Abstract
Objective
In mouse, PGC1-α overexpression in muscle stimulates an increase in expression of FNDC5, a membrane protein that is cleaved and secreted as a newly identified hormone, irisin. One prior study has shown that FNDC5 induces browning of subcutaneous fat in mice and mediates beneficial effects of exercise on metabolism, but a more recent study using gene expression arrays failed to detect a robust increase in FNDC5 mRNA in human muscles from exercising subjects. No prior study has reported on the physiological regulation and role of circulating irisin and FNDC5 in humans.
Materials/Methods
A. FNDC5 gene expression studies: We first examined tissue distribution of FNDC5 in humans. B. Cross-sectional studies: Predictors of FNDC5 mRNA expression levels were examined in muscle tissues from 18 healthy subjects with a wide range of BMI. Assays were optimized to measure circulating FNDC5 and irisin levels, and their associations with anthropometric and metabolic parameters were analyzed in two cross-sectional studies that examined 117 middle-aged healthy women and 14 obese subjects, respectively. C. Interventional studies: The effect of weight loss on FNDC5 mRNA and/or circulating irisin levels was examined in 14 obese subjects before and after bariatric surgery. The effect of acute and chronic exercise was then assessed in 15 young healthy adults who performed intermittent sprint running sessions over an 8 week period.
Results
Tissue arrays demonstrated that in humans, the FNDC5 gene is predominantly expressed in muscle. Circulating irisin was detected in the serum or plasma of all subjects studied, whereas circulating FNDC5 was detected in only a distinct minority of the subjects. Cross-sectional studies revealed that circulating irisin levels were positively correlated with biceps circumference (used as a surrogate marker of muscle mass herein), BMI, glucose, ghrelin, and IGF-1. In contrast, irisin levels were negatively correlated with age, insulin, cholesterol, and adiponectin levels, indicating a possible compensatory role of irisin in metabolic regulation. Multivariate regression analysis revealed that biceps circumference was the strongest predictor of circulating irisin levels underlying the association between irisin and metabolic factors in humans at baseline. Both muscle FNDC5 mRNA levels and circulating irisin levels were significantly downregulated 6 months after bariatric surgery. Circulating irisin levels were significantly upregulated 30 min after acute exercise and were correlated mainly with ATP levels and secondarily with metabolites related to glycolysis and lipolysis in muscle.
Conclusions
Similar to mice, the FNDC5 gene is expressed in human muscle. Age and muscle mass are the primary predictors of circulating irisin, with young male athletes having several fold higher irisin levels than middle-aged obese women. Circulating irisin levels increase in response to acute exercise whereas muscle FNDC5 mRNA and circulating irisin levels decrease after surgically induced weight loss in parallel to decrease in body mass. Further studies are needed to study the regulation of irisin levels and its physiological effects in humans and to elucidate the mechanisms underlying these effects.
Keywords: Irisin, FNDC5, Exercise, Weight loss
1. Introduction
Obesity has reached epidemic proportions; more than two thirds of Americans are overweight (Body mass index (BMI)>25 kg/m2) and one third are obese (BMI>30 kg/m2) [1]. Sedentary lifestyle and lack of exercise are considered as the main causative factors for obesity and obesity-induced metabolic disorders such as chronic inflammation, certain malignancies, type 2 diabetes, and cardiovascular diseases [2,3]. Therefore, the well-recognized beneficial effects of exercise on metabolism are being investigated intensively at both molecular and clinical levels [4,5].
In this context, the muscle tissue has recently been recognized as an endocrine organ that releases a variety of cytokines, termed myokines, which regulate several physiological and metabolic pathways [6]. Discovery of myokines has emphasized the role of muscle as a source of hormones that communicate information and interact with other tissues, including fat, liver, and pancreas, to alter metabolism [7–9].
Irisin is a novel hormone secreted by myocytes that has been proposed to mediate the beneficial effects of exercise on metabolism [10]. Regulated by PPARγ coactivator 1 alpha (PGC1-α), this newly identified myokine is proteolytically processed from the product of the FNDC5 gene prior to being released into the circulation. FNDC5 has been proposed to induce browning of subcutaneous adipocytes and thermo-genesis by increasing uncoupling protein 1 (UCP1) levels, both in culture and in mouse models [10]. Use of adenoviral vectors to express full-length FNDC5 resulted in a 15-fold increase in liver FNDC5 mRNA and 3–4 fold increase in plasma levels of irisin [10], which led to improvement in glucose tolerance of mice fed a high-fat diet. Taken that formation of “beige/brite fat” has been shown to exert anti-obesity, anti-diabetic effects in murine models [11] as well as humans [12], irisin administration has been proposed to be a potentially attractive future therapeutic target for metabolic disorders.
Irisin is highly conserved among species, with mouse and human irisin being 100% identical, supporting the notion for having a highly conserved function in humans; this remains to be shown. In contrast to the increase in human plasma irisin levels measured by western blotting after 10 weeks of endurance exercise training [10], Timmons et al., using gene expression arrays, failed to detect a robust and consistent increase in FNDC5 mRNA in human muscle biopsies from exercising subjects [13].No previous study has examined whether extracellular FNDC5 is detectable in the circulation, and no prior study has quantitated circulating irisin using ELISAs or RIAs to report on the regulation and/or the physiological role of irisin in humans. To gain insights on the role of irisin in humans, we examined its associations with various parameters, especially metabolic markers of obesity and adiposity, by conducting cross-sectional as well as interventional studies in humans. In particular, after examining the expression of FNDC5 gene in various human tissues at the molecular level, we assessed potential correlations of irisin with anthropometric and metabolic parameters in middle-aged women with a wide range of BMI. We then evaluated the response of irisin to acute and chronic exercise in moderately trained young lean subjects and the response to weight loss in morbidly obese men and women.
2. Research design and methods
2.1. Molecular analysis
2.1.1. Tissue distribution of irisin using tissue arrays
FNDC5 gene expression in human tissues were examined using TissueScan qPCR Arrays (OriGene, Rockville, MD). Briefly, TaqMan Gene Expression Assay primer mix and TaqMan PCR Master Mix (Applied Biosystems, Foster City, CA) were added to wells containing equal amounts of cDNA from human tissues. mRNA expression was measured by real-time PCR using the comparative CT method (ABI7500 FAST, Applied Biosystems). Data were normalized to β-actin (id: Hs99999903_m1) in each reaction.
2.1.2. Gene expression analysis in human muscle samples
Total RNA was extracted from human muscles using Trizol (Invitrogen, Carlsbad, CA) according to a standard protocol. mRNA levels of FNDC5, peroxisome proliferator-activated receptor gamma (PPARγ), and PGC1-α (id: Hs00401006_m1, Hs01115513_m1, Hs0106719_m1, respectively) were measured using TaqMan Gene Expression Assays, as described above. Muscles were obtained from the tissue network of NIH (n= 10 kg/m2) and thigh biopsies of obese subjects (n=14) undergoing bariatric surgery, as outlined below.
2.2. Clinical studies
2.2.1. Cross-sectional study of middle-aged healthy women
Plasma from 117 Greek middle-aged women (age 49.3 ± 8.6years, BMI 30.2±5.3kg/m2, mean±SD), were collected as previously described [14]. The study was approved by the Ethics Committee at Harokopio University in Athens, Greece, and the Institutional Review Board of Beth Israel Deaconess Medical Center, Boston, MA. All subjects gave written informed consent before taking part in the study. Subjects completed a questionnaire on general health status, smoking habits, and current medications, as previously described [14]. Both extracellular FNDC5 and irisin levels were measured to determine their correlations with anthropometric and metabolic characteristics.
2.2.2. Interventional study of morbidly obese subjects undergoing bariatric surgery for weight loss
Fourteen subjects (age 53.1±8.9 years, BMI 50.2±10.6 kg/m2) undergoing either laparoscopic adjustable gastric banding (n=9) or Roux-en-Y gastric bypass surgery (n=5) were recruited to this prospective study after they were approved for bariatric surgery. All subjects gave written informed consent before taking part in the study. All participants were pre-screened at Beth Israel Deaconess Medical Center (BIDMC), Boston, MA to qualify for the study. Subjects were examined at baseline and 6 months post-operatively at BIDMC General Clinical Research Center. Blood samples were collected at each visit after an overnight fasting. Anthropometric and body composition measurements were performed using bioelectrical impedance analysis before surgery and 6 months post-operatively. Eight out of the fourteen subjects consented to undergo a left or right thigh biopsy during the baseline and 6-month visit. The biopsy was performed under local anesthesia by a general surgeon. Thigh skeletal muscle tissues were obtained using a biopsy needle and snap frozen until analyzed.
2.2.3. Effect of acute and chronic exercise on serum FNDC5 and irisin levels in young healthy subjects
Fifteen young, moderately trained, healthy males (age 20.5±1.5 years, BMI 21.9±1.6 kg/m2) were assigned to an 8-week long training program involving 3 training sessions per week, as previously described [15]. Briefly, the training included 2 or 3 sets of runs on an indoor track with two 80-m sprint runs in each set. There was a resting period of 20min between sets. During the 1st (week 1) and 24th training sessions (week 8), biopsy from the vastus lateralis muscle was performed before and after the first set, and blood samples were obtained before and 30min after the completion of the exercise protocol. Irisin and FNDC5 levels were assayed in serum, and metabolite contents were examined in muscle.
2.2.4. Effect of a mixed meal on irisin levels
A subgroup of 6 non-diabetic subjects undergoing bariatric surgery completed a 3 hr mixed nutrient stimulation study during their screening visit (mixed meal contained 246 kcal; 6 g fat; 33 g carbohydrate; 15 g protein). Blood samples were collected from a forearm intravenous catheter at 6 time points (baseline, 30, 60, 90, 120, and 180 min) after food intake to evaluate the effect of food on FNDC5 or irisin levels.
2.3. Hormonal analysis
2.3.1. Evaluation of the FNDC5 and irisin assays
Commercially available irisin and soluble (extracellular) FNDC5 enzyme-linked immunosorbent assays (Aviscera Biosciences, Santa Clara, CA) were evaluated before analyzing the clinical samples. Linearity of serial dilutions (1, 1/2, 1/4, 1/8) of both plasma and serum from males and females was tested. The effect of repeated freeze/thaw cycles (0, 2, 4, 8) on concentration and the cross-reactivity between the two assays was also evaluated. The possibility of a “hook effect” was tested in samples with low levels of FNDC5 using serial dilutions (1/10, 1/100, 1/1000).
2.3.2. Other hormonal measurements
Healthy middle-aged women study: Leptin, cortisol, testosterone and estradiol levels, as well as lipid profiles, in the cross-sectional study were measured as previously described [14]. Radioactive immunoassays (RIA) were used for ghrelin (Linco Research Inc., St. Charles, MO), insulin, and adiponectin (Millipore Corporation, Billerica, MA) measurements. Serum insulin-like growth factor 1 (IGF-1)and insulin growth factor binding protein 3 (IGFBP-3) were measured with Siemens Immulite 1000 automated platform immunometric assay (Siemens Healthcare Diagnostics, Norwood, MA).
Weight loss interventional study: Leptin and adiponectin levels were measured using RIA [16]. Serum insulin and cortisol levels were measured with Siemens Immulite 1000 [17].
2.4. Statistical analysis
SPSS versions 11.5 and 18.0 (SPSS Inc., Chicago, IL) were used for the statistical analyses. Data are shown as mean±SD unless stated otherwise. For the molecular analysis on FNDC5 gene expression, mean values obtained from each group were compared by ANOVA with subsequent Fisher’s significant difference method. The cross-sectional study had more than 80% power to detect associations with r≥0.22 at the conventional α=0.05 level. p values of <0.05 were considered as statistically significant for all analyses. Several variables were logarithmically transformed, as needed, to obtain a normal distribution. Associations of circulating irisin levels with various parameters were calculated with Pearson’s (for normally distributed variables) or Spearman’s (for non-normally distributed variables) correlation coefficients, as appropriate. Adjustment for potential confounders was then performed by multivariate regression analysis. For the interventional study on bariatric surgery-induced weight loss, paired t tests were performed. For analyzing the effect of exercise in young healthy adults, Pearson’s correlation and paired t tests were performed.
3. Results
3.1. Distribution of FNDC5 mRNA in human tissues
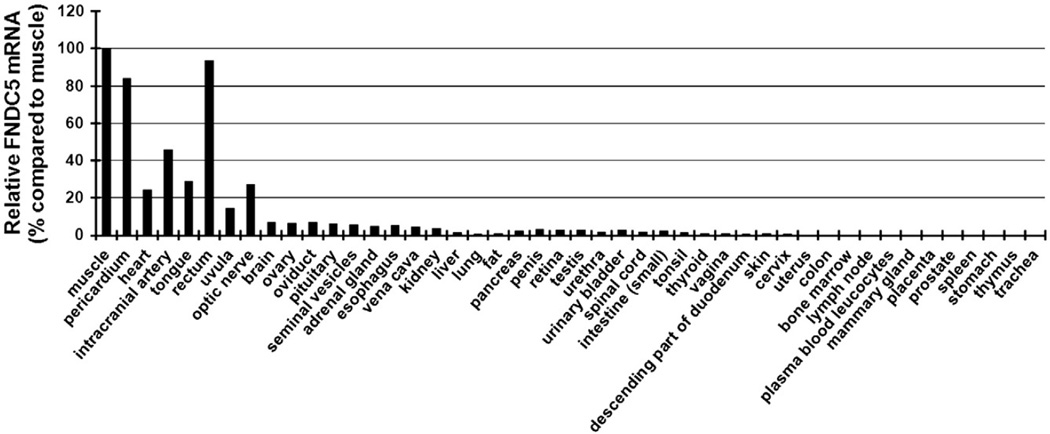
To examine the tissue distribution of irisin in humans, we conducted a quantitative real-time PCR in 47 different human tissues (Fig. 1). As previously reported, FNDC5 mRNA was expressed in high levels in muscles. FNDC5 was also highly expressed in other organs that may contain muscle such as the pericardium and rectum. FNDC5 mRNA was moderately expressed in the heart. Lower expression levels were detected in other major organs, such as kidney, liver, lung, and adipose tissue, as compared to muscle, confirming the role of irisin as amyokine.
Fig. 1.
FNDC5 gene expression in various human tissues. Quantitative real-time PCR was used to analyze the mRNA level of FNDC5 in 47 different human tissues. Data are presented as means of two experiments and as percentage compared to FNDC5 gene expression in muscle.
3.2. Predictors of FNDC5 mRNA expression in human muscle
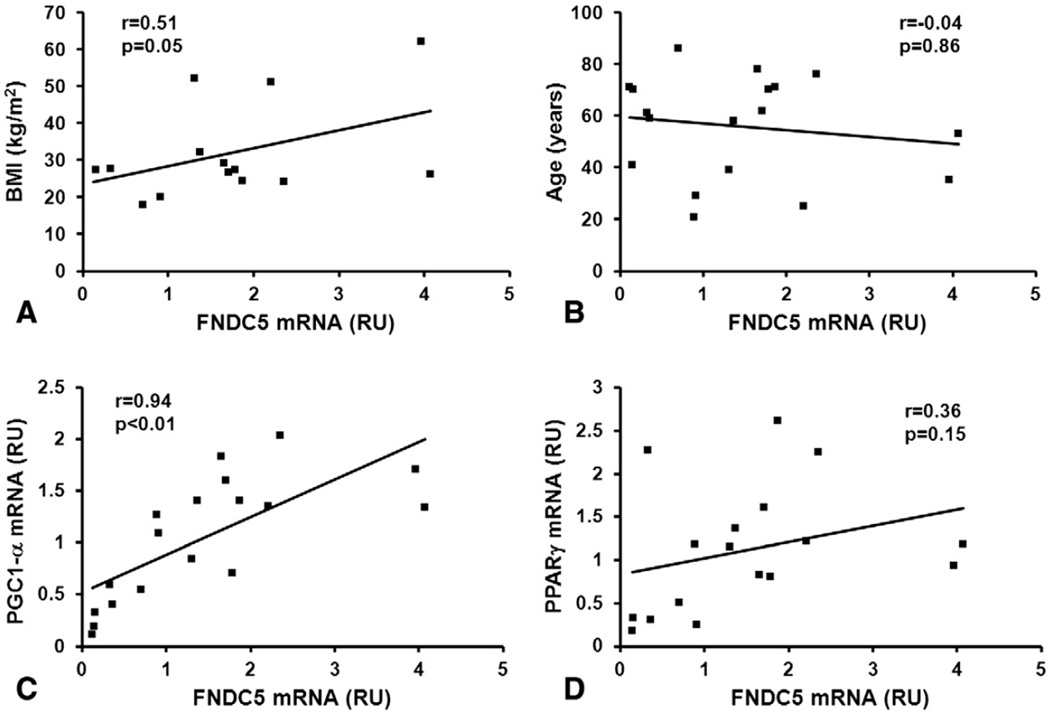
FNDC5 mRNA expression was measured in muscle samples from 18 subjects (10 obtained from the tissue network of NIH and 8 from subjects undergoing bariatric surgery studied at baseline). Bivariate regression analysis revealed that muscle FNDC5 mRNA expression was positively correlated with BMI but, despite a clear trend, it was not significantly associated with age, probably due to the small sample size (Fig. 2A and B). As expected, FNDC5 gene expression was positively and strongly correlated with the expression of PGC1-α (Fig. 2C), which was shown to be the main inducer of irisin in mice [10]. PPARγ mRNA levels were also moderately correlated with FNDC5 mRNA levels (Fig. 2D).
Fig. 2.
Association of FNDC5 gene expression in human muscle. Pearson’s correlation coefficients of muscle FNDC5 mRNA with (A) BMI, (B) age, (C) PGC1-α mRNA, and (D) PPARγ mRNA in healthy subjects (n=14–18). BMI=body mass index, RU=relative unit.
3.3. Evaluation of FNDC5 and irisin assays
To examine the physiology of circulating irisin in humans, we first evaluated several commercially available assay kits. The FNDC5 (extracellular domain molecule: epitope 16–136) and the irisin kits by Aviscera proved to be the most reliable ones. Results from the irisin assay evaluation showed that serum and plasma concentrations of irisin are relatively similar (data not shown). Multiple freezing and thawing of the samples did not cause any significant changes in the irisin levels. Assessment of linearity in serum and plasma irisin levels revealed a 124% recovery for the 1/2 dilution, and higher recovery (up to 213%) in the 1/4 and 1/8 dilutions. The sensitivity of the assay was 0.2 ng/ml and the linear range of the standard was 5 to 500 ng/ml. Results from the mixed-meal tolerance test showed that irisin levels were not affected by food intake (p=0.6). FNDC5 assay evaluation in normal serum and plasma revealed that FNDC5 levels were either very low (in a distinct minority of subjects: 1–20 ng/ml) or undetected. The sensitivity of the assay was 0.3 ng/ml and the linear range of the standard was 10 to 1000 ng/ml. Reanalyzing with 1/10-, 1/100-, and 1/1000-diluted samples confirmed that there was no hook effect. Cross-reactivity with irisin in the FNDC5 kit was 10%–25%, suggesting that the very low levels of FNDC5, where detected, could be due to cross-reactivity with irisin. In contrast to other clinical subjects in this study with low levels of soluble FNDC5, a subpopulation of young athletes showed very high levels of FNDC5 in circulation (over 1000 ng/ml), which might have resulted from exercise-induced muscle damage, a hypothesis that needs to be tested in the future.
3.4. Correlation of circulating irisin with anthropometric, hormonal, and metabolic parameters
3.4.1. Cross-sectional study of healthy subjects
Table 1A summarizes the demographic, anthropometric, metabolic, and hormonal parameters of 117 middle-aged women. The range in BMI was 20.0 to 47.7 kg/m2, including 13 lean subjects with BMI lower than 25 kg/m2. The range in age was 24 to 69 years old. Subjects had a mean plasma irisin concentration of 113.1±20.6 ng/ml, ranging from 50.7 to 166.5 ng/ml.
Table 1.
Descriptive characteristics of the study samples (Mean±SD).
| A. Cross-sectional study of healthy women (n=117) | |
|---|---|
| Demographic characteristics | |
| Age (years) | 49.32±8.63 |
| Gender | |
| Females | n=117 (100%) |
| Menopausal status | |
| -Premenopausal | 43 (37%) |
| -Perimenopausal | 61 (52%) |
| -Postmenopausal | 13 (11%) |
| Smoking status | |
| -Non-smokers | 78 (67%) |
| -Smokers | 34 (29%) |
| -Former-smokers | 5 (4%) |
| Anthropometric characteristics | |
| Weight (kg) | 76.21±14.76 |
| BMI (kg/m2) | 30.23±5.28 |
| Body Fat (kg) | 31.51±9.26 |
| Fat-Free Mass (kg) | 38.27±4.41 |
| Waist-to-Hip Ratio | 0.80±0.06 |
| Biceps Circumference (RU) | 31.54±7.31 |
| Hormonal parameters | |
| Fasting Insulin(µIU/ml) | 3.40±1.55 |
| HOMA-IR | 14.18±6.10 |
| Irisin (ng/ml) | 113.12±20.62 |
| Adiponectin (µg/ml) | 10.55±16.67 |
| Leptin (ng/ml) | 21.80±12.17 |
| Cortisol (µg/dl) | 10.01±4.58 |
| Ghrelin (pg/ml) | 890.94±326.71 |
| Estradiol (pg/ml) | 184.77±115.99 |
| Free Testosterone (pg/ml) | 1.46±0.94 |
| IGF-1 (ng/ml) | 122.35±50.23 |
| IGFBP-3 (µg/ml) | 0.89±0.54 |
| Metabolic parameters | |
| Fasting Glucose (mg/dl) | 97.69±13.54 |
| Total Cholesterol (mg/dl) | 214.54±42.12 |
| LDL Cholesterol (mg/dl) | 140.46±37.68 |
| HDL Cholesterol (mg/dl) | 55.00±12.49 |
| Triglycerides (mg/dl) | 97.48±50.40 |
|
B. Cross-sectional study of obese subjects undergoing bariatric surgery, assessed preoperatively (n=14) | |
|---|---|
| Demographic characteristics | |
| Age (years) | 53.14±8.93 |
| Gender | |
| Females | n = 6 (43%) |
| Males | n = 8 (57%) |
| Anthropometric characteristics | |
| Weight (kg) | 143.81±31.10 |
| BMI (kg/m2) | 50.19±10.63 |
| Body Fat (kg) | 65.66±39.73 |
| Fat Free Mass (kg) | 88.92±33.43 |
| Hormonal parameters | |
| Fasting Insulin (µIU/ml) | 25.49±20.67 |
| HOMA-IR | 114.86±121.20 |
| Irisin (ng/ml) | 112.67±32.19 |
| Adiponectin (µg/ml) | 13.56±8.26 |
| Leptin (ng/ml) | 50.91±28.10 |
| Cortisol (µg/dl) | 9.89±5.27 |
| Ghrelin (pg/ml) | 253.69±211.98 |
| Metabolic parameters | |
| Fasting Glucose (mg/dl) | 95.08±23.90 |
| Total Cholesterol (mg/dl) | 161.81±30.45 |
| LDL Cholesterol (mg/dl) | 100.29±29.14 |
| HDL Cholesterol (mg/dl) | 42.20±12.78 |
| Triglycerides (mg/dl) | 116.38±39.89 |
To evaluate potential predictors of the circulating irisin, we performed bivariate regression analysis of circulating irisin and the anthropometric, metabolic, and hormonal factors (Table 2A). As expected, age was negatively correlated with BMI, fat-free mass, and body fat content. BMI showed positive correlations with biceps circumference, fat-free mass, body fat, and waist-to-hip ratio (WHR). Logarithmically transformed adiponectin levels showed significant negative correlation with body composition or metabolic markers such as BMI, body fat, and WHR and positive correlation with HDL cholesterol. Also, an inverse correlation between age and estradiol levels was observed (Table 2A). These expected associations largely confirmed the validity of our observations.
Table 2.
Correlation matrix of the study variables.
| A | Irisin | Age | BMI | Biceps circum. |
Fat-free mass |
Fat mass |
WHR | Insulin | HOMA -IR |
LnAdiponectin | Leptin | Cortisol | Estradiol | Testosterone | Ghrelin | IGF-1 | IGFBP- 3 |
IGF-1/ IGFBP-3 |
Glucose | Total Cholesterol |
LDL | H HDL |
TG | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Irisin (ng/ml) |
r | 1.00 | ||||||||||||||||||||||
| p | ․ | |||||||||||||||||||||||
| Age (years) |
r | −0.28 | 1.00 | |||||||||||||||||||||
| p | <0.01 | ․ | ||||||||||||||||||||||
| BMI (kg/m2) | r | 0.16 | −0.19 | 1.00 | ||||||||||||||||||||
| p | 0.09 | 0.04 | ․ | |||||||||||||||||||||
| Biceps Circumference (RU) |
r | 0.27 | −0.07 | 0.45 | 1.00 | |||||||||||||||||||
| p | 0.02 | 0.51 | <0.01 | ․ | ||||||||||||||||||||
| Fat-free mass (kg) | r | 0.21 | −0.39 | 0.74 | 0.35 | 1.00 | ||||||||||||||||||
| p | 0.02 | 0.01 | <0.01 | <0.01 | ․ | |||||||||||||||||||
| Fat mass (kg) | r | 0.18 | −0.26 | 0.96 | 0.49 | 0.88 | 1.00 | |||||||||||||||||
| p | 0.06 | <0.01 | <0.01 | <0.01 | <0.01 | ․ | ||||||||||||||||||
| Waist-to-hip ratio (WHR) |
r | 0.16 | −0.09 | 0.39 | 0.27 | 0.31 | 0.41 | 1.00 | ||||||||||||||||
| p | 0.08 | 0.33 | <0.01 | 0.01 | 0.01 | <0.01 | ․ | |||||||||||||||||
| Insulin (µIU/ml) |
r | −0.17 | 0.08 | −0.47 | −0.36 | −0.34 | −0.46 | −0.43 | 1.00 | |||||||||||||||
| p | 0.09 | 0.45 | <0.01 | <0.01 | <0.01 | <0.01 | 0.01 | ․ | ||||||||||||||||
| HOMA-IR | r | −0.15 | 0.01 | −0.34 | −0.23 | −0.23 | −0.33 | −0.38 | 0.84 | 1.00 | ||||||||||||||
| p | 0.14 | 0.96 | <0.01 | 0.05 | 0.03 | 0.01 | 0.01 | <0.01 | ․ | |||||||||||||||
| LnAdiponectin (µg/ml) |
r | −0.27 | 0.24 | −0.23 | −0.05 | −0.24 | −0.23 | −0.37 | 0.32 | 0.25 | 1.00 | |||||||||||||
| p | <0.01 | 0.01 | 0.02 | 0.66 | 0.01 | 0.02 | <0.01 | 0.01 | 0.02 | ․ | ||||||||||||||
| Leptin (ng/ml) | r | 0.09 | −0.18 | 0.69 | 0.48 | 0.62 | 0.72 | 0.26 | −0.53 | −0.42 | −0.04 | 1.00 | ||||||||||||
| p | 0.39 | 0.07 | <0.01 | <0.01 | 0.01 | 0.01 | 0.01 | <0.01 | <0.01 | 0.66 | ․ | |||||||||||||
| Cortisol (µg/dl) | r | −0.12 | −0.09 | 0.11 | −0.09 | 0.09 | 0.12 | 0.10 | −0.09 | −0.16 | −0.01 | 0.18 | 1.00 | |||||||||||
| p | 0.22 | 0.32 | 0.24 | 0.45 | 0.33 | 0.20 | 0.29 | 0.38 | 0.13 | 0.88 | 0.08 | ․ | ||||||||||||
| Estradiol (pg/ml) |
r | 0.24 | −0.35 | 0.33 | 0.11 | 0.26 | 0.29 | 0.12 | −0.19 | −0.15 | −0.29 | 0.25 | 0.03 | 1.00 | ||||||||||
| p | 0.01 | <0.01 | <0.01 | 0.33 | 0.01 | 0.01 | 0.20 | 0.06 | 0.15 | <0.01 | 0.01 | 0.79 | ․ | |||||||||||
| Free Testosterone (pg/ml) |
r | −0.09 | −0.06 | −0.03 | −0.16 | −0.01 | −0.02 | −0.15 | <0.01 | −0.01 | 0.02 | −0.08 | −0.05 | 0.04 | 1.00 | |||||||||
| p | 0.35 | 0.53 | 0.79 | 0.16 | 0.91 | 0.85 | 0.11 | 0.98 | 0.95 | 0.84 | 0.44 | 0.60 | 0.67 | ․ | ||||||||||
| Ghrelin (pg/ml) | r | 0.25 | −0.14 | 0.01 | −0.09 | −0.08 | −0.01 | 0.12 | 0.12 | 0.07 | 0.02 | −0.08 | 0.02 | 0.16 | −0.10 | 1.00 | ||||||||
| p | 0.02 | 0.18 | 0.92 | 0.41 | 0.42 | 0.90 | 0.28 | 0.27 | 0.56 | 0.88 | 0.49 | 0.86 | 0.13 | 0.34 | ․ | |||||||||
| IGF-1 (ng/ml) |
r | 0.32 | −0.27 | −0.09 | 0.03 | −0.01 | −0.07 | 0.10 | −0.20 | −0.10 | −0.12 | −0.04 | 0.01 | 0.12 | −0.11 | −0.02 | 1.00 | |||||||
| p | 0.01 | 0.01 | 0.36 | 0.82 | 0.90 | 0.51 | 0.34 | 0.06 | 0.32 | 0.26 | 0.71 | 0.94 | 0.26 | 0.29 | 0.83 | ․ | ||||||||
| IGFBP-3 (µg/ml) | r | 0.01 | 0.02 | 0.07 | 0.07 | 0.03 | 0.09 | 0.09 | −0.03 | 0.01 | <0.01 | 0.05 | −0.10 | −0.04 | −0.02 | −0.06 | 0.20 | 1.00 | ||||||
| p | 0.95 | 0.81 | 0.47 | 0.57 | 0.74 | 0.40 | 0.36 | 0.75 | 0.90 | 0.97 | 0.63 | 0.33 | 0.70 | 0.86 | 0.60 | 0.04 | ․ | |||||||
| IGF-1/IGFBP-3 Ratio | r | 0.21 | −0.25 | −0.06 | 0.06 | 0.09 | −0.01 | 0.05 | −0.16 | −0.13 | −0.16 | 0.00 | 0.05 | 0.05 | −0.06 | 0.02 | 0.47 | −0.55 | 1.00 | |||||
| p | 0.04 | 0.01 | 0.54 | 0.61 | 0.36 | 0.93 | 0.60 | 0.13 | 0.22 | 0.14 | 0.97 | 0.64 | 0.65 | 0.60 | 0.85 | <0.01 | <0.01 | ․ | ||||||
| Glucose (mg/dl) | r | 0.25 | −0.13 | 0.48 | 0.23 | 0.46 | 0.51 | 0.31 | −0.34 | −0.08 | −0.23 | 0.44 | −0.06 | 0.22 | −0.07 | 0.11 | 0.13 | 0.07 | 0.13 | 1.00 | ||||
| p | 0.01 | 0.17 | <0.01 | 0.05 | <0.01 | <0.01 | <0.01 | <0.01 | 0.46 | 0.02 | <0.01 | 0.54 | 0.02 | 0.49 | 0.32 | 0.20 | 0.51 | 0.22 | ․ | |||||
| Total Cholesterol (mg/dl) |
r | −0.24 | 0.36 | 0.01 | −0.06 | −0.12 | −0.04 | 0.05 | −0.10 | −0.10 | 0.16 | 0.01 | 0.15 | −0.01 | 0.11 | −0.02 | −0.17 | 0.03 | −0.05 | −0.05 | 1.00 | |||
| p | 0.01 | <0.01 | 0.94 | 0.63 | 0.20 | 0.69 | 0.59 | 0.36 | 0.32 | 0.10 | 0.91 | 0.12 | 0.89 | 0.27 | 0.85 | 0.11 | 0.78 | 0.62 | 0.61 | ․ | ||||
| LDL Cholesterol (mg/ dl) |
r | −0.17 | 0.34 | 0.05 | −0.05 | −0.09 | <0.01 | 0.08 | −0.11 | −0.10 | 0.08 | 0.02 | 0.09 | 0.03 | 0.10 | <0.01 | −0.14 | 0.03 | −0.02 | 0.01 | 0.95 | 1.00 | ||
| p | 0.08 | <0.01 | 0.63 | 0.67 | 0.33 | 1.00 | 0.39 | 0.31 | 0.36 | 0.45 | 0.85 | 0.38 | 0.73 | 0.33 | 0.97 | 0.19 | 0.77 | 0.85 | 0.96 | <0.01 | ․ | |||
| HDL Cholesterol (mg/dl) |
r | −0.28 | 0.13 | −0.35 | −0.16 | −0.29 | −0.34 | −0.29 | 0.35 | 0.25 | 0.42 | −0.22 | 0.13 | −0.19 | 0.01 | −0.03 | −0.10 | 0.02 | −0.12 | −0.36 | 0.16 | −0.08 | 1.00 | |
| p | 0.01 | 0.18 | <0.01 | 0.18 | 0.01 | 0.01 | <0.01 | <0.01 | 0.02 | <0.01 | 0.03 | 0.17 | 0.04 | 0.93 | 0.76 | 0.32 | 0.88 | 0.26 | <0.01 | 0.09 | 0.40 | ․ | ||
| Triglycerides (mg/dl) | r | −0.07 | 0.04 | 0.24 | 0.10 | 0.14 | 0.21 | 0.27 | −0.38 | −0.35 | −0.18 | 0.20 | 0.19 | 0.23 | 0.12 | −0.10 | −0.13 | −0.20 | 0.14 | 0.19 | 0.54 | 0.51 | −0.29 | 1.00 |
| p | 0.48 | 0.65 | 0.01 | 0.42 | 0.13 | 0.03 | <0.01 | <0.01 | <0.01 | 0.07 | 0.05 | 0.05 | 0.02 | 0.24 | 0.37 | 0.20 | 0.06 | 0.19 | 0.05 | <0.01 | <0.01 | <0.01 | ․ |
| B | Irisin | Age | BMI | Fat-free mass | Insulin | Adiponectin | Leptin | Cortisol | |
|---|---|---|---|---|---|---|---|---|---|
| Irisin (ng/ml) |
r | 1.00 | |||||||
| p | ․ | ||||||||
| Age (years) |
r | −0.53 | 1.00 | ||||||
| p | 0.05 | ․ | |||||||
| BMI (kg/m2) |
r | −0.02 | −0.17 | 1.00 | |||||
| p | 0.96 | 0.55 | ․ | ||||||
| Fat-free mass (kg) |
r | −0.06 | 0.02 | 0.64 | 1.00 | ||||
| p | 0.84 | 0.94 | 0.02 | ․ | |||||
| Insulin (µIU/ml) |
r | 0.54 | −0.43 | −0.04 | −0.17 | 1.00 | |||
| p | 0.04 | 0.12 | 0.89 | 0.58 | ․ | ||||
| Adiponectin (µg/ml) |
r | −0.32 | 0.38 | −0.09 | −0.37 | −0.52 | 1.00 | ||
| p | 0.26 | 0.18 | 0.77 | 0.22 | 0.06 | ․ | |||
| Leptin (ng/ml) |
r | 0.19 | −0.20 | −0.16 | −0.39 | −0.41 | 0.42 | 1.00 | |
| p | 0.52 | 0.50 | 0.57 | 0.19 | 0.14 | 0.13 | ․ | ||
| Cortisol (µg/dl) |
r | −0.25 | 0.47 | 0.15 | −0.05 | 0.12 | −0.15 | −0.45 | 1.00 |
| p | 0.47 | 0.14 | 0.67 | 0.88 | 0.73 | 0.67 | 0.16 | ․ |
r and p values are shown with significant correlations in bold. (A) Pearson’s correlation coefficients of study variables of the cross-sectional study on healthy subjects. (B) Spearman’s bivariate correlation coefficients of study variables of the study on obese subjects undergoing bariatric surgery (assessed preoperatively), RU: relative unit.
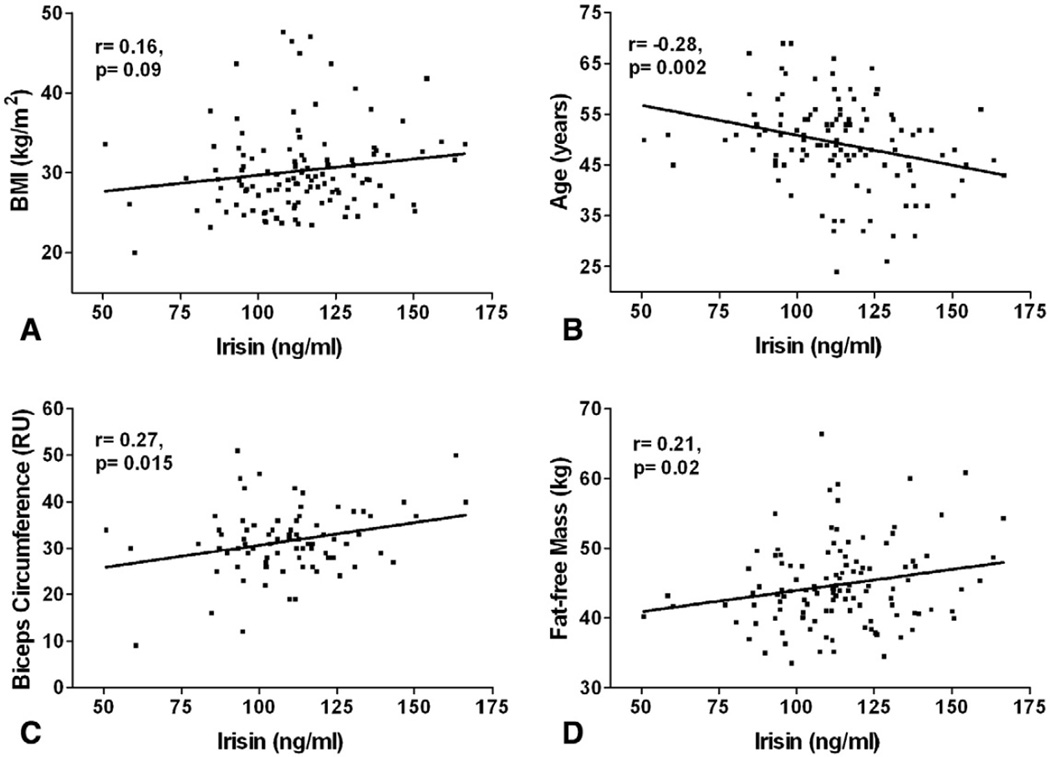
Correlation of irisin levels with anthropometric parameters revealed that circulating irisin had positive associations with biceps circumference, fat-free mass, and BMI (Table 2A and Fig. 3). In contrast, age was negatively associated with irisin levels (Fig. 3B). WHR and body fat in kilograms were not associated with circulating irisin levels in these subjects (Table 2A).
Fig. 3.
Association of circulating irisin with anthropometric parameters in 117 healthy women. Pearson’s correlation coefficients of circulating irisin with (A) BMI, (B) Age, (C) Biceps Circumference, and (D) Fat-free Mass are shown. Pearson’s correlation coefficients and p values are shown in each graph. BMI=body mass index, RU=relative unit.
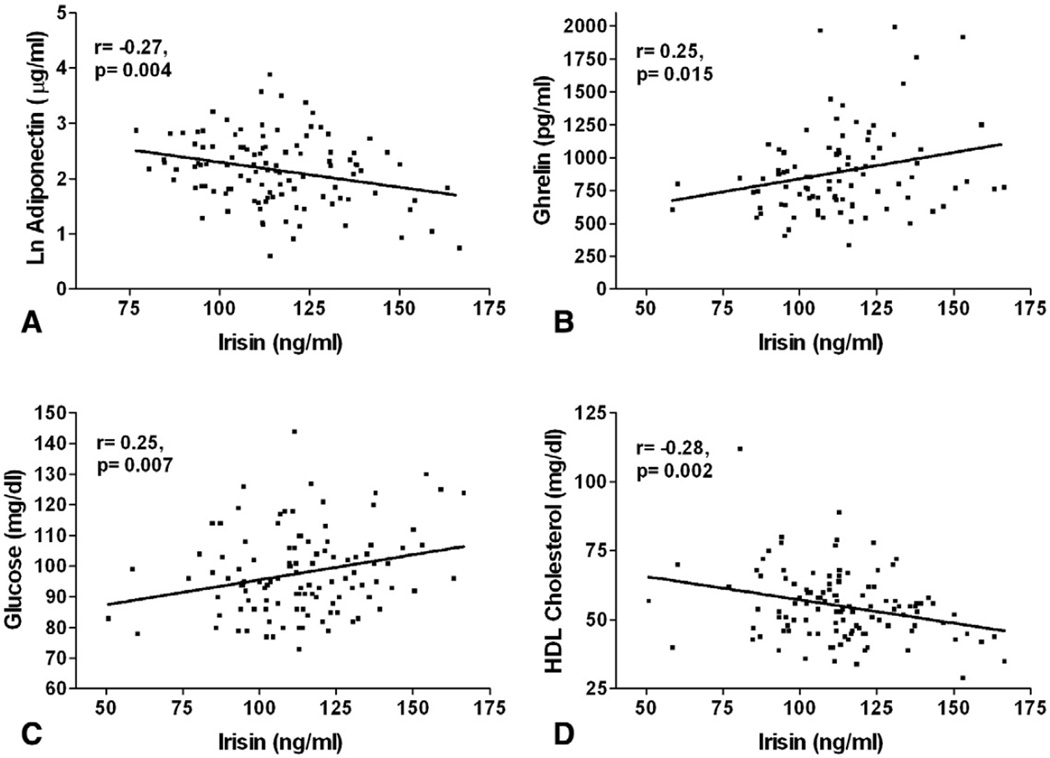
Log adiponectin concentration was negatively correlated with irisin levels whereas the estradiol concentration was positively correlated with the irisin concentration (Table 2A and Fig. 4A). Significant positive associations of circulating irisin with growth hormones including ghrelin and IGF-1 were also found (Table 2A and Fig. 4B). In terms of metabolic variables, irisin was positively correlated with serum glucose and inversely correlated with total and HDL cholesterol (Table 2A, Fig. 4C, and D). LDL cholesterol and triglyceride levels were not associated with irisin. In addition, we found no association of irisin with cortisol and free testosterone (Table 2A).
Fig. 4.
Association of circulating irisin with hormonal and metabolic parameters in 117 healthy women. Pearson’s correlation coefficients of circulating irisin with (A) LnAdiponectin (logarithmically transformed), (B) Ghrelin, (C) Glucose, and (D) HDL Cholesterol in plasma are shown. Pearson’s correlation coefficients and the p values are shown in each graph.
These bivariate associations cannot be considered at face value, since they are not free from confounding. To adjust for potential confounders of the circulating irisin concentration, we performed multivariate linear regression analysis. As shown in Table 3, adjustments for age, menopausal status, smoking status, estradiol levels, fat-free mass, and biceps circumference were made. Significant correlations of irisin with adiponectin, ghrelin, glucose, and HDL cholesterol levels persisted after adjustment for age, menopausal status, and smoking status but were lost when adjusted for estradiol levels and, importantly, biceps circumference. This suggests that muscle mass is the main predictor of the circulating irisin concentration in human subjects and underlies its association with metabolic variables.
Table 3.
Multivariate regression analysis of anthropometric, hormonal, and metabolic factors in relation to circulating irisin levels in the cross-sectional study on healthy subjects.
| β1 | β2 | β3 | β4 | β5 | β6 | β7 | |
|---|---|---|---|---|---|---|---|
| Anthropometric characteristics | |||||||
| Age (yr) | −0.28** | - | - | - | - | - | - |
| BMI(kg/m2) | 0.16 | 0.11 | 0.12 | 0.12 | 0.03 | −0.03 | −0.07 |
| Biceps circumference(RU) | 0.27 * | 0.27* | 0.29** | 0.31** | 0.28* | 0.25* | - |
| Fat free mass(kg) | 0.21 * | 0.12 | 0.12 | 0.13 | 0.09 | - | 0.03 |
| Body fat(kg) | 0.18 | 0.11 | 0.12 | 0.13 | 0.05 | −0.03 | −0.02 |
| Waist-to-hip ratio | 0.16 | 0.14 | 0.15 | 0.15 | 0.13 | 0.11 | −0.04 |
| Hormonal parameters | |||||||
| Leptin a(ng/ml) | 0.16 | 0.11 | 0.10 | 0.11 | 0.07 | 0.04 | 0.08 |
| Adiponectin a(µg/ml) | −0.27 ** | −0.20* | −0.20* | −0.20* | −0.18 | − 0.18 | −0.10 |
| Insulin (µIU/ml) | −0.17 | −0.15 | −0.15 | −0.14 | −0.10 | −0.06 | 0.05 |
| HOMA-IR | −0.15 | −0.16 | −0.16 | −0.15 | −0.12 | −0.09 | −0.04 |
| Cortisol (µg/dl) | −0.12 | −0.14 | −0.14 | −0.14 | −0.15 | −0.16 | −0.15 |
| Estradiol a(pg/ml) | 0.35** | 0.29** | 0.29** | 0.29** | - | - | - |
| Free testosterone(pg/ml) | −0.09 | −0.11 | −0.11 | −0.11 | −0.14 | −0.14 | −0.11 |
| Ghrelin(pg/ml) | 0.25* | 0.22** | 0.21* | 0.21* | 0.19 | 0.22 | 0.16 |
| IGF-1 (ng/ml) | 0.32** | 0.28** | 0.27** | 0.27** | 0.26** | 0.30** | 0.29* |
| IGFBP-3 (µg/ml) | 0.01 | 0.13 | 0.02 | 0.00 | 0.01 | 0.00 | −0.07 |
| IGF-1/IGFBP-3 | 0.21 * | 0.16 | 0.16 | 0.17 | 0.20 | 0.22 | 0.28 |
| Metabolic parameters | |||||||
| Glucose a(µg/ml) | 0.25** | 0.24* | 0.24* | 0.25** | 0.19 | 0.15 | 0.08 |
| Total cholesterol (µg/ml) | −0.24** | −0.16 | −0.16 | −0.16 | −0.18 | −0.18 | −0.25 |
| LDL cholesterol (µg/ml) | −0.17 | −0.08 | −0.08 | −0.07 | −0.10 | −0.10 | −0.17 |
| HDL cholesterol (µg/ml) | −0.28** | −0.25** | −0.26** | −0.27** | −0.25** | −0.23* | −0.21 |
| Triglycerides(µg/ml) | −0.07 | −0.06 | −0.06 | −0.05 | −0.13 | −0.14 | −0.19 |
β1: unadjusted bivariate linear regression coefficient.
β2: multivariate linear regression coefficient adjusted for age.
β3:multivariate linear regression coefficient adjusted for age, menopausal status.
β4: multivariate linear regression coefficient adjusted for age, menopausal status, and smoking status.
β5: multivariate linear regression coefficient adjusted for age, menopausal status, smoking status, and estradiol levels (logarithmically transformed).
β6: multivariate linear regression coefficient adjusted for age, menopausal status, smoking status, estradiol levels (logarithmically transformed), and fat-free mass.
β7: multivariate linear regression coefficient adjusted for age, menopausal status, smoking status, estradiol levels (logarithmically transformed), and biceps circumference.
RU: relative unit, smoking and menopausal status were assessed as described in Table 1A.
Values were logarithmically transformed.
Significant correlation (p <0.05).
Significant correlation (p <0.01).
3.4.2. Cross-sectional study of bariatric surgery subjects at baseline
Pre-operative demographic, anthropometric, metabolic, and hormonal parameters of subjects enrolled in a study on bariatric surgery are presented in Table 1B. The BMI levels were over 37 kg/m2 with 79.6% being over 40k g/m2. Subjects had a mean serum irisin concentration of 112.7±32.2 ng/ml, ranging from 74.7 to 151.2 ng/ml. Circulating levels of irisin appeared to be higher in women than in men (126.9±44.9 vs 102.0±13.5 ng/ml) but the difference did not achieve statistical significance (p=0.39).
To further investigate the potential association of irisin levels with various markers, we conducted a cross-sectional analysis among the subjects at baseline. Correlation analysis matrix confirmed many of our findings in the cross-sectional study of women, albeit with non-significant p values, possibly due to the small size of the study group (Table 2B). Again, irisin was negatively associated with age but, in relation to body composition parameters, there were no significant outcomes. Insulin was positively linked with irisin, suggesting the potential involvement of irisin in insulin-related metabolic pathways. However, there was lack of association between irisin and fasting glucose (data not shown), apparently due to the small number of subjects. Similar to the cross-sectional results from healthy subjects, serum adiponectin was negatively associated with irisin, although this association did not reach significance.
3.5. Interventional study on bariatric surgery subjects
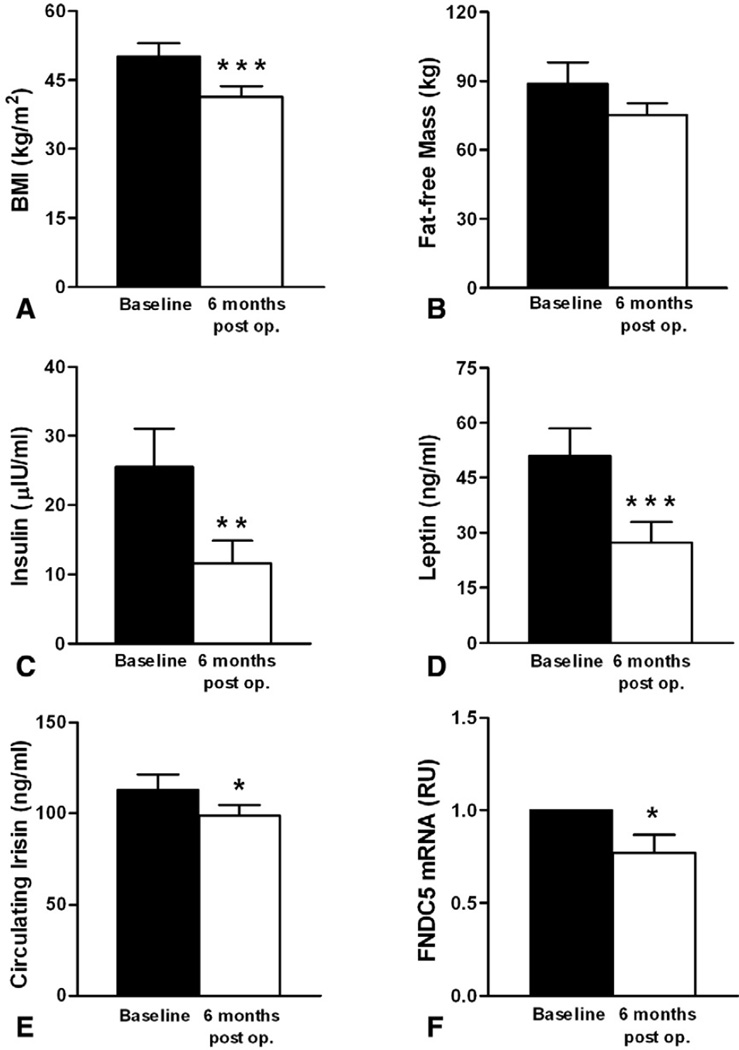
We then examined the effect of bariatric surgery-induced weight loss on circulating irisin levels. BMI was significantly decreased 6 months after surgery (Fig. 5A, 50.2±10.6 vs 41.4± 8.5 kg/m2) and, as expected, this was associated with decreased insulin and leptin levels (Fig. 5C and D) [18,19]. Interestingly, bariatric surgery-induced weight loss resulted in significant decrease in circulating irisin levels (Fig. 5E, 112.7±32.2 vs 98.6±22.1 ng/ml), along with a tendency to decrease in fat-free mass (Fig. 5B). The statistical significance of the decrease in circulating irisin level was lost when adjusted with fat-free mass (p=0.48), consistent with its association with biceps circumference observed in the cross-sectional study. In parallel, FNDC5 mRNA levels in muscle were significantly reduced (Fig. 5F), confirming the connection between muscle irisin expression and its circulating concentration.
Fig. 5.
Change in (A) BMI, (B) Fat-free Mass, (C) Insulin, (D) Leptin, (E) Circulating Irisin, and (F) muscle FNDC5 mRNA expression in obese subjects (n=8) before and 6 months after bariatric surgery. Data are shown as means±SE. *p<0.05, **p<0.01, ***p<0.001. 6 months post op. indicates 6 months after operation. BMI=body mass index, RU=relative unit.
3.6. Effects of acute and chronic exercise on circulating irisin levels of young healthy subjects
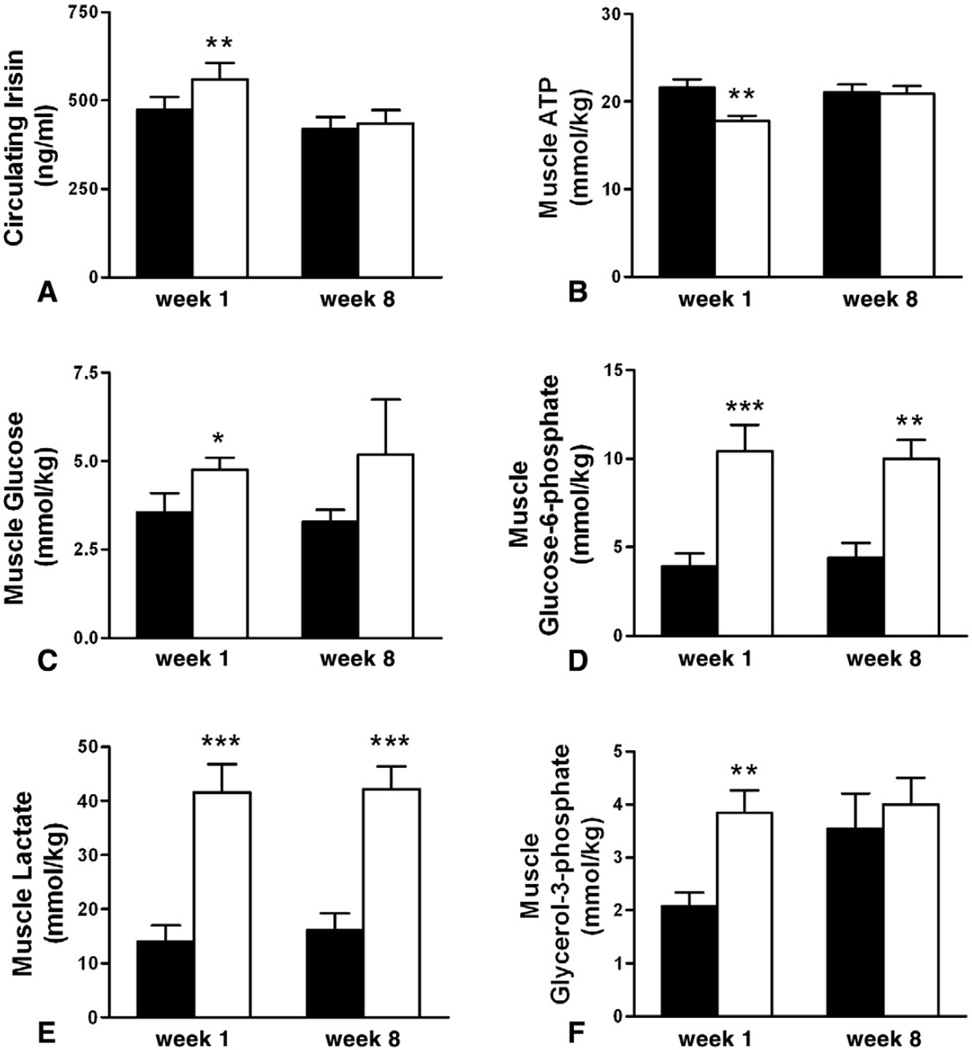
Correlation analysis between circulating irisin and muscle metabolite contents in young healthy subjects before exercise (Table 4), showed that irisin has a positive, but not significant due to small numbers, correlation with ATP. To assess the effect of exercise, we analyzed circulating irisin levels, before and after 2 or 3 sets of double sprints at first week and after 8 weeks of training, as described in the methods. As previously reported [15], analysis of muscle metabolite contents at week 1 showed that ATP levels were acutely decreased in response to exercise, along with decreased phospho-creatine levels (57.8±3.4 vs 31.8±3.5, mean±SE, p <0.01), and increased creatine, glucose, glucose-6-phosphate, glycerol-3-phosphate, fructose-6-phosphate (0.9±0.3 vs 1.9±0.3, p=0.02), and lactate (Fig. 6). There were no effects on pyruvate (2.6±0.5 vs 3.7±1.1, p=0.26), ADP (3.1± 0.3 vs 3.1±0.4, p=0.88), and glucose-1-phosphate (0.6±0.1 vs 0.7±0.1, p=0.31). In line with the decreased muscle ATP content as well as increased glycolytic and lipolytic metabolism, circulating irisin levels were significantly induced 30min after the exercise (473.4±36.4 vs 560.4±46.3, p=0.001, Fig. 6A). Training for 8 weeks mitigated the exercise-induced decrease in muscle ATP and also glucose and glycerol-3-phosphate levels, whereas lactate, creatine, glucose-6-phosphate, and fructose-6-phosphate levels showed similar increases after the sprints. Interestingly, similar to ATP, irisin levels remained unchanged by exercise after 8 weeks of training (420.3±32.7 vs 435.1 ±38.5, p=0.5).
Table 4.
Correlation matrix of the study variables in young healthy subjects at week 1 at baseline (Pearson’s coefficient).
| Irisin | ATP | Phospho creatine |
Creatine | Glucose | Glucose-1- phosphate |
Glucose-6- phosphate |
Fructose-6- phosphate |
Glycerol-3- phosphate |
Pyruvate | Lactate | ADP | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Irisin (ng/ml) | r | 1.00 | |||||||||||
| p | ․ | ||||||||||||
| ATP (mmol/kg) | r | 0.49 | 1.00 | ||||||||||
| p | 0.09 | ․ | |||||||||||
| Phosphocreatine (mmol/kg) |
r | −0.45 | 0.15 | 1.00 | |||||||||
| p | 0.12 | 0.62 | ․ | ||||||||||
| Creatine (mmol/kg) | r | 0.31 | −0.12 | −0.45 | 1.00 | ||||||||
| p | 0.31 | 0.69 | 0.12 | ․ | |||||||||
| Glucose (mmol/kg) | r | 0.41 | −0.07 | −0.70 | 0.16 | 1.00 | |||||||
| p | 0.16 | 0.83 | <0.01 | 0.61 | ․ | ||||||||
| Glucose-1-phosphate (mmol/kg) |
r | 0.34 | 0.42 | −0.32 | 0.35 | 0.26 | 1.00 | ||||||
| p | 0.25 | 0.16 | 0.28 | 0.24 | 0.39 | ․ | |||||||
| Glucose-6-phosphate (mmol/kg) |
r | 0.23 | 0.07 | −0.54 | 0.61 | 0.49 | 0.66 | 1.00 | |||||
| p | 0.45 | 0.83 | 0.05 | 0.03 | 0.09 | 0.01 | ․ | ||||||
| Fructose-6-phosphate (mmol/kg) |
r | 0.19 | 0.25 | −0.35 | 0.21 | 0.22 | 0.72 | 0.73 | 1.00 | ||||
| p | 0.54 | 0.41 | 0.24 | 0.50 | 0.48 | <0.01 | <0.01 | ․ | |||||
| Glycerol-3-phosphate (mmol/kg) |
r | 0.04 | 0.33 | −0.15 | −0.17 | 0.27 | 0.00 | 0.28 | 0.34 | 1.00 | |||
| p | 0.89 | 0.27 | 0.62 | 0.59 | 0.37 | 0.99 | 0.36 | 0.26 | ․ | ||||
| Pyruvate (mmol/kg) | r | −0.45 | 0.06 | 0.19 | −0.35 | −0.09 | −0.21 | 0.08 | 0.08 | 0.38 | 1.00 | ||
| p | 0.12 | 0.84 | 0.53 | 0.23 | 0.76 | 0.48 | 0.78 | 0.80 | 0.21 | ․ | |||
| Lactate (mmol/kg) | r | 0.20 | 0.25 | −0.35 | 0.34 | 0.32 | 0.32 | 0.77 | 0.67 | 0.63 | 0.17 | 1.00 | |
| p | 0.51 | 0.41 | 0.24 | 0.25 | 0.28 | 0.28 | 0.00 | 0.01 | 0.02 | 0.59 | ․ | ||
| ADP (mmol/kg) | r | −0.04 | 0.26 | −0.23 | 0.46 | −0.04 | 0.49 | 0.71 | 0.66 | 0.26 | 0.15 | 0.79 | 1.00 |
| p | 0.89 | 0.39 | 0.45 | 0.11 | 0.90 | 0.09 | <0.01 | 0.01 | 0.40 | 0.63 | <0.01 | ․ |
Irisin concentrations were measured in serum and metabolites were measured in muscle. r- and p-values are shown with significant correlations in bold.
Fig. 6.
Change in (A) Circulating Irisin, muscle content of (B) ATP (C) Glucose, (D) Glucose-6-phosphate, (E) Lactate, (F) Glycerol-3-phosphate in young healthy subjects pre- (black) and post-exercise (white bar) at weeks 1 and 8 of training. Data are shown as means±SE. *p<0.05, **p<0.01, ***p<0.001.
Correlation between changes in irisin concentrations with changes in muscle metabolite contents in week 1 showed strong in magnitude but non-significant, correlations between circulating irisin and ATP, ADP, and lactate(data not shown), suggesting a relationship between circulating irisin expression with ATP levels and muscle metabolism after exercise. The fact that circulating irisin concentrations increased significantly when ATP levels in muscle dropped but remained unchanged when the ATP remained unchanged, apparently due to conditioning with continued participation in the prescribed exercise regimen, suggests that defective metabolism in muscle may represent a strong signal for irisin secretion.
4. Discussion
Irisin is a novel myokine, an extracellularly cleaved and, possibly, post-translationally modified, product of the transmembrane protein transcribed from the FNDC5 gene [10]. FNDC5 overexpression in mice, resulting in several fold increased irisin levels in the circulation, has been shown to induce browning and to increase thermogenic function of subcutaneous white adipose tissue and to improve glucose homeostasis [10]. FNDC5/irisin levels are induced by PGC1-α and exercise in mice [10], and thus it has been suggested that irisin could prove to be a therapeutic agent against metabolic disease [20]. However, the beneficial role of irisin has been challenged [13] on the basis of gene expression data showing no consistent increase in FNDC5 mRNA in response to exercise training in humans.
Here, we present the first study on the distribution of FNDC5 expression in human tissues. We also provide, for the first time, a detailed evaluation of assays to measure circulating FNDC5 and irisin levels and report that although FNDC5 is largely undetectable, irisin is detectable and correlates closely with muscle mass, as expressed by fat-free mass or biceps circumference, in humans. We failed to find direct evidence supporting a beneficial role for irisin in metabolic regulation in humans, in agreement with the findings by Timmons et al., who used Affymetrix array data in relation to BMI, fasting insulin, fasting glucose, and 2-h post-glucose load glucose levels [13]. We also extend these previous findings by systemically analyzing the anthropometric, hormonal, and metabolic parameters of subjects in association with circulating irisin levels in subjects with a wide range of BMI and fat mass using both cross-sectional and interventional studies, and we study irisin gene expression in muscle in the context of an interventional study. Finally, we show for the first time that acute exercise can increase the irisin concentration in human serum.
Tissue distribution analysis has confirmed that FNDC5 gene is predominantly expressed in human muscles. By relatively high expression also shown in pericardium, intracranial artery, and rectum, FNDC5 is likely to be expressed not only in skeletal muscles but also in cardiac and smooth muscles. Low expression of FNDC5 mRNA was observed in major organs such as kidney, liver, lung, and fat. Although subcutaneous white adipose tissue has been suggested to be largely affected by irisin [10], the expression of FNDC5 gene in fat was about 1/100 of expression in muscle, which implies that the effect of irisin on adipose tissue is probably endocrine in nature. The distinct possibility exists that after being released into the circulation, irisin may have endocrine actions by binding to cell surface receptor(s) yet to be discovered. Irisin may also have paracrine and autocrine actions.
We found significant correlations between FNDC5 and PGC1-α mRNA levels in human muscle, which suggests that, similar to mice [10], PGC1-α is the major factor for irisin regulation in humans. PPARγ and PGC1-α are both induced by physical training in human muscle [21]. Therefore, irisin could also be partly regulated by PPARγ activity. In our study, there was a marginal correlation between PPARγ and FNDC5 mRNA levels, which may become significant with larger data groups. In human subcutaneous fat, PGC1-α and PPARγ mRNA levels are also upregulated by physical training [21], and deficiency in adipose tissue PGC1-α in mice leads to systemic insulin resistance. Although irisin is expressed in low levels in fat (Fig. 1), further studies are needed to find the underlying mechanism in regulation of FNDC5 expression in muscle as well as its role in adipose tissue.
To gain insights into the predictors and physiological regulation of circulating irisin at the whole organism level, we conducted cross-sectional and interventional studies. Correlation analysis between circulating irisin levels and anthropometric parameters showed that age-related muscle loss leads to lower irisin levels in blood (Fig. 3 and Table 2A). A strong positive correlation between irisin and biceps circumference, a marker of muscle mass, persisted after adjusting for age, menopausal status, smoking, estradiol levels, and fat-free mass, indicating that muscle mass is the main predictor of circulating irisin levels in humans (Fig. 3, Table 3). Considering that reduction in BMI leads to restoration of metabolic balance including decreased leptin and insulin levels (Fig. 5A–D), one would expect irisin levels to be increased by bariatric surgery-induced weight loss if it is to mediate such beneficial effects on metabolism. In this study, circulating irisin levels as well as muscle FNDC5 gene expression were significantly downregulated 6 months post-operation (Fig. 5E and F). Although fat-free mass did not change significantly, adjustment for changes in fat-free mass rendered the significant decrease of irisin concentrations null suggesting that the latter may simply reflect changes in muscle mass. Whether decreasing FNDC5 gene expression and circulating irisin levels are due to decreasing, albeit non-significantly, muscle mass, or whether surgically induced weight loss has a direct effect to reduce irisin levels remains to be confirmed and conclusively shown by future larger studies. In any case, the decreasing irisin concentrations cannot be directly responsible for the increasing energy expenditure and improvement of insulin resistance observed in response to weight loss or the improved metabolic profile after bariatric surgery [18,19,22].
Additional factors that were significant predictors of circulating irisin herein included ghrelin and IGF-1. Ghrelin is known to stimulate the release of growth hormone (GH) from the pituitary gland and thus mediate the growth of muscle mass and breakdown of fat [23]. In addition to its direct effects, GH stimulates secretion of IGF-1, which conveys growth actions in the periphery [24,25]. In our cross-sectional study in healthy women, irisin was positively correlated with ghrelin, IGF-1 and the IGF-1/IGFBP-3 ratio, implying that growth hormones may be responsible for the secretion of irisin through a positive effect on muscle mass and/or beyond their effect to increase muscle mass, as indicated by our multivariable models. Estradiol and testosterone also induce protein anabolism in muscle [26,27]. In healthy women, estradiol levels were significantly and positively correlated with circulating irisin levels. Estradiol may either directly induce irisin secretion or act through anabolic pathways to increase muscle mass and upregulate irisin or simply mediate the effects of aging, since estradiol levels decrease with advanced age. Decrease in anabolic steroids during aging results in decreased muscle mass [28] and, therefore, the negative correlation of irisin with age can also be explained by age-induced muscle loss. Whether testosterone, along with the aforementioned hormones, has similar effects on irisin needs to be investigated in future interventional studies. In obese subjects undergoing bariatric surgery, irisin levels were higher in females compared to males, although not significantly, implying that the physiological role of irisin may differ by gender.
To our surprise, circulating adiponectin levels were inversely correlated with irisin levels; moreover, irisin had positive correlations with glucose, total cholesterol, and several GHs (Table 2A and Fig. 4). The fact that the circulating irisin levels are positively associated with hormones and parameters reflecting dysregulated metabolism may suggest a compensatory role for irisin in response to deterioration of insulin sensitivity and glucose/lipid metabolism. Increased irisin in response to low adiponectin levels observed in central obesity may create a positive feedback on metabolic enzymes to consume more energy in adipocytes. In the previous study [10], adiponectin was also decreased by FNDC5 treatment in primary subcutaneous adipocytes. Although the authors did not suggest any possible mechanism for this phenomenon, it is possible that irisin may exert a direct effect on white adipocyte metabolism other than browning of subcutaneous adipose tissue. This important topic needs to be clarified by future interventional studies.
The interventional studies we report herein have unveiled the effect of acute exercise and drastic weight loss by bariatric surgery on irisin levels. As shown in Fig. 6, acute sprint training, an anaerobic exercise, induced ATP deprivation followed by activation of glycolysis for prompt ATP resynthesis. Circulating irisin levels were relatively higher in these young healthy adults compared to other populations shown in this study, and an increase in the serum irisin concentration was observed in as little as 30min after the end of exercise, but not after 8 weeks of training. These data raise the hypothesis that when the ATP concentration in muscle is decreased, irisin production is upregulated. Whether increased secretion of irisin contributes to enhanced ATP production through glycolysis and lipolysis in mitochondria remains to be seen. Thus, irisin may have a short term effect to restore ATP homeostasis but may return to baseline soon after ATP levels are restored. This hypothesis remains to be tested by time course experiments after acute and chronic aerobic and anaerobic exercise. Moreover, any effects of physiological concentrations of irisin, possibly through PGC1-α/AMPK axis, on ATP production by adipocytes or myocytes should be directly tested in the future. If these hypotheses are confirmed, the role of irisin may prove to be no different than that of other myokines, such as IL-6, which increase immediately after exercise to regulate thermogenesis and metabolism but are negatively associated with metabolic variables in the long term [29]. In any case, if the proposed beneficial effects of irisin are proven, the distinct possibility exists that irisin could be synthesized using recombinant DNA technology and tested as a possible novel therapeutic agent for metabolic disorders including diabetes and obesity associated malignancies [30].
This study strongly demonstrates clinically significant associations of circulating irisin and muscle irisin expression with anthropometric, metabolic, and hormonal parameters to gain a wide perspective on the physiological role of irisin in humans. As much as our study has provided valuable and novel information about irisin, it has also opened the door to many questions yet to be answered. The causality of the observed associations between study parameters should be more intensively studied in prospective cohort and interventional studies. Identification of the receptor system for irisin and relevant signaling pathways is necessary to advance an in-depth understanding of irisin in humans.
In summary, we have presented molecular and clinical data from cross-sectional and interventional studies to elucidate the physiology of irisin in humans, and we have demonstrated that muscle mass is the primary predictor of the circulating irisin concentration, underlying the association between irisin and metabolic factors. Circulating irisin levels increased in response to acute exercise and decreased after surgically induced weight loss. Further studies are needed to discover the mechanisms underlying the regulation of irisin levels as well as its physiological effects in humans.
Acknowledgments
The authors would like to thank Drs Yannakouris and Kontogianni for contributions in early phases of the cross-sectional study and to Daria Lisicki for editorial assistance.
Funding
The Mantzoros Laboratory is supported by the National Institute of Diabetes and Digestive and Kidney Diseases grants 58785, 79929 and81913 as well as Award Number 1I01CX000422-01A1 from the Clinical Science Research and Development Service of the VA Office of Research and Development.
Abbreviations
- BMI
body mass index
- GH
growth hormone
- IGF-1
insulin-like growth factor 1
- IGFBP-3
insulin-like growth factor binding protein 3
- PGC1-α
PPARγ coactivator 1 alpha
- PPARγ
peroxisome proliferator-activated receptor gamma
- UCP1
uncoupling protein 1
- WHR
waist-to-hip ratio.
Footnotes
Author contributions
JYH and GP performed experiments and statistical evaluations, VM, MB, BS and MV collected data, CSM designed the study and oversaw its performance. All authors contributed to writing the manuscript.
Conflict of interest
The authors do not have any conflict of interest related to this manuscript.
REFERENCES
- 1.Ogden CL, Carroll MD, Kit BK, et al. Prevalence of obesity in the United States, 2009–2010. NCHS Data Brief. 2012;82:1–8. [PubMed] [Google Scholar]
- 2.Tuomilehto J, Lindstrom J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344(18):1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 3.Nocon M, Hiemann T, Muller-Riemenschneider F, et al. Association of physical activity with all-cause and cardiovascular mortality: a systematic review and meta-analysis. Eur J Cardiovasc Prev Rehabil. 2008;15(3):239–246. doi: 10.1097/HJR.0b013e3282f55e09. [DOI] [PubMed] [Google Scholar]
- 4.Thompson D, Karpe F, Lafontan M, et al. Physical activity and exercise in the regulation of human adipose tissue physiology. Physiol Rev. 2012;92(1):157–191. doi: 10.1152/physrev.00012.2011. [DOI] [PubMed] [Google Scholar]
- 5.Pinto A, Di Raimondo D, Tuttolomondo A, et al. Effects of physical exercise on inflammatory markers of atherosclerosis. Curr Pharm Des. 2012 doi: 10.2174/138161212802481192. [epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 6.Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol. 2012;8(8):457–465. doi: 10.1038/nrendo.2012.49. [DOI] [PubMed] [Google Scholar]
- 7.Pedersen BK. The diseasome of physical inactivity — and the role of myokines in muscle-fat cross talk. J Physiol. 2009;587(Pt 23):5559–5568. doi: 10.1113/jphysiol.2009.179515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Febbraio MA, Hiscock N, Sacchetti M, et al. Interleukin-6 is a novel factor mediating glucose homeostasis during skeletal muscle contraction. Diabetes. 2004;53(7):1643–1648. doi: 10.2337/diabetes.53.7.1643. [DOI] [PubMed] [Google Scholar]
- 9.Handschin C, Choi CS, Chin S, et al. Abnormal glucose homeostasis in skeletal muscle-specific pgc-1alpha knockout mice reveals skeletal muscle-pancreatic beta cell crosstalk. J Clin Invest. 2007;117(11):3463–3474. doi: 10.1172/JCI31785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bostrom P, Wu J, Jedrychowski MP, et al. A pgc1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481(7382):463–468. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seale P, Conroe HM, Estall J, et al. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J Clin Invest. 2011;121(1):96–105. doi: 10.1172/JCI44271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Enerback S. Human brown adipose tissue. Cell Metab. 2010;11(4):248–252. doi: 10.1016/j.cmet.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 13.Timmons JA, Baar K, Davidsen PK, et al. Is irisin a human exercise gene? Nature. 2012;488(7413):E9–E10. doi: 10.1038/nature11364. [discussion E-1]. [DOI] [PubMed] [Google Scholar]
- 14.Lee JH, Chan JL, Yiannakouris N, et al. Circulating resistin levels are not associated with obesity or insulin resistance in humans and are not regulated by fasting or leptin administration: cross-sectional and interventional studies in normal, insulin-resistant, and diabetic subjects. J Clin Endocrinol Metab. 2003;88(10):4848–4856. doi: 10.1210/jc.2003-030519. [DOI] [PubMed] [Google Scholar]
- 15.Saraslanidis P, Petridou A, Bogdanis GC, et al. Muscle metabolism and performance improvement after two training programmes of sprint running differing in rest interval duration. J Sports Sci. 2011;29(11):1167–1174. doi: 10.1080/02640414.2011.583672. [DOI] [PubMed] [Google Scholar]
- 16.Vamvini MT, Aronis KN, Chamberland JP, et al. Energy deprivation alters in a leptin- and cortisol-independent manner circulating levels of activin a and follistatin but not myostatin in healthy males. J Clin Endocrinol Metab. 2011;96(11):3416–3423. doi: 10.1210/jc.2011-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moragianni VA, Aronis KN, Chamberland JP, et al. Short-term energy deprivation alters activin a and follistatin but not inhibin b levels of lean healthy women in a leptin-independent manner. J Clin Endocrinol Metab. 2011;96(12):3750–3758. doi: 10.1210/jc.2011-1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nijhuis J, Van Dielen FM, Buurman WA, et al. Ghrelin, leptin and insulin levels after restrictive surgery: a 2-year follow-up study. Obes Surg. 2004;14(6):783–787. doi: 10.1381/0960892041590980. [DOI] [PubMed] [Google Scholar]
- 19.Ballantyne GH, Gumbs A, Modlin IM. Changes in insulin resistance following bariatric surgery and the adipoinsular axis: role of the adipocytokines, leptin, adiponectin and resistin. Obes Surg. 2005;15(5):692–699. doi: 10.1381/0960892053923789. [DOI] [PubMed] [Google Scholar]
- 20.Villarroya F. Irisin, turning up the heat. Cell Metab. 2012;15(3):277–278. doi: 10.1016/j.cmet.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 21.Ruschke K, Fishbein L, Dietrich A, et al. Gene expression of ppargamma and pgc-1alpha in human omental and subcutaneous adipose tissues is related to insulin resistance markers and mediates beneficial effects of physical training. Eur J Endocrinol. 2010;162(3):515–523. doi: 10.1530/EJE-09-0767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vijgen GH, Bouvy ND, Teule GJ, et al. Increase in brown adipose tissue activity after weight loss in morbidly obese subjects. J Clin Endocrinol Metab. 2012;97(7):E1229–E1233. doi: 10.1210/jc.2012-1289. [DOI] [PubMed] [Google Scholar]
- 23.Van Der Lely AJ, Tschop M, Heiman ML, et al. Biological, physiological, pathophysiological, and pharmacological aspects of ghrelin. Endocr Rev. 2004;25(3):426–457. doi: 10.1210/er.2002-0029. [DOI] [PubMed] [Google Scholar]
- 24.Salvatori R. Growth hormone and igf-1. Rev Endocr Metab Disord. 2004;5(1):15–23. doi: 10.1023/B:REMD.0000016121.58762.6d. [DOI] [PubMed] [Google Scholar]
- 25.Welle S. Growth hormone and insulin-like growth factor-i as anabolic agents. Curr Opin Clin Nutr Metab Care. 1998;1(3):257–262. doi: 10.1097/00075197-199805000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Greising SM, Carey RS, Blackford JE, et al. Estradiol treatment, physical activity, and muscle function in ovarian-senescent mice. Exp Gerontol. 2011;46(8):685–693. doi: 10.1016/j.exger.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herbst KL, Bhasin S. Testosterone action on skeletal muscle. Curr Opin Clin Nutr Metab Care. 2004;7(3):271–277. doi: 10.1097/00075197-200405000-00006. [DOI] [PubMed] [Google Scholar]
- 28.Brown M. Skeletal muscle and bone: effect of sex steroids and aging. Adv Physiol Educ. 2008;32(2):120–126. doi: 10.1152/advan.90111.2008. [DOI] [PubMed] [Google Scholar]
- 29.Pedersen BK, Febbraio MA. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol Rev. 2008;88(4):1379–1406. doi: 10.1152/physrev.90100.2007. [DOI] [PubMed] [Google Scholar]
- 30.Sanchis-Gomar F, Lippi G, Mayero S, et al. Irisin: a new potential hormonal target for the treatment of obesity and type 2 diabetes. J Diabetes. 2012;4(3):196. doi: 10.1111/j.1753-0407.2012.00194.x. [DOI] [PubMed] [Google Scholar]