Abstract
Direct brain control of a prosthetic system is the subject of much popular and scientific news. Neural technology and science have advanced to the point that proof-of-concept systems exist for cortically-controlled prostheses in rats, monkeys, and even humans. However, realizing the dream of making such technology available to everyone is still far off. Fortunately today there is great public and scientific interest in making this happen, but it will only occur when the functional benefits of such systems outweigh the risks. In this article, the authors briefly summarize the state of the art and then highlight many issues that will directly limit clinical translation, including system durability, system performance, and patient risk. Despite the challenges, scientists and clinicians are in the desirable position of having both public and fiscal support to begin addressing these issues directly. The ultimate challenge now is to determine definitively whether these prosthetic systems will become clinical reality or forever unrealized.
Keywords: brain-machine interface, brain-computer interface, motor, communication, prostheses, performance, risk
Recently there have been several reports of humans directly interfacing with computers and controlling a cursor or performing other simple prosthetic control tasks. This has led to public interest in the prospect of fulfilling the predictions of a future where direct man and machine communication will be commonplace. Although we have made tremendous advancements in the development of electronic systems known as BMIs or BCIs, we find ourselves at an important threshold of determining whether their clinical potential will be realized or not. With the public eye on this field, and significant fiscal support and encouragement, the field is now in a unique position to answer this question. In short, translation of human cortical prostheses will only result when their benefits clearly outweigh the risks. Here we discuss some of the obstacles that currently concern human cortical prostheses, which if unaddressed, may doom their realization.
Motor and Communication Prostheses
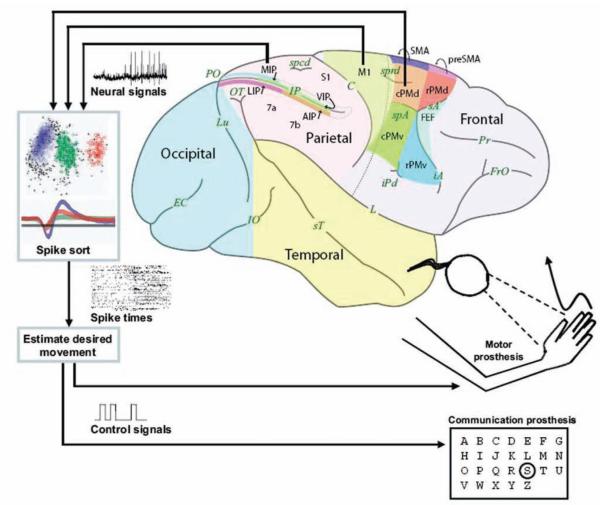
Existing BMIs strive to restore normal function in patients suffering from neurological impairments. Two successful examples include cochlear prostheses, which provide surrogate electrical signals to the nerves processing auditory stimuli, and DBS devices that alter activity in the motor circuits to disrupt movement gone awry. Emerging BMIs aim to help paralyzed patients, such as those with spinal cord injuries, who generally have intact cortical motor signals that are unable to arrive at end effectors such as the arm. Much research has been dedicated to studying the neural activity from various brain regions involved in movement, with the result that desired movements can now be estimated from cortical activity. Two types of prostheses, motor and communication, have taken root (Fig. 1). Both types are similar, but differ in their details. A motor prostheses taps into brain signals to continuously guide a paralyzed or prosthetic arm through space. This could be used to reach for objects or to paint a picture where the movement path and speed are important. A communication prosthesis again uses brain signals to guide a prosthesis, but in this case emphasizes just the desired endpoint without concern for the movement path or speed. Selecting keys on a keyboard or dialing a phone are examples of where how you move is not as important as what you accomplish. Both functions are important for paralyzed patients, but most authors have focused on one or the other type of prosthesis.
Fig. 1.
Concept sketch of cortical motor and communication prostheses. Neural signals are obtained from arrays of electrodes implanted in various possible cortical areas (PMd, M1, PRR, MIP). These are then processed and interpreted to generate control signals. These can be used to reconstruct an arm trajectory (motor prosthesis) or to select a target from a menu (communication prostheses). MIP = medial intraparietal area; PMd = dorsal premotor cortex; PMv = ventral premotor cortex;s PRR = parietal reach region; SMA = supplementary motor area. Figure reproduced from Shenoy et al., Increasing the performance of corticallycontrolled prostheses, in Proceedings of the 28th IEEE EMBS Annual International Conference. New York City, USA, Aug 30-Sept 3, 2006. IEEE, 2006, pp 6652–6655. Reproduced with permission, from IEEE, 2006. Copyright 2006, IEEE.
A Brief History of Cortical Prostheses
In the late 1960s, Fetz and collegues12–14 discovered that nonhuman primates could learn to regulate the firing rate of individual cortical neurons, which suggested their use to control a prosthesis.26 By the late 1990s, technological advances and a considerably better understanding of how cortical neurons contribute to limb movement15,49 sparked renewed interest in developing clinically viable systems. Isaacs and colleagues27 demonstrated that 2D and 3D hand location could be reconstructed from the activity of M1 neurons in rhesus monkeys, and others reported similar reconstructions from parietal cortex, dorsal premotor cortex, and M1.20,35,65
In 1999 Chapin and colleagues7 reported a study in trained rats that could move a lever to receive a reward through brain activity alone. In 2002, Serruya and colleagues51 demonstrated 2D cursor control by rhesus monkeys using M1 neurons recorded from an implanted electrode array. Targets on a computer screen could be hit within 1–2 seconds using brain control only. That same year, Schwartz’s group60 demonstrated 3D cursor control by rhesus monkeys using M1 neurons. Targets could be hit on 70–80% of trials within 1.5–2.0 seconds. This group has since gone on to demonstrate that nonhuman primates can use these 3D control signals to feed themselves with a robotic arm and also open and close a hand- like gripper.57,63 Carmena and colleagues6 demonstrated 2D cursor control and hand grasping force control with rhesus monkeys. These are examples of reconstructing arm trajectory as appropriate for motor prostheses.
Other groups have reasoned that if the desired target location can be estimated directly from neural plan activity, the cursor can be positioned immediately on the desired key (in communication prostheses). Recent reports suggest that there may be considerable performance benefit afforded by looking at the desired target and not the intermediate trajectory.20,38,46,53
The Human Cortical Prostheses Experience
In the late 1990s, Kennedy and colleagues30,31 demonstrated a communication prosthesi that used one or two neurons from the motor cortex of locked-in human patients with amyotrophic lateral sclerosis to move a cursor across a virtual keyboard to type out messages. These patients had glass cone electrodes implanted in the cortex that had been treated with patient-derived neurotrophic factors to encourage assimilation. Patients were able to slide cursors in 1D and 2D to type messages.
There are also several implementations of communication prostheses that enable target/menu selection by modulating EEG waves.5,22,36,50,66 These techniques are attractive for their low risk. Electrocorticography recording from grids placed during epilepsy surgery have been used as a potential source of signals for BCIs.34,48 Another group described recording from a 32-wire microwire array in patients undergoing subthalamic nucleus stimulation. Ensembles from the subthalamic and thalamic motor nuclei could be used to control grip strength.41
The first implanted cortical electrode array human prosthesis was reported by Kim and colleagues23,32 who, in collaboration with Cyberkinetics Neurotechnology Inc., demonstrated 2D cursor control using M1 recordings. Performance was similar to their previous results using the same electrode array technology in nonhuman primates.51 This work was additionally encouraging in that it demonstrated for the first time that studies in normal (uninjured) nonhuman primates could transfer to human patients with injuries.
Lost in Translation?
Taken together, these investigations provide compelling proof-of-concept demonstrations arguing that cortically controlled motor and communication prostheses are possible. Although none of these devices have demonstrated a level of performance likely to be considered adequate for widespread use in humans, it was high enough to motivate next-generation experiments and technological designs. Prostheses will be clinically viable when the anticipated quality of life improvement outweighs the potential risks. Only when prosthetic performance substantially surpasses what is possible with other techniques, and surgical risk is sufficiently mitigated, will invasive electrodebased cortical prostheses find more widespread use.
We believe that there are 3 general areas that must be addressed immediately: system durability, system performance, and patient risks. Otherwise, these will likely become serious obstacles to clinical translation, potentially serious enough to halt the progress of human cortical prostheses.
System Durability
A prosthetic system must be durable to be clinically viable. Durability implies that it can be used in everyday life and not just within the confines of a controlled laboratory environment. To do this, the prosthetic system must: 1) have a neural interface that will work consistently for as long as possible; 2) be robust enough to be able to adapt to changes in the population of neurons it is recording; and 3) physically tolerate any real-life conditions to which it may be subjected.
Neural Interface Issues
The brain interface is the point of contact between engineered and biological worlds. Electrode arrays are actively pursued as brain interfaces for the high quality electrical signals they provide, but they must be surgically implanted into the brain tissue. These fine needle-like arrays of electrodes can be fashioned from individual wires or on a micromachined substrate such as silicon (see the “Utah” array, Fig. 2). Many different electrodes have been successfully used for cortical recording; however, most of these were used for short spans of time, only several months of data collection. As more long-term implants are being used, we are beginning to understand the lifespan of the current technologies.
Fig. 2.
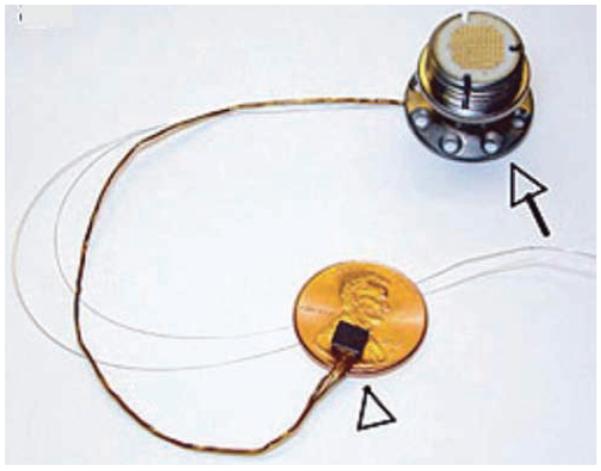
Photograph of the Utah electrode array (Blackrock Microsystems, Inc.), showing a silicon micromachined electrode array (arrowhead) with 96 needle-like electrodes. This is connected via a wire bundle to a connector port (arrow) that must be anchored to the subject. This port is then used to externally connect to each electrode. The thin silver wires are for grounding (reference) purposes. Reprinted by permission from from Macmillan Publishers Ltd: Nature,23 2006.
Some authors have reported obtaining good quality recordings from a few months to over 1 year from silicon electrode arrays in nonhuman primates.59 In our experience with more than 15 Utah electrode array implants in nonhuman primate frontal cortex, we achieved up to 3 years (with 1 outlier array), but much more typically only 6–12 months before signal quality on most electrodes diminished substantially. Over 2 years of recording was reported in 1 human trial participant with the same array technology.24 The cause of this limited lifespan is not well understood. Nonhuman primate pathology studies have shown reactivity and gliosis around implanted electrodes ranging from mild to concerning.16,42 We have noted fibroblastic tissue development encapsulating the explanted array in some animals. Comparison of implantation techniques, including a pneumatic array inserter (commonly used with Utah electrode arrays) and manual insertion (commonly used with microwire and Michigan arrays), has received some attention. In one study, mechanically inserted microwire arrays in rat auditory cortex functioned for 6 weeks while no manually inserted arrays did.43 To address these possible biological and mechanical issues limiting electrode lifetime, there is considerable development aimed at producing advanced arrays with novel materials, biocompatible coatings, and optimized geometries.33,52 Without more durable interfaces, cortical prosthetic systems will be severely impaired.
System Robustness
Even with initially high-quality electrode interfaces, we can probably safely assume that the number of electrodes with excellent signal quality will degrade over time. Prosthetic systems are being designed to counter or otherwise contend with this signal loss, else system performance will also degrade. Another issue regards dynamic physical changes at the neural interface itself. The arrays are typically rigid structures that are grossly fixed in the brain. However, because the brain deforms, there is micromotion between the electrode tip and brain tissue.45 This gives rise to variations in the recordings that may be the result of tissue volume changes or abrupt shifts associated with tissue acceleration/deceleration (such as a violent sneeze). We are currently characterizing these changes in recordings obtained 24 hours a day, 7 days a week from implanted arrays in nonhuman primates.8,9,45 This will provide the data needed to design adaptive systems capable of compensating for these effects. (Fig. 3)
Fig. 3.
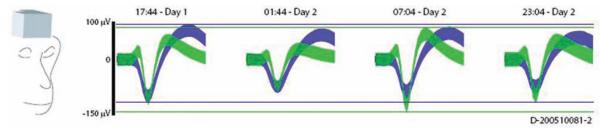
Diagram showing neural recordings obtained from an unrestrained nonhuman primate over a 48-hour period after implantation of a chronic electrode array. The picture illustrates a box of recording electronics that was mounted on the subject’s head. Spike waveforms from these recordings show that waveforms changed from day to day which could not be explained by fluctuations in the signal path, indicating that the neurons seen were different. Figure reproduced from Shenoy et al., Increasing the performance of cortically-controlled prostheses, in Proceedings of the 28th IEEE EMBS Annual International Conference. New York City, USA, Aug 30-Sept 3, 2006. IEEE, 2006, pp 6652–6655. Copyright 2006, IEEE.
Physical Durability
Much of the research done to date is with very delicate equipment inside a laboratory. A real prosthetic system must be physically durable. Most neurosurgeons have seen an implant migrate to an inprobable location and had to deal with traumatized implants. Clinical trials created NeuroPort (Blackrock Microsystems, Inc. ), a reasonably durable system, for human use, but it proved too bulky and unwieldy for practical use. Other current systems are large devices that must be miniaturized for any practical use, but this brings up new considerations that have not been addressed. Drawing from other examples, DBS devices and cardiac pacers rely on electronics and power supplies remote to the electrode, necessitating hardy but nevertheless accident-prone wires as well as a need for routine replacement. Permanent linkages for power supplies for cochlear and phrenic nerve implants can be difficult to maintain. Programmable shunt valves are placed in strategic locations to allow for interrogation and adjustment. Finally, the unexpected should be expected in patients who inadvertently fall, trip, and otherwise push the limits of manufacturing tolerances.
System Performance
The performance of prostheses is a hot topic currently because it is difficult to quantify. Performance is measured by how well the motor prosthesis replicates a movement, while performance is measured in communication prostheses by how well information can be transmitted; there is no standard performance metric, however. Motor prosthetics can be quantified by how closely they replicate true movements. In addition to the path taken, they must be quantified in terms of how quickly they move. Communication prosthetics could be quantified by a commonly used information transfer metric known as bps. It also turns out that goal-oriented motor prosthetics (those which are essentially selecting targets) can also be roughly quantified with bps. Most current reported motor prosthetics operate roughly in the 1.0–1.5 bps range (Table 1). Quantification is vital because current noninvasive EEG-based BCIs operate in a similar range, but these systems incur little clinical risk. Though it is generally agreed that the invasive electrodes provide better neural signals and from a smaller group of neurons, it is not settled that such signals are superior. Improved performance must be objectively demonstrated.
TABLE 1.
Comparison of roughly normalized performance of systems performing target selection tasks*
| Performer | Bps | Words Per Minute |
|---|---|---|
| human typist (typical) | 10 | 25 |
| human typist (professional) | 25+ | 60+ |
| EEG/ECoG prostheses | 0.5–1.3 | 1.2–3.1 |
| electrode-based prostheses (early) | 1.0–1.5 | 2.5–3.8 |
| electrode-based prostheses (fastest reported) | 6.5 | 16 |
| electrode-based prostheses (current theoretical) | 10 | 25 |
As mentioned in the text, the determination of these numbers is by no means definitive because there are no standards; however, it is useful to look at these numbers to gauge desired performance. ECoG = electrocorticography.
Here we describe some recent research in pushing the performance limits of current prostheses. In addition, we discuss interesting optimizations that have developed from our research. Finally, we explore autonomous system design that may allow for “natural” operation of a prosthetic system.
Maximizing Performance
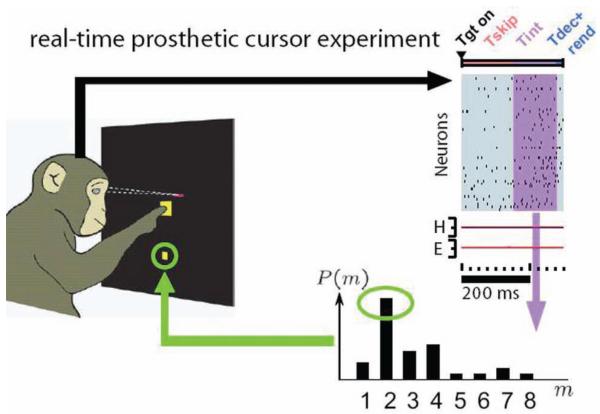
We have devoted much of our recent research effort to understanding the fundamental performance limits of electrode-based neural prostheses and to establishing a principled design methodology to develop prostheses that can operate at these limits.46 We conducted a series of experiments and computational simulations to investigate how quickly and accurately a communication prosthesis could be driven by cortical activity. We used signals from the dorsal premotor cortex (PMd), as those signals can be correlated with the endpoint of a reach or selection. The subject was trained to plan to reach for targets on the screen, which we could accurately and quickly determine based on neural activity. (Fig. 4) We optimized the algorithm and ultimately realized 6.5 bps performance – this is 4 times faster than previous prosthetic systems. This is equivalent to typing at a rate of ~16 words per minute. This level of performance is very encouraging as it helps supports both continuing prosthetic research and the potential of invasive electrode technology. We believe that even better performance can be achieved, based on recent biological and algorithmic insights.
Fig. 4.
Diagram showing an overview of our nonhuman primate high-performance communication prosthesis experiment. The real-time prosthetic cursor placement task begins by fixating and touching central targets, followed by the appearance of a peripheral target to which the subject plans (but does not execute) a reach. A period of neural data following this target onset (Tgt on) is set aside (Tskip). A period of neural data (Tint) is then analyzed to estimate the desired target; (P(m) refers to the target-m with the highest probability; here, m = 2), which could have appeared in one of 8 locations in this task, and after a brief computational decode and display rendering “overhead” period (Tdec+rend), the predicted target is encircled. Figure reproduced from Shenoy et al., Increasing the performance of cortically-controlled prostheses, in Proceedings of the 28th IEEE EMBS Annual International Conference. New York City, USA, Aug 30-Sept 3, 2006. IEEE, 2006, pp 6652–6655. Copyright 2006, IEEE.
Neural Optimizations
In the above study, we noted that our performance fell short by about 1.2 bps of our theoretically predicted performance. We discovered that this was due in part to response changes of the individual neurons. Firing rates became more or less intense depending on when in a sequence of trials the responses were measured. In other words, if this were a system used with human patients, depending on where in a word the subject was while typing a given letter, a neuron could respond slightly differently. We referred to this as neural response nonstationarity as opposed to having the same response for any specific condition. The underlying cause of this response modulation is not entirely clear, but presumably it is due in part to pushing the speed of planning in our prostheses experiments to some limit (that is, planning 3–4 reaches per second is perhaps close to the neurobiologically dictated limits). However, we can work around this by using mathematical techniques to infer these effects from the measured data, and then mitigate them. Our group introduced the use of factor analysis–based algorithms and was able to decrease errors by up to 75% when applied to data from our previous experiment.47
Optimal Target Placement
In the 1870s, Christopher Sholes developed the QWERTY keyboard to optimize for minimal mechanical dysfunction and maximum typing efficiency. Most keyboards or targets used in BCI research experiments have been created arbitrarily by the scientist—these layouts tended to be a circle of targets. However, both of these layouts are independent of the response properties of the particular neurons under observation and therefore could limit performance. Not surprisingly, any randomly selected population of neurons would have areas and directions to which they would respond better or worse. By adjusting the location of the targets to optimize for these spatial considerations, could we improve performance? We developed a method to optimally place targets to maximize the accuracy of the prosthesis. (Fig. 5) By just altering the location of the targets, we could increase performance accuracy by several percent.10 This has further utility as target layouts can be modified over the life of the array to adjust for neural population drift.
Fig. 5.
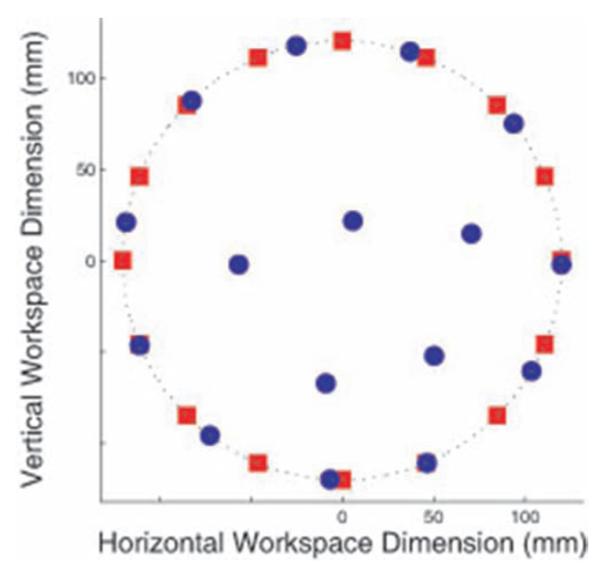
Sixteen target optimal target placement (OTP) example. Red squares are uniformly spaced targets around a circle, as an experimenter might use without OTP methods. Blue circles show an OTP solution. Figure from Cunningham et al., 200810 used with permission.
Autonomous System Design
An often overlooked performance issue has to do with system autonomy. We naturally spend time thinking of moving and not moving at all. An ideal prosthetic should be able to autonomously determine when an active prosthetic signal is present, or not, achieve “natural” function as opposed to having to turn the prostheses on and off. This can be done by determining the state of the brain—“idling,” “thinking of moving/planning to move,” and “executing a movement”—and activating the prostheses based on this information. (Fig. 6) Such a system would have to deal with plans that are not executed and long stretches of inactivity. We have developed hidden Markov model and maximum likelihood decoders to do just this.1,29 We have been able to determine the state of the brain accurately by observing the neural signals in various states. In this way, we created a mechanism by which to switch a system on and off automatically.
Fig. 6.
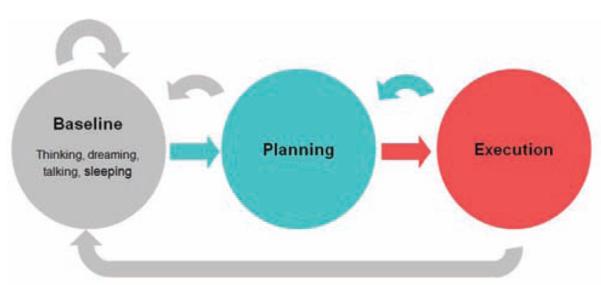
Illustration of brain states involved in autonomous reaching. We spend most of our time in the idle, or baseline state (gray). From here, we switch to a different state where we plan a movement (blue). The movement can then be executed which is another brain state (red). Once completed we can then plan another move (blue arrow) or return to the idling state (long gray arrow). One can also start planning and then abort the move as indicated by the gray short return arrow. Automating a prosthetic involves determining the unique neural characteristics of these states to determine when these transitions occur. Our early computational results suggest that this can be done. See the studies by Achtman et al., 2007, and Kemere et al., 2008.
Given these recent advances, we believe it possible to demonstrate, in future cortical prosthetic experiments, 10-bps performance (~ 25 words per minute). Performance quantification is a very important factor in assessing the value of the device, but is not the only critical factor to take into account. Other factors such as autonomous function must be considered. One additional exciting frontier is the ability of the patient to improve system performance—something that has been untestable until recently.
Patient Risks
A person’s well-being should never take second priority to the pursuit of interesting science. With increased electrode lifespan and durable devices, and as performance approaches acceptable functional restoration, we must seriously address patient risks. This discussion of risk is not about another factor in making BCI a reality, so much as a frank discussion regarding whether invasive BCIs are even worth pursuing as a practical solution.
It is difficult to quantify the risk of these proposed procedures. However, we can explore the potential issues for consideration. The risks involved with invasive BCIs fall into 2 categories, direct and indirect. The direct risks are related to the nature of the implant and any associated procedures. The indirect risks are related to the additional patient risk due to the lack of durability and performance of the system.
Surgical Risk
Surgeons have implanted foreign objects into various parts of the body with varying success for many decades. Neurosurgeons are familiar with the implantation of shunt tubing, vascular clips, pedicle screws, and neurostimulators. We can best estimate implanted cortical prosthetic systems risks based on our experience with subdural grid placement for epilepsy and DBS for movement disorders. Complications from subdural recording grids for subacute recording (up to 21 days) have been described. These include CSF leaks (in up to 31% of patients), hemorrhagic complications (in up to 14%), cerebral edema (in up to 14%), transient neurological deficits, and symptomatic pneumocephalus.2,4,28,39,56 A review of 179 DBS electrode implantations in 109 patients noted 16 serious adverse events related to surgery in 14 patients (12.8%). There were 2 perioperative deaths (1.8%). There were also pulmonary embolisms, subcortical hemorrhages, chronic subdural hematomas, venous infarction, seizure, infections, CSF leaks, and skin erosion. The incidence of permanent injury was 4.6%.11,62 Implanted cortical prosthetics will probably incur a similar morbidity profile which further emphasizes the need for a clear clinical benefit as is the case in DBS for movement disorders.
Infectious Complications
Infection is the greatest long-term concern for hardware implantation, and can range in severity from simple skin infections to deep infections of the bone and brain. Although treatable, these infections may require prolonged antibiotic therapy and system explantation. Another concern is the ability of infection to track down hardware, resulting in brain infections which could directly damage the viable cortex. Infection risk can never be completely eliminated. Fortunately, we can learn how to mitigate such risks from the research in similar systems.
Subdural electrode grids are short-term implants tunneled out of the scalp. These have been associated with an infection incidence of up to 8.7%, including wound infections (2.5%), meningitis (2.5%), osteomyelitis (0.8%), epdidural abscess, and empyema.28 Deep brain stimulators, in contrast, are completely internal systems that are implanted for the long term. In published reports of hardware-related infections, the incidence ranges from 4.5 to 6.1%.18,55 Although these infections are treatable, many of the infected patients required complete explantation of the system, resulting in loss of benefit.
Until a completely implantable device is created, prosthetic systems may rely on a skull-mounted interface port (such as the NeuroPort); this skin interface is of concern for infection. There is another device called the bone-anchored hearing aid which requires attachment of a metal post to the skull behind the ear, and is similar to such a port. The authors of a long-term review of 165 patients with these implants noted a 21% incidence of local skin reactions, 18% loosening of the hardware, 8% severe reactions requiring treatment, and 1% skin necrosis. Overall 34% of the patients required revision surgery.3
Implant-Related Risks
Implanted hardware must be durable, and there are practical considerations regarding wires and power as mentioned above. Clearly a small, fully implantable, self-powered device is ideal to mitigate the risk of infection (Fig. 7). However, direct hardware complications have been reported for DBS devices, despite the small electrode and pacer package. A review of the literature identified a 5.1% incidence of electrode migration, 5.0% lead fracture, and 1.3% skin erosion.18,62
Fig. 7.
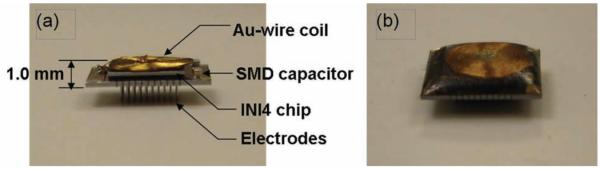
A working fully implantable miniaturized implant that integrates an electrode array with amplification and telemetric circuits; shown in profile (a) and after being encased in polymer (b). Such a small implant would be necessary for long-term chronic recordings as well as to contribute to overall durability and feasibility of the system. Figure reproduced from Harrison et al., A wireless neural interface for chronic recording, in Biomedical Circuits and Systems Conference, 2008. IEEE, 2008, pp 125–128. Copyright 2008, IEEE.
Implantation of an electrode array into the brain tissue is not without risks. The probes must be forced through the pia mater into tissues of the brain. The authors of an initial study of the histological impact of electrode arrays implanted for 3–28 days in resected epileptogenic tissue noted no tissue injury or inflammatory response.64 However, they did note suboptimal wound closure because of the array connector pedestal profile. The authors of another study investigated the results of mechanical implantation of silicon electrode arrays in the human brain using a commercially available array impactor, and noted mild cortical deformity and small focal hemorrhages several millimeters below the electrode tines, motivating the design of human-specific array impactors.25
Although it is difficult to implant an array into nonhuman primate cortex, the greatly expanded real estate of the human brain, and the ability to access areas not easily studied in primates (such as the intrasulcal areas) may present new and as yet unencountered risks.
Long-Term Neural Tissue Injury
Another consideration is long-term damage to brain tissues caused by implanted devices. This concern has been reported in the DBS literature. Although transient MR imaging signal changes have been noted within 3 months of electrode placement,44 most studies have indicated that the neural tissue exhibits few or no permanent tissue changes.16,21 The most significant findings relate to mild gliosis and limited foreign body giant cell reactions.17,37,58 The human brain seems to tolerate the large polyurethane-coated DBS implants well, and hopefully, this will carry over to the arrays.
Indirect Risks
Indirect risks are related to the previously described issues of durability and performance. An ideal system will run forever, delivering complete functional restoration. The reality is that these devices will need upkeep, replacement, and possible upgrading. Would a patient want a device that would last a year with perfect functional replacement? Or would a 5-year implant with good-enough replacement suffice? Different patient populations are likely to have different need considerations. A patient with terminal neurodystrophy would probably not mind a short device lifespan, but this would be unacceptable to a young patient rendered a high tetraplegic in a motor vehicle accident. The risk-to-benefit ratio will also differ between the early human studies (high risk with little benefit) and commercial production (low risk with high benefit). Such tradeoffs are important to consider in the overall system design.
Ethical Considerations
Human cortical prosthetic trials must adequately address the ethical issues of the nature of the proposed research and of informed consent. Clinical research by definition formally investigates a clinical intervention involving humans to yield scientific knowledge that may not benefit the subject. In fact, the risks may be suspected, and could harm the patient. As such, physicians must actively balance beneficence, our duty to help our patients, and nonmaleficence, our duty to do no harm when creating research protocols. If harm to the patient is clear, such as implants that lead to destruction of normal cortex, the research cannot be undertaken. If implant infection results exceed expectations, ongoing research must be stopped.
The use of unwilling research subjects is ethically and legally unacceptable today. Informed consent, voluntary participation by a subject after a clear dialogue regarding the nature of the intervention, the risks and benefits, and any alternatives, is mandatory. Research is clearly different from treatment, and physicians must understand this distinction. There can be no confusion, no coercion, no unrealistic expectations, and no promise of safety. Many patients and investigators may be enamored with the future potential of cortical prosthetics, but currently there is very little that is attractive to the average paralyzed patient.
We have endeavored to point out the limitations to the research and advancement to date to help put in perspective the expectations and risks associated with human cortical research. Performance results in animal models ethically justify the pursuit of human trials. A discussion of possible risks satisfies the requirement for informed consent. Any research that could severely damage normal brain tissue would also be unethical until compelling evidence of benefit is accumulated. We can also imagine more complex situations of patients desperate for any intervention and willing to ignore the risks, or of other patients who may benefit but are neurologically incapacitated (such as locked-in syndrome), thus complicating informed consent. Fortunately there is growing interest in neuroethics, and because of this, in conjunction with institutional review boards, any human trials will be closely monitored for ethical and legal compliance and more light will be shed on these very important topics.
Regulatory Considerations
A barrier to entry for many human studies relates to the regulatory hurdles required to translate research into clinical application. Although laboratory research is heavily overseen, especially at the institutional level, human experimentation is even more tightly regulated at the national level. The FDA requires submission and approval of almost any investigational devices, as well as for the slightest changes in protocol. Fortunately, sufficient prior work has been done with their approval, making translational experiments in cortical prostheses quite possible today.
In the late 1990s, Kennedy and colleagues received FDA approval to develop and place glass electrodes into the cortex of impaired patients. In the 2000s, Cyberkinetics Neurotechnology, Inc. obtained 510(k) regulatory approval for implantation of an electrode array and signal processing system (NeuroPort System, Blackrock Microsystems, Inc.), which is still available for use in acute inpatient applications of < 30 days’ duration. This approval led to the FDA approval of the BrainGate trial, a Phase I FDA study approved in 2004 to explore the feasibility and safety of long-term human cortical recording. Several patients were recruited, and the first pilot clinical trial of cortical prostheses in human patients was performed.23,32,61 These studies set the groundwork for a favorable regulatory environment to pursue more translational research.
Additionally, the NIH and other funding sources are creating a favorable funding environment to pursue more translational research. The NIH Neural Prosthesis Program was initiated in the 1970s to support basic, translational, and clinical neuroengineering projects. Today, because of all the recent results and the therapeutic potential of the technology, several NIH institutes are involved in specifically encouraging and funding translational and clinical pilot studies with the express desire of developing a clinically useful neural prosthetic.40
Conclusions
We are currently at an exciting and vital crossroads in the field of human cortical prostheses. The promise of this technology has captured the interest of the public and scientific community. It is imperative to capitalize on this support to make these prostheses a reality lest they be relegated to becoming another scientific curiosity. Clearly there have been many exciting advances to put us on the threshold of developing a viable clinical human prosthetic, but much needs to be done in collaboration with members of many disciplines. The neurosurgeon is in a unique position to become involved in these endeavors, providing insight into the overall impact on patients, and becoming the ultimate provider of therapy. We cannot assume that cortical prostheses are an eventuality. We have been provided a precious opportunity to determine whether these systems will become a reality, and this opportunity must not be squandered.
Acknowledgments
Disclosure
Support from the Christopher and Dana Reeve Foundation was given to Dr. Ryu. Dr. Shenoy received support from the Buroughs Wellcome Fund Career Award in the Biomedical Sciences, Center for Integrated Systems at Stanford, Christopher and Dana Reeve Foundation, McKnight Foundation, NIH-NINDS, and the Weston Havens Foundation.
Abbreviations used in this paper
- BCI
brain-computer interface
- BMI
brain-machine interface
- bps
bits per second
- DBS
deep brain stimulating
- EEG
electroencephalography
- M1
primary motor cortex
- NIH
National Institutes of Health
References
- 1.Achtman N, Afshar A, Santhanam G, Yu B, Ryu SI, Shenoy KV. Free-paced high performance brain-computer interfaces. J Neural Eng. 2007;4:336–347. doi: 10.1088/1741-2560/4/3/018. [DOI] [PubMed] [Google Scholar]
- 2.Adelson PD, Black PM, Madsen JR, Kramer U, Rockoff MA, Riviello JJ, et al. Use of subdural grids and strip electrodes to identify a seizure focus in children. Pediatr Neurosurg. 1995;22:174–180. doi: 10.1159/000120898. [DOI] [PubMed] [Google Scholar]
- 3.Badran K, Arya AK, Bunstone D, Mackinnon N. Long-term complications of bone-anchored hearing aids: a 14-year experience. J Laryngol Otol. 2009;123:170–176. doi: 10.1017/S0022215108002521. [DOI] [PubMed] [Google Scholar]
- 4.Bauman JA, Feoli E, Romanelli P, Doyle WK, Devinsky O, Weiner HL. Multistage epilepsy surgery: safety, efficacy, and utility of a novel approach in pediatric extratemporal epilepsy. Neurosurgery. 2005;56:318–334. doi: 10.1227/01.neu.0000148908.71296.fa. [DOI] [PubMed] [Google Scholar]
- 5.Birbaumer N, Ghanayim N, Hinterberger T, Iversen I, Kotchoubey B, Kubler A, et al. A spelling device for the paralysed. Nature. 1999;398:297–298. doi: 10.1038/18581. [DOI] [PubMed] [Google Scholar]
- 6.Carmena JM, Lebedev MA, Crist RE, O’Doherty JE, Santucci DM, Dimitrov DF, et al. Learning to control a brain-machine interface for reaching and grasping by primates. PLoS Biol. 2003;1:193–208. doi: 10.1371/journal.pbio.0000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chapin JK, Moxon KA, Markowitz RS, Nicolelis MAL. Realtime control of a robot arm using simultaneously recorded neurons in the motor cortex. Nat Neurosci. 1999;2:664–670. doi: 10.1038/10223. [DOI] [PubMed] [Google Scholar]
- 8.Chestek CA, Gilja V, Nuyujukian P, Kier R, Solzbacher F, Ryu SI, et al. Hermes C: Low-power wireless neural recording system for freely moving primates. IEEE Trans Neural Syst Rehabil Eng. 2009 doi: 10.1109/TNSRE.2009.2023293. [in press] [DOI] [PubMed] [Google Scholar]
- 9.Chestek CA, Gilja V, Nuyujukian P, Ryu SI, Kier RJ, Solzbacher F, et al. HermesC: RF wireless low-power neural recording system for freely behaving primates. Proc IEEE ISCAS. 2008:1752–1755. [Google Scholar]
- 10.Cunningham JP, Yu BM, Gilja V, Ryu SI, Shenoy KV. Toward optimal target placement for neural prosthetic devices. J Neurophysiol. 2008;100:3445–3457. doi: 10.1152/jn.90833.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deogaonkar A, Avitsian R, Henderson JM, Schubert A. Venous air embolism during deep brain stimulation surgery in an awake supine patient. Stereotact Funct Neurosurg. 2005;83:32–35. doi: 10.1159/000085024. [DOI] [PubMed] [Google Scholar]
- 12.Fetz EE. Operant conditioning of cortical unit activity. Science. 1969;163:955–957. doi: 10.1126/science.163.3870.955. [DOI] [PubMed] [Google Scholar]
- 13.Fetz EE, Baker MA. Operantly conditioned patterns of precentral unit activity and correlated responses in adjacent cells and contralateral muscles. J Neurophysiol. 1972;36:179–204. doi: 10.1152/jn.1973.36.2.179. [DOI] [PubMed] [Google Scholar]
- 14.Fetz EE, Finocchio DV. Operant conditioning of specific patterns of neural and muscular activity. Science. 1971;174:431–435. doi: 10.1126/science.174.4007.431. [DOI] [PubMed] [Google Scholar]
- 15.Georgopoulos AP, Schwartz AB. Kettner: Neuronal population coding of movement direction. Science. 1986;233:1416–1419. doi: 10.1126/science.3749885. [DOI] [PubMed] [Google Scholar]
- 16.Griffith RW, Humphrey DR. Long-term gliosis around chronically implanted platinum electrodes in the Rhesus macaque motor cortex. Neurosci Lett. 2006;406:81–86. doi: 10.1016/j.neulet.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 17.Haberler C, Alesch F, Mazal PR, Pilz P, Jellinger K, Pinter MM, et al. No tissue damage by chronic deep brain stimulation in Parkinson’s disease. Ann Neurol. 2000;48:372–376. [PubMed] [Google Scholar]
- 18.Hamani C, Lozano AM. Hardware-related complications of deep brain stimulation: a review of the published literature. Stereotact Funct Neurosurg. 2006;84:248–251. doi: 10.1159/000096499. [DOI] [PubMed] [Google Scholar]
- 19.Harrison RR, Kier RJ, Chestek CA, Gilja V, Nuyujukian P, Kim S, et al. A wireless neural interface for chronic recording. Biomedical Circuits and Systems Conference, 2008; IEEE; 2008. pp. 125–128. [Google Scholar]
- 20.Hatsopoulos N, Joshi J, O’Leary JG. Decoding continuous and discrete motor behaviors using motor and premotor cortical ensembles. J Neurophysiol. 2004;92:1165–1174. doi: 10.1152/jn.01245.2003. [DOI] [PubMed] [Google Scholar]
- 21.Henderson JM, Pell M, O’Sullivan DJ, McCusker EA, Fung VS, Hedges P, et al. Postmortem analysis of bilateral subthalamic electrode implants in Parkinson’s disease. Mov Disord. 2002;17:133–137. doi: 10.1002/mds.1261. [DOI] [PubMed] [Google Scholar]
- 22.Hinterberger T, Schmidt S, Neumann N, Mellinger J, Blankertz B, Curio G, et al. Brain-computer communication and slow cortical potentials. IEEE Trans Biomed Eng. 2004;51:1011–1018. doi: 10.1109/TBME.2004.827067. [DOI] [PubMed] [Google Scholar]
- 23.Hochberg LR, Serruya MD, Friehs GM, Mukand JA, Saleh M, Caplan AH, et al. Neuronal ensemble control of prosthetic devices by a human with tetraplegia. Nature. 2006;442:164–171. doi: 10.1038/nature04970. [DOI] [PubMed] [Google Scholar]
- 24.Hochberg LR, Simeral JD, Kim SP. 2008 Abstract Viewer/Itinerary Planner. Society for Neuroscience; Washington DC: 2008. More than two years of intracortically based cursor control via a neural interface system (program no. 673.15) [Google Scholar]
- 25.House PA, MacDonald JD, Tresco PA, Normann RA. Acute microelectrode array implantation into human neocortex: preliminary technique and histological considerations. Neurosurg Focus. 2006;20(5):E4. doi: 10.3171/foc.2006.20.5.5. [DOI] [PubMed] [Google Scholar]
- 26.Humphrey DR, Schmidt EM, Thompson WD. Predicting measures of motor performance from multiple cortical spike trains. Science. 1970;170:758–762. doi: 10.1126/science.170.3959.758. [DOI] [PubMed] [Google Scholar]
- 27.Isaacs RE, Weber DJ, Schwartz AB. Work toward real-time control of a cortical neural prosthesis. IEEE Trans Rehabil Eng. 2000;8:196–198. doi: 10.1109/86.847814. [DOI] [PubMed] [Google Scholar]
- 28.Johnston JM, Jr, Mangano FT, Ojemann JG, Park TS, Trevathan E, Smyth MD. Complications of invasive subdural electrode monitoring at St. Louis Children’s Hospital, 1994–2005. J Neurosurg. 2006;105:343–347. doi: 10.3171/ped.2006.105.5.343. [DOI] [PubMed] [Google Scholar]
- 29.Kemere C, Santhanam G, Yu BM, Afshar A, Ryu SI, Meng TH, et al. Detecting neural state transitions using hidden markov models for motor cortical prostheses. J Neurophysiol. 2008;100:2441–2452. doi: 10.1152/jn.00924.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kennedy PR, Bakay RAE. Restoration of neural output from a paralyzed patient by a direct brain connection. Neuroreport. 1998;9:1707–1711. doi: 10.1097/00001756-199806010-00007. [DOI] [PubMed] [Google Scholar]
- 31.Kennedy PR, Bakay RAE, Moore MM, Adams K, Goldwaithe J. Direct control of a computer from the human central nervous system. IEEE Trans Rehabil Eng. 2000;8:198–202. doi: 10.1109/86.847815. [DOI] [PubMed] [Google Scholar]
- 32.Kim SP, Simeral JD, Hochberg LR, Donoghue JP, Black MJ. Neural control of computer cursor velocity by decoding motor cortical spiking activity in humans with tetraplegia. J Neural Eng. 2008;5:455–476. doi: 10.1088/1741-2560/5/4/010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kipke DR, Shain W, Buzsaki G, Fetz E, Henderson JM, Hetke JF, et al. Advanced neurotechnologies for chronic neural interfaces: new horizons and clinical opportunities. J Neurosci. 2008;28:11830–11838. doi: 10.1523/JNEUROSCI.3879-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leuthardt EC, Schalk G, Wolpaw JW, Ojemann JG, Moran DW. A brain computer interface using electrocorticographic signals in humans. J Neural Eng. 2004;1:63–71. doi: 10.1088/1741-2560/1/2/001. [DOI] [PubMed] [Google Scholar]
- 35.Maynard EM, Hatsopoulos NG, Ojakangas CL, Acuna BD, Sanes JN, Normann RA, et al. Neuronal interactions improve cortical population coding of movement direction. J Neurosci. 1999;19:8083–8093. doi: 10.1523/JNEUROSCI.19-18-08083.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McFarland DJ, Sarnacki WA, Wolpaw JR. Brain-computer interface (BCI) operation: optimizing information transfer rates. Biol Psychol. 2003;63:237–251. doi: 10.1016/s0301-0511(03)00073-5. [DOI] [PubMed] [Google Scholar]
- 37.Moss J, Ryder T, Aziz TZ, Graeber MB, Bain PG. Electron microscopy of tissue adherent to explanted electrodes in dystonia and Parkinson’s disease. Brain. 2004;127:2755–2763. doi: 10.1093/brain/awh292. [DOI] [PubMed] [Google Scholar]
- 38.Musallam S, Corneil BD, Greger B, Scherberger H, Andersen RA. Cognitive control signals for neural prosthetics. Science. 2004;305:258–262. doi: 10.1126/science.1097938. [DOI] [PubMed] [Google Scholar]
- 39.Önal C, Otsubo H, Araki T, Chitoku S, Ochi A, Weiss S, et al. Complications of invasive subdural grid monitoring in children with epilepsy. J Neurosurg. 2003;98:1017–1026. doi: 10.3171/jns.2003.98.5.1017. [DOI] [PubMed] [Google Scholar]
- 40.Pancrazio JJ, Peckham PH. Neuroprosthetic device: how far are we from recovering movement in paralyzed patients? Expert Rev Neurother. 2009;9 doi: 10.1586/ern.09.12. [in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patil PG, Carmena JM, Nicolelis MA, Turner DA. Ensemble recordings of human subcortical neurons as a source of motor control signals for a brain-machine interface. Neurosurgery. 2004;55:27–35. [PubMed] [Google Scholar]
- 42.Polikov VS, Tresco PA, Reichert WM. Response of brain tissue to chronically implanted neural electrodes. J Neurosci Methods. 2005;148:1–18. doi: 10.1016/j.jneumeth.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 43.Rennaker RL, Street S, Ruyle AM, Sloan AM. A comparison of chronic multi-channel cortical implantation techniques: manual versus mechanical insertion. J Neurosci Methods. 2005;142:169–176. doi: 10.1016/j.jneumeth.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 44.Ryu SI, Romanelli P, Heit G. Asymptomatic transient MRI signal changes after unilateral deep brain stimulation electrode implantation for movement disorder. Stereotact Funct Neurosurg. 2004;82:65–69. doi: 10.1159/000077402. [DOI] [PubMed] [Google Scholar]
- 45.Santhanam G, Linderman MD, Gilja V, Afshar A, Ryu SI, Meng TH, et al. A continuous neural recording system for freely behaving primates. IEEE Trans Biomed Eng. 2007;54:2037–2050. doi: 10.1109/TBME.2007.895753. [DOI] [PubMed] [Google Scholar]
- 46.Santhanam G, Ryu SI, Yu BM, Afshar A, Shenoy KV. A highperformance brain-computer interface. Nature. 2006;442:195–198. doi: 10.1038/nature04968. [DOI] [PubMed] [Google Scholar]
- 47.Santhanam G, Yu BM, Gilja V, Afshar A, Ryu SI, Sahani M, et al. Factor-analysis methods for higher-performance neural prostheses. J Neurophysiol. 2009 doi: 10.1152/jn.00097.2009. [in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schalk G, Miller KJ, Anderson NR, Wilson JA, Smyth MD, Ojemann JG, et al. Two-dimensional movement control using electrocorticographic signals in humans. J Neural Eng. 2008;5:75–84. doi: 10.1088/1741-2560/5/1/008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schwartz AB. Direct cortical representation of drawing. Science. 1994;265:540–542. doi: 10.1126/science.8036499. [DOI] [PubMed] [Google Scholar]
- 50.Serby H, Yom-Tov E, Inbar GF. An improved p300-based brain-computer interface. IEEE Trans Neural Syst Rehabil Eng. 2005;13:89–98. doi: 10.1109/TNSRE.2004.841878. [DOI] [PubMed] [Google Scholar]
- 51.Serruya MD, Hatsopoulos NG, Paninski L, Fellows MR, Donoghue J. Instant neural control of a movement signal. Nature. 2002;416:141–142. doi: 10.1038/416141a. [DOI] [PubMed] [Google Scholar]
- 52.Seymour JP, Kipke DR. Neural probe design for reduced tissue encapsulation in CNS. Biomaterials. 2007;28:3594–3607. doi: 10.1016/j.biomaterials.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 53.Shenoy KV, Meeker D, Cao S, Kureshi SA, Pesaran B, Mitra P, et al. Neural prosthetic control signals from plan activity. Neuroreport. 2003;14:591–596. doi: 10.1097/00001756-200303240-00013. [DOI] [PubMed] [Google Scholar]
- 54.Shenoy KV, Santhanam G, Ryu SI, Afshar A, Yu BM, Gilja V, et al. Increasing the performance of cortically-controlled prostheses. Proceedings of the 28th IEEE EMBS Annual International Conference; New York City, USA. Aug 30-Sept 3, 2006; IEEE; 2006. pp. 6652–6655. [DOI] [PubMed] [Google Scholar]
- 55.Sillay KA, Larson PS, Starr PA. Deep brain stimulator hardware-related infections: incidence and management in a large series. Neurosurgery. 2008;62:360–366. doi: 10.1227/01.neu.0000316002.03765.33. [DOI] [PubMed] [Google Scholar]
- 56.Simon SL, Telfeian A, Duhaime AC. Complications of invasive monitoring used in intractable pediatric epilepsy. Pediatr Neurosurg. 2003;38:47–52. doi: 10.1159/000067555. [DOI] [PubMed] [Google Scholar]
- 57.Spalding MC, Velliste M, Jarosiewicz B, Schwartz A. 2005 Abstract Viewer/ Itinerary Planner. Society for Neuroscience; Washington, DC: 2005. 3-D cortical control of an anthropomorphic robotic arm for reaching and retrieving (program no. 401.3) [Google Scholar]
- 58.Sun DA, Yu H, Spooner J, Tatsas AD, Davis T, Abel TW, et al. Postmortem analysis following 71 months of deep brain stimulation of the subthalamic nucleus for Parkinson disease. J Neurosurg. 2008;109:325–329. doi: 10.3171/JNS/2008/109/8/0325. [DOI] [PubMed] [Google Scholar]
- 59.Suner S, Fellows MR, Vargas-Irwin C, Nakata GK, Donoghue JP. Reliability of signals from a chronically implanted, silicon-based electrode array in non-human primate primary motor cortex. IEEE Trans Neural Syst Rehabil Eng. 2005;13:524–541. doi: 10.1109/TNSRE.2005.857687. [DOI] [PubMed] [Google Scholar]
- 60.Taylor DM, Tillery SIH, Schwartz AB. Direct cortical control of 3D neuroprosthetic devices. Science. 2002;296:1829–1832. doi: 10.1126/science.1070291. [DOI] [PubMed] [Google Scholar]
- 61.Truccolo W, Friehs GM, Donoghue JP, Hochberg LR. Primary motor cortex tuning to intended movement kinematics in humans with tetraplegia. J Neurosci. 2008;28:1163–1178. doi: 10.1523/JNEUROSCI.4415-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Umemura A, Jaggi JL, Hurtig HI, Siderowf AD, Colcher A, Stern MB, et al. Deep brain stimulation for movement disorders: morbidity and mortality in 109 patients. J Neurosurg. 2003;98:779–784. doi: 10.3171/jns.2003.98.4.0779. [DOI] [PubMed] [Google Scholar]
- 63.Velliste M, Perel S, Spalding MC, Whitford AS, Schwartz AB. Cortical control of a prosthetic arm for self-feeding. Nature. 2008;453:1098–1101. doi: 10.1038/nature06996. [DOI] [PubMed] [Google Scholar]
- 64.Waziri A, Schevon CA, Cappell J, Emerson RG, McKhann GM, II, Goodman RR. Initial surgical experience with a dense cortical microarray in epileptic patients undergoing craniotomy for subdural electrode implantation. Neurosurgery. 2009;64:540–545. doi: 10.1227/01.NEU.0000337575.63861.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wessberg J, Stambaugh CR, Kralik JD, Beck PD, Laubach M, Chapin JK, et al. Real-time prediction of hand trajectory by ensembles of cortical neurons in primates. Nature. 2000;408:361–365. doi: 10.1038/35042582. [DOI] [PubMed] [Google Scholar]
- 66.Wolpaw JR, McFarland D. Control of a two-dimensional movement signal by a noninvasive brain-computer interface in humans. Proc Natl Acad Sci U S A. 2004;101:17849–17854. doi: 10.1073/pnas.0403504101. [DOI] [PMC free article] [PubMed] [Google Scholar]