2010;13:550–558. doi: 10.1375/twin.13.6.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le SY, Ramoz N, Horwood J, Falissard B, Hassler C, Romo L, Choquet M, Fergusson D, Gorwood P. First positive reactions to cannabis constitute a priority risk factor for cannabis dependence. Addiction. 2009;104:1710–1717. doi: 10.1111/j.1360-0443.2009.02680.x. [DOI] [PubMed] [Google Scholar]
- Lynskey MT, Agrawal A, Henders A, Nelson EC, Madden PA, Martin NG. An Australian twin study of cannabis and other illicit drug use and misuse, and other psychopathology. Twin Res Hum Genet. 2012:1–11. doi: 10.1017/thg.2012.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynskey MT, Fergusson DM, Horwood LJ. The origins of the correlations between tobacco, alcohol, and cannabis use during adolescence. J Child Psychol Psychiatry. 1998;39:995–1005. [PubMed] [Google Scholar]
- Lyons MJ, Toomey R, Meyer JM, Green AI, Eisen SA, Goldberg J, True WR, Tsuang MT. How do genes influence marijuana use? The role of subjective effects. Addiction. 1997;92:409–417. [PubMed] [Google Scholar]
- Neale MC, Harvey E, Maes HH, Sullivan PF, Kendler KS. Extensions to the modeling of initiation and progression: applications to substance use and abuse. Behav Genet. 2006;36:507–524. doi: 10.1007/s10519-006-9063-x. [DOI] [PubMed] [Google Scholar]
- Pomerleau OF, Pomerleau CS, Namenek RJ. Early experiences with tobacco among women smokers, ex-smokers, and never-smokers. Addiction. 1998;93:595–599. doi: 10.1046/j.1360-0443.1998.93459515.x. [DOI] [PubMed] [Google Scholar]
- Rabinoff M, Caskey N, Rissling A, Park C. Pharmacological and chemical effects of cigarette additives. Am J Public Health. 2007;97:1981–1991. doi: 10.2105/AJPH.2005.078014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAS Institute. SAS v9.2. Cary, NC: 2012. [Google Scholar]
- Scherrer JF, Grant JD, Duncan AE, Sartor CE, Haber JR, Jacob T, Bucholz KK. Subjective effects to cannabis are associated with use, abuse and dependence after adjusting for genetic and environmental influences. Drug Alcohol Depend. 2009;105:76–82. doi: 10.1016/j.drugalcdep.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons MS, Tashkin DP. The relationship of tobacco and marijuana smoking characteristics. Life Sci. 1995;56:2185–2191. doi: 10.1016/0024-3205(95)00206-l. [DOI] [PubMed] [Google Scholar]
- Stata Corp. STATA. College Station, TX: 2003. [Google Scholar]
- Storr CL, Wagner FA, Chen CY, Anthony JC. Childhood predictors of first chance to use and use of cannabis by young adulthood. Drug Alcohol Depend. 2011;117:7–15. doi: 10.1016/j.drugalcdep.2010.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Mitchell JM, Besson MJ, Caboche J, Maldonado R. Behavioural and biochemical evidence for interactions between Delta 9-tetrahydrocannabinol and nicotine. Br J Pharmacol. 2002;135:564–578. doi: 10.1038/sj.bjp.0704479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner FA, Anthony JC. Into the world of illegal drug use: exposure opportunity and other mechanisms linking the use of alcohol, tobacco, marijuana, and cocaine. Am J Epidemiol. 2002;155:918–925. doi: 10.1093/aje/155.10.918. [DOI] [PubMed] [Google Scholar]
- Zeiger JS, Haberstick BC, Corley RP, Ehringer MA, Crowley TJ, Hewitt JK, Hopfer CJ, Stallings MC, Young SE, Rhee SH. Subjective effects to marijuana associated with marijuana use in community and clinical subjects. Drug Alcohol Depend. 2010;109:161–166. doi: 10.1016/j.drugalcdep.2009.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeiger JS, Haberstick BC, Corley RP, Ehringer MA, Crowley TJ, Hewitt JK, Hopfer CJ, Stallings MC, Young SE, Rhee SH. Subjective effects for alcohol, tobacco, and marijuana association with cross-drug outcomes. Drug Alcohol Depend. 2012;123:S52–S58. doi: 10.1016/j.drugalcdep.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]