Abstract
Objectives
As part of ongoing efforts by the Columbia University College of Dental Medicine to devise community-based models of health promotion and care for local residents, we sought to answer the following query: “What contributes to self-rated oral health among community-dwelling older adults?”
Methods
The present study is cross sectional in design and centrally concerned with baseline data collected during community-based screenings of adults aged 50 years and older who agreed to participate in the ElderSmile program in northern Manhattan, New York City. The primary outcome measure of interest is self-rated oral health, which was assessed as follows: “Overall, how would you rate the health of your teeth and gums – excellent, good, fair, or poor?”
Results
More than a quarter (28.5 percent) of ElderSmile participants aged 50 years and older reported that their oral health was poor. After adjustment for age (in years), place of birth, educational level, and dental insurance status in a logistic regression model, recent visits to the dentist (within the past year versus more than a year ago) contributed to better self-rated oral health and non-Hispanic Black race/ethnicity, dentate (versus edentulous) status, tooth decay as measured by decayed missing filled teeth, and severe periodontal inflammation contributed to worse self-rated oral health in this population.
Conclusions
Recent dental care contributed to better self-rated oral health among community-dwelling older adults living in northern Manhattan. Significant gradients were evident in the caries experience and periodontal inflammation of dentate adults by self-rated oral health, suggesting that untreated oral disease contributes to poor self-rated oral health.
Keywords: self-rated oral health, older adults, community-based oral health care, edentulism, untreated dental caries, periodontal inflammation, oral pain, access to oral health care, oral health-related quality of life, oral health disparities
Introduction
The presence of extensive dental caries, periodontal disease, and tooth loss among older adults in the United States indicates that a sizable proportion of the aged population lacks access to or fails to use interventions that are effective in preventing and controlling oral disease (1). In advanced states, caries can involve the pulp of the tooth and destroy the tooth structure (2). Untreated gingivitis can advance to periodontitis, and the associated chronic inflammatory response leads to loss of tissues and bone that support the teeth, resulting in clinical inflammation, abscesses, and tooth mobility (3). If left untreated, pain from oral conditions can restrict activities of daily living and disturb sleep (4), and caries and periodontitis lead to tooth loss (5).
Unfortunately, there are few models of community-based interventions targeted to vulnerable older adults that are designed to both assess and more importantly meet their oral health needs. A notable exception is the ElderSmile clinical program of the Columbia University College of Dental Medicine (CDM); it currently consists of 27 prevention centers that are located at senior centers and other locations in which older adults gather in the largely impoverished communities of Harlem and Washington Heights/Inwood in northern Manhattan, New York City (6). The CDM elected to create a network of community-based prevention centers, in part, to access a local population of older adults who are not centrally interested in obtaining oral health care in order to intervene before disease is severe (7). The prevention centers host a combination of services, including the following: a) general presentations and discussions in both English and Spanish of oral health promotion in later life (e.g., potential oral health problems, how to choose oral health care products, and access to oral health care, including transportation issues); b) demonstrations of brushing and flossing techniques and care of prosthetic devices; and c) oral cancer and oral health examinations for older adults who elect to participate. Services are provided by two faculty dentists of the CDM, who were trained by the project director (6,7); dental students serve as recorders.
Self-perceptions of health status, as measured by general and oral health, have been shown to be independent predictors of access to and utilization of health care, functional ability, and mortality (8–12). In a community where pain is not central, self-perceptions of oral health status can potentially play an important role in influencing the extent to which older adults perceive access to oral health care and maintenance of oral health as important objectives. While dental research has traditionally focused on dental status and outcomes of treatment as assessed by oral health care providers, it is increasingly recognized that the perspective of patients is also important in understanding the burden of oral disease in the population (13). Even as previous research in population-based samples has examined sociodemographic differences in self-rated oral health (9,12), there is a dearth of local data on the self-rated oral health of older adults with limited resources that may be used in program planning. Hence, as part of ongoing efforts by the CDM to devise community-based models of health promotion and care for local residents, we sought to answer the following query: “What contributes to self-rated oral health among community-dwelling older adults?”
Methods
The present study is cross sectional in design and centrally concerned with baseline data collected during community-based screenings of adults aged 50 years and older who agreed to participate in the ElderSmile program. While the program targeted older adults (especially those aged 65 years and older), no one who sought services on the basis of age was turned away. Hence, middle-aged adults (those aged 50–64 years) are included in these analyses, and for the sake of brevity, all adults aged 50 years and older are referred to as older adults in this paper to distinguish them from younger adults (aged 18–49 years). Self-reported sociodemographic characteristics and health and health care information were provided by ElderSmile participants who took part in community-based oral health education and completed a screening questionnaire in either English or Spanish, according to their language preference. Oral health screenings were conducted by dentists in partnering prevention centers among ElderSmile participants who agreed to be clinically examined.
The primary outcome measure of interest is self-rated oral health, which was assessed by the following query: “Overall, how would you rate the health of your teeth and gums – excellent, good, fair, or poor?” As a first step, the self-rated oral health of each participant with teeth was compared with the assessment of her/his oral hygiene as rated by an ElderSmile dentist via a clinical examination (excellent, good, fair, or poor). The criteria for dentist-rated dental hygiene were based on plaque and calculus levels as follows: excellent = no visible plaque or calculus; good = minimal levels of plaque and calculus in only a few locations; fair = plaque and calculus throughout the mouth; and poor = extensive plaque and calculus throughout mouth.
The presence or absence of teeth was assessed by an ElderSmile dentist based upon a dentition of 28 teeth. Third molars were excluded from the analysis as they are often missing because of reasons other than dental caries or other oral diseases. Edentulism was defined as having no natural permanent teeth in the mouth (1). The following conditions were measured and calculated based upon the clinical examinations of ElderSmile participants: a) DT – number of decayed permanent teeth as assessed by gross lesions clearly visible to the unaided eye (radiographs were not used); b) MT –number of missing permanent teeth (14); c) FT – number of restored permanent teeth; d) decayed missing filled teeth (DMFT) – number of decayed, missing, and filled permanent teeth; e) DFT – number of decayed and filled permanent teeth; and f) ST – number of sound permanent teeth. No radiographs or periodontal probing was involved, which may lead to an underestimation of dental caries but not missing teeth. Finally, periodontal inflammation of each participant with teeth who was clinically examined by an ElderSmile dentist was assessed (severe, moderate, slight, and none). The criteria for dentist-rated periodontal inflammation were based on the color and texture of periodontal tissues as follows: none = pink, stippled tissue throughout the mouth; slight = inflammation in a few areas of the mouth; moderate = inflammation in multiple areas of the mouth; and severe = inflammation throughout the mouth or areas of significant inflammation in the mouth.
Sociodemographic characteristics examined included the following: age group (50–64, 65–74, or 75+ years), gender (female or male), race/ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic, or other), place of birth (mainland United States, Dominican Republic, Puerto Rico, or other), and highest level of education (primary, high school, or college). Health and health care characteristics examined included the following: smoking status (current smoker, former smoker, or never smoked), health insurance (yes or no) and type (Medicaid, Medicare, or private), time of last visit to doctor (≤1 year, 1–3 years, more than 3 years, or never), dental insurance (yes or no) and type (Medicaid or private), and time of last visit to dentist (≤1, 1–3, or more than 3 years).
Statistical analyses
Weighted kappa is widely used for ordered categorical data, such as self-rated oral health and dentist-rated oral hygiene (15,16). Whereas unweighted kappa does not distinguish among degrees of disagreement (17), weighted kappa incorporates the magnitude of each disagreement and provides partial credit for disagreements when agreement is not complete (18). The usual approach is to assign weights to each disagreement pair with larger weights indicating greater disagreement. Thus, a weighted kappa was calculated comparing self-rated oral health with dentist-rated oral hygiene.
Means and standard deviations were computed for continuous variables, and counts and percentages were computed for categorical variables (19). In addition to descriptive statistics, tests for comparison of means across categories of self-rated oral health were conducted for continuous variables, and tests of linear trend for ordinal data (Cochran–Mantel–Haenszel chi square) were conducted for categorical variables (19). Bivariable and multivariable logistic regression analyses were conducted with self-rated oral health (at least fair versus poor) as the outcome variable, and age (in years), race/ethnicity, place of birth, highest education level, dental insurance status, time of last visit to dentist, edentulism status, DMFT, and periodontal inflammation status as the predictor variables. All analyses were conducted in SAS version 9.1 (20).
Results
The characteristics of the ElderSmile program participants, overall and by age category (50–64 and 65+ years), are available in Table 1.
Table 1.
Sociodemographic, Health, and Health Care Characteristics of Adults Aged 50 Years and Older Who Participated in Community-Based Oral Health Education and Completed a Screening Questionnaire by Age Category and Overall (n = 870): The ElderSmile Program, New York, NY, August 2006 to March 2009
| Characteristic | Age category in years
|
||
|---|---|---|---|
| 50–64 (n = 141) | 65+ (n = 729) | Overall, 50+ (n = 870) | |
| Gender (n = 869) | |||
| Female | 103 (73.0%) | 478 (65.7%) | 581 (66.9%) |
| Male | 38 (27.0%) | 250 (34.3%) | 288 (33.1%) |
| Race/ethnicity (n = 763) | |||
| Non-Hispanic White | 10 (8.1%) | 108 (16.9%) | 118 (15.5%) |
| Non-Hispanic Black | 49 (39.5%) | 264 (41.3%) | 313 (41.0%) |
| Hispanic | 61 (49.2%) | 239 (37.4%) | 300 (39.3%) |
| Other | 4 (3.2%) | 28 (4.4%) | 32 (4.2%) |
| Place of birth (n = 865) | |||
| Mainland United States | 58 (41.1%) | 337 (46.6%) | 395 (45.7%) |
| Dominican Republic | 33 (23.4%) | 107 (14.8%) | 140 (16.2%) |
| Puerto Rico | 29 (20.6%) | 136 (18.8%) | 165 (19.1%) |
| Other | 21 (14.9%) | 144 (19.9%) | 165 (19.1%) |
| Highest education (n = 840) | |||
| Primary | 45 (32.4%) | 228 (32.5%) | 273 (32.5%) |
| High school | 58 (41.7%) | 269 (38.4%) | 327 (38.9%) |
| College | 36 (25.9%) | 204 (29.1%) | 240 (28.6%) |
| Smoking status (n = 752) | |||
| Current smoker | 25 (19.5%) | 64 (10.3%) | 89 (11.8%) |
| Former smoker | 38 (29.7%) | 201 (32.2%) | 239 (31.8%) |
| Never smoked | 65 (50.8%) | 359 (57.5%) | 424 (56.4%) |
| Health insurance (n = 856) | |||
| Yes | 118 (84.9%) | 677 (94.4%) | 795 (92.9%) |
| No | 21 (15.1%) | 40 (5.6%) | 61 (7.1%) |
| Time of last visit to doctor (n = 844) | |||
| ≤1 year | 105 (76.6%) | 572 (80.9%) | 677 (80.2%) |
| 1–3 years | 17 (12.4%) | 58 (8.2%) | 75 (8.9%) |
| >3 years | 5 (3.6%) | 41 (5.8%) | 46 (5.4%) |
| Never | 10 (7.3%) | 36 (5.1%) | 46 (5.4%) |
| Dental insurance (n = 828) | |||
| Yes | 78 (58.2%) | 324 (46.7%) | 402 (48.6%) |
| No | 56 (41.8%) | 370 (53.3%) | 426 (51.4%) |
| Time of last visit to dentist (n = 833) | |||
| ≤1 year | 57 (41.3%) | 317 (45.6%) | 374 (44.9%) |
| 1–3 years | 58 (42.0%) | 212 (30.5%) | 270 (32.4%) |
| >3 years | 23 (16.7%) | 166 (23.9%) | 189 (22.7%) |
Two-thirds (66.9 percent) of the sample were women, the large majority self-identified as non-Hispanic Black (41.0 percent) or Hispanic (39.3 percent), and over half were born outside of the mainland United States (54.3 percent), largely in Puerto Rico (19.1 percent) or the Dominican Republic (16.2 percent).
Agreement between self-rated oral health and dentist-rated oral hygiene was calculated using Cohen’s kappa statistic (15,16). The weighted kappa of 0.13 (95 percent confidence interval 0.07, 0.19) obtained is significantly greater than zero, implying a certain level of agreement between self-rated oral health and dentist-rated oral hygiene, even as this value of kappa is not considered very high (see Table 2 for the analysis and interpretation of agreement measured by Cohen’s kappa statistic) (17–19).
Table 2.
Analysis of Self-Rated Oral Health (in Rows) versus Dentist-Rated Oral Hygiene (in Columns) among Adults Aged 50 Years and Older with Teeth Who Completed a Baseline Questionnaire and Were Clinically Examined by a Dentist (n = 566): The ElderSmile Program, New York, NY, August 2006 to March 2009
| Frequency
|
Dentist-rated oral hygiene
|
|||||
|---|---|---|---|---|---|---|
| Row percent
|
Excellent | Good | Fair | Poor | Total | |
| Column percent | ||||||
| Self-rated oral health | Excellent | 1 | 6 | 1 | 1 | 9 |
| 11.11% | 66.67% | 11.11% | 11.11% | |||
| 4.76% | 2.47% | 0.45% | 1.23% | |||
| Good | 8 | 105 | 66 | 22 | 202 | |
| 3.98% | 52.24% | 32.84% | 10.95% | |||
| 38.10% | 43.21% | 29.86% | 27.16% | |||
| Fair | 6 | 71 | 82 | 22 | 181 | |
| 3.31% | 39.23% | 45.30% | 12.15% | |||
| 28.57% | 29.22% | 37.10% | 27.16% | |||
| Poor | 6 | 61 | 72 | 36 | 175 | |
| 3.43% | 34.86% | 41.14% | 20.57% | |||
| 28.57% | 25.10% | 32.58% | 44.44% | |||
| Total | 21 | 243 | 221 | 81 | 566 | |
| Agreement measured by Cohen’s kappa statistic
| ||||||
|---|---|---|---|---|---|---|
| Simple kappa statistic
| ||||||
| Value | Standard error | 95% CI | Testing H0: kappa = 0
|
|||
| Standard error under H0 | Z-score | One-sided P-value | Two-sided P-value | |||
| 0.1086 | 0.0288 | (0.0520, 0.1651) | 0.0276 | 3.9384 | <0.0001 | <0.0001 |
| Weighted kappa statistic
| ||||||
|---|---|---|---|---|---|---|
| Value | Standard error | 95% CI | Testing H0: kappa = 0
|
|||
| Standard error under H0 | Z-score | One-sided P-value | Two-sided P-value | |||
| 0.1312 | 0.0304 | (0.0716, 0.1908) | 0.0294 | 4.4624 | <0.0001 | <0.0001 |
A positive value of kappa indicates inter-rater agreement; kappa = 1 stands for perfect agreement, kappa = 0 means lack of agreement (agreement by pure chance), and a negative value of kappa indicates negative agreement. In the present analysis, kappa (both simple and weighted) is “significantly greater than zero,” implying some agreement between self-rated oral health and dentist-rated oral hygiene, although not very high agreement. “Weighted kappa may be more suitable here because the categories are ordered.”
CI, confidence interval.
The major difference was that patients were much more likely to self-report their oral health as poor (30.9 percent) than dentists were to rate of the oral hygiene of these same patients as poor (14.3 percent).
Next, the distribution of self-rated oral health over selected sociodemographic, health, and health care characteristics was examined (Table 3).
Table 3.
Distribution of Self-Rated Oral Health over Sociodemographic, Health, and Health Care Characteristics of Adults Aged 50 Years and Older Who Participated in Community-Based Oral Health Education and Completed a Screening Questionnaire (n = 776): The ElderSmile Program, New York, NY, August 2006 to March 2009
| Characteristic (n*) | Self-rated oral health
|
||||
|---|---|---|---|---|---|
| n (%) excellent | n (%) good | n (%) fair | n (%) poor | P-value† | |
| Age in years (n = 776) | |||||
| 50–64 | 2 (1.5) | 48 (36.6) | 45 (34.4) | 36 (27.5) | 0.29 |
| 65–74 | 7 (2.3) | 103 (34.0) | 101 (33.3) | 92 (30.4) | |
| 75+ | 11 (3.2) | 134 (39.2) | 104 (30.4) | 93 (27.2) | |
| Gender (n = 775) | |||||
| Female | 12 (2.3) | 196 (37.5) | 156 (29.8) | 159 (30.4) | 0.41 |
| Male | 8 (3.2) | 89 (35.3) | 93 (36.9) | 62 (24.6) | |
| Race/ethnicity (n = 687) | |||||
| Non-Hispanic White | 2 (1.9) | 47 (43.9) | 33 (30.8) | 25 (23.4) | 0.04 |
| Non-Hispanic Black | 8 (2.9) | 79 (28.2) | 92 (32.9) | 101 (36.1) | |
| Hispanic | 9 (3.3) | 112 (40.6) | 95 (34.4) | 60 (21.7) | |
| Other | 0 (0.0) | 11 (45.8) | 4 (16.7) | 9 (37.5) | |
| Place of birth (n = 773) | |||||
| Mainland United States | 5 (1.4) | 113 (32.1) | 115 (32.7) | 119 (33.8) | <0.01 |
| Dominican Republic | 4 (3.1) | 45 (35.2) | 50 (39.1) | 29 (22.7) | |
| Puerto Rico | 5 (3.4) | 68 (45.6) | 40 (26.8) | 36 (24.2) | |
| Other | 6 (4.2) | 58 (40.3) | 44 (30.6) | 36 (25.0) | |
| Highest education (n = 754) | |||||
| Primary | 8 (3.3) | 97 (39.6) | 84 (34.3) | 56 (22.9) | 0.01 |
| High school | 8 (2.7) | 107 (35.9) | 97 (32.6) | 86 (28.9) | |
| College | 4 (1.9) | 71 (33.6) | 62 (29.4) | 74 (35.1) | |
| Smoking status (n = 681) | |||||
| Current smoker | 2 (2.5) | 30 (37.0) | 24 (29.6) | 25 (30.9) | 0.12 |
| Former smoker | 3 (1.4) | 70 (31.8) | 77 (35.0) | 70 (31.8) | |
| Never smoked | 12 (3.2) | 150 (39.5) | 115 (30.3) | 103 (27.1) | |
| Health insurance (n = 765) | |||||
| Yes | 19 (2.7) | 263 (36.8) | 235 (32.9) | 198 (27.7) | 0.11 |
| No | 1 (2.0) | 17 (34.0) | 10 (20.0) | 12 (38.7) | |
| Type of health insurance (n = 703) | |||||
| Medicaid | 10 (3.4) | 111 (38.1) | 100 (34.4) | 70 (24.0) | 0.18 |
| Medicare | 7 (1.9) | 136 (36.8) | 114 (30.8) | 113 (30.5) | |
| Private | 2 (4.8) | 13 (31.0) | 16 (38.1) | 11 (26.2) | |
| Time of last visit to doctor (n = 758) | |||||
| Less than 1 year | 17 (2.8) | 231 (37.8) | 198 (32.4) | 166 (27.1) | 0.28 |
| 1–3 years | 0 (0.0) | 24 (36.4) | 22 (33.3) | 20 (30.3) | |
| More than 3 years | 0 (0.0) | 13 (33.3) | 12 (30.8) | 14 (35.9) | |
| Never | 2 (4.9) | 13 (31.7) | 14 (34.2) | 12 (29.3) | |
| Dental insurance (n = 744) | |||||
| Yes | 14 (3.8) | 144 (38.9) | 119 (32.2) | 93 (25.1) | 0.01 |
| No | 6 (1.6) | 129 (34.5) | 120 (32.1) | 119 (31.8) | |
| Type of dental insurance (n = 319) | |||||
| Medicaid | 11 (5.0) | 88 (39.6) | 76 (34.2) | 47 (21.2) | 0.08 |
| Private | 2 (2.1) | 35 (36.1) | 31 (32.0) | 29 (29.9) | |
| Time of last visit to dentist (n = 748) | |||||
| Less than 1 year | 12 (3.5) | 142 (41.3) | 103 (29.9) | 87 (25.3) | <0.01 |
| 1–3 years | 3 (1.2) | 79 (32.5) | 90 (37.0) | 71 (29.2) | |
| More than 3 years | 5 (3.1) | 50 (31.1) | 47 (29.2) | 59 (36.6) | |
| Overall | 20 (2.6) | 285 (36.7) | 250 (32.2) | 221 (28.5) | |
Differences where p ≤ 0.05 are highlighted in bold.
Sample sizes (hence denominators used to compare percentages) may vary across characteristics because of missing values.
P-values correspond to testing of linear trend for ordinal data using the Cochran–Mantel–Haenszel chi-square test.
Findings were that those who were non-Hispanic Black or of other race/ethnicity (versus non-Hispanic White or Hispanic), born in the mainland United States (versus the Dominican Republic, Puerto Rico, or elsewhere), attained a college education (versus primary or high school), lacked dental insurance (versus had dental insurance), and had not visited a dentist in more than 3 years (versus less than 3 years) were statistically significantly more likely to self-report their oral health as poor (Table 3).
To further understand the contributors to self-rated oral health in this population, clinical measures obtained through community-based oral examinations by dentists were used. First, participants were grouped into those with teeth (dentate) and those without teeth (edentulous). Results were that edentulous adults reported significantly better self-rated health than their dentate counterparts (Table 4).
Table 4.
Distribution of Self-Rated Oral Health for Dentate (with Some Teeth) and Edentate (with No Natural Teeth) Adults Aged 50 Years and Older Who Participated in Community-Based Oral Health Examinations Conducted by Dentists (n = 708): The ElderSmile Program, New York, NY, August 2006 to March 2009
| Self-reported oral health | Dentate adults (n = 586) | Edentate adults (n = 122) | P-value* |
|---|---|---|---|
| Excellent | 9 (1.5%) | 10 (8.2%) | <0.01 |
| Good | 208 (35.5%) | 49 (40.2%) | |
| Fair | 185 (31.6%) | 44 (36.1%) | |
| Poor | 184 (31.4%) | 19 (15.6%) |
P-value corresponds to the test of linear trend for ordinal data using the Cochran–Mantel–Haenszel chi-square test.
Thus, edentulous adults were removed from the next set of analyses to better understand the contributors to poor self-rated oral health among dentate adults. In Table 5, the dental caries experience of ElderSmile participants with teeth by self-rated oral health is presented using a variety of indicators.
Table 5.
Means and Standard Errors (SE) of Decayed Teeth (DT), Missing Teeth (MT), Filled Teeth (FT), Decayed Missing Filled Teeth (DMFT), Decayed Filled Teeth (DFT), and Sound Teeth (ST) by Self-Rated Oral Health of Adults Aged 50 Years and Older with Teeth (n = 597*) Who Participated in Community-Based Oral Health Examinations Conducted by Dentists: The ElderSmile Program, New York, NY, August 2006 to March 2009
| Self-rated oral health | Mean DT (SE) | P-value† | Mean MT (SE) | P-value† | Mean FT (SE) | P-value† | Mean DMFT (SE) | P-value† | Mean DFT (SE) | P-value† | Mean ST (SE) | P-value† |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Excellent (n = 9) | 0.7 (0.3) | <0.01 | 5.3 (2.1) | <0.01 | 8.3 (1.4) | <0.01 | 14.3 (2.1) | <0.01 | 9.0 (1.4) | 0.46 | 12.7 (2.1) | <0.01 |
| Good (n = 208) | 0.7 (0.1) | 10.5 (0.6) | 7.1 (0.4) | 18.2 (0.4) | 7.8 (0.4) | 9.4 (0.4) | ||||||
| Fair (n = 185) | 1.0 (0.1) | 10.9 (0.5) | 6.5 (0.4) | 18.3 (0.4) | 7.4 (0.4) | 9.4 (0.4) | ||||||
| Poor (n = 184) | 1.9 (0.2) | 13.0 (0.5) | 5.1 (0.3) | 20.1 (0.4) | 7.1 (0.4) | 7.8 (0.4) |
Differences where p ≤ 0.05 are highlighted in bold.
Sample sizes may vary because of missing values.
P-values correspond to comparisons of means across categories of self-rated oral health.
Findings were that mean DT (decayed teeth), MT (missing teeth), and DMFT were significantly higher in those with poor (versus better) self-rated oral health, and mean FT (filled teeth) and ST (sound teeth) were significantly higher in those with excellent (versus worse) self-rated oral health. That is, untreated dental caries were associated with lower self-rated oral health, and treated dental caries were associated with higher self-rated oral health among ElderSmile participants.
In Table 6, the contribution of periodontal inflammation to self-rated oral health among ElderSmile participants with teeth is presented.
Table 6.
Distribution of Periodontal Inflammation over Self-Rated Oral Health of Adults Aged 50 Years and Older with Teeth (n = 568*) Who Participated in Community-Based Oral Health Examinations Conducted by Dentists: The ElderSmile Program, New York, NY, August 2006 to March 2009
| Self-rated oral health | Periodontal inflammation
|
P-value* | |||
|---|---|---|---|---|---|
| Severe (n = 21) | Moderate (n = 115) | Slight (n = 282) | None (n = 150) | ||
| Excellent | 0 (0.0%) | 0 (0.0%) | 5 (1.8%) | 4 (2.7%) | <0.01 |
| Good | 4 (19.1%) | 32 (27.8%) | 105 (37.2%) | 60 (40.0%) | |
| Fair | 7 (33.3%) | 38 (33.0%) | 86 (30.5%) | 46 (30.7%) | |
| Poor | 10 (47.6%) | 45 (39.1%) | 86 (30.5%) | 40 (26.7%) | |
P-value corresponds to the test of linear trend for ordinal data using the Cochran–Mantel–Haenszel chi-square test.
Note that among adults with severe periodontal inflammation, fully 47.6 percent self-rated their oral health as poor (versus 26.7 percent for those with no periodontal inflammation).
To investigate the joint contribution of multiple factors on self-rated oral health, logistic regression was used. A binary version of self-rated oral health (at least fair versus poor) was consistent with the cutpoint indicated in the stratified analyses and yielded the models with the best goodness of fit. Unadjusted and adjusted results are presented in Table 7.
Table 7.
Unadjusted and Adjusted Odds Ratios (ORs) and 95% Confidence Intervals (CIs) of the Binary Version of Self-Rated Oral Health (At Least Fair versus Poor) by Age, Race/Ethnicity, Place of Birth, Education Level, Dental Insurance Status, Time of Last Visit to Dentist, Edentulism Status, Decayed Missing Filled Teeth (DMFT), and Periodontal Inflammation Status of Adults Aged 50 Years and Older (n = 7081) Who Participated in Community-Based Oral Health Examinations Conducted by Dentists: The ElderSmile Program, New York, NY, August 2006 to March 2009
| Unadjusted OR (95% CI) | Adjusted OR (95% CI) | |
|---|---|---|
| Age (in years) | 0.99 (0.97,1.01) | 0.98 (0.96,1.00) |
| Race/ethnicity | ||
| Non-Hispanic White | 1.00 | 1.00 |
| Non-Hispanic Black | 0.63 (0.37,1.08) | 0.52 (0.28,0.95) |
| Hispanic | 1.28 (0.73,2.25) | 1.13 (0.51,2.49) |
| Other | 0.70 (0.25,1.97) | 0.41 (0.13,1.27) |
| Place of birth | ||
| Mainland United States | 1.00 | 1.00 |
| Dominican Republic | 1.50 (0.86,2.59) | 0.63 (0.26,1.51) |
| Puerto Rico | 1.82 (1.03,3.21) | 0.86 (0.37,2.01) |
| Other | 1.74 (1.03,2.92) | 1.42 (0.77,2.63) |
| Education level | ||
| Primary | 1.00 | 1.00 |
| High school | 0.76 (0.47,1.21) | 0.87 (0.51,1.46) |
| College | 0.61 (0.38,0.99) | 0.67 (0.38,1.17) |
| Dental insurance status | ||
| No | 1.00 | 1.00 |
| Yes | 1.17 (0.81,1.69) | 1.12 (0.74,1.68) |
| Time of last visit to dentist | ||
| ≥3 years | 1.00 | 1.00 |
| 1–3 years | 1.59 (0.97,2.63) | 1.65 (0.97,2.83) |
| ≤1 year | 1.78 (1.12,2.83) | 2.02 (1.20,3.39) |
| Edentulism status | ||
| No natural teeth | 1.00 | 1.00 |
| Some natural teeth | 0.43 (0.23,0.81) | 0.25 (0.11,0.58) |
| DMFT | 0.97 (0.94,1.00) | 0.93 (0.90,0.97) |
| Periodontal inflammation status | ||
| None | 1.00 | 1.00 |
| Slight | 0.66 (0.42,1.02) | 0.72 (0.43,1.20) |
| Moderate | 0.45 (0.26,0.75) | 0.58 (0.32,1.05) |
| Severe | 0.33 (0.12,0.91) | 0.46 (0.15,1.40) |
Differences where p ≤ 0.05 are highlighted in bold.
P-value corresponds to the test of linear trend for ordinal data using the Cochran-Mantel-Haenszel chi-square test.
Even after adjustment for the other factors in the model, recent visits to the dentist (within the past year versus more than a year ago) contributed to better self-rated oral health and non-Hispanic Black race/ethnicity, dentate (versus edentulous) status, tooth decay as measured by DMFT, and severe periodontal inflammation contributed to worse oral health among ElderSmile participants.
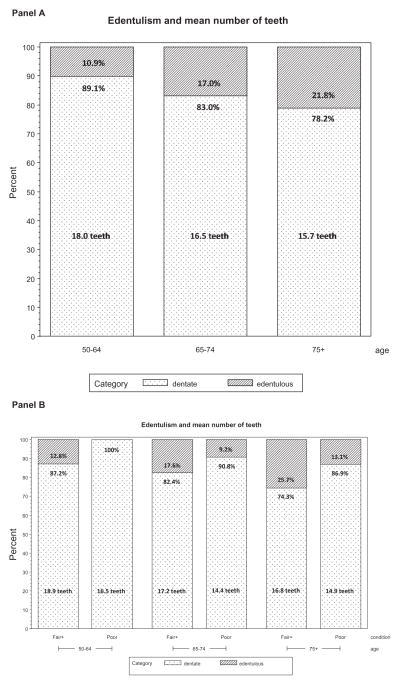
Finally, Figure 1 graphically displays the relationship between tooth loss and self-reported oral health.
Figure 1.
Tooth loss and self-reported oral health (at least fair versus poor) by age group (50–64, 65–74, and 75+ years) among adults aged 50 years and older who participated in community-based oral health examinations conducted by dentists (n = 708): The ElderSmile Program, New York, NY, August 2006 to March 2009. The mean number of teeth for dentate adults is specified in the lightly shaded region of each bar.
As highlighted in panel A, edentulism increases with age, but even in the group aged 75 years and older, four of five adults still retain an average of 15 teeth. In panel B, it is evident that in all age groups, the percentage of edentulous adults is higher in those reporting at least fair versus poor self-rated oral health. Nonetheless, among the dentate, the number of teeth for those with poor self-rated oral health is less than the number of teeth for those with at least fair self-rated oral health in all age groups.
Discussion
This study contributes to a better understanding of the self-rated oral health of community-dwelling older adults who were examined at senior centers and other venues where older adults gather, rather than those who were seeking oral health care per se. Overall, more than a quarter (28.5 percent) of participants aged 50 years and older reported that their oral health was poor. This appears to be an important cutpoint (i.e., at least fair versus poor) in this community-based sample of older adults based upon the stratified analyses and the goodness of fit of the logistic regression models. It may be that what matters most for underserved older adults in rating their oral health is pain related to untreated disease, rather than aesthetics or oral hygiene, and thus there appears to be a lower cutpoint in the ElderSmile program sample than that found in other population-based samples (i.e., excellent or good versus fair or poor).
An important take-home message from this study is that older adults were perceptive regarding their self-rated oral health. Edentulous adults were significantly more likely to rate their oral health as fair or better in comparison with dentate adults. When edentulous adults were removed from the analyses, significant gradients were evident in the caries experience of dentate adults by self-rated oral health. This suggests that untreated oral disease influences self-reported oral health among older adults. Conversely, the highest mean value of filled teeth – a measure of dental care received – was found among adults with excellent self-rated oral health. In the same way, the degree of periodontal inflammation among dentate adults was significantly related to self-rated oral health. The discomfort and bleeding that accompany severe and moderate periodontal inflammation may be responsible for the high percentages of poor self-rated oral health in the subsets of participating adults with these conditions (47.6 percent and 39.1 percent, respectively).
The different pattern of results between dentate and edentulous participants is not unexpected, as the two groups are clearly distinct and there is considerably more variation in self-reported oral health among dentate in comparison with edentulous older adults. The former are a broader group that includes people with a range of 1–28 teeth, whereas the latter are a more homogeneous group of people without any natural teeth. More importantly, edentulism is a crude and aggregate oral health status measure that reflects the accumulation of oral disease and experience of dental treatment throughout the life course. Once it occurs, it is irreversible. In essence, then, edentulism is a robust measure of total tooth mortality. When people become edentulous, too often they believe that they need to adapt to this condition and adjust their perceptions and expectations accordingly (21,22). Instead, a well-fitting prosthesis – especially if retained by an implant – is able to restore function, prevent bone resorption, and improve aesthetics. Evaluation for muscosal diseases and oral squamous cell carcinoma remains priorities for edentulous older adults; efforts are thus needed to counter the mis-perception that they no longer need dental care.
Among the strengths of this study is that it is part of the larger ElderSmile program at the CDM. This context permitted the leveraging of resources in the form of faculty and students to assist in the dental screenings and health promotion activities and better ensured high cooperation rates for the involved community partners, given ongoing relationships between the CDM and the administrators and staff at the involved prevention sites. In addition, few local data are available on the self-rated oral health of community-dwelling older adults, so this study adds to the public health literature regarding the utility and robustness of this measure. Among the limitations are that the assessments reported here were restricted by the field nature of the study. Second, older adults who were home-bound or institutionalized were not included in the study. Third, while DMFT is a useful summary measure for caries experience in the multivariable model, it does not take into account trauma (15) and thus future studies ought to explore the relationship between trauma and self-rated oral health. Finally, self-rated oral health was based on a single measure, namely, “Overall, how would you rate the health of your teeth and gums – excellent, good, fair, or poor?” While self-rated oral health has been found to have the strongest association with having frequent problems among factors examined in relation to oral health-related quality of life (23), it may fail to capture other dimensions of quality of life in older adults.
Nearly three decades ago, Mossey and Shapiro found convincing empirical support for the long held, but until then inadequately substantiated, belief that the way a person views her or his health is importantly related to subsequent health outcomes, including mortality (24). Recently, Peker and Bermek reported that self-rated oral health, sociodemographic factors, and oral health behaviors were significantly associated with oral health control beliefs, which may be useful for planning oral health promotion programs and for formulating advice given by oral health professionals (25). Of special interest is that Ostberg and Hall-Lord found that older persons with reported oral pain experienced very low oral and general health-related quality of life (26). This is consistent with the results presented here that untreated dental caries and severe periodontal inflammation are related to poor self-rated oral health in community-dwelling older adults.
Locker et al. sought to understand the referents and meanings that underlie self-ratings of oral health. Results were that biomedical and behavioral referents were the most common, including current oral problems and dental visiting patterns in concert with the current findings (27). There was some variation in the referents used according to sociodemographic characteristics, with age being the main source of variation, which was not found in the present study, likely due to higher numbers of edentulous adults at older ages. Further investigation exploring the nature and extent of discomfort and pain experienced in older adults, as well as more understanding of the frames of reference used with respect to self-ratings of oral health among immigrant populations and diverse racial and ethnic groups (28), is warranted. In the present study, participants who were born in Puerto Rico or in countries other than the Dominican Republic had better self-rated oral health than those who were born in the mainland United States in the unadjusted but not the adjusted logistic regression model. Meanwhile, participants who were non-Hispanic Black (largely from the mainland United States and the Dominican Republic) were more likely than other racial/ethnic groups to self-report poor oral health in the adjusted logistic regression model, perhaps due to fewer oral health providers in the communities where they reside (Harlem and the south Bronx versus Washington Heights and Inwood).
Conclusions
As the proportion of edentulous older adults decreases over time, there will be increased need for oral health services for dentate older adults (1). Programs such as ElderSmile that target underserved communities are needed to address the high levels of poor self-reported oral health among older adults. Jamieson and Thomson found that edentulism, poor self-rated oral health, and two or more years since last dental visit were most prevalent among those from low-socioeconomic status (SES) households who were resident in high-deprivation areas (29). On the other hand, respondents from high-SES households located in the least deprived areas had the lowest prevalence of edentulism, poor self-reported oral health, and two or more years since their last dental visit. In seeking to eliminate socioeconomic disparities in oral health, it may be most effective to target resources and clinical effort on people living in low-SES households in underserved areas rather than those living in low-SES households in wealthier areas (29). Unfortunately, programs and professionals that now comprise the dental safety net are insufficient to meet the burgeoning needs of older adults (30). If they are to continue to operate and be scaled up to cover larger numbers of impoverished older adults in underserved communities, policy reform that enhances federal and state financing of dental insurance and educational reform that includes community-based training for oral health professionals are key components of requisite comprehensive solutions.
Acknowledgments
The authors thank The Fan Fox and Leslie R. Samuels Foundation, Stella and Charles Guttman Foundation, and The Jean & Louis Dreyfus Foundation, Inc. for their financial support of the project, “The ElderSmile Dental Network: Delivering Dental Services to the Elderly in Northern Manhattan,” which provided major funding for the educational and screening components of the ElderSmile program. The authors were supported in the research, analysis, and writing of this paper by the National Institute for Dental and Craniofacial Research (grant 1R21DE021187-02) through the project, “Leveraging Opportunities to Improve Oral Health in Older Adults.” Finally, the authors thank Luisa N. Borrell, DDS, PhD, for helpful advice and feedback on the revised paper.
References
- 1.Dye BA, Tan S, Smith V, Lewis BG, Barker LK, Thornton-Evans G, Eke PI, Beltrán-Aguilar ED, Horowitz AM, Li CH. Trends in oral health status: United States, 1988–1994 and 1999–2004. Vital Health Stat 11. 2007;248:1–92. [PubMed] [Google Scholar]
- 2.Monse B, Heinrich-Weltzien R, Benzian H, Holmgren C, van Palenstein Helderman W. PUFA – an index of clinical consequences of untreated dental caries. Community Dent Oral Epidemiol. 2010;38(1):77–82. doi: 10.1111/j.1600-0528.2009.00514.x. [DOI] [PubMed] [Google Scholar]
- 3.Herrera D, Roldán S, González I, Sanz M. The periodontal abscess (I). Clinical and microbiological findings. J Clin Periodontol. 2000;27(6):387–94. doi: 10.1034/j.1600-051x.2000.027006387.x. [DOI] [PubMed] [Google Scholar]
- 4.US Department of Health and Human Services. Oral health in America: a report of the surgeon general. Rockville, Maryland: National Institute of Dental and Craniofacial Research; 2000. [Google Scholar]
- 5.Burt BA, Eklund SA. Dentistry, dental practice, and the community. 6. St. Louis: Elsevier Saunders; 2005. [Google Scholar]
- 6.Marshall S, Northridge ME, De La Cruz LD, Vaughan RD, O’Neil-Dunne J, Lamster IB. ElderSmile: a comprehensive approach to improving oral health for older adults. Am J Public Health. 2009;99(4):595–9. doi: 10.2105/AJPH.2008.149211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Northridge ME, Ue F, Borrell LN, Bodnar S, De La Cruz L, Marshall S, Lamster IB. Tooth loss and dental caries in community-dwelling older adults in northern Manhattan. Gerodontology. 2011 doi: 10.1111/j.1741-2358.2011. 00502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ries P. Americans assess their health: United States, 1987. Vital Health Stat 10. 1990;174:1–63. [PubMed] [Google Scholar]
- 9.Atchison KA, Gift HC. Perceived oral health in a diverse sample. Adv Dent Res. 1997;11(2):272–80. doi: 10.1177/08959374970110021001. [DOI] [PubMed] [Google Scholar]
- 10.Idler EL, Kasl SV. Self-ratings of health: do they also predict change in functional ability? J Gerontol B Psychol Sci Soc Sci. 1995;50(6):S344–353. doi: 10.1093/geronb/50b.6.s344. [DOI] [PubMed] [Google Scholar]
- 11.Idler EL, Benyamini Y. Self-rated health and mortality: a review of twenty-seven community studies. J Health Soc Behav. 1997;38(1):21–37. [PubMed] [Google Scholar]
- 12.Borrell LN, Baquero MC. Self-rated general and oral health in New York City adults: assessing the effect of individual and neighborhood social factors. Community Dent Oral Epidemiol. 2011 Jan 11;39:361–71. doi: 10.1111/j.1600-0528.2010.00603.x. [DOI] [PubMed] [Google Scholar]
- 13.Dahl KE, Wang NJ, Holst D, Ohrn K. Oral health-related quality of life among adults 68–77 years old in Nord-Trøndelag, Norway. Int J Dent Hyg. 2011;9(1):87–92. doi: 10.1111/j.1601-5037.2010.00445.x. [DOI] [PubMed] [Google Scholar]
- 14.Klein H, Palmer CE, Knutson JW. I. Dental status and dental needs of elementary school children. Public Health Rep. 1938;53:751–65. [Google Scholar]
- 15.Spitzer RL, Cohen J, Fleiss JL, Endicott J. Quantification of agreement in psychiatric diagnosis. Arch Gen Psychiatry. 1967;17(1):83–7. doi: 10.1001/archpsyc.1967.01730250085012. [DOI] [PubMed] [Google Scholar]
- 16.Cohen J. Weighted kappa: nominal scale agreement with provision for scaled disagreement or partial credit. Psychol Bull. 1968;70(4):213–20. doi: 10.1037/h0026256. [DOI] [PubMed] [Google Scholar]
- 17.Cohen J. A coefficient of agreement for nominal scales. Educ Psychol Meas. 1960;20(1):37–46. [Google Scholar]
- 18.Maclure M, Willett WC. Misinterpretation and misuse of the kappa statistic. Am J Epidemiol. 1987;126(2):161–9. doi: 10.1093/aje/126.2.161. [DOI] [PubMed] [Google Scholar]
- 19.Fleiss JL, Levin B, Paik MC. Statistical methods for rates and proportions. 3. Hoboken, NJ: John Wiley & Sons; 2003. [Google Scholar]
- 20.SAS Institute, Inc. SAS/STAT 9.1 user’s guide. Cary, NC: SAS Institute, Inc; 2004. [Google Scholar]
- 21.Carr AJ, Gobson B, Robinson PG. Measuring quality of life: is quality of life determined by expectations or experience? BMJ. 2001;322:1240–3. doi: 10.1136/bmj.322.7296.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacEntee MI, Hole R, Stolar E. The significance of the mouth in old age. Soc Sci Med. 1997;45:1449–58. doi: 10.1016/s0277-9536(97)00077-4. [DOI] [PubMed] [Google Scholar]
- 23.Dahl KE, Wang NJ, Skau I, Ohrn K. Oral health-related quality of life and associated factors in Norwegian adults. Acta Odontol Scand. 2011;69:208–14. doi: 10.3109/00016357.2010.549502. Early Online:1–7. [DOI] [PubMed] [Google Scholar]
- 24.Mossey JM, Shapiro E. Self-rated health: a predictor of mortality among the elderly. Am J Public Health. 1982;72:800–8. doi: 10.2105/ajph.72.8.800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peker K, Bermek G. Oral health: locus of control, health behavior, self-rated oral health and sociodemographic factors in Istanbul adults. Acta Odontol Scand. 2011;69:54–64. doi: 10.3109/00016357.2010.535560. [DOI] [PubMed] [Google Scholar]
- 26.Ostberg AL, Hall-Lord ML. Oral health-related quality of life in older Swedish people with pain problems. Scand J Caring Sci. 2011 Jan 5;25:510–6. doi: 10.1111/j.1471-6712.2010.00857.x. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 27.Locker D, Maggirias J, Wexler E. What frames of reference underlie self-ratings of oral health? J Public Health Dent. 2009;69(2):78–89. doi: 10.1111/j.1752-7325.2008.00103.x. [DOI] [PubMed] [Google Scholar]
- 28.Self-rated health among Hispanics vs non-Hispanic white adults: the San Luis Valley Health and Aging Study. Am J Public Health. 1996;86(12):1798–801. doi: 10.2105/ajph.86.12.1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jamieson LM, Thomson WM. Adult oral health inequalities described using area-based and household-based socioeconomic status measures. J Public Health Dent. 2006;66(2):104–9. doi: 10.1111/j.1752-7325.2006.tb02564.x. [DOI] [PubMed] [Google Scholar]
- 30.Edelstein B. The dental safety net, its workforce, and policy recommendations for its enhancement. J Public Health Dent. 2010;70:S32–9. doi: 10.1111/j.1752-7325.2010.00176.x. [DOI] [PubMed] [Google Scholar]