SUMMARY
The most severe form of human malaria is caused by the protozoan parasite Plasmodium falciparum. The primary antigenic and virulence determinant expressed on the surface of infected red blood cells is PfEMP1, a protein that mediates adhesion and sequestration of the parasites in deep tissue vascular beds. Different forms of PfEMP1 are encoded by different members of the multi-copy var gene family. Expression of var genes is mutually exclusive, and by switching which gene is expressed, parasites alter both their antigenic and virulence phenotypes. Regulation of var gene expression involves gene activation, silencing and cellular memory, and the details of the mechanisms that control this process are not understood. Here we provide evidence that active transcription is required for the maintenance of the cellular memory that marks a specific var gene to be stably expressed through numerous cell cycles. Forcing transfected parasites to express increasing numbers of unregulated episomal var promoters led to a corresponding down-regulation of the active var gene in the parasite’s genome, presumably by competing for the transcriptional machinery of the parasite and suggesting the existence of a limited nuclear factor that is required for var gene activation. This process allowed us to repress transcription of the active var gene without acting through the mechanism that controls mutually exclusive expression, and thus to investigate the role of transcription itself in maintaining epigenetic memory. When the competing episomes were removed, the parasites did not return to their previous var gene expression pattern, but rather displayed random var gene activation, demonstrating that the epigenetic imprint that controls var gene expression had been completely erased and thus linking active transcription to the maintenance of cellular memory.
Keywords: antigenic variation, virulence, transcription, gene expression, epigenetics
INTRODUCTION
Malaria caused by the parasite Plasmodium falciparum is known for its persistent infections as well as its potentially lethal complications. Both of these characteristics are linked to expression of a variable antigen displayed on the surface of infected red blood cells called Plasmodium falciparum erythrocyte membrane protein one (PfEMP1)1. This protein functions as a surface receptor that mediates binding and cytoadherence of infected cells to various molecules found on the surface of the endothelial cells of the microvasculature. This cytoadherence results in both obstruction of blood flow and a localized immune response that are thought to be responsible for such severe complications as cerebral malaria and pregnancy associated malaria2. Each individual parasite is capable of expressing many different forms of PfEMP1, each differing in both its antigenic and cytoadherent characteristics. By switching which PfEMP1 is expressed over the course of an infection, the parasite is able to avoid the antibody response generated by the host to previously expressed forms of PfEMP13.
PfEMP1 is encoded by the multi-copy var gene family4–6. The genome of each parasite contains approximately 60 different var genes, each coding for a different form of PfEMP17. The genes are expressed in a mutually exclusive fashion such that only a single gene is transcriptionally active at a time while the remainder of the var gene complement is maintained in a transcriptionally silent state8,9. This strict regulatory paradigm ensures that only a single form of PfEMP1 is found on the surface of an infected red blood cell, thus the expressed var gene determines both the cell’s antigenic as well as cytoadherence phenotype. Switches in var gene expression result in antigenic variation by changing the form of PfEMP1 exposed to the immune system, thereby allowing parasites to maintain a persistent, long-term infection that is difficult to clear. Proper regulation of var gene expression requires recognition of all var genes as members of a single family as well as coordinated activation and silencing of individual genes to maintain mutually exclusive expression in the event of a switch. In addition, a form of cellular memory allows parasites to display a relatively low var gene switching rate over the course of many cell cycles, thus preventing premature expenditure of the antigenic repertoire10,11. This low switching rate reflects the fact that once activated, a var gene tends to remain active, suggesting the possibility that the act of transcription itself might help to reinforce the maintenance of the epigenetic marks that control cellular memory. Consistent with this hypothesis, it has recently been shown in other eukaryotic systems that certain chromatin modifying proteins directly associate with RNA polymerase II and exert their influence on chromatin structure during transcriptional elongation, thus linking transcription to chromatin modification and epigenetic memory12,13. However, the molecular basis of var gene recognition, silencing and activation, as well as the how switching is coordinated, remain obscure.
Each var gene contains two separate promoters, one upstream of the first exon that is responsible for expression of the PfEMP1 encoding mRNA and a second found within a conserved intron that separates the two exons of each gene14. This intron promoter drives expression of a “sterile” noncoding transcript and has been implicated in var gene silencing6. We have recently found that “unpairing” a var upstream promoter from its adjacent intron promoter renders it constitutively active and unrecognized by the mechanism controlling mutually exclusive expression15,16. This resulted in the unusual situation of individual parasites expressing two var promoters simultaneously. This observation raised the question as to whether there is any limit to how many var promoters an individual parasite nucleus can express at one time, and whether expressing high numbers of unregulated var promoters has any effect on the expression of the endogenous, properly regulated var genes.
Here we show that by forcing parasites to express increasing numbers of episomal “unpaired” var promoters, it is possible to down-regulate and ultimately silence all endogenous var genes in the parasite genome. This ability to specifically repress var gene transcription without affecting the rest of the genome or the viability of the parasites provided us with the opportunity to investigate the role that active transcription plays in maintaining var gene epigenetic memory. When parasites were released from var transcriptional repression, they again expressed endogenous, chromosomal var genes in a mutually exclusive manner, however individual var genes were activated at random, indicating that the epigenetic marks regulating cellular memory that had previously controlled var gene expression had effectively been erased from the genome. These results have implications for understanding both var gene activation as well as the role of active transcription in maintaining cellular memory in malaria parasites.
RESULTS
Forced expression of multiple, unregulated var promoters represses the transcription of endogenous var genes
Mutually exclusive var gene expression requires pairing of the two promoters found in each gene15,16. In order to create transgenic parasite lines that simultaneously expressed multiple var promoters, it was essential to use “unpaired” var promoters that were constitutively active and not recognized by the mechanism that controls mutually exclusive expression. We therefore utilized the plasmid pVBH in which a var promoter that is free from the influence of any adjacent promoter activity and not recognized by the mechanism that controls mutually exclusive expression is used to drive expression of the blasticidin S deaminase gene (bsd)16. This plasmid was used to transfect an NF54 parasite population (called C3) that predominantly expressed a single var gene (PFD1005c). Expression of this particular var gene is remarkably stable over time due to its low off rate17. Replication of episomes in malaria parasites creates multi-copy concatamers18,19 and we hypothesized that by increasing drug pressure we would be able to select for parasites that carried correspondingly increasing numbers of active var promoters on larger multi-copy pVBH concatamers. Parasite were transfected as described 20,21 and initially selected with 2 μg/ml blasticidin. Once a resistant population was established, the parasites were exposed to increasing concentrations of blasticidin (Fig 1A), thus selecting for parasite populations that carried increasing numbers of active episomal var promoters. Selection with higher levels of blasticidin resulted in an initial delayed growth phenotype (Fig 1A), however once established, all parasite populations grew at comparable rates. Using Q-RT-PCR it was possible to quantify the exact copy number of the transgenic, episomal var promoters in each parasite population. As expected, the number of episomal var promoters increased as we increased drug concentration and varied from around a single copy in parasites selected using the lower blasticidin concentration to approximately 18 copies in those selected with the highest dose (Fig 1B). The increased number of promoters resulted in an overall increase in the total expression of bsd in the different transgenic lines (Fig 1C) indicating that they are transcriptionally active and express bsd.
Figure 1.
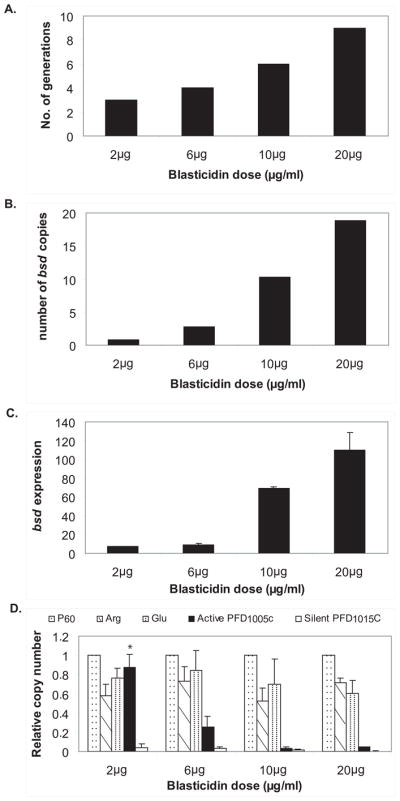
Transcriptional repression of endogenous var genes by forced expression of multiple episomal var promoters. (A) Selection for parasite populations that carry the episome pVBH using increasing concentrations of blasticidin. The number of generations to reach 5% parasitemia increases in correlation to the increase in blasticidin concentration. (B) Parasite populations carry increasing copy numbers of pVBH with respect to blasticidin pressure. The number of bsd copies measured by Q-RT-PCR using gDNA as template is presented for each of the parasite populations grown under different concentrations of blasticidin. (C) bsd expression is correlated with the number of copies of pVBH. Total bsd expression was measured by Q-RT-PCR using cDNA as template from each of the parasite populations carrying increasing copy numbers of pVBH. Total expression is presented as relative copy numbers with respect to the control housekeeping gene seryl-tRNA synthetase (PF07_0073). (D) Transcriptional repression of the endogenous var PFD1005c in parasite populations expressing high copy numbers of pVBH. Expression levels of the active var gene PFD1005c and the silent var gene PFD1015c are presented for each of the parasite populations grown under different blasticidin concentrations with respect to the 3 control housekeeping genes: P60 - seryl-tRNA synthetase (PF07_0073), Arg - arginyl-tRNA synthetase (PFL0900c) and Glu -glutaminyl-tRNA synthetase (PF13_0170), (* significant down regulation, F = 10.49, P < 0.01).
We then were interested in measuring the level of expression of the endogenous var gene (PFD1005c) that was actively transcribed in the original NF54 culture that was transfected. PFD1005c was the only var gene transcribed significantly above background in this population of parasites, and this gene had previously displayed a particularly low off rate, remaining stably expressed for over 6 months of continuous culture after cloning17. We observed that as we increased the number of active exogenous var promoters, the level of transcription of PFD1005c was significantly down-regulated (P < 0.01), and in parasites populations that carried large numbers of the active transgenic “unpaired” var promoters, the level of expression of PFD1005c was repressed to a level equivalent to a silent var gene (Fig 1D). The data suggest that parasites have an inherent capability of expressing up to ~20 var promoters simultaneously, nonetheless in a normal situation, the mechanism that controls mutually exclusive expression limits expression to a single gene. However, in the experiment described here, by forcing expression of an increasing number of unregulated, episomal var promoters, we have saturated the parasite’s ability to actively transcribe var genes, leading to repression of the entire genomic complement. It is worth noting that this is unlike previous genetic manipulations that resulted in silencing of the entire var gene repertoire22. In our previous work, parasites were forced to express a single, properly regulated transgenic var promoter, thus silencing of the var complement in that instance was achieved by infiltrating the mechanism that controls mutually exclusive expression.
A specific nuclear element is a limiting factor for var gene expression
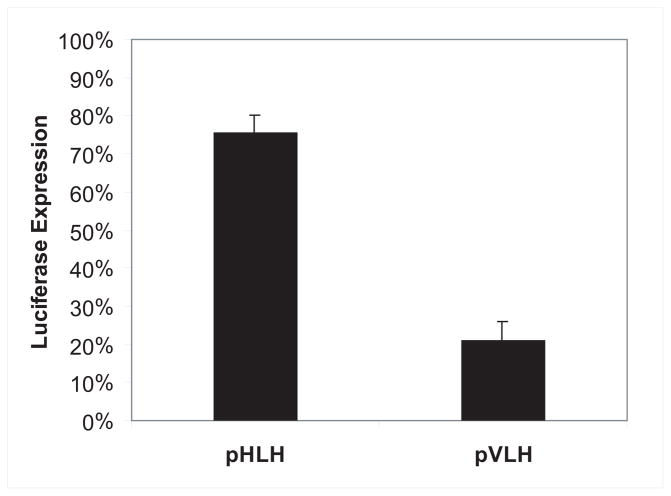
Interestingly, the observed down-regulation of PFD1005c seemed to be var-specific and did not affect parasite growth or the expression of housekeeping genes (Fig 1D). To further test the hypothesis that the observed down-regulation is specific for var promoters, we performed transient transfection assays in two transgenic cultures that were stably carrying pVBH concatamers. Parasites grown under low blasticidin pressure (2μg/ml) and therefore carrying low numbers of active var promoters, and the culture grown under high blasticidin pressure (20μg/ml) and carrying a high number of active var promoters, were used in the experiment. Both cultures were transfected separately with the constructs pVLH and pHLH that express the luciferase reporter gene under the control of either a var promoter (pVLH) or an hrp3 promoter (pHLH). Transient tranfections were performed as described21 and luciferase expression was measured for each of the transfected cultures to determine the effect of the number of active transgenic var promoters in each parasite on its ability to express luciferase from either an additional var promoter or the hrp3 promoter. The expression of luciferase by a var promoter was significantly suppressed (~80%, P < 0.01) in the culture that was already saturated with large numbers of active var promoters stably carried on the multi-copy pVBH episomes when compared to luciferase expression in the parasite line that carried a small number of episomal var promoters (Fig 2). Interestingly, expression of luciferase by the hrp3 promoter was down-regulated only by 26% in the culture carrying the large numbers of active episomal var promoters when compared to its expression in parasites that carried a small number of active episomal var promoters. It is not clear if this small reduction is a result of the increased number of active var promoters or perhaps a side effect of the higher level of blasticidin in the media. Nonetheless, these results confirmed that the transcriptional repression caused by saturation with episomal var promoters has a much stronger effect on var genes than on other genes in the genome.
Figure 2.
A specific nuclear element is required for expression of var promoters. The histogram shows levels of luciferase expression from parasites transiently transfected with plasmids carrying either a var promoter (pVLH) or the unrelated hrp3 promoter (pHLH). The values show luciferase expression by transgenic parasites that stably a carry high copy number of pVBH episomes (grown under 20 μg/ml blasticidin) as a percentage of that expressed by parasites carrying a low copy number (under 2 μg/ml blasticidin). Error bars represent calculated standard errors.
Down-regulation of endogenous var gene expression results in erasing of var epigenetic memory
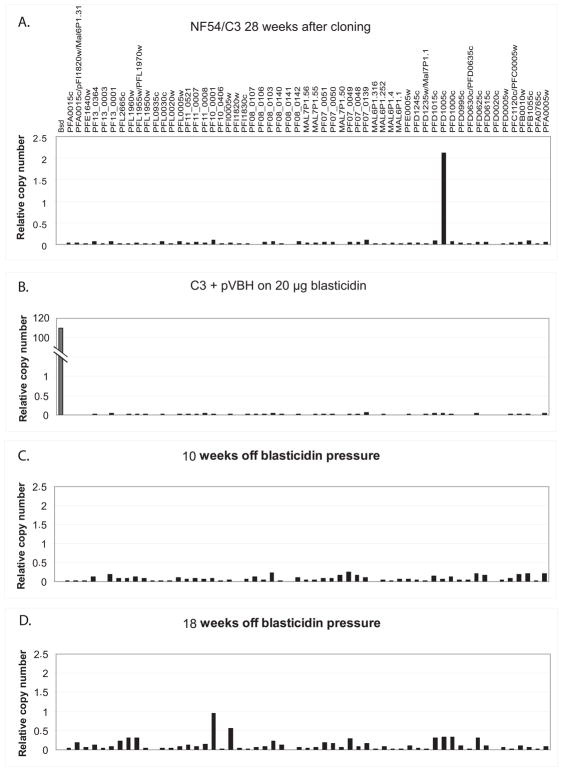
Two recent studies showed that regulation of var expression involves a type of “molecular memory” that includes specific histone modifications10,11. This memory ensures that once activated, a var gene remains active in the vast majority of subsequent cell cycles, thus resulting in the relatively low off rate per generation that is necessary to avoid premature expenditure of the var gene repertoire. Without this memory, var genes would be activated at random after each cell cycle and would not display the measurable switching dynamics observed over time in long-term cultures17. We were interested in whether preventing transcription of PFD1005c by forced expression of high numbers of unregulated episomal var promoters would influence the epigenetic memory that had marked this gene as the only active var gene for over 7 months in the original culture (Fig 3A). To test this, we removed the drug pressure from the transfected culture and let the parasites grow for a month in regular media, providing enough time for the parasites to shed the pVBH episomes. Plasmid rescue and PCR assays verified that the parasites had shed all the episomes. The level of transcription of each individual var gene in the genome was then measured using Q-RT-PCR as described22,23.
Figure 3.
Induced repression of an endogenous var gene erases its epigenetic memory. Steady state mRNA levels expressed from each var gene (labeled by annotation number) as well as bsd are shown for each population of parasites. Analysis of the levels of transcription shows that PFD1005c is the dominant gene expressed by parasites in the NF54/C3 population (A). Expression of high copy numbers of pVBH results in silencing of all var genes in the family (B). Removal of blasticidin pressure leads to loss of the episomes and a random activation pattern after a period of 10 and 18 weeks (C and D, respectively). The parasite populations in C and D have become heterogenous, with numerous subpopulations present, each expressing a different individual var gene.
The gene expression assays demonstrated that all var genes were silent in the parasites that were growing under high levels of blasticidin pressure (Fig 3B). However, 10 weeks after drug pressure was removed the parasites did not return to their previous var gene expression pattern, but rather displayed activation of many var genes (Fig 3C). We were unable to detect any preference in var gene expression at this point, indicating that activation was likely random. In particular PFD1005c, the var gene that was dominant in the original population of parasites as well as in the parasites grown under low doses of blasticidin, was now barely detectable among the actively transcribed genes. Instead, a pattern of low transcription levels from many var genes was evident, consistent with a heterogeneous culture in which numerous subpopulations of parasites exist, all expressing different var genes. We again monitored the expression pattern 8 weeks later and again could detect transcripts from numerous endogenous var genes. At this time point, a subset of genes became more dominant and the detectable levels were higher than the first time point, suggesting active var gene expression switching (Fig 3D). In order to verify that this pattern represented true switching we isolated several clones from this culture by limiting dilution and measured the transcription pattern of the whole var gene family of these early clones by Q-RT-PCR. We found that each new clone displayed the typical pattern of a single dominant transcript (data not shown), as would be expected for normal var gene switching patterns17 and indicating that they are once again being expressed in a mutually exclusive manner. These results demonstrate that down-regulating transcription of PFD1005c through competition with high numbers of “unpaired” var promoters results in this gene switching to the silent state, and that this silent state persists even after removal of the competing “unpaired” promoters. Thus the epigenetic memory that had previously marked PFD1005c as the only active gene had been completely erased.
Unpaired var promoters cannot be epigenetically silenced
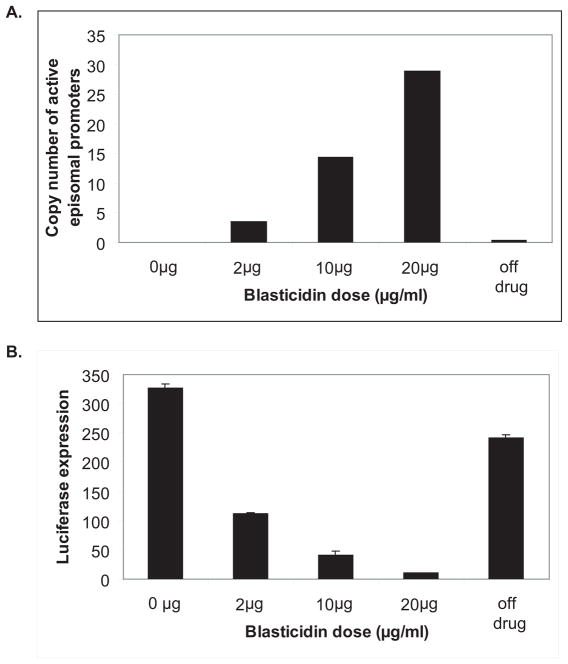
As shown above, repression of transcription of an active var gene through competition with high numbers of “unpaired” promoters resulted in epigenetic silencing of this gene. Previous work had shown that “unpaired” promoters were by default transcriptionally active and unrecognized by the mechanism that controls mutually exclusive expression 15,16,22,24. We were therefore interested to determine if an “unpaired” var promoter that had been integrated into the genome could similarly be repressed by competition with high numbers of episomal “unpaired” var promoters, and if so, would it then become epigenetically silenced. To answer this question, we used a transgenic parasite line (D10/inte) in which an “unpaired” var promoter expressing the luciferase reporter gene had been integrated into the genome at the hrp2 locus15. In these parasites the var promoter driving luciferase expression (referred to here as varLUC) is constitutively active and not recognized by the mechanism that controls mutually exclusive expression. These parasites were transfected with pVBH and selected with 2, 10 and 20μg/ml blasticidin. In this way we were able to generate 3 parasite lines that carried increasing numbers of active episomal “unpaired” var promoters (Fig 4A).
Figure 4.
An unregulated var promoter cannot be epigenetically marked for silencing. (A) Selection for varLUC parasite populations carrying increasing numbers of pVBH episomes. The number of bsd copies measured by Q-RT-PCR is presented for varLUC parasites transfected with pVBH and selected on increasing concentrations of blasticidin. Removal of blasticidin pressure resulted in almost complete shedding the pVBH episomes (off drug). (B) Repression of the unregulated varLUC promoter does not reach complete epigenetic silencing. The luciferase expression of each of the different parasite populations is presented in luminescence units. Parasite populations in which blasticidin pressure was removed and pVBH episomes shed returned to expressing high levels of luciferase (off drug).
Interestingly, while the level of luciferase expression was significantly down-regulated (P < 0.05) in correlation with the increasing number of active transgenic var promoters (Fig 4B) similar to that observed for expression of the endogenous var gene PFD1005c, we were not able to reach complete silencing under either 10 or 20μg/ml blasticidin. Under both of these concentrations the levels of expression of the intact PFD1005c gene were extremely low (Figure 1), suggesting it had switched to the silenced state. In contrast, the varLUC gene was never completely silenced and continued to display a low level of expression. 5 weeks after blasticidin pressure was removed and the parasites had shed most of the episomes, luciferase expression levels returned to levels similar to that observed before transfection. These results show that high numbers of competing episomal “unpaired” var promoters can significantly repress the expression of the chromosomal “unpaired” var promoter, however, they also demonstrate that unlike PFD1005c, this var promoter rapidly reverts to the active state once the competing promoters are removed, suggesting that it is unable to assume an epigenetically silent state. These data provide further support for a model of var gene regulation that depends on a cooperative interaction between var upstream promoters and the promoter found within var introns.
DISCUSSION
The ability to compete for a limiting factor and thus artificially repress transcription of an active var gene as described here provided a unique opportunity to investigate the role of transcription in maintaining a var gene in the epigenetically determined “on” state. The importance of transcription in establishing and maintaining cellular memory has been well documented in fruit fly development 25. Recent work in several model systems has demonstrated that the carboxy-terminal domain of RNA polymerase II can associate with histone modifying enzymes, specifically histone methyltransferases, and thus modify the chromatin structure of a gene during transcription elongation12,13. This process can therefore “mark” genes that have been recently transcribed. It is possible that a similar mechanism is utilized by malaria parasites to mark the active var gene, thus resulting in a positive feedback loop ensuring that once activated, a particular var gene remains active for many cell cycles. Such a system would contribute to cellular memory and explain why artificially preventing an active var gene from being transcribed results in it reverting to the silent state.
Shedding of high-copy number episomes by parasites in which the entire var gene complement had been silenced led to random var gene activation (Figure 3), suggesting that when no var genes are in the epigenetically determined active state, all have an approximately equal probability of being activated. This artificial situation may be analogous to the point in the life cycle when parasites first exit the infected liver after transmission from a mosquito. Such parasites are leaving a stage of development in which var genes are presumably not expressed, and thus the entire gene family is thought to be silent. As the merozoites leave the liver and invade circulating RBCs, random var gene activation by each individual parasite would ensure an antigenically diverse population, thereby preventing complete clearance by any pre-existing anti-PfEMP1 antibodies and increasing the chances for a successful blood stage infection. Examination of experimental human infections have detected activation of all var genes immediately after parasite leave the liver, consistent with this model26.
Previous work regarding the regulation of var gene expression has resulted in the proposal of different, somewhat conflicting models, particularly with regard to the role of the intron in silencing and mutually exclusive expression15,16,27,28. The work presented here demonstrates that when working with transgenic lines of parasites, if the concept of mutually exclusive expression (i.e. the idea that only a single var promoter is active at a time) is to be accurately assessed, it is imperative that the concatameric nature of replicating episomes be considered. For example, in parasites transfected with the plasmid pVBH, high doses of blasticidin resulted in silencing of all chromosomal var genes and limited expression to only the var promoters found on the concatameric pVBH episome. Nonetheless, this should not be interpreted as silencing through the mechanism that controls mutually exclusive expression, as the parasites were expressing a very high number of episomal var promoters (perhaps as many as 20) and therefore silencing in this case was rather through saturation of the nuclei’s ability to transcribe var genes. In contrast, we have previously shown silencing through the pathway that controls mutually exclusive expression by utilizing “promoter trapping”22,29 to ensure that only a single transgenic var promoter is functional. In this instance the transgenic var promoter was paired with an intron and therefore recognized by the regulatory mechanism.
The var gene family can be divided into several subgroups based on sequence homology and chromosomal position30,31. Although sequence similarities between subgroups of promoters have been described30, no general motif was found to be involved in var gene activation. Nonetheless, in the present study we demonstrated that multiple copies of one promoter type (upsC) were able to keep all var genes in the genome in a silent state, therefore all var promoter types must share requirements for certain limited nuclear factors. The evidence described here suggests that var-specific factor or factors are plentiful enough to permit the simultaneous expression of only a limited number of var promoters, in this instance ~20. It is conceivable that the limiting factor is simply the space within a specific, subnuclear expression site. Previous work from several laboratories has shown that var genes seem to move into a specific sub-nuclear location upon transcriptional activation16,27,32,33. The space within this site may have a limited capacity for promoters and might have reached the highest limits of its capacity with the inclusion of multiple copies of episomal var promoters that are forced to be active. Thus the observed repression of the endogenous var promoter PFD1005c and the transgenic varLUC gene might due to the fact that they are simply forced out of the expression site where active var gene transcription occurs.
Alternatively, the limiting factor may not be space per se, but a regulatory element or protein complex that resides within this nuclear compartment and is essential for var gene activation by interacting with basal transcription factors, chromatin-remodeling factors or histone modifying enzymes. Supporting evidence for this hypothesis is that as the number of competing episomes increased, the endogenous var gene PFD1005c did not simply display continuously falling levels of expression, but rather reverted to a completely silenced state, and this state persisted even after the competing episomes were removed, suggesting that the epigenetic memory for this gene was erased. Similar patterns of repression were observed in other eukaryotic systems and shown to involve transcription initiation or suppression through limiting amounts of promoter regulatory elements34. A better comprehension of the components that control nuclear architecture, chromatin assembly and epigenetic memory in P. falciparum will greatly improve our understanding of the process of antigenic variation as well as parasite gene regulation in general.
MATERIALS AND METHODS
Parasite culture and transfection
All experiments utilized the Plasmodium falciparum NF54 line or transgenic derivatives15,17 cultivated at 5% hematocrit in RPMI 1640 medium, 0.5% Albumax II (Invitrogen), 0.25% sodium bicarbonate, and 0.1 mg/ml gentamicin. Parasites were incubated at 37°C in an atmosphere of 5% oxygen, 5% carbon dioxide and 90% nitrogen. Parasites were transfected by using “DNA loaded” red blood cells as previously described21. Briefly, 0.2cm electroporation cuvettes were loaded with 0.175ml of erythrocytes and 50 μg of plasmid DNA in incomplete cytomix solution. Transient assay with the plasmids pVLH, and pHLH was performed as described21. Parasite populations that stably carry the construct pVBH16 were selected by culturing in the presence increasing concentrations (2–20 μg/ml) of Blasticidin S HCl (Invitrogen, USA). The constructs pVLH35, pHLH20 and pVBH16 have been previously described, however a brief description is provided here. In pVLH and pHLH, expression of a firefly luciferase gene is driven by either the var7b promoter (pVLH) or the promoter of the hrp3 gene (pHLH). pVBH is identical to pVLH, except the luciferase coding region has been replaced by the gene encoding blasticidin S deaminase.
Clonal cultures originating from a single parasite were created by limiting dilution using 96 well microtiter plates as previously described36. Individual plates were screened for parasites during media changes on days 21, 25 and 30. Individual parasite cultures were expanded to 20 ml cultures and used for DNA and RNA extraction.
Genomic DNA extraction
Infected RBCs were pelleted by centrifugation at 3000 × g. After discarding the supernatant the pellet was divided into 2 microcentrifuge tubes followed by resuspension in 500 μl phosphate buffered Saline (PBS) and 20 μl 10% Saponin. Parasites were pelleted by centrifugation and washed twice with 1000μl PBS. The parasite pellet was then taken up in 200 μl TSE buffer (100 mM NaCl, 50 mM EDTA, 20 mM Tris, pH 8) to which 40 μl of 10% SDS and 20 μl 6M NaClO4 were added. This suspension was placed on a rocker overnight and the DNA extracted with phenol/chloroform the next morning. The DNA was precipitated from the final aqueous phase with ethanol and resuspended in 100 μl sterile dH2O.
RNA extraction and Realtime RT-PCR for assaying expression of the var gene family
RNA extraction and Q-RT-PCR were performed as previously described22. Briefly, RNA was extracted from synchronized ring stage parasites 16–18 hours post invasion. RNA extraction was performed with the TRIZOL LS Reagent® as previously described37. RNA to be used for cDNA synthesis was purified on PureLink column (Invitrogen) according to manufacturer’s protocol. Isolated RNA was then treated with Deoxyribonuclease I® (Invitrogen) to degrade contaminating gDNA. cDNA synthesis was performed from 800 ng total RNA with Superscript II Rnase H reverse transcriptase ® (Invitrogen) with random primers ® (Invitrogen) as described by the manufacturer. For Q-RT-PCR reactions to detect transcription from all var genes present in the 3D7 genome, we employed the primer set of Salanti et. al.23 with the modifications described in15,17,22. Copy numbers of transgenes were calculated using Q-RT-PCR of gDNA and comparing the Ct of the transgene to that of the single copy housekeeping gene seryl-tRNA synthetase (PF07_0073) as described15.
Luciferase assays
Transient assays were performed as described38. Luciferase expression by parasites that stably express luciferase was performed as described by Frank et al.15. For both assays, the presented luminesence units are the average of triplicates and normalized to 1% parasitemia.
Statistical analysis
All assays were performed at least in triplicates and the average between replicates is presented together with its standard deviation. The data was sorted using Microsoft Excel and analyzed with the Sigma Stat (SPSS Science) statistical software package with significant levels set at P < 0.05. The significance of different promoters on the levels of down regulation of luciferase expression in the transient assay was tested using the independent samples t-test. Comparisons of genes expression levels between and within the different parasite population was performed using one way analysis of variance (ANOVA) followed by post hoc testing of the different groups using the least significant (LSD) test (Fisher LSD test).
Acknowledgments
This work was supported by a grant from the National Institutes of Health [AI 52390]. The authors would like to thank Drs. Matthias Frank and Christian Epp for discussions regarding experimental design and for critical reading of the manuscript. We also thank Roy Faiman for his help in the statistical analysis. The Department of Microbiology and Immunology at Weill Medical College of Cornell University acknowledges the support of the William Randolph Hearst Foundation. KWD is a Stavros S. Niarchos Scholar. RD is a Golda Meir Scholar and supported by the Marie Curie International Reintegration Grant (IRG) [203675] and the German Israeli Foundation [2163-1725.11/2006].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kyes S, Horrocks P, Newbold C. Antigenic variation at the infected red cell surface in malaria. Annu Rev Microbiol. 2001;55:673–707. doi: 10.1146/annurev.micro.55.1.673. [DOI] [PubMed] [Google Scholar]
- 2.Berendt AR, Ferguson DJP, Newbold CI. Sequestration in Plasmodium falciparum Malaria: Sticky Cells and Sticky Problems. Parasitology Today. 1990;6:247–254. doi: 10.1016/0169-4758(90)90184-6. [DOI] [PubMed] [Google Scholar]
- 3.Miller LH, Good MF, Milon G. Malaria Pathogenesis. Science. 1994;264:1878–1883. doi: 10.1126/science.8009217. [DOI] [PubMed] [Google Scholar]
- 4.Smith JD, Chitnis CE, Craig AG, Roberts DJ, Hudson-Taylor DE, Peterson DS, Pinches R, Newbold CI, Miller LH. Switches in expression of Plasmodium falciparum var genes correlate with changes in antigenic and cytoadherent phenotypes of infected erythrocytes. Cell. 1995;82:101–110. doi: 10.1016/0092-8674(95)90056-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baruch DI, Pasloske BL, Singh HB, Bi X, Ma XC, Feldman M, Taraschi TF, Howard RJ. Cloning the P. falciparum gene encoding PfEMP1, a malarial variant antigen and adherence receptor on the surface of parasitized human erythrocytes. Cell. 1995;82:77–87. doi: 10.1016/0092-8674(95)90054-3. [DOI] [PubMed] [Google Scholar]
- 6.Su X, Heatwole VM, Wertheimer SP, Guinet F, Herrfeldt JV, Peterson DS, Ravetch JV, Wellems TE. A large and diverse gene family (var) encodes 200–350 kD proteins implicated in the antigenic variation and cytoadherence of Plasmodium falciparum-infected erythrocytes. Cell. 1995;82:89–100. doi: 10.1016/0092-8674(95)90055-1. [DOI] [PubMed] [Google Scholar]
- 7.Gardner MJ, Hall N, Fung E, White O, Berriman M, Hyman RW, Carlton JM, Pain A, Nelson KE, Bowman S, Paulsen IT, James K, Eisen JA, Rutherford K, Salzberg SL, Craig A, Kyes S, Chan MS, Nene V, Shallom SJ, Suh B, Peterson J, Angiuoli S, Pertea M, Allen J, Selengut J, Haft D, Mather MW, Vaidya AB, Martin DM, Fairlamb AH, Fraunholz MJ, Roos DS, Ralph SA, McFadden GI, Cummings LM, Subramanian GM, Mungall C, Venter JC, Carucci DJ, Hoffman SL, Newbold C, Davis RW, Fraser CM, Barrell B. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature. 2002;419:498–511. doi: 10.1038/nature01097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scherf A, Hernandez-Rivas R, Buffet P, Bottius E, Benatar C, Pouvelle B, Gysin J, Lanzer M. Antigenic variation in malaria: in situ switching, relaxed and mutually exclusive transcription of var genes during intra-erythrocytic development in Plasmodium falciparum. EMBO J. 1998;17:5418–5426. doi: 10.1093/emboj/17.18.5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Q, Fernandez V, Sundstrom A, Schlichtherle M, Datta S, Hagblom P, Wahlgren M. Developmental selection of var gene expression in Plasmodium falciparum. Nature. 1998;394:392–395. doi: 10.1038/28660. [DOI] [PubMed] [Google Scholar]
- 10.Chookajorn T, Dzikowski R, Frank M, Li F, Jiwani AZ, Hartl DL, Deitsch KW. Epigenetic memory at malaria virulence genes. Proc Natl Acad Sci U S A. 2007;104:899–902. doi: 10.1073/pnas.0609084103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lopez-Rubio JJ, Gontijo AM, Nunes MC, Issar N, Hernandez RR, Scherf A. 5’ flanking region of var genes nucleate histone modification patterns linked to phenotypic inheritance of virulence traits in malaria parasites. Mol Microbiol. 2007;66:1296–1305. doi: 10.1111/j.1365-2958.2007.06009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hampsey M, Reinberg D. Tails of intrigue: phosphorylation of RNA polymerase II mediates histone methylation. Cell. 2003;113:429–432. doi: 10.1016/s0092-8674(03)00360-x. [DOI] [PubMed] [Google Scholar]
- 13.Eissenberg JC, Shilatifard A. Leaving a mark: the many footprints of the elongating RNA polymerase II. Curr Opin Genet Dev. 2006;16:184–190. doi: 10.1016/j.gde.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 14.Calderwood MS, Gannoun-Zaki L, Wellems TE, Deitsch KW. Plasmodium falciparum var genes are regulated by two regions with separate promoters, one upstream of the coding region and a second within the intron. Journal of Biological Chemistry. 2003;278:34125–34132. doi: 10.1074/jbc.M213065200. [DOI] [PubMed] [Google Scholar]
- 15.Frank M, Dzikowski R, Constantini D, Amulic B, Burdougo E, Deitsch K. Strict pairing of var promoters and introns is required for var gene silencing in the malaria parasite plasmodium falciparum. J Biol Chem. 2006;281:9942–9952. doi: 10.1074/jbc.M513067200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dzikowski R, Li F, Amulic B, Eisberg A, Frank M, Patel S, Wellems TE, Deitsch KW. Mechanisms underlying mutually exclusive expression of virulence genes by malaria parasites. EMBO Rep. 2007;8:959–965. doi: 10.1038/sj.embor.7401063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frank M, Dzikowski R, Amulic B, Deitsch K. Variable switching rates of malaria virulence genes are associated with chromosomal position. Mol Microbiol. 2007;64:1486–1498. doi: 10.1111/j.1365-2958.2007.05736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williamson DH, Janse CJ, Moore PW, Waters AP, Preiser PR. Topology and replication of a nuclear episomal plasmid in the rodent malaria Plasmodium berghei. Nucleic Acids Research. 2002;30:726–731. doi: 10.1093/nar/30.3.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Donnell RA, Freitas LH, Preiser PR, Williamson DH, Duraisingh M, McElwain TF, Scherf A, Cowman AF, Crabb BS. A genetic screen for improved plasmid segregation reveals a role for Rep20 in the interaction of Plasmodium falciparum chromosomes. Embo Journal. 2002;21:1231–1239. doi: 10.1093/emboj/21.5.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu Y, Sifri CD, Lei HH, Su X, Wellems TE. Transfection of Plasmodium falciparum within human red blood cells. Proceedings of the National Academy of Sciences USA. 1995;92:973–977. doi: 10.1073/pnas.92.4.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deitsch KW, Driskill CL, Wellems TE. Transformation of malaria parasites by the spontaneous uptake and expression of DNA from human erythrocytes. Nucleic Acids Research. 2001;29:850–853. doi: 10.1093/nar/29.3.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dzikowski R, Frank M, Deitsch K. Mutually Exclusive Expression of Virulence Genes by Malaria Parasites Is Regulated Independently of Antigen Production. PLoS Pathog. 2006;2:e22. doi: 10.1371/journal.ppat.0020022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salanti A, Staalsoe T, Lavstsen T, Jensen ATR, Sowa MPK, Arnot DE, Hviid L, Theander TG. Selective upregulation of a single distinctly structured var gene in chondroitin sulphate A-adhering Plasmodium falciparum involved in pregnancy-associated malaria. Molecular Microbiology. 2003;49:179–191. doi: 10.1046/j.1365-2958.2003.03570.x. [DOI] [PubMed] [Google Scholar]
- 24.Gannoun-Zaki L, Jost A, Mu JB, Deitsch KW, Wellems TE. A silenced Plasmodium falciparum var promoter can be activated in vivo through spontaneous deletion of a silencing element in the intron. Eukaryotic Cell. 2005;4:490–492. doi: 10.1128/EC.4.2.490-492.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmitt S, Prestel M, Paro R. Intergenic transcription through a polycomb group response element counteracts silencing. Genes Dev. 2005;19:697–708. doi: 10.1101/gad.326205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lavstsen T, Magistrado P, Hermsen CC, Salanti A, Jensen AT, Sauerwein R, Hviid L, Theander TG, Staalsoe T. Expression of Plasmodium falciparum erythrocyte membrane protein 1 in experimentally infected humans. Malar J. 2005;4:21. doi: 10.1186/1475-2875-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Voss TS, Healer J, Marty AJ, Duffy MF, Thompson JK, Beeson JG, Reeder JC, Crabb BS, Cowman AF. A var gene promoter controls allelic exclusion of virulence genes in Plasmodium falciparum malaria. Nature. 2006;439:1004–1008. doi: 10.1038/nature04407. [DOI] [PubMed] [Google Scholar]
- 28.Voss TS, Tonkin CJ, Marty AJ, Thompson JK, Healer J, Crabb BS, Cowman AF. Alterations in local chromatin environment are involved in silencing and activation of subtelomeric var genes in Plasmodium falciparum. Mol Microbiol. 2007;66:139–150. doi: 10.1111/j.1365-2958.2007.05899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang P, Wang Q, Sims PF, Hyde JE. Rapid positive selection of stable integrants following transfection of Plasmodium falciparum. Mol Biochem Parasitol. 2002;123:1–10. doi: 10.1016/s0166-6851(02)00105-6. [DOI] [PubMed] [Google Scholar]
- 30.Lavstsen T, Salanti A, Jensen ATR, Arnot DE, Theander TG. Sub-grouping of Plasmodium falciparum 3D7 var genes based on sequence analysis of coding and non-coding regions. Malaria Journal. 2003;2:27. doi: 10.1186/1475-2875-2-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kraemer SM, Smith JD. Evidence for the importance of genetic structuring to the structural and functional specialization of the Plasmodium falciparum var gene family. Molecular Microbiology. 2003;50:1527–1538. doi: 10.1046/j.1365-2958.2003.03814.x. [DOI] [PubMed] [Google Scholar]
- 32.Duraisingh MT, Voss TS, Marty AJ, Duffy MF, Good RT, Thompson JK, Freitas-Junior LH, Scherf A, Crabb BS, Cowman AF. Heterochromatin silencing and locus repositioning linked to regulation of virulence genes in Plasmodium faiciparum. Cell. 2005;121:13–24. doi: 10.1016/j.cell.2005.01.036. [DOI] [PubMed] [Google Scholar]
- 33.Ralph SA, Scheidig-Benatar C, Scherf A. Antigenic variation in Plasmodium falciparum is associated with movement of var loci between subnuclear locations. Proc Natl Acad Sci U S A. 2005;102:5414–5419. doi: 10.1073/pnas.0408883102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stein GS, Lian JB, van Wijnen AJ, Stein JL, Javed A, Montecino M, Zaidi SK, Young D, Choi JY, Gutierrez S, Pockwinse S. Nuclear microenvironments support assembly and organization of the transcriptional regulatory machinery for cell proliferation and differentiation. J Cell Biochem. 2004;91:287–302. doi: 10.1002/jcb.10777. [DOI] [PubMed] [Google Scholar]
- 35.Deitsch KW, del Pinal A, Wellems TE. Intra-cluster recombination and var transcription switches in the antigenic variation of Plasmodium falciparum. Mol Biochem Parasitol. 1999;101:107–116. doi: 10.1016/s0166-6851(99)00062-6. [DOI] [PubMed] [Google Scholar]
- 36.Kirkman LA, Su XZ, Wellems TE. Plasmodium falciparum: isolation of large numbers of parasite clones from infected blood samples. Exp Parasitol. 1996;83:147–149. doi: 10.1006/expr.1996.0058. [DOI] [PubMed] [Google Scholar]
- 37.Kyes S, Pinches R, Newbold C. A simple RNA analysis method shows var and rif multigene family expression patterns in Plasmodium falciparum. Mol Biochem Parasitol. 2000;105:311–315. doi: 10.1016/s0166-6851(99)00193-0. [DOI] [PubMed] [Google Scholar]
- 38.Deitsch KW, Calderwood MS, Wellems TE. Malaria. Cooperative silencing elements in var genes. Nature. 2001;412:875–876. doi: 10.1038/35091146. [DOI] [PubMed] [Google Scholar]