Synopsis
Altered mental status is a common chief complaint among older emergency department (ED) patients. Acute changes in mental status are more concerning and are usually secondary to delirium, stupor, and coma. These forms of acute brain dysfunction are commonly precipitated by an underlying medical illness that can be potentially life-threatening and are associated with a multitude of adverse outcomes. Though stupor and coma are easily identifiable, the clinical presentation of delirium can be subtle and is often missed without actively screening for it. For patients with acute brain dysfunction, the ED evaluation should focus on searching for the underlying etiology. Infection is one of the most common precipitants of delirium, but multiple etiologies may exist concurrently.
Keywords: delirium, coma, stupor emergency department, epidemiology, diagnosis, management
Introduction
Altered mental status is a common chief complaint among older emergency department (ED) patients. Despite the frequency of this complaint, the term “altered mental status” is vague and has several synonyms such as confusion, not acting right, altered behavior, generalized weakness, lethargy, agitation, psychosis, disorientation, inappropriate behavior, inattention, and hallucination.1 Such lack of standardized terminology not only hinders the assessment and appropriate management of patients with altered mental status, but also the advancement of knowledge through research.
Altered mental status has varying time courses and degrees of severity. Acute changes in mental status are usually secondary to delirium, stupor, and coma, which are forms of acute brain dysfunction. These changes occur over a period of hours or days and are usually precipitated by an underlying medical illness that is potentially life threatening. Chronic alterations in mental status (e.g. dementia) occur over a period of months and years and are less likely to be precipitated by a life-threatening illness.2 For these reasons, acute changes in mental status will be the focus of this review. Altered mental status is rarely caused by psychiatric illnesses such as depression or schizophrenia, and in elder patients, these should be diagnoses of exclusion. Acute brain dysfunction (delirium, stupor, and coma) and their underlying etiology should be ruled out prior to considering any psychiatric diagnoses, especially in patients without a previous history of psychiatric illness.
The ED plays a critical role in the evaluation and management of older patients with altered mental status. The ED is often the initial point of entry for geriatric hospital admissions,3 and it is tasked with rapidly identifying those who are critically ill, while efficiently diagnosing the underlying etiology, and promptly initiating life saving therapies. In the United States alone, the ED sees approximately 18 million patients who are 65 years and older each year.4 Because of the projected exponential growth of the US aging population over the next several decades, the number of elder ED patient visits will likely grow at a similar pace.5 Hence, the emergency physician must be adept in evaluating and managing patients with acute alterations in mental status. This review will discuss the epidemiology of delirium, stupor and coma in the ED along with their effect on patient outcomes. This chapter will also review the mental status assessment of the patient with acute brain dysfunction, as well as their diagnostic workup and treatment. Of all the forms of acute brain dysfunction, delirium is probably the most well-studied and will be the focus of this review. However, the concepts pertinent to delirium can be generalized to stupor and coma, because there is significant overlap.
The Spectrum of Acute Brain Dysfunction
Delirium, stupor, and coma represent a broad spectrum of acute brain dysfunction (Figure 1) and are associated with an impairment of consciousness. There are two interrelated domains of neurologic function that are related to consciousness: content and level (also known as arousal) of consciousness.6 The content of consciousness has many components such as orientation, perception, executive function, and memory, and is mediated at the cortical level. The level (or arousal) of consciousness signifies the patient’s wakeful state and reactivity to surrounding stimuli. This is mediated at the ascending reticular activating system located in the brainstem.6 Traditionally, terms such as lethargic, drowsy, or somnolent have been used to describe level or arousal of consciousness. Because these descriptors can have different meanings for different clinicians, using a structured arousal scale such as the Richmond Agitation Sedation Scale (RASS) may be a more reliable method to describe altered level of consciousness. This scale ranges from −5 (unresponsive to pain and voice) to +4 (extreme combativeness).7,8 As the patient’s level of consciousness becomes more disturbed, the concern for an underlying life threatening acute medical illness should similarly increase. Patients with acute brain dysfunction can not only fluctuate between different RASS scores, but can also transition between delirium, stupor, and coma.
Figure 1.
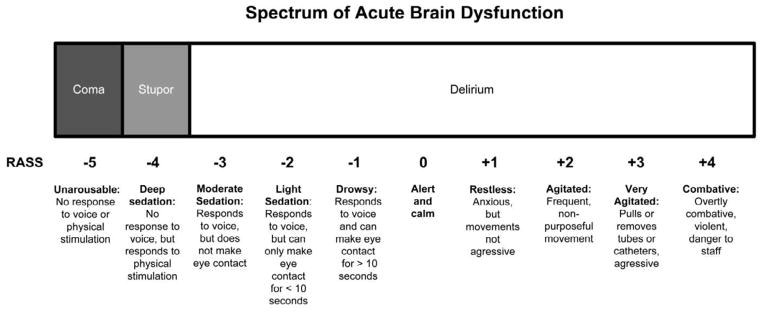
Spectrum of acute brain dysfunction based upon the Richmond Agitation Sedation Scale (RASS). Courtesy of Vanderbilt University, Nashville, TN. Copyright © 2012. Used with Permission.
Stupor and Coma: Definitions and their Epidemiology in the Emergency Department
Stupor and coma occurs in 5 to 9% of older ED patients and when present, are considered to be medical emergencies that require immediate evaluation.9,10 These two forms of acute brain dysfunction occur over a period of hours to days and represent the most severe disruptions in both the level and content of consciousness. Stupor (RASS −4) is a condition of deep sleep or similar behavioral unresponsiveness from which the patient can be aroused only with vigorous and continuous stimulation. Coma (RASS −5) is defined as a state of unresponsiveness in which the patient cannot be aroused with any stimuli.
Delirium: Definitions and its Epidemiology in the Emergency Department
Delirium is an acute disturbance of consciousness (i.e. attention) that is accompanied by an acute loss in cognition that is not better explained by a preexisting dementia.11 This form of acute brain dysfunction occurs in 8 to 10% of patients of older ED patients.10,12–17 Similar to stupor and coma, delirium occurs over a period of hours and days, and its course tends to wax and wane throughout the day. In contrast to stupor and coma, however, some elements of the level and content of consciousness are maintained in patients with delirium. The degree of impairment in the level of consciousness can be variable, ranging from moderate sleepiness (RASS −3) to extreme combativeness (RASS +4). Patients with delirium also have inattention which is considered a cardinal feature of delirium.18,19 The impairment of content of consciousness is similarly variable and leads to an acute loss in cognition. Examples of such impairments observed in delirious patients are disorganized thought, perceptual disturbances, and disorientation (See Box).18
Box. Examples of impairments in the cognitive domains that can be seen in patients with delirium.
| Disorganized thinking |
| The patient’s thought process is disorganized and incoherent. The patient may ramble, make irrelevant statements, or have illogical flow of ideas. The following conversation is an example of disorganized thinking: |
| Physician: “Mr. B, how are feeling today?” |
| Mr. B: “I’m feeling horrible today. It reminds me of the day I visited Italy when I was younger… those were the days of yesterday, today, and the future. The future is irrelevant to the past, and this makes me happy.” |
| Perceptual disturbances |
| The patient may be seeing things that aren’t their (visual hallucinations) or hearing things that no one else can hear (auditory hallucinations). For example, a delirious patient with visual hallucinations is seen picking at his blanket thinking that he is picking up bugs, when in reality, none are present. |
| Disorientation |
| The patient may not know where he is or what the date is. |
The Psychomotor Subtypes of Delirium
Delirium can be further classified into three psychomotor subtypes: hypoactive, hyperactive, and mixed.20 Hypoactive (RASS < 0) delirium is described as “quiet” delirium and is characterized by psychomotor retardation; delirious patients with this subtype can appear drowsy, somnolent, or even lethargic. Because the clinical presentation can be very subtle, hypoactive delirium is frequently undetected by health care providers,21 and is often attributed to other etiologies such as depression or fatigue.22,23 To the contrary, patients with hyperactive delirium (RASS > 0) have increased psychomotor activity and may appear restless, anxious, agitated, or combative. Hyperactive delirium is more easily recognized by health care providers. Mixed-type delirium exhibits fluctuating levels of psychomotor activity; the patient can exhibit hypoactive symptomatology at one moment and hyperactive symptomatology several hours or even seconds later. Hypoactive delirium and mixed-type delirium appear to be the predominant subtypes in older patients regardless of the clinical setting.14,24–29 In the ED specifically, hyperactive delirium is the least common subtype.14
It is hypothesized that each psychomotor subtype has different underlying pathophysiological mechanisms.20,30 Though the mechanisms are unclear, it is hypothesized that each delirium subtypes has differential neurotransmitter activity (cholinergic, dopamine, serotonin, and gamma-aminobutyric acid).20 Each psychomotor subtype may also be cause by different etiologies. Delirium caused by an infection or metabolic derangement is more likely to be the hypoactive subtype, whereas delirium caused by alcohol or benzodiazepine withdrawal is more likely to be the hyperactive subtype.31 The psychomotor subtypes of delirium may also have a differential effect on clinical course and outcomes.32 In 225 older patients admitted to a post acute care facility, Kiely at al. observed that patients with hypoactive delirium had the highest 1-year mortality rate compared with the other subtypes.33
Delirium versus Dementia
Delirium is distinct from dementia (Table 1), yet many clinicians use these terms interchangeably. It is important to note, however, that dementia is an important predisposing factor to delirium, and patients can have both conditions concurrently. As previously mentioned, the loss of cognition observed in delirium tends to occur rapidly, and its course tends to fluctuate throughout the day. The loss of cognition observed in dementia is usually gradual (over months to years), and its course tends to be stable. Patients with delirium also have inattention, which is considered the cardinal feature of delirium where as attention is usually preserved in patients with dementia. Altered level of consciousness, disorganized thinking, sleep-wake cycle disturbances, and perceptual disturbances are also commonly observed in delirium, whereas these characteristics are typically absent in dementia.
Table 1.
Key Differences between delirium and dementia.
| Characteristic | Delirium | Dementia |
|---|---|---|
| Onset | Rapid over a period of hours or days | Gradual over months and years |
| Course | Waxing and waning | Stable |
| Inattention | Present | * Absent |
| Altered of level of consciousness | Usually present | * Typically absent |
| Disorganized thinking present | May be present | * Typically absent |
| Sleep-wake cycle disturbance | Present | * Typically absent |
| Perceptual disturbances and hallucinations | May be present | * Typically absent |
| Is cognitive decline reversible? | Usually reversible | Rarely reversible |
May be present in patients with severe dementia.
There are instances when the clinical features of delirium and dementia overlap, making them difficult to distinguish from each other. This is especially the case in patients with end-stage dementia, where they can exhibit symptoms of inattention, altered level of consciousness, disorganized thinking, sleep-wake cycle disturbances, and perceptual disturbances in the absence of delirium.34 When patients with end-stage dementia develop delirium, an acute change in mental status is still observed, and any pre-existing abnormalities in cognition and level of consciousness will likely worsen. For this reason, diagnosing delirium can be extremely challenging in patients with severe dementia and establishing their baseline mental status is critical to the diagnosis.
Delirium is classically thought of as reversible and is usually precipitated by an underlying medical illness. However, there are also a proportion of patients whose delirium is not transient, and their symptoms can persist for months or even years.35,36 Dementia is thought of as irreversible and not secondary to an underlying medical illness. However, there are circumstances in which dementia may be reversible. Hypothyroidism, normal pressure hydrocephalus, vitamin B12 deficiency, and depression are examples of illnesses that can cause reversible dementia or a dementia-like illness (pseudodementia). One meta-analysis comprised of 39 articles reported that 9% of dementia were potentially reversible, but only 0.6% of the dementia cases showed any improvement in cognition after the reversible cause was addressed.2
Dementia with Lewy bodies is the second most common type of dementia (after Alzheimer’s) and deserves special mention because it can be very challenging to distinguish from delirium.37 Similar to delirium, the loss of cognition observed in dementia with Lewy bodies can be rapid, and it can fluctuate over several hours or days. Perceptual disturbances are also commonly observed in dementia with Lewy bodies. Patients with dementia with Lewy bodies, however, have Parkinsonian motor symptoms such as cog wheeling, shuffling gait, stiff movements, and reduced arm-swing during walking; these motor symptoms are usually absent in patients with delirium. Differentiating between dementia with Lewy bodies and delirium can be difficult in the ED and may require a detailed evaluation by a neurologist or psychiatrist.
Other Illnesses That Can Mimic Delirium, Stupor, and Coma
In patients with altered mental status, the emergency physician must also consider other neurologic diagnoses. In patients who appear unresponsive, locked-in syndrome should be considered and is caused by focal injury to the ventral pons secondary to an infarct, hemorrhage, or trauma. If secondary to an infarct, the distal basilar artery is usually occluded. Multiple sclerosis and central pontine myelinosis can also cause locked-in syndrome. Though the clinical presentation is variable, quadriplegia and anarthria is usually present, but vertical eye gaze and upper eyelid movement are usually retained.38 Despite having the outward appearance of being unresponsive, patients with locked-in syndrome have normal levels of consciousness and are fully aware of their surroundings. If locked-in syndrome is suspected, then prompt neuroimaging and neurology consultation are warranted. Patients with a suspected thromboembolic cause of locked-in syndrome should immediately go to interventional radiology for intra-arterial thrombolytic therapy, even if the symptoms have being ongoing for more than the traditional 3-hour window.39 Without emergent intervention, survival and neurological recovery for these patients can be poor.
Non-convulsive status epilepticus (NCSE) should also be considered in patients who have altered mental status, especially if no obvious cause for their mental status changes is found. This diagnosis should strongly be considered if the patient has a seizure history or had a seizure prior to arriving to the ED. One systematic review reported that NCSE occurred in 8% – 30% of patients with altered mental status (mean prevalence = 22%).40 This systematic review consisted of 5 studies that predominantly enrolled patients in the hospital setting.40 NCSE is an underrecognized and important form of altered mental status that can only be diagnosed with electroencephalography (EEG). If this cause of altered mental status is considered, then neurology consultation should be obtained promptly; the treatment of NSCE (benzodiazepine and anti-epileptic medications) is significantly different from other causes of altered mental status.
Risk Factors for Developing Acute Brain Dysfunction
The etiology of delirium (and other forms of acute brain dysfunction) involves a complex interplay between patient vulnerability (or predisposing) factors and precipitating factors (Figure 2).41–43 Patients who are highly vulnerable (e.g. 92 year old with severe dementia, poor functional status, and multiple comorbidities) will require a relatively benign insult to develop delirium. For these patients, a relatively benign insult such as a simple urinary tract infection or small dose of narcotic medication can precipitate delirium. Because elderly patients are more likely to have multiple vulnerability factors, they are more susceptible to becoming delirious compared with their younger counterparts. Nursing home patients are particularly vulnerable.44 For patients who are less vulnerable (e.g. 67 year old with no dementia, little comorbidity burden, and who is still functionally independent), higher doses of noxious stimuli such as severe sepsis are required to develop delirium. Consequently, when a patient with little or no vulnerability factors presents to the ED with delirium, stupor, or coma, the clinician should have more concern for an underlying life threatening illness. To develop stupor of coma, even higher doses of noxious stimuli are required.
Figure 2.
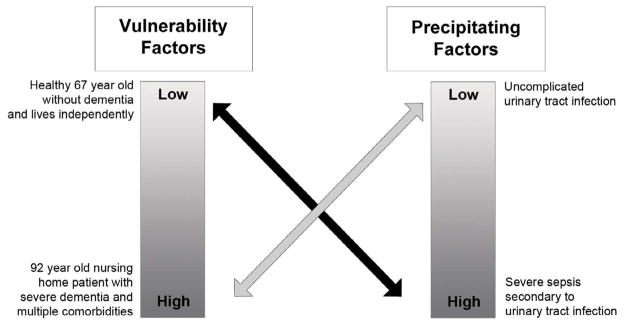
Interrelationship between patient vulnerability and precipitating factors for developing acute brain dysfunction such as delirium. Patients who not vulnerable require significant noxious stimuli to develop acute brain dysfunction (black arrow). Patients who are highly vulnerable require only minor noxious stimuli to develop acute brain dysfunction (gray arrow). Adapted from Inouye et al. JAMA 1996;275:852–857.
Patient Vulnerability Factors for Acute Brain Dysfunction
A multitude of patient vulnerability factors for delirium have been identified in the hospital literature (Table 2) and can likely be extrapolated to stupor and coma.45–48 Dementia is the most consistently observed vulnerability factor for delirium regardless of clinical setting.41,45,49–55 A dose-response relationship seems to exist; as the severity of dementia worsens, the risk of developing delirium increases.56 Similarly, low education attainment also increases the patient’s susceptibility to developing delirium.57 Both dementia and education attainment may be indicative of poor cognitive reserve and reflect the inability of the brain to adequately compensate for any noxious or stressful physiological insult. Other commonly observed vulnerability factors for delirium include poor functional status,50,55 advanced age,49,54 home psychoactive medication use such as narcotics, benzodiazepines,49,58 and medications with anticholinergic properties,51 history of alcohol abuse,50,55 visual impairment,41 high comorbidity burden,55 and malnutrition.42 There are limited data from the ED setting, but one study identified dementia, premorbid functional impairment, and hearing impairment as risk factors for delirium in the ED.14 Another ED study also identified dementia as a risk factor for delirium.59 They also observed that patients with advanced age, or a past history of cerebrovascular disease and seizure disorder were more likely to be delirious in the ED.59
Table 2.
Patient Vulnerability Factors for Delirium
| Vulnerability Factors | |
|---|---|
Demographics
|
Medications and Drugs
|
Increased severity of these vulnerability factors will increase the patient’s susceptibility to delirium. Modified from Pun et al, Fearing et al., and the American Psychiatry Association Delirium Guidelines.46–48
Psychoactive medications include benzodiazepines, opioids, and medications with anticholinergic properties.
Precipitating Factors for Acute Brain Dysfunction
There are numerous precipitating factors of delirium that have been reported in the hospital literature (Table 3).46–48 Generally speaking, patients with higher severities of illness are more likely to develop delirium,41,58 and even higher doses are required to cause stupor and coma. Infections are probably the most common causes of delirium occurring in 16 to 67% of cases;49,52–54,60–63 urinary tract infection and pneumonia are common infectious etiologies. Other precipitants for delirium include electrolyte abnormalities such as hyponatremia, hypernatremia, hypercalcemia, and hypocalcemia,58,64 organ failure,58,64 Wernicke’s encephalopathy, thyroid dysfunction,65 central nervous system insults such as cerebrovascular accidents, intracerebral hemorrhage, epidural and subdural hematomas, and subarachnoid hemorrhage,52,53,60,63 ethanol and benzodiazepine withdrawal,66–68 dehydration,41 and cardiovascular illnesses such as congestive heart failure,53,54 and acute myocardial infarction.69 Poorly controlled somatic pain (i.e. extremity fracture patients) can also precipitate delirium.70–72 Vital sign abnormalities (hypertensive encephalopathy, hypotension, hyperthermia or hypothermia, or hypoxia), endocrine disorders (hyperglycemia, hypoglycemia, adrenal insufficiency), and hypercarbia can also precipitate delirium.73,74 More often than not, multiple delirium precipitants will exist concurrently,58 but in 13% of cases, no obvious etiologic agent will be found.63 Delirium can also be precipitated by iatrogenic events in the ED. Inouye et al. observed that the use of physical restraints, bladder catheters, or the addition of more than three medications were associated with delirium development.42
Table 3.
Precipitating factors for delirium.
| Precipitating Causes for Delirium | |
|---|---|
Systemic
|
Metabolic
|
CNS
|
Cardiopulmonary
|
Medications and Drugs
|
Iatrogenic
|
Medication Risk Factors for Delirium
Medications are important vulnerability and precipitating risk factors factor for delirium, because polypharmacy is highly prevalent in the older patient population. Clegg et al. performed a systematic review and observed that benzodiazepines, opioids, dihydropyridines (e.g. nifedpine), and antihistamines may increase the risk for delirium.75 Of the opioids, meperidine is probably the most deliriogenic.72,76–79 These medications, especially benzodiazepines and opioids, can also induce stupor and coma at higher doses.
Medications with anticholinergic properties are thought to be frequent causes of delirium. There are over 600 medications with anticholinergic properties, and of these, 11% are frequently prescribed to the older patients.80 Some examples of commonly prescribed medications with anticholinergic properties are diphenhydramine, promethazine, hydroxyzine, meclizine, lomotil, and heterocyclic antidepressants (e.g. amitriptyline, nortriptyline, doxepin). In acute stroke patients, Caerio et al. found that that patients on home medications with anticholinergic properties were more susceptible to developing delirium during hospitalization.81 In 278 older medical patients, Han et al. observed that anticholinergic medications were associated with increased delirium severity.82 However, the evidence linking medications with anticholinergic properties and delirium is not consistently observed. Agostini et al. observed a trend towards increase risk (relative risk = 2.1, 95%CI: 0.9 – 4.7) of developing delirium in older hospitalized patients when diphenhydramine was used.83 Luukkanen et al. found that older patients who used more than one medication with anticholinergic properties were more likely to have delirium in the unadjusted analysis (27.0% versus 16.7%, p-value = 0.05). However, this relationship became non-significant after adjusting for age, gender, and comorbidity.84 In 147 hospitalized older patients, Campbell et al. observed that anticholinergic medications were not associated with delirium that developed in the hospital. 85 These discrepant observations may be a result of patient characteristics (stroke versus non-stroke, race, etc.,) or the method in which anticholinergic burden was measured. Despite these discrepant findings, the general consensus among geriatric and psychiatric experts is that medications with anticholinergic properties in older patients should be avoided, especially if safer alternatives exist.86,87
The Effect of Acute Brain Dysfunction in the Emergency Department on Outcomes
There is a dearth of data with regard to acute brain dysfunction in the ED and its effect on patient outcomes. Much of what is known is based upon studies conducted in older hospitalized patients. Studies investigating the role of stupor and coma are mainly limited to the intensive care unit setting. It is clear that the development of stupor and coma portend adverse outcomes; multiple studies have observed that these patients are more likely to die and have poor functional outcomes regardless of underlying etiology.88–92
The link between delirium and patient outcomes is well established in older hospitalized patients. A recent meta-analysis that included 21 studies observed that hospitalized patients with delirium are twice as likely to die, have a 2-fold increased odds of being institutionalized, and have a 12-fold increased odds of developing dementia compared with patients without delirium.93 Delirium also accelerates the rate of cognitive decline in patients with pre-existing dementia, and it is hypothesized that delirium may cause irreversible brain injury.94,95 In addition, delirium is associated with accelerated functional decline,96 prolonged hospitalizations,58 and higher health care expenditures.97 The duration of delirium appears to be an important prognostic indicator as for every 48 hours of delirium increases the risk of 3-month death by 11%.98 Furthermore, many patients with delirium can remember their delirious experience and this can cause significant distress in 70 to 80% patients.99,100
The consequences of acute brain dysfunction in the ED remain unclear. Although older patient cohorts are similar between the ED and hospital settings, caution must be taken when attempting to generalize the results of studies from one setting to another especially with delirium. ED patients who are discharged home represent a significant proportion of older ED patients and are not represented in hospital-based studies. Up to 25% of delirious older ED patients are discharged home,12,101 and likely represent a unique population. Additionally, most hospital-based delirium studies enrolled patients 24 to 48 hours after admission,27,55,96,102,103 and this may not reflect what the patient’s delirium status was in the ED. Delirium can rapidly fluctuate and can quickly resolve in less than one day in 20% – 51% of patients;104–106 hospitalized patients who were classified as non-delirious in the hospital may have been delirious in the ED. Conversely, patients who were not delirious in the ED may have become delirious in the hospital at the time of enrollment. Such misclassification can also occur in stupor and coma studies that enrolled patients in the hospital; such patients may have been delirious or had normal mental status in the ED.
Though there are no studies investigating the role of stupor and coma in the ED on outcomes, there are limited data on the effect of ED delirium. Delirium in older ED patients appear to be a predictor of long-term mortality, and this relationship is independent of age, comorbidity burden, severity of illness, dementia, functional dependence, and nursing home residence.107 Delirium is also a marker for death for those who are discharged from the ED. Kakuma et al. observed that in older ED patients who were discharged from the ED, delirium was independently associated with 6-month mortality.17 Older ED patients with delirium are also more likely to have prolonged hospitalizations.101 Only one ED study has investigated the relationship between delirium and long-term functional outcomes. Vida et al. observed that delirium in the ED was associated with accelerated functional decline in patients without pre-existing dementia in the unadjusted analysis only.108 However, this relationship disappeared after potential confounders were adjusted for in the multivariable model.108 Even with the relatively small number of ED studies, it is likely that delirium in the ED is a marker for adverse patient outcomes.
Under-recognition of Delirium in the Emergency Department
Because of the severity of impairment observed, emergency physicians readily recognize stupor and coma with little difficulty. However, emergency physicians miss delirium in 57 to 83% of the cases,10,12–17 because its clinical presentation can be subtle and can be missed if it is not actively sought. Missing delirium is considered by many to be a medical error and may have important downstream implications for clinical care.109 Patients with delirium may be unable to provide an accurate reason of why they are in the ED,110 and this may lead to inappropriate or inadequate diagnostic workups. Delirium may be ascribed to another psychiatric illness such as depression.23 These patients may be erroneously admitted to a psychiatric ward and there may be delay in the diagnosis of their underlying medical illness.111 Up to 25% of delirious older ED patients are discharged home,12,101 a significant proportion may not be able to comprehend their discharge instructions,110 leading to non-compliance.112 Older ED patients who are discharged home with unrecognized delirium are more likely to die at 6-months compared with those whose delirium was recognized.17 Hustey et al. observed that out of the five older ED patients who were discharged home with delirium, one patient fell and two patients returned to the ED within three days and were eventually hospitalized.12 Lastly, if the patient is admitted, over 90% of delirium that is missed in the ED will also be missed in the hospital setting.14
Assessment of the Dysfunctional Brain in the Emergency Department
In any ED patient with acute alterations in mental status, the first step is to assess for the level of consciousness using a validated arousal scale such as the RASS. If the patient in a sleep-like state, then it is necessary to determine the intensity of stimulation that is needed to arouse the patient.6 If a patient is unarousable to loud voice and vigorous shaking, then a painful stimulus should be introduced, but every effort should be made to avoid causing tissue damage. Painful stimuli can be introduced by moderate compression of the nail beds, the supraorbital ridge, or the temporomandibular joint.6 Patients with a RASS −4 and −5 are considered to be stuporous or comatose, respectively; these patients should then be assessed with a validated coma scale (See Assessment of Stupor and Coma). Patients with a RASS of −3 and above should be assessed for delirium (See Assessment of Delirium).
Assessment of Stupor and Coma
If the patient’s RASS is −4 or −5, then the patient is likely to be stuporous or comatose for which several scoring assessments exist. The Glasgow Coma Scale (GCS) is probably the most widely used coma scale, and rates eye, verbal, and motor response to verbal and painful stimuli (Table 4).113 Scores range from 3 (unresponsive) to 15 (normal). The GCS has prognostic significance as it predicts mortality,114,115 but has several limitations as the verbal score is difficult to obtain in patients who are intubated or aphasic. The GCS can also have low inter-rater reliability because it is difficult to remember.116 The Full Outline of Unresponsiveness (FOUR) is another coma score that assesses for eye response, motor response, pupillary reflexes, and breathing.117 Because the FOUR Score does not evaluate verbal response, this scale can be performed in patients who are intubated or aphasic. However, the FOUR score is relatively complicated and is also difficult to remember. Hence, the FOUR score may have limited utility in the ED.
Table 4.
Glasgow Coma Scale
| Points | Scale Elements |
|---|---|
|
| |
| Eyes | |
| 4 | Opens eyes spontaneously |
| 3 | Opens eyes in response to voice |
| 2 | Opens eyes in response to painful stimuli |
| 1 | Does not open eyes |
|
| |
| Verbal | |
| 5 | Oriented, converses normally |
| 4 | Confused, disoriented |
| 3 | Utters inappropriate words |
| 2 | Incomprehensible sounds |
| 1 | Makes no sounds |
|
| |
| Motor | |
| 6 | Obeys commands |
| 5 | Localizes painful stimuli |
| 4 | Flexion/withdrawal to painful stimuli |
| 3 | Abnormal flexion to painful stimuli (decorticate response) |
| 2 | Extension to painful stimuli (decerebrate response) |
| 1 | Makes no movements |
The AVPU is another commonly used scale to help describe impaired level of consciousness. It stands for alert, responsive to verbal stimuli, responsive to painful stimuli, and unresponsive. The benefit of the AVPU scale is that it is easy to remember and allows for quick communication of the patient’s global picture of level of responsiveness. However, it lacks granularity to detect subtle impairments.118
Assessment of Delirium
For patients with altered mental status with a RASS of -3 or greater (arousable to verbal stimuli), the patient should be assessed for delirium. It is important to remember that the minority of patients with delirium will have the chief complaint of “altered mental status” and the clinical manifestation of delirium can be subtle especially if it is the hypoactive subtype. As a result, many cases of delirium will be missed unless it is actively screened for using validated delirium assessments. Recently, delirium screening has been proposed to be one of the key quality indicators for emergency geriatric care,119 and a core competency for emergency medicine resident education.120
A psychiatrist evaluation using Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR) criteria is considered to be the gold standard for delirium diagnosis.11 To meet DSM-IV-TR criteria for delirium, a patient must have all of the following: 1) a disturbance of consciousness (i.e., reduced clarity of awareness of the environment) with reduced ability to focus, sustain or shift attention, 2) a change in cognition or the development of a perceptual disturbance that is not better accounted for by a preexisting, established or evolving dementia, 3) the disturbance develops over a short period of time (usually hours to days) and tends to fluctuate during the course of the day, and 4) there is evidence that the disturbance is caused by the direct physiological consequence of a general medical condition or substance. Determining these criteria require a comprehensive neurocognitive testing and a detailed interview with a person who knows the patient well (i.e. proxy or caregiver).
Obtaining routine psychiatric consultation to perform these DSM-IV-TR delirium evaluations may not be feasible for most hospitals and these criteria are not routinely applied by non-psychiatrists. The Confusion Assessment Method (CAM) was developed for non-psychiatrists and is probably the most widely used delirium assessment in the medical setting,121 and has undergone the most extensive validation.122 The CAM operationalizes the Diagnostic and Statistical Manual of Mental Disorders criteria for delirium and consists of 4 features: 1) altered mental status and fluctuating course, 2) inattention, 3) disorganized thinking and 4) altered level of consciousness. Feature 1 (altered mental status and fluctuating course) is obtained through collateral history from a person who knows the patient’s baseline cognition (i.e. family member, caregiver, nursing home, or primary care provider). Time of onset of the change in mental status should be established as well as the presence fluctuations (“Did the symptoms you noticed tend to come and go, or get worse and better over time”). In the ED, approximately 40% of older patients may not have a proxy available in the ED, and they may need to be contacted by telephone.123 If the patient is grossly altered and a proxy is not readily available, information from the patient’s medical record may be useful to help establish their mental status baseline. For example, if a patient is unable to follow simple commands and barely able to stay awake to verbal stimuli (RASS −3), but was able to provide a detailed history in a recent primary care provider clinic visit, this patient likely has an acute change in mental status. Feature 2 is inattention and considered to be a cardinal feature of delirium.18,19,124 Patients with inattention are easily distractible to irrelevant stimuli and can have difficulty maintaining a conversation. Questions often have to be repeated to the patient when inattention is present. Patients who are inattentive may also fall asleep during the interview when disengaged. Inattention is also commonly assessed for by asking the patient to recite the months of year backwards, recite the days of the week backwards, or count backwards from 20 to 1.124,125 Patients with disorganized thinking (feature 3) exhibit incoherent thought processes; they may ramble or have irrelevant conversations. They may also display illogical flow or of ideas, circumstantiality, or tangential thoughts. Feature 4 is altered level of consciousness and is patient who is drowsy, lethargic, anxious, restless, or agitated (RASS other than 0).
For a patient to meet criteria for delirium, both features 1 and 2, and either 3 or 4 must be present. The original CAM validation study was conducted in hospitalized patients, and was found to have excellent sensitivity (94 – 100%) and specificity (90 – 95%) compared with a psychiatrist’s assessment using DSM-III-R criteria.121 The CAM is the only delirium assessment to be validated in the ED setting, albeit in patients from Brazil.126 In 100 patients who were 60 years or older, the CAM’s sensitivity was 94.1% and its specificity was 97.4% when performed by a geriatrician.126 The psychiatrist’s DSM-IV assessment was the reference standard for this study. In order to assess all of the CAM’s features, the training manual recommends performing the Modified Mini-Cog and the Digit Span test, and can take approximately 5 minutes to complete.127 If the patient is delirious or demented, the CAM may take even longer.128 The CAM requires significant training and may be less sensitive when used by raters with less clinical experience.129,130
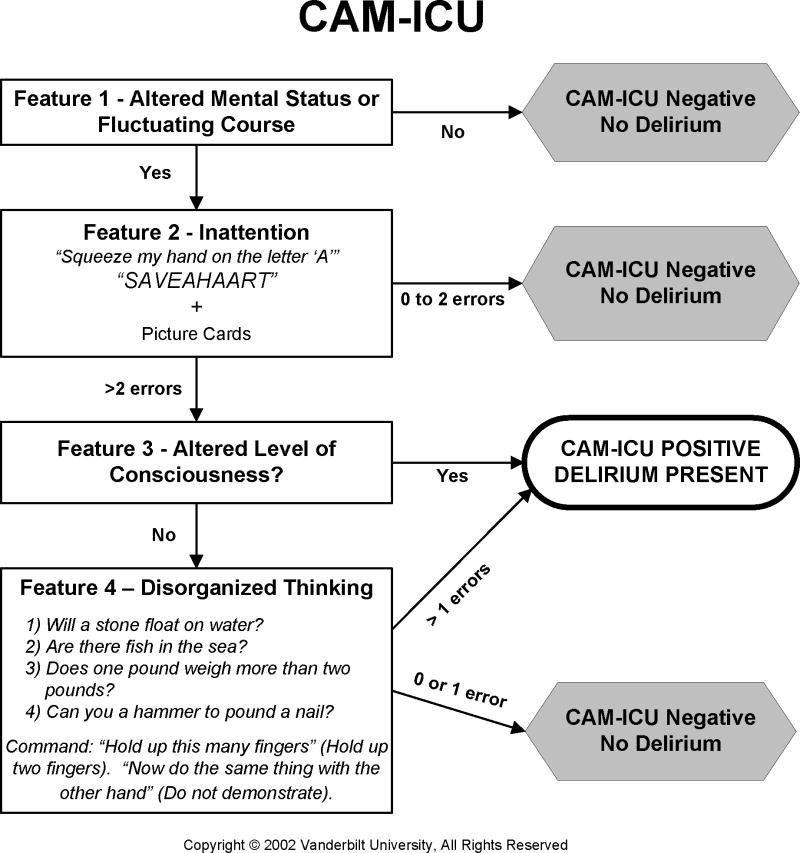
Detecting delirium in the ED can be challenging, because it is fast paced and the ED staff is usually under immense time constraints. As a result, spending 5 minutes performing a delirium assessment may not be feasible and briefer (<2 minutes) delirium assessments may be needed in this difficult setting. The Confusion Assessment Method of the Intensive Care Unit (CAM-ICU) is based upon the CAM and may be feasible to perform in the ED, because it takes less than 2 minutes to complete.131 The CAM-ICU (Figure 3) has been recently restructured and allows for early stoppage. For a patient to meet criteria for feature 1, a patient must have either altered mental status or fluctuating course; this is slightly different than the CAM which requires both to be present. The CAM-ICU uses brief objective assessments to test for inattention and disorganized thinking. To test for inattention (feature 2), the CAM-ICU rater gives a series of 10 letters (“SAVEAHAART”) and asks the patient to squeeze the rater’s hand whenever the letter “A” is heard. A picture recognition test, which tests the patient’s short term memory, is also used to test for inattention. To test for disorganized thinking, four yes/no questions are asked as well as a simple command. The CAM-ICU incorporates the RASS to assess for altered level of consciousness (Feature 3); a score other than 0 indicates altered level of consciousness. Additional details are available in www.icudelirium.org.
Figure 3.
Confusion Assessment Method for the Intensive Care Unit. Adapted from www.icudelirium.org. Courtesy of Dr. Wes Ely and Vanderbilt University, Nashville, TN. Copyright © 2002. Used with Permission.
The CAM-ICU has excellent diagnostic performance in the critically-ill patient population, but there are limited data evaluating the CAM-ICU’s diagnostic performance in those who are non-critically ill. Neufeld et al. evaluated the CAM-ICU performed by a layperson in 139 noncritically ill medical oncology inpatients where the median age was 57 years old. They found that its sensitivity was 18% and its specificity was 99% compared with a psychiatrist’s DSM-IV assessment.132 In 129 patients with acute stroke and a median age of 72.5 years, the CAM-ICU’s sensitivity and specificity was 76% and 98%, respectively.133 It is unclear why the sensitivities are so discrepant, but it may be secondary to differences in median age, underlying disease, and severities of illness. Recently, the CAM-ICU was modified into the Brief Confusion Assessment Method (B-CAM) to improve its sensitivity. The B-CAM and CAM-ICU primarily differ on how they test for inattention. The B-CAM simply asks the patient to recite the months of the year backwards from December to July. The CAM-ICU, B-CAM, and other brief delirium assessments specifically designed for the ED are currently being validated in the older ED patients.
Several very brief delirium assessments have been validated in other settings and may have promise for the ED. The Delirium Diagnostic Tool-Provisional (DDT-Pro) is brief delirium assessment that assesses for disorganized thinking, inattention, and sleep-wake cycle disturbances. In 36 patients with traumatic brain injury, the DDT-Pro was 100% sensitive and 94% specific when performed by a layperson compared with a psychiatrist’s assessment.134 However, the median age was 44 years old, and patients were recruited from a rehabilitation hospital, indicating significant brain injury; the diagnostic performance may be different in older ED patients without head injury. The Single Question in Delirium (SQiD) is another promising delirium assessment that asks the patient’s family or friend the following question: “‘Do you think [name of patient] has been more confused lately?” In 33 oncology inpatients, the SQiD was 80% sensitive and 71% specific compared with a psychiatrist’s assessment.135 Lastly, the modified Richmond Agitation and Sedation Scale (mRASS) may also have clinical utility in the ED; this assessment takes less than 30 seconds to complete and can easily be integrated into the ED clinical workflow.135 The mRASS is based upon the RASS, but also incorporates the rater’s observations of the patient’s attentiveness. Using the psychiatrist’s assessment as the reference standard, a mRASS other than 0 (altered level of consciousness or inattentive) is 64% sensitive and 93% specific for delirium in older hospitalized patients.135 Performing serial mRASS assessments increases the sensitivity to 74% with a specificity of 92%.135
Initial Management of Patients with Altered Mental Status in the Emergency Department
When a patient with altered mental status arrives to the ED, the first step is to determine whether this patient is critically ill or not. As the level of consciousness becomes more disturbed (i.e. RASS −5 or RASS +4), the index of suspicion for a life threatening illness that precipitated the acute change in mental status should similarly increase. This is particularly the case for patients who are stuporous or comatose. These patients should become the emergency physician’s immediate priority, and the primary goal should be to promptly assess and stabilize the patient with as little delay as possible.
The initial evaluation and management for ED patients with altered mental status is summarized in Table 5. The patient’s airway, breathing, circulation, and disability should be rapidly evaluated and addressed; the patient should be fully exposed to facilitate evaluation and treatment. Concomitantly, the patient should be placed on pulse oximeter and cardiac monitor, and an intravenous line should be established; if the patient is hemodynamically unstable, then two large bore intravenous lines should be started in the antecubital fossa. Finger stick blood glucose should be obtained immediately to rule out hypoglycemia. Vital signs should also be rapidly measured and if possible, a rectal temperature should be performed to accurately rule out hypo- or hyperthermia.
Table 5.
Initial Assessment and Management of a Patient with Altered Mental Status
| Assessment | Intervention | |
|---|---|---|
| Airway |
|
|
| Breathing |
|
|
| Circulation |
|
|
| Disability |
|
|
| Exposure |
|
|
In patients who have chronic obstructive pulmonary disease, high flow oxygen may remove their respiratory drive especially in patients with chronic respiratory failure. Oxygen saturation should be titrated to the low 90s%.
Bedside ultrasounds are commonly used in emergency departments and intensive care units to rapidly rule out causes of hypotension such as cardiac tamponade and intrabdominal blood. The inferior vena cava can also be assessed using the bedside ultrasound to assess whether a patient is hypovolemic (dehydration, hemorrhage) or hypervolemic (heart failure).
GCS, Glasgow Coma Scale
If opioid overdose is suspected, then intravenous nalaxone should be given especially if the patient is comatose or stuporous. The initial dose of nalaxone is not well established, but is generally considered to be 0.4mg; this initial dose should be diluted in 10cc of normal saline and be administered slowly over several minutes to avoid severe withdrawal symptoms. If there is no response, then the dose can be escalated to 2mg and up to 10mg intravenously; higher doses may be needed if the patient is on a long-acting opioid medication such as methadone. Transdermal fentanyl patches should also be looked for while exposing the patient and if present, it should be removed immediately. Reversing benzodiazepine overdoses with flumazenil is not routinely recommended because it may elicit life threatening withdrawal and seizures in patients who are chronic users. If the finger stick blood sugar indicates hypoglycemia, one ampule (50cc) of D50 should be administered intravenously; if intravenous access is difficult to obtain, 2mg glucagon can given intramuscularly. Thiamine 100mg given parenterally or intramuscularly can also be considered if Wernicke’s encephalopathy is suspected; clinicians should have a high index of suspicion of this in patients who have a history of ethanol abuse or appear malnourished. Thiamine should be given prior to glucose administration. Based upon animal models, there is a theoretical risk that glucose administration may worsen encephalopathy in those who are thiamine deficient.136
The Diagnostic Evaluation of Patients with Acute Brain Dysfunction
In patients with delirium, stupor, or coma, the diagnostic evaluation should be focused on uncovering the underlying etiology. Though other causes of acute brain dysfunction are common, the emergency physician’s first priority is to consider life threatening causes. Life threatening causes of acute brain dysfunction can be remembered using the mnemonic device “WHHHHIMPS” (Table 6).73 Many of these life-threatening causes such as hypoglycemia or hypoxemia can be ruled out within the initial assessment mentioned in the previous section. Other diagnoses, such as meningitis, require a more extensive evaluation and should be considered if no other cause for the patient’s acute brain dysfunction is found. Once these life threatening causes have been considered, the ED evaluation can then focus on ruling out other less life threatening causes of acute brain dysfunction as listed in Table 3.
Table 6. Life-threatening causes of delirium.
Adapted from Caplan GA et al. Delirium. In: Stern TA, ed. Massachusetts General Hospital comprehensive Clinical Psychiatry. 1st ed. Philadelphia, PA: Mosby/Elsevier; 2008.73
| Wernicke’s disease or ethanol withdrawal |
| Hypoxia or hypercarbia |
| Hypoglycemia |
| Hypertensive encephalopathy |
| Hyperthermia or hypothermia |
| Intracerebral hemorrhage |
| Meningitis/encephalitis |
| Poisoning (whether exogenous or iatrogenic) |
| Status epilepticus |
History
Because a patient with acute brain dysfunction, including delirium, is unlikely to provide an accurate history,110 obtaining a detailed history from a proxy is critical to uncovering the underlying etiology. This may be from a family member, caregiver, or a nursing home staff if the patient resides in one. Because so many medications can be deliriogenic, it is important to carefully review the patient’s medication history. Medication histories obtained from triage or from electronic medical record are notoriously inaccurate and should be confirmed with the caregiver or their pharmacy.137,138 A history of any recent changes or additions to the patient’s home medication regimen should be elicited as well as increased dosages; the clinician should determine if these changes are temporally related to the development of symptoms. In addition to prescribed medications, history of taking over the counter medications and alternative medications should also be obtained. A careful substance history should also be obtained, preferably from a proxy. A significant proportion of elderly patients are abusers of ethanol and sedative-hypnotics;139,140 significant ingestion or withdrawal from these substances can precipitate delirium.
Physical Examination
The detailed physical examination (Figure 4) is also crucial to uncovering the underlying etiology of delirium, especially if no collateral history is available. A head examination should look for any signs of recent trauma; the presence of trauma should increase one’s suspicion for a subdural hematoma, subarachnoid hemorrhage, or other traumatic intracranial injury. A pupillary examination should also be performed looking for the presence of fixed or dilated pupils. The presence of miosis and mydriasis may also indicate opioid and anticholinergic medication toxicity, respectively. The pupillary exam may be difficult to assess in elderly patients with eye diseases, prior surgery, or in those who use opthalmic eye drops. A fundoscopic examination can also be considered looking for papilledema suggestive of increased intracranial pressure or retinal subhyaloid hemorrhage suggestive of subarachnoid hemorrhage. If possible, an extraocular muscle examination should also be performed especially in patients who appear to be unresponsive; the presence of vertical extraocular eye movements may suggest locked-in syndrome. Ophthalmoplegia can also be seen in patients with Wernicke’s encephalopathy or increased intracranial pressure. The presence of nystagmus may indicate intoxication with alcohol or other drugs.
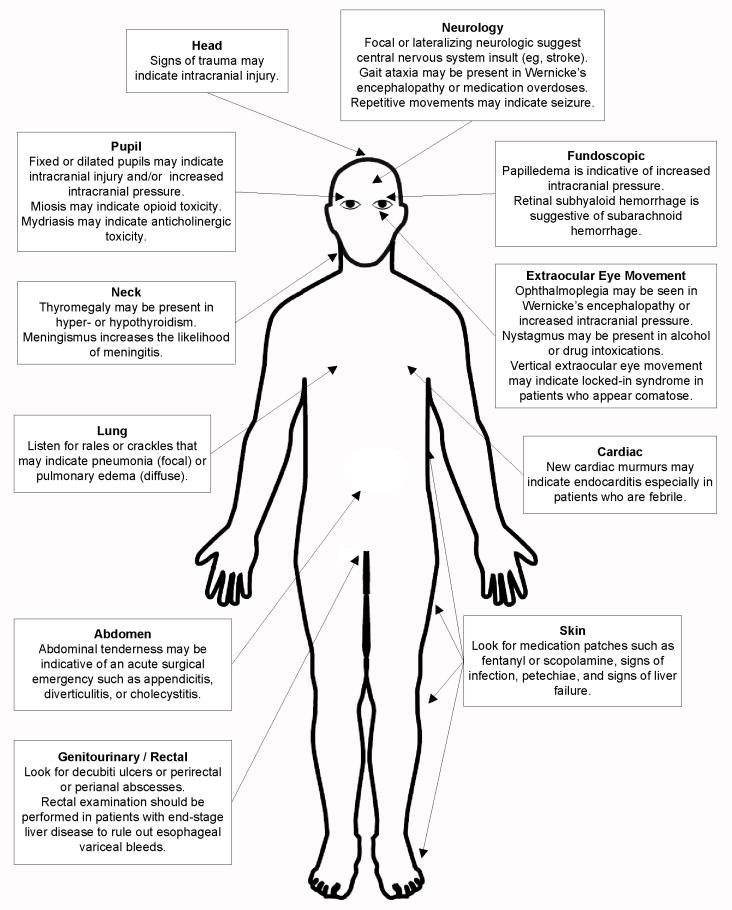
Figure 4.
The physical examination of the patient with delirium, stupor, or coma.
A neck examination should look for thyromegaly and meningismus. Though the presence of meningismus greatly increases the likelihood of meningitis, its absence does not reliably rule it out. A lung examination should look for any signs of pulmonary edema or pneumonia. If febrile, the cardiac exam should be focused on looking for new murmurs that may suggest endocarditis. An abdominal examination is needed to rule out any acute surgical emergencies such as acute appendicitis, diverticulitis, or cholecystitis. The patient should also be completely exposed and the skin should be examined for medication patches (e.g. fentanyl or scopolamine), signs of infection, petechiae, and sequelae of liver failure. A genitourinary examination should be performed to look for infected decubiti ulcers or perirectal or perianal abscesses. A rectal examination should be performed in patients with end stage liver disease as esophageal variceal bleeds can precipitate hepatic encephalopathy.
A focused neurological examination should be also performed. The presence of focal, lateralizing neurological symptoms is suggestive of a central nervous system insult (e.g. cerebrovascular accident, intraparenchymal hemorrhage, or mass effect). Additionally, the presence of repetitive movement of the eyelids, eyes, or extremities may be suggestive of seizure. The presence of gait ataxia may also be seen in patients with Wernicke’s encephalopathy or medication overdoses.
Laboratory Testing
Laboratory tests are routinely performed in patients with delirium, stupor, and coma. Because urinary tract infections are common delirium precipitants, a urinalysis should be performed in all patients. Serum electrolytes should also be routinely obtained to rule-out electrolyte abnormalities such as hypernatremia, hyponatremia, hypercalcemia, or hypocalcemia. Because uremia can precipitate delirium, a blood urea nitrogen and serum creatinine should be ordered. In patients with respiratory complaints or issues, an arterial or venous blood gas should be considered if hypercarbia is suspected. In patients with physical findings suggestive of end stage liver disease, transaminases and ammonia levels can also be ordered. Thyroid stimulating hormone and free T4 can also be considered to rule out hyperthyroidism and hypothyroidism. Serum drug levels should also be ordered if the patient is on medications that can be measured in the serum (i.e. anticonvulsants, theophylline, and digoxin). Occasionally, patients with acute myocardial infarction can also present with delirium without chest pain;69,141 hence, a 12-lead electrocardiogram and cardiac biomarkers should be considered, but their diagnostic yield in ED patients with delirium remains unknown. A lumbar puncture, though not routinely performed, should be strongly considered if there is a clinical suspicion for meningitis or encephalitis or if the patient has a fever or leukocytosis without an obvious source.142,143 This diagnostic procedure can also be considered if no other etiologies for delirium, stupor, or coma are found. A urine drug screen can also be ordered but should be interpreted with caution as it can mislead the clinician into thinking that the patient has a toxicological etiology for their acute brain dysfunction, when in fact another underlying illness, such as meningitis, exists. A patient who is on home opioids and benzodiazepines will likely have a positive urine drug screen for these substances. It also does not provide serum levels, and false positive and negative results can occur.144
Radiographic and Other Testing
With regard to radiological testing, a chest x-ray can be considered to rule out pneumonia or pulmonary edema, especially in the setting of a history of cough and dyspnea, hypoxemia, or tachypnea. An abdominal ultrasound or abdomen and pelvis computed tomography (CT) should be considered if the patient has abdominal pain. For patients who are stuporous or comatose, they should promptly receive a head CT emergently to rule out any structural lesions. For delirious patients, however, there is little evidence based guidance to when a head CT is appropriate; their routine use is not recommended as its diagnostic yield may be low.9 Based upon two studies, a head CT’s diagnostic yield is increased when performed in delirious patients with impaired level consciousness, a recent history of a fall or head trauma, or a focal neurological deficit.9,145 It can also be considered when no other cause for delirium is found. Clinical judgment should be used when deciding whether or not a delirious patient requires a head CT.
Magnetic resonance imaging of the brain (brain MRI) and electroencephalography (EEG) are occasionally performed in patients with acute brain dysfunction including delirium, but their optimal role in the ED is yet to be determined. In addition, these diagnostic modalities may not be readily available in all settings further limiting their use. Patients with cerebrovascular accidents involving the right parietal lobe can present with delirium as the sole manifestation and without any focal neurological findings.146 Early in the clinical course (several hours), the head CT may be non-diagnostic, and a brain MRI can help rule in this diagnosis. Non-convulsive status epilepticus can also manifest as unusual mental status changes including delirium, but making this diagnosis can be challenging without an EEG. An early EEG should be considered in patients who had a reported seizure prior to ED arrival or have a seizure history. Though EEGs are difficult to obtain in the ED, the development of abbreviated (5-minute) EEG protocols147 and portable EEG monitoring devices may improve the feasibility of obtaining this diagnostic modality quickly.148 Early diagnosis of cerebrovascular accidents or non-convulsive status epilepticus is important, because earlier intervention may improve patient outcomes. If either of these diagnoses is being considered as a cause for altered mental status, then prompt neurologic consultation in the ED is recommended to facilitate both diagnostic evaluation and therapeutic intervention.
Emergency Department Management of Patients with Delirium
The single most effective treatment for acute brain dysfunction is to diagnose and treat the underlying etiology. Beyond this, the clinical management of acute brain dysfunction is unclear especially for delirium and is secondary to the limited evidence available. Delirium care is slowly evolving, and non-pharmacologic and pharmacologic interventions currently exist, especially for those who are agitated. In general, non-pharmacologic interventions are favored as the initial management and pharmacologic means should be used as a last resort. The following sections can also be applied to patients in stupor or coma, as many of these patients will eventually transition into delirium.
Non-Pharmacologic Management
Though hyperactive delirium occurs less frequently in older ED patients, its management can be challenging if they are agitated or combative, and especially if patient, caregiver, and ED staff safety is a concern. Non-pharmacologic interventions should be attempted prior to considering pharmacologic interventions. Initial steps would be to modify the environment and may involve dimming or turning off lights, minimizing auditory stimulation from beeping cardiac monitors or intravenous infusion pumps, and having family members and familiar objects from home at the patient’s bedside.47,149
Flaherty et al. recommend the “TADA” approach as a non-pharmacologic means to managing and preventing agitation. It stands for “Tolerate, Anticipate, Don’t Agitate”.150 Tolerating behaviors that may appear to be potentially dangerous is the first step and is contrary to the nature of health care providers. For example, a delirious patient may attempt to get out of bed without assistance or pull on intravenous lines, oxygen tubing or cardiac monitoring devices. However, tolerating such behaviors allows patients to respond naturally to their circumstances and may provide them a sense of control while in their confused state. These behaviors may also be a cue that something is bothering them; they are so cognitively impaired that they are unable to communicate what’s wrong. For example, a patient who is agitated and getting out bed may really need to go to the bathroom. Tolerating behaviors require close supervision to maintain patient safety.150 Anticipating behaviors is where the health care provider prepares for what the patient might do and avoids inciting agents that may exacerbate agitation. This includes avoiding unnatural attachments or tethers that are not absolutely necessary for clinical care.150 Examples of tethers are multiple intravenous lines, nasal cannula oxygen, and monitoring devices. Instead of giving intravenous normal saline continuously for maintenance fluids, intermittent boluses should be considered. Supplemental oxygen is not needed if the patient is not hypoxic or in respiratory compromise. Blood pressure cuffs, pulse oximeter, and cardiac monitoring devices should also be minimized if continuous monitoring is not necessary; intermittent vital sign measurements should be used whenever possible.150 Getting out of bed is also anticipated and encouraged by this approach as long as patient’s safety can be ensured. Don’t Agitate is the final step and considered the golden rule of this approach.150 Some agitators are obvious (i.e. urinary bladder catheters) and some are not. Reorientation can be unpredictable as it can occasionally worsen agitation and should only be attempted if the patient is amenable to it.
Indwelling urinary bladder catheters deserve special mention because they are frequently placed in patients with acute brain dysfunction. They should be avoided in delirious patients when there is no clear medical indication. Urinary bladder catheters can not only agitate the patient further, but their use is associated with increased in-hospital and 90-day mortality, longer hospital lengths of stays, and a higher risk of developing delirium.42,151 If the delirious patient has urinary incontinence and urine is needed for a urinalysis, straight catheterization should be used. Avoiding or removing urinary bladder catheters can not only reduce agitation, but can also help minimize urethral trauma from forced self-removal and reduce the risk of catheter associated infections.
Similarly, physical restraints should be avoided as they are associated with a four-fold increase in delirium development during hospitalization and is associated with increased delirium severity.42 If placed for patient or provider safety, physical restraints should be a temporary measure and should be removed once non-pharmacological or pharmacological alternatives have taken effect. Having family members in the room or using sitters can help reduce the need for physical restraints; they can provide feedback to patients when they initiate dangerous behaviors such as climbing out of bed or attempting to go the bathroom without assistance.
Pharmacologic Management
Pharmacologic management is mainly focused on the agitated delirious patient (hyperactive) and can be considered if non-pharmacologic interventions fail. Benzodiazepines should be avoided as monotherapy in delirious patients whenever possible,47,152 because they have high side effect profiles and can exacerbate delirium. Breitbart et al. performed a randomized control trial that compared antipsychotic medications with lorazepam, and observed a higher prevalence of treatment limiting side effects such as over-sedation and increased confusion in the lorazepam arm.153
The National Institute of Health and Clinical Excellence (NICE) delirium guidelines recommend using antipsychotic medications in patients with agitation and who are at high risk for harming themselves and others.152 However, these medications should be used only if verbal and non-verbal de-escalation techniques have failed, and if used, the lowest dose possible should be given.152 Antipsychotic medications are also used in delirious patients with behavioral disturbances and overt psychotic manifestations (i.e. visual hallucinations and delusions). However, their routine use is controversial because most studies investigating the use of antipsychotics are limited by their non-blinded trial design, poor randomization, or inadequate power. Two systematic reviews investigated the role of antipsychotic medications in delirium management, and they concluded that there was little evidence to support their routine use and that additional studies using more rigorous methodology were needed.154,155
Haloperidol is one of the most studied and widely used antipsychotic medication because its lacks anticholinergic properties and can be administered orally, intramuscularly, or intravenously.47 Hu et al. compared haloperidol with placebo, and observed that 70.4% of delirious patients who received haloperidol showed improvement in their delirium severity at the end of one week compared with 29.7% of the placebo group.156 For agitated elderly patients, 0.25 – 1.0 mg of haloperidol can be given every 30 to 60 minutes in the intravenous or intramuscular form. Special care must be taken when given in the intravenous formulation, because torsades de point has been reported when given in this form.157 If possible, a 12-lead electrocardiogram should be obtained prior to intravenous haloperidol administration to evaluate the patient’s QTc interval, and should be avoided if the QTc interval is greater than 500ms. The benefit of the intravenous haloperidol is that it is the least likely to cause extrapyramidal symptoms whereas the intramuscular formulation is associated with the highest incidence.
Atypical antipsychotic medications such as olanzapine, risperidone, and quetiapine are also commonly used by psychiatrists and geriatricians for the treatment of delirium. The advantage of these medications is the decreased incidence of extrapyramidal side effects compared with haloperidol.158 Most atypical antipsychotics are given orally, but recently, olanzapine, risperidone, ziprasidone, and aripiprazole have been made available in intramuscular formulations. Unfortunately, these forms may not be readily available in many EDs. Similar to haloperidol, there is limited evidence on the effectiveness of atypical antipsychotics and few have been compared to placebo. Compared with placebo, olanzapine has been shown to improve delirium severity in one randomized control trial,156 but its effectiveness may be attenuated in patients who are 70 years and older.159 Tahir et al. performed a double-blinded randomized control trial in 42 delirious patients and observed that patients in the quetiapine arm recovered faster than those who received placebo.160
In patients with Diffuse Lewis Body dementia or Parkinson’s disease, antipsychotic medications should be should be avoided or used with extreme caution.161 These patients are highly sensitive to these medications and easily develop extrapyramidal side effects.152 If antipsychotic medications must be given in these patients, quetiapine is the least likely to have extrapyramidal side effects.162 Regardless, if these patients are agitated and non-pharmacologic measures have failed, psychiatric and / or geriatric consultation may be warranted.
As previously mentioned, benzodiazepines should be avoided for the management of delirious patients. The exception to this recommendation is for patients who are withdrawing from ethanol (delirium tremens) or benzodiazepines. 47,163 Giving benzodiazepines to these patients will improve delirium severity and may also improve mortality and morbidity.163 Because poorly controlled somatic pain can precipitate delirium, 70–72 using opioid medications in this subgroup may be beneficial and help reduce agitation.71 Pain control can also be achieved by using regional anesthesia such as femoral nerve blocks in patients with lower extremity fractures.
Medications to Avoid in Patients with Delirium
Part of the management of patients with acute brain dysfunction is to avoid medications known to be precipitate or worsen delirium. Recently, the Beers Criteria for Potentially Inappropriate Medication Use in Older Adults was updated. Based upon “moderate quality evidence and strong strength of recommendation” from the panel, they recommend avoiding tricyclic antidepressants, medications with anticholinergic properties, benzodiazepines and other sedative-hypnotics, corticosteroids, and histamine-2 receptor antagonists in older patients because they may worsen or exacerbate delirium.86 If the patient is on multiple medications with anticholinergic properties, reducing these medications may improve delirium severity; in a study of 34 delirious nursing home patients who were on at least one anticholinergic medication, subjects were randomly assigned to receive an intervention that reduced their anticholinergic load by 25%.164 Those who received the intervention had significant improvements in delirium symptomatology.164 There several reports of histamine-2 blockers (cimetidine, ranitidine, and famotidine) precipitating delirium,165 and they should be avoided in delirious patients since safer alternatives such as proton pump inhibitors are available.
Disposition
Patients who are stuporous or comatose need a hospital admission and likely require an intensive care unit. With delirium, however, there is little evidence-based guidance regarding the appropriate disposition of older ED patients. Most delirious patients will require hospitalization, especially if they have severe symptoms, have poor social support at home, or poor access to follow-up care. There is also evidence to suggest that older ED patients with delirium who are discharged home are more likely to die than their non-delirious counterparts; this relationship is further magnified when the delirium is missed by the ED.17 However, for a small minority of delirious patients with reliable caregivers and accessible transportation, ED discharge can be considered. This is if the etiology is unequivocally obvious and the delirium symptoms resolve (e.g. accidental opioid overdose reversed with nalaxone) or are mild, the patient can be closely supervised at home, and close outpatient follow-up can be arranged.
If admitted to the hospital, delirious patients should preferably be admitted to specialized geriatric unit such as an Acute Care for Elderly unit or a Delirium Room.166,167 These units typically have multidisciplinary team of physicians, nurses, and social workers or case managers who specialize in geriatrics care and have expertise in managing delirious patients. They also implement non-pharmacologic, multi-component delirium interventions which 1) minimize the use of psychoactive medications such as benzodiazepines and medications with anticholinergic properties, 2) maximize mobility and limit the use of urinary bladder catheters and physical restraints, 3) implement the TADA approach as described above, 4) reduce sensory deprivation by offering eye-glasses or hearing devices, 5) provide cognitive stimulation and reorientation, and 5) encourage normal sleep-wake cycles (e.g. minimize nighttime noise, use ear plugs at night).149,167,168 However, the efficacy of these interventions is equivocal, especially for delirious medical inpatients.169 Pitkala et al. found that a multi-component delirium intervention enhanced delirium resolution in the hospital and improved cognition and health related quality of life at 6-months.170,171 However, they did not observe any differences in long-term mortality or institutionalization.170,171 Regardless of which inpatient unit delirious patients are admitted to, their time spent in the ED should probably be minimized. Inouye et al. observed that patients who were in the ED for longer than 12 hours were two-times more likely to develop delirium in the hospital setting.42
Communication During Transitions of Care
Regardless of the patient’s disposition, the patient’s mental status in the ED should be communicated to the physician at the next level of care. The patient’s delirium status and the delirium assessment used to make the diagnosis, the suspected underlying etiology, and treatments administered should be communicated. Communicating the patient’s level of consciousness using an arousal scale such as the RASS may also be useful provide information on the patient’s psychomotor status (normal, hypoactive, or hyperactive). Additionally, knowing a baseline RASS may be useful as changes in RASS increases the likelihood that the patient has delirium.135 If the delirious patient is being admitted, then this information should be conveyed to the admitting physician. If the patient is being discharged home, then his or her primary care provider should be notified. Similar communication should occur between ED physicians and nurses during shift change.
Improving Delirium Recognition in the Emergency Department - Challenges and Future Research
Delirium is currently missed in the majority of older ED patients,10,12–17 because EDs do not screen for this form of acute brain dysfunction. Improving delirium recognition in the ED will be challenging. Emergency physicians are usually under huge time constraints and have a limited amount of time to spend with the patient. They often take care of large numbers of patients at once and their patient evaluations are also frequently interrupted (i.e. radiological testing or a higher acuity patient arrives). This limits the feasibility of a prolonged mental status examination. Brief and efficient approaches to delirium monitoring that are specifically tailored for the ED setting are needed. Future research should focus developing these approaches. This may entail validating brief (< 2 minutes) delirium assessments in older ED patients. These assessments should balance brevity with diagnostic accuracy, be easy to use, and be reliable when performed by non-physicians such as nurses, paramedics, or patient care technicians. Non-physicians may play a more instrumental role in ED delirium monitoring, because they may also have more time to spend with the patient at multiple time points compared with physicians.172
In addition, the utility serial measurements of global tests of cognition should be investigated. Decreasing scores in these cognitive assessments, such as the Mini-Mental State Examination, may be diagnostic of delirium.173 However, the Mini-Mental State Examination can take 5 – 10 minutes to perform and may not be feasible to perform in the ED.128 Brief cognitive assessments such as the Six-Item Screener174,175 Mini-Cog,175 Ottawa 3DY,176 and Brief Alzheimer’s Screen exist176 but it is unclear how sensitive these assessments are in detecting changes in cognition observed in delirium. This would also require that routine cognitive screening be performed in outpatient clinic and possibly the ED setting during non-delirious episodes in order to establish a baseline.
Delirium surveillance in the ED can further be optimized by performing assessments on patients who are higher risk for having delirium. Ideally, the process of identifying of high risk patients should be automated and incorporated into the electronic medical record system in order to minimize ED staff workload. Currently, there are little data to suggest what risk factors should be used to identify older ED patients at high risk for delirium. Future research should focus on developing and validating such clinical decision rules; these rules should use patient characteristics that are immediately and easily available to the clinician.
Perhaps the most significant obstacle to routine ED delirium monitoring is the absence of cost-effective interventions for delirious ED patients. Thus far, uncovering the underlying etiology remains the most effective treatment for delirium. Several non-pharmacological multi-component interventions have been developed (see Disposition), but as previously mentioned, their efficacy is questionable in medical delirious patients. Perhaps initiating these interventions in the ED instead of the hospital setting would improve their efficacy. Additionally, the role of antipsychotic medications in older ED patients with delirium remains unknown. It is also possible that there is a subgroup of delirious of patients that may more benefit from these non-pharmacologic and pharmacologic interventions. Future studies using randomized control trial methodology are needed to test these hypotheses.
The American Delirium Society
Delirium remains an underappreciated geriatric syndrome among clinicians outside of geriatrics, psychiatry, and neurology. To increase delirium’s awareness, recognition, and advance its science, the American Delirium Society was recently created. The overall mission of this society is to “foster research, education, quality improvement, advocacy and implementation science to minimize the impact of delirium on short and long-term health and well being, and the effects of delirium on the health care system as a whole.”177 This organization consists of an interdisciplinary group of physicians, nurses, pharmacists, and social workers in psychiatry, geriatrics, emergency medicine, internal medicine, and critical care. Additional information can be found at www.americandeliriumsociety.org.
Conclusion
Altered mental status is a common complaint in older ED patients. Acute changes are more concerning because they are usually caused by an underlying medical illness and can be life threatening. Delirium, stupor, and coma are common causes of altered mental status, and these forms of acute brain dysfunction are associated with a multitude of adverse outcomes including higher death rates. Their etiology multifactorial and are a result of a complex interplay between patient vulnerability and precipitating factors. Patients with acute brain dysfunction can fluctuate and transition from one state to another. A patient who is initially comatose in the ED frequently transitions into delirium. The initial evaluation of a patient with acute brain dysfunction should begin by assessing the level of consciousness using a arousal scale such as the RASS. If the patient is unresponsive (RASS −5) or responsive to painful stimuli (RASS −4) only, then the patient is considered to be comatose or stuporous, respectively; a validated coma scale such as the GCS should be used. If the patient has a RASS of −3 and above, a delirium assessment such as the CAM should be used, but it takes 5 minutes to perform and may not be feasible for some EDs. Several briefer delirium assessments exist (CAM-ICU, B-CAM, SQiD, and mRASS) but require validation in older ED patients and such studies are ongoing. Once acute brain dysfunction is detected in the ED, the primary goal is to find and treat the underlying cause. For agitated patients, non-pharmacologic interventions, such as the TADA approach, should be attempted initially. If non-pharmacologic interventions fail, antipsychotic medications such as haloperidol can be considered. Benzodiazepines should be avoided as monotherapy unless the patient is withdrawing from ethanol or benzodiazepines. Patients with delirium, stupor, or coma generally require admission. The optimal method for delirium monitoring and treatment in the ED remains unclear and additional research is needed.
Key Points.
Altered mental status is a common chief complaint among older emergency department (ED) patients.
Patients with acute changes in mental status are likely to have delirium, stupor, and coma, and these changes are commonly precipitated by an underlying medical illness that can be potentially life-threatening.
Though stupor and coma are easily identifiable, the clinical presentation of delirium can be subtle and is often missed without actively screening for it.
The ED management of patients with altered mental status should initially be focused on stabilizing the patient (airway, breathing, circulation) while searching for the underlying etiology.
Acknowledgments
Jin H. Han is supported by a National Institute on Aging K23AG032355.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jin H. Han, Email: jin.h.han@vanderbilt.edu, Center for Quality Aging, Assistant Professor of Emergency Medicine, Vanderbilt University School of Medicine, Department of Emergency Medicine, 703 Oxford House, Nashville, TN 37232-4700, Phone: 615-936-1434, Fax: 615-936-1316.
Scott T. Wilber, Email: wilbers@summahealth.org, Emergency Medicine Research Center, Associate Professor of Emergency Medicine, Summa Akron City Hospital, Northeastern Ohio Medical University, 525 East Market Street, Akron, Ohio 44309, Phone: 330-375-7530, Fax: 330-375-7564.
References
- 1.Morandi A, Pandharipande P, Trabucchi M, et al. Understanding international differences in terminology for delirium and other types of acute brain dysfunction in critically ill patients. Intensive Care Med. 2008;34(10):1907–1915. doi: 10.1007/s00134-008-1177-6. [DOI] [PubMed] [Google Scholar]
- 2.Clarfield AM. The decreasing prevalence of reversible dementias: an updated meta-analysis. Arch Intern Med. 2003;163(18):2219–2229. doi: 10.1001/archinte.163.18.2219. [DOI] [PubMed] [Google Scholar]
- 3.Schuur JD, Venkatesh AK. The growing role of emergency departments in hospital admissions. N Engl J Med. 2012;367(5):391–393. doi: 10.1056/NEJMp1204431. [DOI] [PubMed] [Google Scholar]
- 4.Niska R, Bhuiya F, Xu J. National Hospital Ambulatory Medical Care Survey: 2007 emergency department summary. Natl Health Stat Report. 2010;(26):1–31. [PubMed] [Google Scholar]
- 5.He W, Sengupta M, Velkoff VA, DeBarros KA. US Census Bureau, Current Population Reports, P23–209, 65+ in the United States: 2005. U.S. Government Printing Office; 2005. [Google Scholar]
- 6.Posner JB, Plum F. Plum and Posner’s diagnosis of stupor and coma. 4. Oxford ; New York: Oxford University Press; 2007. [Google Scholar]
- 7.Sessler CN, Gosnell MS, Grap MJ, et al. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166(10):1338–1344. doi: 10.1164/rccm.2107138. [DOI] [PubMed] [Google Scholar]
- 8.Ely EW, Truman B, Shintani A, et al. Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation-Sedation Scale (RASS) JAMA. 2003;289(22):2983–2991. doi: 10.1001/jama.289.22.2983. [DOI] [PubMed] [Google Scholar]
- 9.Naughton BJ, Moran M, Ghaly Y, Michalakes C. Computed tomography scanning and delirium in elder patients. Acad Emerg Med. 1997;4(12):1107–1110. doi: 10.1111/j.1553-2712.1997.tb03690.x. [DOI] [PubMed] [Google Scholar]
- 10.Naughton BJ, Moran MB, Kadah H, Heman-Ackah Y, Longano J. Delirium and other cognitive impairment in older adults in an emergency department. Ann Emerg Med. 1995;25(6):751–755. doi: 10.1016/s0196-0644(95)70202-4. [DOI] [PubMed] [Google Scholar]
- 11.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders : DSM-IV-TR. 4. Washington, DC: American Psychiatric Association; 2000. Task Force on DSM-IV. [Google Scholar]
- 12.Hustey FM, Meldon SW, Smith MD, Lex CK. The effect of mental status screening on the care of elderly emergency department patients. Ann Emerg Med. 2003;41(5):678–684. doi: 10.1067/mem.2003.152. [DOI] [PubMed] [Google Scholar]
- 13.Lewis LM, Miller DK, Morley JE, Nork MJ, Lasater LC. Unrecognized delirium in ED geriatric patients. Am J Emerg Med. 1995;13(2):142–145. doi: 10.1016/0735-6757(95)90080-2. [DOI] [PubMed] [Google Scholar]
- 14.Han JH, Zimmerman EE, Cutler N, et al. Delirium in older emergency department patients: recognition, risk factors, and psychomotor subtypes. Acad Emerg Med. 2009;16(3):193–200. doi: 10.1111/j.1553-2712.2008.00339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elie M, Rousseau F, Cole M, Primeau F, McCusker J, Bellavance F. Prevalence and detection of delirium in elderly emergency department patients. CMAJ. 2000;163(8):977–981. [Google Scholar]
- 16.Hustey FM, Meldon SW. The prevalence and documentation of impaired mental status in elderly emergency department patients. Ann Emerg Med. 2002;39(3):248–253. doi: 10.1067/mem.2002.122057. [DOI] [PubMed] [Google Scholar]
- 17.Kakuma R, du Fort GG, Arsenault L, et al. Delirium in older emergency department patients discharged home: effect on survival. J Am Geriatr Soc. 2003;51(4):443–450. doi: 10.1046/j.1532-5415.2003.51151.x. [DOI] [PubMed] [Google Scholar]
- 18.Meagher DJ, Moran M, Raju B, et al. Phenomenology of delirium. Assessment of 100 adult cases using standardised measures. Br J Psychiatry. 2007;190:135–141. doi: 10.1192/bjp.bp.106.023911. [DOI] [PubMed] [Google Scholar]
- 19.Meagher DJ, Maclullich AM, Laurila JV. Defining delirium for the International Classification of Diseases, 11th Revision. J Psychosom Res. 2008;65(3):207–214. doi: 10.1016/j.jpsychores.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 20.Meagher DJ, Trzepacz PT. Motoric subtypes of delirium. Semin Clin Neuropsychiatry. 2000;5(2):75–85. doi: 10.153/SCNP00500075. [DOI] [PubMed] [Google Scholar]
- 21.Inouye SK, Foreman MD, Mion LC, Katz KH, Cooney LM., Jr Nurses’ recognition of delirium and its symptoms: comparison of nurse and researcher ratings. Arch Intern Med. 2001;161(20):2467–2473. doi: 10.1001/archinte.161.20.2467. [DOI] [PubMed] [Google Scholar]
- 22.Nicholas LM, Lindsey BA. Delirium presenting with symptoms of depression. Psychosomatics. 1995;36(5):471–479. doi: 10.1016/S0033-3182(95)71628-0. [DOI] [PubMed] [Google Scholar]
- 23.Farrell KR, Ganzini L. Misdiagnosing delirium as depression in medically ill elderly patients. Arch Intern Med. 1995;155(22):2459–2464. [PubMed] [Google Scholar]
- 24.Peterson JF, Pun BT, Dittus RS, et al. Delirium and its motoric subtypes: a study of 614 critically ill patients. J Am Geriatr Soc. 2006;54(3):479–484. doi: 10.1111/j.1532-5415.2005.00621.x. [DOI] [PubMed] [Google Scholar]
- 25.Liptzin B, Levkoff SE. An empirical study of delirium subtypes. Br J Psychiatry. 1992;161:843–845. doi: 10.1192/bjp.161.6.843. [DOI] [PubMed] [Google Scholar]
- 26.O’Keeffe ST. Clinical subtypes of delirium in the elderly. Dement Geriatr Cogn Disord. 1999;10(5):380–385. doi: 10.1159/000017174. [DOI] [PubMed] [Google Scholar]
- 27.Kelly KG, Zisselman M, Cutillo-Schmitter T, Reichard R, Payne D, Denman SJ. Severity and course of delirium in medically hospitalized nursing facility residents. Am J Geriatr Psychiatry. 2001;9(1):72–77. [PubMed] [Google Scholar]
- 28.Marcantonio E, Ta T, Duthie E, Resnick NM. Delirium severity and psychomotor types: their relationship with outcomes after hip fracture repair. J Am Geriatr Soc. 2002;50(5):850–857. doi: 10.1046/j.1532-5415.2002.50210.x. [DOI] [PubMed] [Google Scholar]
- 29.Pandharipande P, Cotton BA, Shintani A, et al. Motoric subtypes of delirium in mechanically ventilated surgical and trauma intensive care unit patients. Intensive Care Med. 2007;33(10):1726–1731. doi: 10.1007/s00134-007-0687-y. [DOI] [PubMed] [Google Scholar]
- 30.Ross CA. CNS arousal systems: possible role in delirium. Int Psychogeriatr. 1991;3(2):353–371. doi: 10.1017/s1041610291000819. [DOI] [PubMed] [Google Scholar]
- 31.Ross CA, Peyser CE, Shapiro I, Folstein MF. Delirium: phenomenologic and etiologic subtypes. Int Psychogeriatr. 1991;3(2):135–147. doi: 10.1017/s1041610291000613. [DOI] [PubMed] [Google Scholar]
- 32.O’Keeffe ST, Lavan JN. Clinical significance of delirium subtypes in older people. Age Ageing. 1999;28(2):115–119. doi: 10.1093/ageing/28.2.115. [DOI] [PubMed] [Google Scholar]
- 33.Kiely DK, Jones RN, Bergmann MA, Marcantonio ER. Association between psychomotor activity delirium subtypes and mortality among newly admitted post-acute facility patients. J Gerontol A Biol Sci Med Sci. 2007;62(2):174–179. doi: 10.1093/gerona/62.2.174. [DOI] [PubMed] [Google Scholar]
- 34.Boller F, Verny M, Hugonot-Diener L, Saxton J. Clinical features and assessment of severe dementia. A review. Eur J Neurol. 2002;9(2):125–136. doi: 10.1046/j.1468-1331.2002.00356.x. [DOI] [PubMed] [Google Scholar]
- 35.Marcantonio ER, Simon SE, Bergmann MA, Jones RN, Murphy KM, Morris JN. Delirium symptoms in post-acute care: prevalent, persistent, and associated with poor functional recovery. J Am Geriatr Soc. 2003;51(1):4–9. doi: 10.1034/j.1601-5215.2002.51002.x. [DOI] [PubMed] [Google Scholar]
- 36.Levkoff SE, Evans DA, Liptzin B, et al. Delirium. The occurrence and persistence of symptoms among elderly hospitalized patients. Arch Intern Med. 1992;152(2):334–340. doi: 10.1001/archinte.152.2.334. [DOI] [PubMed] [Google Scholar]
- 37.McKeith IG, Galasko D, Kosaka K, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology. 1996;47(5):1113–1124. doi: 10.1212/wnl.47.5.1113. [DOI] [PubMed] [Google Scholar]
- 38.Smith E, Delargy M. Locked-in syndrome. BMJ. 2005;330(7488):406–409. doi: 10.1136/bmj.330.7488.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wijdicks EF, Nichols DA, Thielen KR, et al. Intra-arterial thrombolysis in acute basilar artery thromboembolism: the initial Mayo Clinic experience. Mayo Clin Proc. 1997;72(11):1005–1013. doi: 10.4065/72.11.1005. [DOI] [PubMed] [Google Scholar]
- 40.Zehtabchi S, Abdel Baki SG, Malhotra S, Grant AC. Nonconvulsive seizures in patients presenting with altered mental status: an evidence-based review. Epilepsy Behav. 2011;22(2):139–143. doi: 10.1016/j.yebeh.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Inouye SK, Viscoli CM, Horwitz RI, Hurst LD, Tinetti ME. A predictive model for delirium in hospitalized elderly medical patients based on admission characteristics. Ann Intern Med. 1993;119(6):474–481. doi: 10.7326/0003-4819-119-6-199309150-00005. [DOI] [PubMed] [Google Scholar]
- 42.Inouye SK, Charpentier PA. Precipitating factors for delirium in hospitalized elderly persons. Predictive model and interrelationship with baseline vulnerability. JAMA. 1996;275(11):852–857. [PubMed] [Google Scholar]
- 43.Inouye SK. Predisposing and precipitating factors for delirium in hospitalized older patients. Dement Geriatr Cogn Disord. 1999;10(5):393–400. doi: 10.1159/000017177. [DOI] [PubMed] [Google Scholar]
- 44.Han JH, Morandi A, Ely W, et al. Delirium in the nursing home patients seen in the emergency department. J Am Geriatr Soc. 2009;57(5):889–894. doi: 10.1111/j.1532-5415.2009.02219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Elie M, Cole MG, Primeau FJ, Bellavance F. Delirium risk factors in elderly hospitalized patients. J Gen Intern Med. 1998;13(3):204–212. doi: 10.1046/j.1525-1497.1998.00047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pun BT, Ely EW. The importance of diagnosing and managing ICU delirium. Chest. 2007;132(2):624–636. doi: 10.1378/chest.06-1795. [DOI] [PubMed] [Google Scholar]
- 47.Practice guideline for the treatment of patients with delirium. American Psychiatric Association. Am J Psychiatry. 1999;156(5 Suppl):1–20. [PubMed] [Google Scholar]
- 48.Fearing MA, Inouye SK. Delirium. In: Blazer DG, Steffens DC, editors. The American Psychiatric Publishing Textbook of Geriatric Psychiatry. 4. Washington, D. C: American Psychiatric Publishing; 2009. [Google Scholar]
- 49.Schor JD, Levkoff SE, Lipsitz LA, et al. Risk factors for delirium in hospitalized elderly. JAMA. 1992;267(6):827–831. [PubMed] [Google Scholar]
- 50.Marcantonio ER, Goldman L, Mangione CM, et al. A clinical prediction rule for delirium after elective noncardiac surgery. JAMA. 1994;271(2):134–139. [PubMed] [Google Scholar]
- 51.Gustafson Y, Berggren D, Brannstrom B, et al. Acute confusional states in elderly patients treated for femoral neck fracture. J Am Geriatr Soc. 1988;36(6):525–530. doi: 10.1111/j.1532-5415.1988.tb04023.x. [DOI] [PubMed] [Google Scholar]
- 52.Jitapunkul S, Pillay I, Ebrahim S. Delirium in newly admitted elderly patients: a prospective study. Q J Med. 1992;83(300):307–314. [PubMed] [Google Scholar]
- 53.Kolbeinsson H, Jonsson A. Delirium and dementia in acute medical admissions of elderly patients in Iceland. Acta Psychiatr Scand. 1993;87(2):123–127. doi: 10.1111/j.1600-0447.1993.tb03342.x. [DOI] [PubMed] [Google Scholar]
- 54.Rockwood K. Acute confusion in elderly medical patients. J Am Geriatr Soc. 1989;37(2):150–154. doi: 10.1111/j.1532-5415.1989.tb05874.x. [DOI] [PubMed] [Google Scholar]
- 55.Pompei P, Foreman M, Rudberg MA, Inouye SK, Braund V, Cassel CK. Delirium in hospitalized older persons: outcomes and predictors. J Am Geriatr Soc. 1994;42(8):809–815. doi: 10.1111/j.1532-5415.1994.tb06551.x. [DOI] [PubMed] [Google Scholar]
- 56.Voyer P, Cole MG, McCusker J, Belzile E. Prevalence and symptoms of delirium superimposed on dementia. Clin Nurs Res. 2006;15(1):46–66. doi: 10.1177/1054773805282299. [DOI] [PubMed] [Google Scholar]
- 57.Jones RN, Yang FM, Zhang Y, Kiely DK, Marcantonio ER, Inouye SK. Does educational attainment contribute to risk for delirium? A potential role for cognitive reserve. J Gerontol A Biol Sci Med Sci. 2006;61(12):1307–1311. doi: 10.1093/gerona/61.12.1307. [DOI] [PubMed] [Google Scholar]
- 58.Francis J, Martin D, Kapoor WN. A prospective study of delirium in hospitalized elderly. JAMA. 1990;263(8):1097–1101. [PubMed] [Google Scholar]
- 59.Kennedy M, Enander RA, Wolfe RE, Marcantonio ER, Shapiro NI. Identification of delirium in elderly emergency department patients. Acad Emerg Med. 2012;19(Suppl 1 s1):S147. [Google Scholar]
- 60.Rahkonen T, Makela H, Paanila S, Halonen P, Sivenius J, Sulkava R. Delirium in elderly people without severe predisposing disorders: etiology and 1-year prognosis after discharge. Int Psychogeriatr. 2000;12(4):473–481. doi: 10.1017/s1041610200006591. [DOI] [PubMed] [Google Scholar]
- 61.George J, Bleasdale S, Singleton SJ. Causes and prognosis of delirium in elderly patients admitted to a district general hospital. Age Ageing. 1997;26(6):423–427. doi: 10.1093/ageing/26.6.423. [DOI] [PubMed] [Google Scholar]
- 62.Wahlund L, Bjorlin GA. Delirium in clinical practice: experiences from a specialized delirium ward. Dement Geriatr Cogn Disord. 1999;10(5):389–392. doi: 10.1159/000017176. [DOI] [PubMed] [Google Scholar]
- 63.Koponen H, Stenback U, Mattila E, Soininen H, Reinikainen K, Riekkinen PJ. Delirium among elderly persons admitted to a psychiatric hospital: clinical course during the acute stage and one-year follow-up. Acta Psychiatr Scand. 1989;79(6):579–585. doi: 10.1111/j.1600-0447.1989.tb10306.x. [DOI] [PubMed] [Google Scholar]
- 64.Foreman MD. Confusion in the hospitalized elderly: incidence, onset, and associated factors. Res Nurs Health. 1989;12(1):21–29. doi: 10.1002/nur.4770120105. [DOI] [PubMed] [Google Scholar]
- 65.El-Kaissi S, Kotowicz MA, Berk M, Wall JR. Acute delirium in the setting of primary hypothyroidism: the role of thyroid hormone replacement therapy. Thyroid. 2005;15(9):1099–1101. doi: 10.1089/thy.2005.15.1099. [DOI] [PubMed] [Google Scholar]
- 66.Isbell H, Fraser HF, Wikler A, Belleville RE, Eisenmen AJ. An experimental study of the etiology of rum fits and delirium tremens. Q J Stud Alcohol. 1955;16(1):1–33. [PubMed] [Google Scholar]
- 67.Fleischhacker WW, Barnas C, Hackenberg B. Epidemiology of benzodiazepine dependence. Acta Psychiatr Scand. 1986;74(1):80–83. doi: 10.1111/j.1600-0447.1986.tb06231.x. [DOI] [PubMed] [Google Scholar]
- 68.Fruensgaard K. Withdrawal psychosis: a study of 30 consecutive cases. Acta Psychiatr Scand. 1976;53(2):105–118. doi: 10.1111/j.1600-0447.1976.tb00065.x. [DOI] [PubMed] [Google Scholar]
- 69.Bayer AJ, Chadha JS, Farag RR, Pathy MS. Changing presentation of myocardial infarction with increasing old age. J Am Geriatr Soc. 1986;34(4):263–266. doi: 10.1111/j.1532-5415.1986.tb04221.x. [DOI] [PubMed] [Google Scholar]
- 70.Lynch EP, Lazor MA, Gellis JE, Orav J, Goldman L, Marcantonio ER. The impact of postoperative pain on the development of postoperative delirium. Anesth Analg. 1998;86(4):781–785. doi: 10.1097/00000539-199804000-00019. [DOI] [PubMed] [Google Scholar]
- 71.Vaurio LE, Sands LP, Wang Y, Mullen EA, Leung JM. Postoperative delirium: the importance of pain and pain management. Anesth Analg. 2006;102(4):1267–1273. doi: 10.1213/01.ane.0000199156.59226.af. [DOI] [PubMed] [Google Scholar]
- 72.Morrison RS, Magaziner J, Gilbert M, et al. Relationship Between Pain and Opioid Analgesics on the Development of Delirium Following Hip Fracture. J Gerontol A Biol Sci Med Sci. 2003;58(1):M76–81. doi: 10.1093/gerona/58.1.m76. [DOI] [PubMed] [Google Scholar]
- 73.Caplan GA, Cassem NH, Murray GB. Delirium. In: Stern TA, editor. Massachusetts General Hospital comprehensive clinical psychiatry. 1. Philadelphia, PA: Mosby/Elsevier; 2008. p. xvii.p. 1273. [Google Scholar]
- 74.Fearing MA, Inouye SK. Delirium. In: Blazer DG, Steffens DC, editors. The American Psychiatric Publishing textbook of geriatric psychiatry. 4. Washington, DC: American Psychiatric Pub; 2009. [Google Scholar]
- 75.Clegg A, Young JB. Which medications to avoid in people at risk of delirium: a systematic review. Age Ageing. 2011;40(1):23–29. doi: 10.1093/ageing/afq140. [DOI] [PubMed] [Google Scholar]
- 76.Marcantonio ER, Juarez G, Goldman L, et al. The relationship of postoperative delirium with psychoactive medications. JAMA. 1994;272(19):1518–1522. [PubMed] [Google Scholar]
- 77.Adunsky A, Levy R, Heim M, Mizrahi E, Arad M. Meperidine analgesia and delirium in aged hip fracture patients. Arch Gerontol Geriatr. 2002;35(3):253–259. doi: 10.1016/s0167-4943(02)00045-6. [DOI] [PubMed] [Google Scholar]
- 78.Pandharipande P, Shintani A, Peterson J, et al. Lorazepam is an independent risk factor for transitioning to delirium in intensive care unit patients. Anesthesiology. 2006;104(1):21–26. doi: 10.1097/00000542-200601000-00005. [DOI] [PubMed] [Google Scholar]
- 79.Dubois MJ, Bergeron N, Dumont M, Dial S, Skrobik Y. Delirium in an intensive care unit: a study of risk factors. Intensive Care Med. 2001;27(8):1297–1304. doi: 10.1007/s001340101017. [DOI] [PubMed] [Google Scholar]
- 80.Tune LE. Anticholinergic effects of medication in elderly patients. J Clin Psychiatry. 2001;62 (Suppl 21):11–14. [PubMed] [Google Scholar]
- 81.Caeiro L, Ferro JM, Claro MI, Coelho J, Albuquerque R, Figueira ML. Delirium in acute stroke: a preliminary study of the role of anticholinergic medications. Eur J Neurol. 2004;11(10):699–704. doi: 10.1111/j.1468-1331.2004.00897.x. [DOI] [PubMed] [Google Scholar]
- 82.Han L, McCusker J, Cole M, Abrahamowicz M, Primeau F, Elie M. Use of Medications With Anticholinergic Effect Predicts Clinical Severity of Delirium Symptoms in Older Medical Inpatients. Archives of Internal Medicine. 2001;161(8):1099–1105. doi: 10.1001/archinte.161.8.1099. [DOI] [PubMed] [Google Scholar]
- 83.Agostini JV, Leo-Summers LS, Inouye SK. Cognitive and other adverse effects of diphenhydramine use in hospitalized older patients. Arch Intern Med. 2001;161(17):2091–2097. doi: 10.1001/archinte.161.17.2091. [DOI] [PubMed] [Google Scholar]
- 84.Luukkanen MJ, Uusvaara J, Laurila JV, et al. Anticholinergic Drugs and Their Effects on Delirium and Mortality in the Elderly. Dementia and Geriatric Cognitive Disorders Extra. 2011;1(1):43–50. doi: 10.1159/000322883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Campbell N, Perkins A, Hui S, Khan B, Boustani M. Association between prescribing of anticholinergic medications and incident delirium: a cohort study. J Am Geriatr Soc. 2011;59 (Suppl 2):S277–281. doi: 10.1111/j.1532-5415.2011.03676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60(4):616–631. doi: 10.1111/j.1532-5415.2012.03923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Alagiakrishnan K, Wiens CA. An approach to drug induced delirium in the elderly. Postgrad Med J. 2004;80(945):388–393. doi: 10.1136/pgmj.2003.017236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Teres D, Brown RB, Lemeshow S. Predicting mortality of intensive care unit patients. The importance of coma. Crit Care Med. 1982;10(2):86–95. doi: 10.1097/00003246-198202000-00004. [DOI] [PubMed] [Google Scholar]
- 89.Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet. 1975;1(7905):480–484. doi: 10.1016/s0140-6736(75)92830-5. [DOI] [PubMed] [Google Scholar]
- 90.Levy DE, Caronna JJ, Singer BH, Lapinski RH, Frydman H, Plum F. Predicting outcome from hypoxic-ischemic coma. JAMA. 1985;253(10):1420–1426. [PubMed] [Google Scholar]
- 91.Tuhrim S, Dambrosia JM, Price TR, et al. Prediction of intracerebral hemorrhage survival. Ann Neurol. 1988;24(2):258–263. doi: 10.1002/ana.410240213. [DOI] [PubMed] [Google Scholar]
- 92.Booth CM, Boone RH, Tomlinson G, Detsky AS. Is this patient dead, vegetative, or severely neurologically impaired? Assessing outcome for comatose survivors of cardiac arrest. JAMA. 2004;291(7):870–879. doi: 10.1001/jama.291.7.870. [DOI] [PubMed] [Google Scholar]
- 93.Witlox J, Eurelings LS, de Jonghe JF, Kalisvaart KJ, Eikelenboom P, van Gool WA. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta-analysis. JAMA. 2010;304(4):443–451. doi: 10.1001/jama.2010.1013. [DOI] [PubMed] [Google Scholar]
- 94.Fong TG, Jones RN, Shi P, et al. Delirium accelerates cognitive decline in Alzheimer disease. Neurology. 2009;72(18):1570–1575. doi: 10.1212/WNL.0b013e3181a4129a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gross AL, Jones RN, Habtemariam DA, et al. Delirium and long-term cognitive trajectory among persons with dementia. Archives of Internal Medicine. 2012:1–8. doi: 10.1001/archinternmed.2012.3203. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Inouye SK, Rushing JT, Foreman MD, Palmer RM, Pompei P. Does delirium contribute to poor hospital outcomes? A three-site epidemiologic study. J Gen Intern Med. 1998;13(4):234–242. doi: 10.1046/j.1525-1497.1998.00073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Leslie DL, Marcantonio ER, Zhang Y, Leo-Summers L, Inouye SK. One-year health care costs associated with delirium in the elderly population. Arch Intern Med. 2008;168(1):27–32. doi: 10.1001/archinternmed.2007.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gonzalez M, Martinez G, Calderon J, et al. Impact of delirium on short-term mortality in elderly inpatients: a prospective cohort study. Psychosomatics. 2009;50(3):234–238. doi: 10.1176/appi.psy.50.3.234. [DOI] [PubMed] [Google Scholar]
- 99.Grover S, Shah R. Distress due to delirium experience. Gen Hosp Psychiatry. 2011;33(6):637–639. doi: 10.1016/j.genhosppsych.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 100.Bruera E, Bush SH, Willey J, et al. Impact of delirium and recall on the level of distress in patients with advanced cancer and their family caregivers. Cancer. 2009;115(9):2004–2012. doi: 10.1002/cncr.24215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Han JH, Eden S, Shintani A, et al. Delirium in older emergency department patients is an independent predictor of hospital length of stay. Acad Emerg Med. 2011;18(5):451–457. doi: 10.1111/j.1553-2712.2011.01065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.O’Keeffe S, Lavan J. The prognostic significance of delirium in older hospital patients. J Am Geriatr Soc. 1997;45(2):174–178. doi: 10.1111/j.1532-5415.1997.tb04503.x. [DOI] [PubMed] [Google Scholar]
- 103.McCusker J, Cole M, Abrahamowicz M, Primeau F, Belzile E. Delirium predicts 12-month mortality. Arch Intern Med. 2002;162(4):457–463. doi: 10.1001/archinte.162.4.457. [DOI] [PubMed] [Google Scholar]
- 104.Manos PJ, Wu R. The duration of delirium in medical and postoperative patients referred for psychiatric consultation. Ann Clin Psychiatry. 1997;9(4):219–226. doi: 10.1023/a:1022300309496. [DOI] [PubMed] [Google Scholar]
- 105.Sirois F. Delirium: 100 cases. Can J Psychiatry. 1988;33(5):375–378. doi: 10.1177/070674378803300512. [DOI] [PubMed] [Google Scholar]
- 106.McCusker J, Cole M, Dendukuri N, Han L, Belzile E. The course of delirium in older medical inpatients: a prospective study. J Gen Intern Med. 2003;18(9):696–704. doi: 10.1046/j.1525-1497.2003.20602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Han JH, Shintani A, Eden S, et al. Delirium in the emergency department: an independent predictor of death within 6 months. Ann Emerg Med. 2010;56(3):244–252. doi: 10.1016/j.annemergmed.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Vida S, Galbaud du Fort G, Kakuma R, Arsenault L, Platt RW, Wolfson CM. An 18-month prospective cohort study of functional outcome of delirium in elderly patients: activities of daily living. Int Psychogeriatr. 2006;18(4):681–700. doi: 10.1017/S1041610206003310. [DOI] [PubMed] [Google Scholar]
- 109.Sanders AB. Missed delirium in older emergency department patients: a quality-of-care problem. Ann Emerg Med. 2002;39(3):338–341. doi: 10.1067/mem.2002.122273. [DOI] [PubMed] [Google Scholar]
- 110.Han JH, Bryce SN, Ely EW, et al. The effect of cognitive impairment on the accuracy of the presenting complaint and discharge instruction comprehension in older emergency department patients. Ann Emerg Med. 2011;57(6):662–671. e662. doi: 10.1016/j.annemergmed.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Reeves RR, Parker JD, Burke RS, Hart RH. Inappropriate psychiatric admission of elderly patients with unrecognized delirium. South Med J. 2010;103(2):111–115. doi: 10.1097/SMJ.0b013e3181c99423. [DOI] [PubMed] [Google Scholar]
- 112.Clarke C, Friedman SM, Shi K, Arenovich A, Culligan C. Emergency department discharge instructions comprehension and compliance study. CJEM. 2005;7(1):5–11. doi: 10.1017/s1481803500012860. [DOI] [PubMed] [Google Scholar]
- 113.Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2(7872):81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- 114.Sacco RL, VanGool R, Mohr JP, Hauser WA. Nontraumatic coma. Glasgow coma score and coma etiology as predictors of 2-week outcome. Arch Neurol. 1990;47(11):1181–1184. doi: 10.1001/archneur.1990.00530110035013. [DOI] [PubMed] [Google Scholar]
- 115.Bastos PG, Sun X, Wagner DP, Wu AW, Knaus WA. Glasgow Coma Scale score in the evaluation of outcome in the intensive care unit: findings from the Acute Physiology and Chronic Health Evaluation III study. Crit Care Med. 1993;21(10):1459–1465. doi: 10.1097/00003246-199310000-00012. [DOI] [PubMed] [Google Scholar]
- 116.Gill MR, Reiley DG, Green SM. Interrater reliability of Glasgow Coma Scale scores in the emergency department. Ann Emerg Med. 2004;43(2):215–223. doi: 10.1016/s0196-0644(03)00814-x. [DOI] [PubMed] [Google Scholar]
- 117.Wijdicks EF, Bamlet WR, Maramattom BV, Manno EM, McClelland RL. Validation of a new coma scale: The FOUR score. Ann Neurol. 2005;58(4):585–593. doi: 10.1002/ana.20611. [DOI] [PubMed] [Google Scholar]
- 118.McNarry AF, Goldhill DR. Simple bedside assessment of level of consciousness: comparison of two simple assessment scales with the Glasgow Coma scale. Anaesthesia. 2004;59(1):34–37. doi: 10.1111/j.1365-2044.2004.03526.x. [DOI] [PubMed] [Google Scholar]
- 119.Terrell KM, Hustey FM, Hwang U, Gerson LW, Wenger NS, Miller DK. Quality indicators for geriatric emergency care. Acad Emerg Med. 2009;16(5):441–449. doi: 10.1111/j.1553-2712.2009.00382.x. [DOI] [PubMed] [Google Scholar]
- 120.Hogan TM, Losman ED, Carpenter CR, et al. Development of geriatric competencies for emergency medicine residents using an expert consensus process. Acad Emerg Med. 2010;17(3):316–324. doi: 10.1111/j.1553-2712.2010.00684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12):941–948. doi: 10.7326/0003-4819-113-12-941. [DOI] [PubMed] [Google Scholar]
- 122.Wong CL, Holroyd-Leduc J, Simel DL, Straus SE. Does this patient have delirium?: value of bedside instruments. JAMA. 2010;304(7):779–786. doi: 10.1001/jama.2010.1182. [DOI] [PubMed] [Google Scholar]
- 123.Carpenter CR, DesPain B, Keeling TN, Shah M, Rothenberger M. The Six-Item Screener and AD8 for the detection of cognitive impairment in geriatric emergency department patients. Ann Emerg Med. 2011;57(6):653–661. doi: 10.1016/j.annemergmed.2010.06.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Blazer DG, van Nieuwenhuizen AO. Evidence for the diagnostic criteria of delirium: an update. Curr Opin Psychiatry. 2012;25(3):239–243. doi: 10.1097/YCO.0b013e3283523ce8. [DOI] [PubMed] [Google Scholar]
- 125.Inouye SK. Delirium in hospitalized older patients. Clin Geriatr Med. 1998;14(4):745–764. [PubMed] [Google Scholar]
- 126.Fabbri RM, Moreira MA, Garrido R, Almeida OP. Validity and reliability of the Portuguese version of the Confusion Assessment Method (CAM) for the detection of delirium in the elderly. Arq Neuropsiquiatr. 2001;59(2-A):175–179. doi: 10.1590/s0004-282x2001000200004. [DOI] [PubMed] [Google Scholar]
- 127.Inouye SK. The Confusion Assessment Method (CAM): Training Manual and Coding Guide. Yale University School of Medicine; 2009. [Google Scholar]
- 128.Young RS, Arseven A. Diagnosing delirium. JAMA. 2010;304(19):2125–2126. doi: 10.1001/jama.2010.1617. author reply 2126–2127. [DOI] [PubMed] [Google Scholar]
- 129.Wei LA, Fearing MA, Sternberg EJ, Inouye SK. The Confusion Assessment Method: a systematic review of current usage. J Am Geriatr Soc. 2008;56(5):823–830. doi: 10.1111/j.1532-5415.2008.01674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lemiengre J, Nelis T, Joosten E, et al. Detection of delirium by bedside nurses using the confusion assessment method. J Am Geriatr Soc. 2006;54(4):685–689. doi: 10.1111/j.1532-5415.2006.00667.x. [DOI] [PubMed] [Google Scholar]
- 131.Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU) JAMA. 2001;286(21):2703–2710. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 132.Neufeld KJ, Hayat MJ, Coughlin JM, et al. Evaluation of two intensive care delirium screening tools for non-critically ill hospitalized patients. Psychosomatics. 2011;52(2):133–140. doi: 10.1016/j.psym.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 133.Mitasova A, Kostalova M, Bednarik J, et al. Poststroke delirium incidence and outcomes: validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) Crit Care Med. 2012;40(2):484–490. doi: 10.1097/CCM.0b013e318232da12. [DOI] [PubMed] [Google Scholar]
- 134.Kean J, Trzepacz PT, Murray LL, Abell M, Trexler L. Initial validation of a brief provisional diagnostic scale for delirium. Brain Inj. 2010;24(10):1222–1230. doi: 10.3109/02699052.2010.498008. [DOI] [PubMed] [Google Scholar]
- 135.Chester JG, Beth Harrington M, Rudolph JL. Serial administration of a modified richmond agitation and sedation scale for delirium screening. J Hosp Med. 2011 doi: 10.1002/jhm.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zimitat C, Nixon PF. Glucose loading precipitates acute encephalopathy in thiamin-deficient rats. Metab Brain Dis. 1999;14(1):1–20. doi: 10.1023/a:1020653312697. [DOI] [PubMed] [Google Scholar]
- 137.Mazer M, Deroos F, Hollander JE, McCusker C, Peacock N, Perrone J. Medication history taking in emergency department triage is inaccurate and incomplete. Acad Emerg Med. 2011;18(1):102–104. doi: 10.1111/j.1553-2712.2010.00959.x. [DOI] [PubMed] [Google Scholar]
- 138.Staroselsky M, Volk LA, Tsurikova R, et al. An effort to improve electronic health record medication list accuracy between visits: patients’ and physicians’ response. Int J Med Inform. 2008;77(3):153–160. doi: 10.1016/j.ijmedinf.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 139.Finlayson RE, Davis LJ., Jr Prescription drug dependence in the elderly population: demographic and clinical features of 100 inpatients. Mayo Clin Proc. 1994;69(12):1137–1145. doi: 10.1016/s0025-6196(12)65764-4. [DOI] [PubMed] [Google Scholar]
- 140.Blazer DG, Wu LT. The epidemiology of substance use and disorders among middle aged and elderly community adults: national survey on drug use and health. Am J Geriatr Psychiatry. 2009;17(3):237–245. doi: 10.1097/JGP.0b013e318190b8ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Uguz F, Kayrak M, Cicek E, Kayhan F, Ari H, Altunbas G. Delirium following acute myocardial infarction: incidence, clinical profiles, and predictors. Perspect Psychiatr Care. 2010;46(2):135–142. doi: 10.1111/j.1744-6163.2010.00249.x. [DOI] [PubMed] [Google Scholar]
- 142.Warshaw G, Tanzer F. The effectiveness of lumbar puncture in the evaluation of delirium and fever in the hospitalized elderly. Arch Fam Med. 1993;2(3):293–297. doi: 10.1001/archfami.2.3.293. [DOI] [PubMed] [Google Scholar]
- 143.Metersky ML, Williams A, Rafanan AL. Retrospective analysis: are fever and altered mental status indications for lumbar puncture in a hospitalized patient who has not undergone neurosurgery? Clin Infect Dis. 1997;25(2):285–288. doi: 10.1086/514531. [DOI] [PubMed] [Google Scholar]
- 144.Moeller KE, Lee KC, Kissack JC. Urine drug screening: practical guide for clinicians. Mayo Clin Proc. 2008;83(1):66–76. doi: 10.4065/83.1.66. [DOI] [PubMed] [Google Scholar]
- 145.Hardy JE, Brennan N. Computerized tomography of the brain for elderly patients presenting to the emergency department with acute confusion. Emerg Med Australas. 2008;20(5):420–424. doi: 10.1111/j.1742-6723.2008.01118.x. [DOI] [PubMed] [Google Scholar]
- 146.Mesulam MM, Waxman SG, Geschwind N, Sabin TD. Acute confusional states with right middle cerebral artery infarctions. J Neurol Neurosurg Psychiatry. 1976;39(1):84–89. doi: 10.1136/jnnp.39.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ziai WC, Schlattman D, Llinas R, et al. Emergent EEG in the emergency department in patients with altered mental states. Clin Neurophysiol. 2012;123(5):910–917. doi: 10.1016/j.clinph.2011.07.053. [DOI] [PubMed] [Google Scholar]
- 148.Agarwal N, Nagananda MS, Rahman SM, Sengupta A, Santhosh J, Anand S. Portable cost-effective EEG data acquisition system. J Med Eng Technol. 2011;35(3–4):185–190. doi: 10.3109/03091902.2011.560701. [DOI] [PubMed] [Google Scholar]
- 149.Inouye SK, Bogardus ST, Jr, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340(9):669–676. doi: 10.1056/NEJM199903043400901. [DOI] [PubMed] [Google Scholar]
- 150.Flaherty JH, Little MO. Matching the environment to patients with delirium: lessons learned from the delirium room, a restraint-free environment for older hospitalized adults with delirium. J Am Geriatr Soc. 2011;59 (Suppl 2):S295–300. doi: 10.1111/j.1532-5415.2011.03678.x. [DOI] [PubMed] [Google Scholar]
- 151.Holroyd-Leduc JM, Sen S, Bertenthal D, et al. The relationship of indwelling urinary catheters to death, length of hospital stay, functional decline, and nursing home admission in hospitalized older medical patients. J Am Geriatr Soc. 2007;55(2):227–233. doi: 10.1111/j.1532-5415.2007.01064.x. [DOI] [PubMed] [Google Scholar]
- 152.National Institute of Heath and Clinical Excellence. [Accessed June 21, 2012.];Delirium: diagnosis, prevention, and management (Clinical guideline 103) 2010 http://publications.nice.org.uk/delirium-cg103.
- 153.Breitbart W, Marotta R, Platt MM, et al. A double-blind trial of haloperidol, chlorpromazine, and lorazepam in the treatment of delirium in hospitalized AIDS patients. Am J Psychiatry. 1996;153(2):231–237. doi: 10.1176/ajp.153.2.231. [DOI] [PubMed] [Google Scholar]
- 154.Flaherty JH, Gonzales JP, Dong B. Antipsychotics in the Treatment of Delirium in Older Hospitalized Adults: A Systematic Review. J Am Geriatr Soc. 2011;59:S269–S276. doi: 10.1111/j.1532-5415.2011.03675.x. [DOI] [PubMed] [Google Scholar]
- 155.Lonergan E, Britton AM, Luxenberg J, Wyller T. Antipsychotics for delirium. Cochrane Database Syst Rev. 2007;(2):CD005594. doi: 10.1002/14651858.CD005594.pub2. [DOI] [PubMed] [Google Scholar]
- 156.Hu H, Deng W, Yang H. A prospective random control study comparison of olanzapine and haloperidol in senile delirium. Chongging Medical Jounral. 2004;8:1234–1237. [Google Scholar]
- 157.Hassaballa HA, Balk RA. Torsade de pointes associated with the administration of intravenous haloperidol:a review of the literature and practical guidelines for use. Expert Opin Drug Saf. 2003;2(6):543–547. doi: 10.1517/14740338.2.6.543. [DOI] [PubMed] [Google Scholar]
- 158.Ozbolt LB, Paniagua MA, Kaiser RM. Atypical antipsychotics for the treatment of delirious elders. J Am Med Dir Assoc. 2008;9(1):18–28. doi: 10.1016/j.jamda.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 159.Breitbart W, Tremblay A, Gibson C. An open trial of olanzapine for the treatment of delirium in hospitalized cancer patients. Psychosomatics. 2002;43(3):175–182. doi: 10.1176/appi.psy.43.3.175. [DOI] [PubMed] [Google Scholar]
- 160.Tahir TA, Eeles E, Karapareddy V, et al. A randomized controlled trial of quetiapine versus placebo in the treatment of delirium. J Psychosom Res. 2010;69(5):485–490. doi: 10.1016/j.jpsychores.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 161.Ballard C, Kahn Z, Corbett A. Treatment of dementia with Lewy bodies and Parkinson’s disease dementia. Drugs Aging. 2011;28(10):769–777. doi: 10.2165/11594110-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 162.Komossa K, Rummel-Kluge C, Schmid F, et al. Quetiapine versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev. 2010;(1):CD006625. doi: 10.1002/14651858.CD006625.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Mayo-Smith MF, Beecher LH, Fischer TL, et al. Management of alcohol withdrawal delirium. An evidence-based practice guideline. Arch Intern Med. 2004;164(13):1405–1412. doi: 10.1001/archinte.164.13.1405. [DOI] [PubMed] [Google Scholar]
- 164.Tollefson GD, Montague-Clouse J, Lancaster SP. The relationship of serum anticholinergic activity to mental status performance in an elderly nursing home population. J Neuropsychiatry Clin Neurosci. 1991;3(3):314–319. doi: 10.1176/jnp.3.3.314. [DOI] [PubMed] [Google Scholar]
- 165.Catalano G, Catalano MC, Alberts VA. Famotidine-associated delirium. A series of six cases. Psychosomatics. 1996;37(4):349–355. doi: 10.1016/S0033-3182(96)71548-7. [DOI] [PubMed] [Google Scholar]
- 166.Naughton BJ, Saltzman S, Ramadan F, Chadha N, Priore R, Mylotte JM. A multifactorial intervention to reduce prevalence of delirium and shorten hospital length of stay. J Am Geriatr Soc. 2005;53(1):18–23. doi: 10.1111/j.1532-5415.2005.53005.x. [DOI] [PubMed] [Google Scholar]
- 167.Flaherty JH, Steele DK, Chibnall JT, Vasudevan VN, Bassil N, Vegi S. An ACE unit with a delirium room may improve function and equalize length of stay among older delirious medical inpatients. J Gerontol A Biol Sci Med Sci. 2010;65(12):1387–1392. doi: 10.1093/gerona/glq136. [DOI] [PubMed] [Google Scholar]
- 168.Lundstrom M, Edlund A, Karlsson S, Brannstrom B, Bucht G, Gustafson Y. A multifactorial intervention program reduces the duration of delirium, length of hospitalization, and mortality in delirious patients. J Am Geriatr Soc. 2005;53(4):622–628. doi: 10.1111/j.1532-5415.2005.53210.x. [DOI] [PubMed] [Google Scholar]
- 169.Milisen K, Lemiengre J, Braes T, Foreman MD. Multicomponent intervention strategies for managing delirium in hospitalized older people: systematic review. J Adv Nurs. 2005;52(1):79–90. doi: 10.1111/j.1365-2648.2005.03557.x. [DOI] [PubMed] [Google Scholar]
- 170.Pitkala KH, Laurila JV, Strandberg TE, Kautiainen H, Sintonen H, Tilvis RS. Multicomponent geriatric intervention for elderly inpatients with delirium: effects on costs and health-related quality of life. J Gerontol A Biol Sci Med Sci. 2008;63(1):56–61. doi: 10.1093/gerona/63.1.56. [DOI] [PubMed] [Google Scholar]
- 171.Pitkala KH, Laurila JV, Strandberg TE, Tilvis RS. Multicomponent geriatric intervention for elderly inpatients with delirium: a randomized, controlled trial. J Gerontol A Biol Sci Med Sci. 2006;61(2):176–181. doi: 10.1093/gerona/61.2.176. [DOI] [PubMed] [Google Scholar]
- 172.Vasilevskis EE, Morandi A, Boehm L, et al. Delirium and sedation recognition using validated instruments: reliability of bedside intensive care unit nursing assessments from 2007 to 2010. J Am Geriatr Soc. 2011;59 (Suppl 2):S249–255. doi: 10.1111/j.1532-5415.2011.03673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.O’Keeffe ST, Mulkerrin EC, Nayeem K, Varughese M, Pillay I. Use of Serial Mini-Mental State Examinations to Diagnose and Monitor Delirium in Elderly Hospital Patients. J Am Geriatr Soc. 2005;53(5):867–870. doi: 10.1111/j.1532-5415.2005.53266.x. [DOI] [PubMed] [Google Scholar]
- 174.Wilber ST, Carpenter CR, Hustey FM. The Six-Item Screener to detect cognitive impairment in older emergency department patients. Acad Emerg Med. 2008;15(7):613–616. doi: 10.1111/j.1553-2712.2008.00158.x. [DOI] [PubMed] [Google Scholar]
- 175.Wilber ST, Lofgren SD, Mager TG, Blanda M, Gerson LW. An evaluation of two screening tools for cognitive impairment in older emergency department patients. Acad Emerg Med. 2005;12(7):612–616. doi: 10.1197/j.aem.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 176.Carpenter CR, Bassett ER, Fischer GM, Shirshekan J, Galvin JE, Morris JC. Four sensitive screening tools to detect cognitive dysfunction in geriatric emergency department patients: brief Alzheimer’s Screen, Short Blessed Test, Ottawa 3DY, and the caregiver-completed AD8. Acad Emerg Med. 2011;18(4):374–384. doi: 10.1111/j.1553-2712.2011.01040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. [Accessed September 10, 2012.]; http://www.americandeliriumsociety.org/About_ADS.html.