Abstract
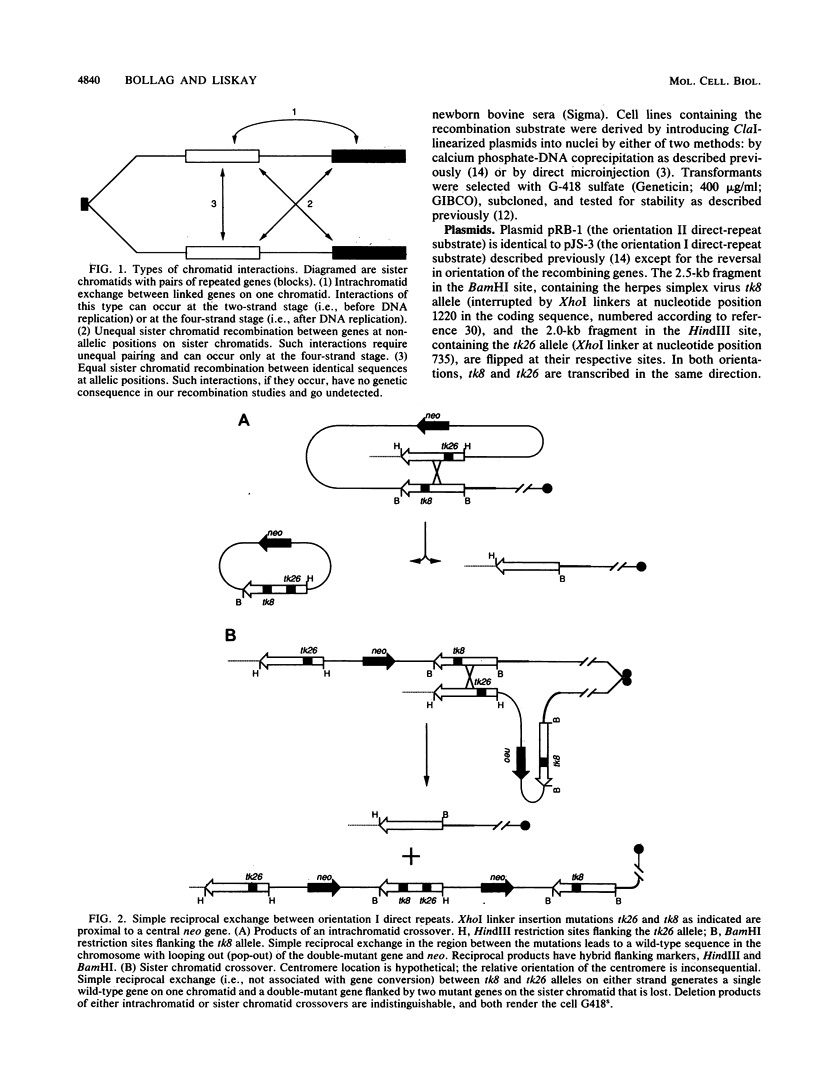
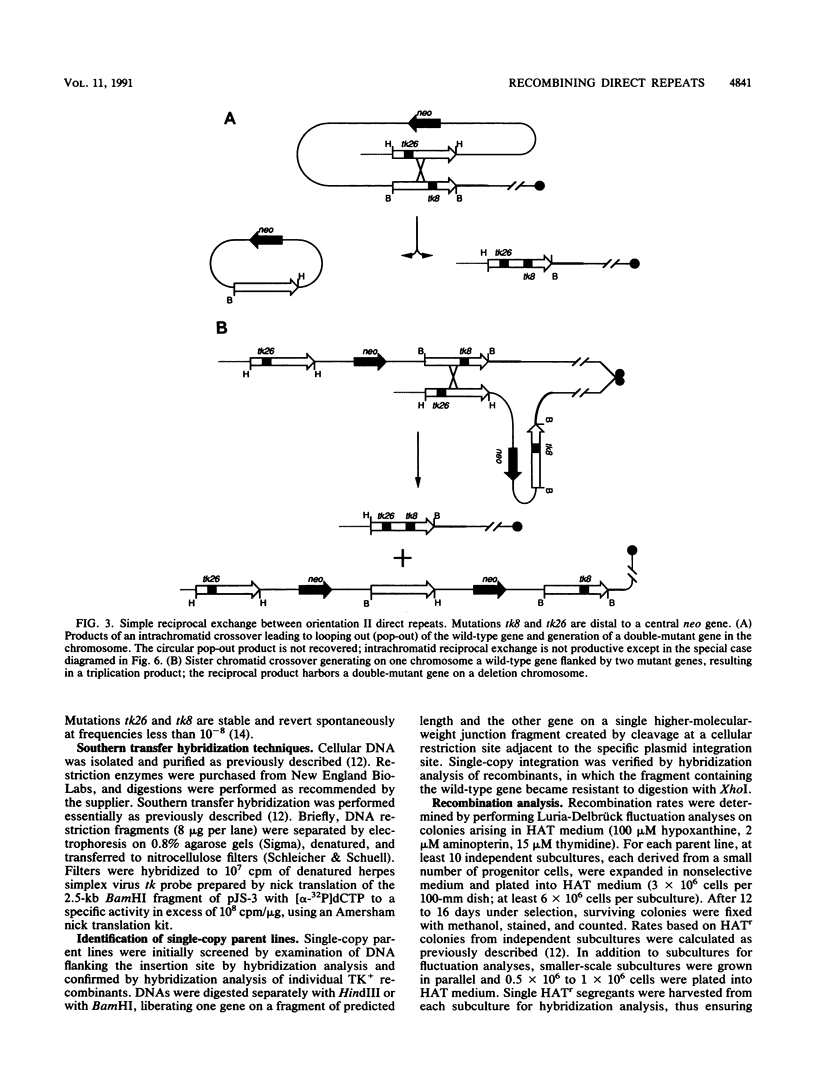
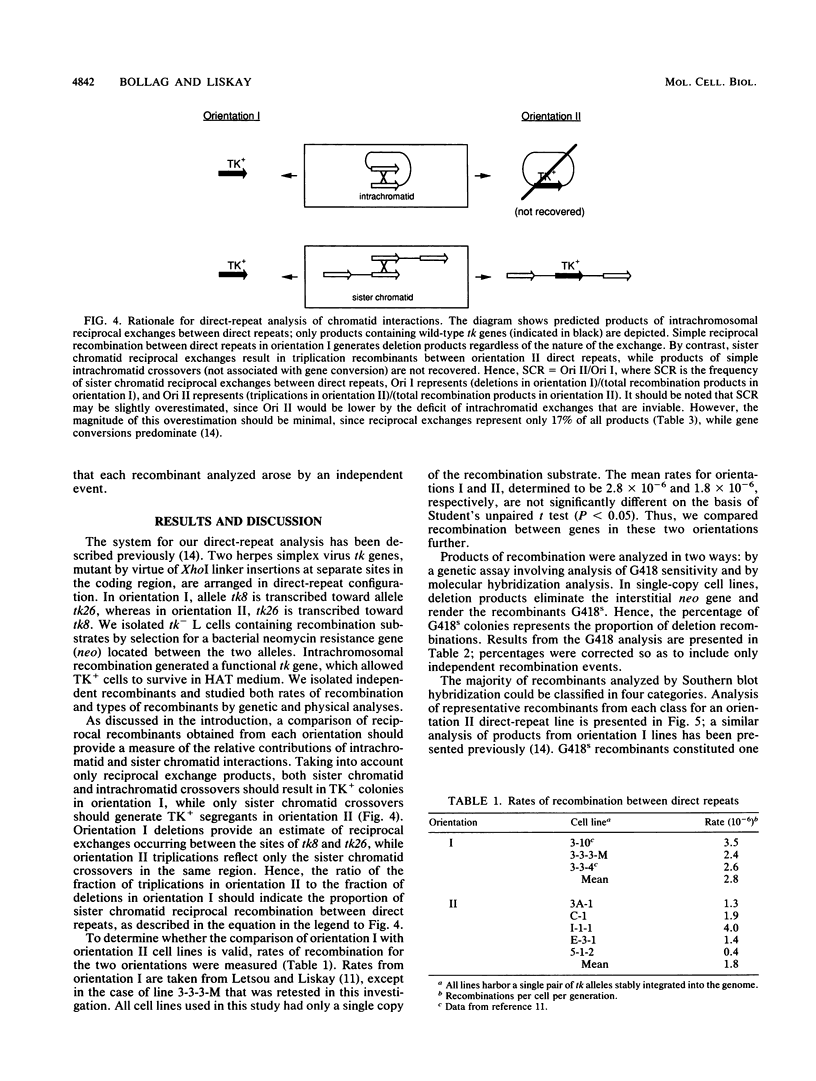
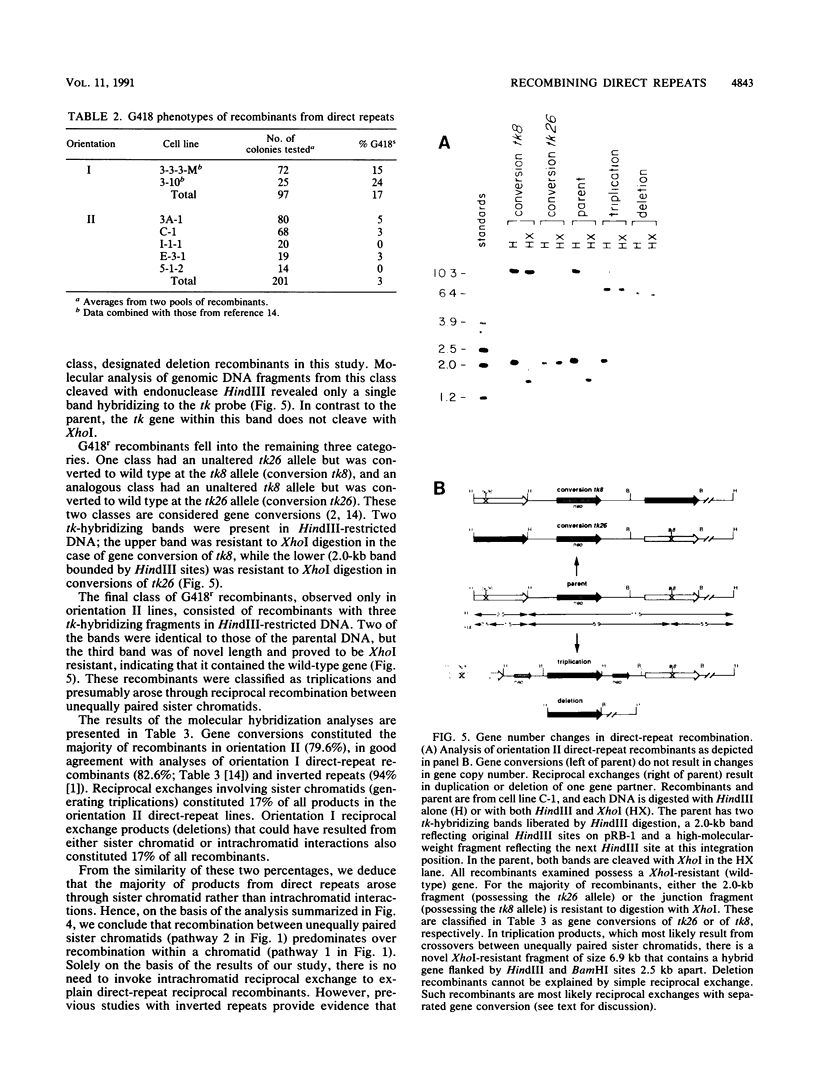
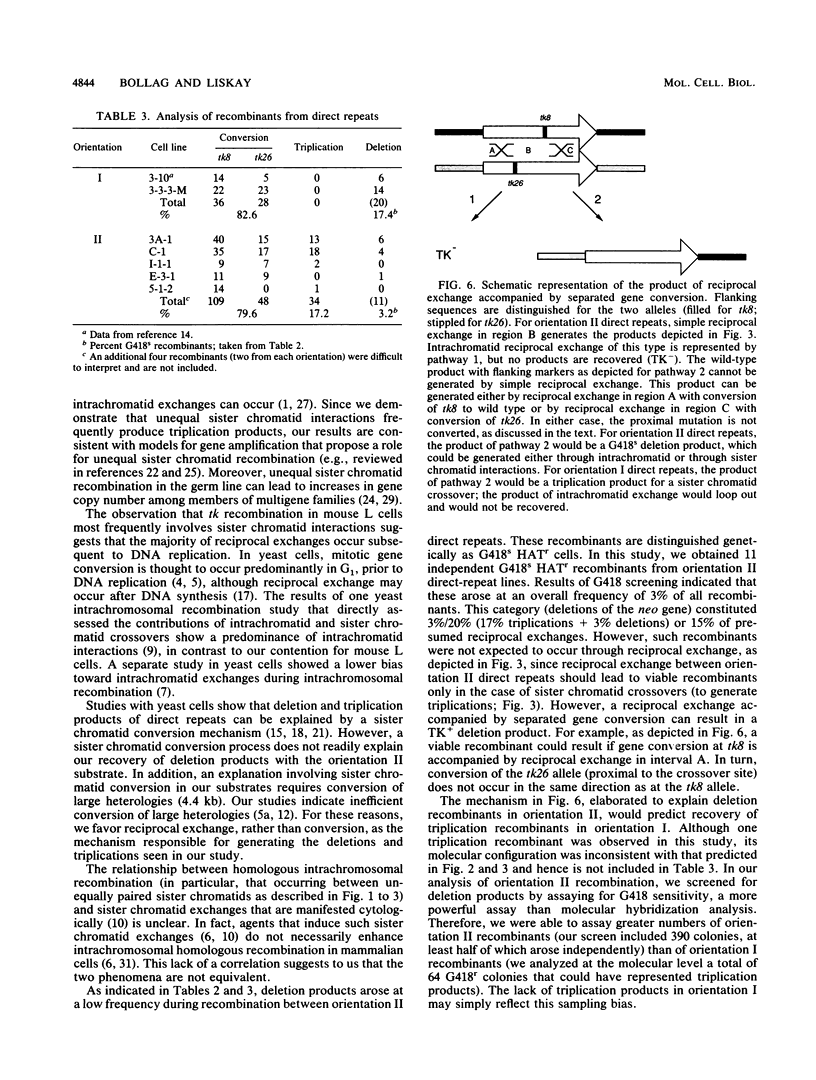
Homologous intrachromosomal recombination between linked genes can involve interactions that are either intramolecular (intrachromatid) or intermolecular (sister chromatid). To assess the relative proportions of chromatid interactions, we report studies of intrachromosomal recombination in mouse L cells containing herpes simplex virus thymidine kinase genes in two alternative configurations of direct repeats. By comparing products of reciprocal exchanges between these two configurations, we conclude that the majority of interactions that give rise to crossover products involve unequally paired sister chromatids after DNA replication. Analyses of an additional class of crossover products that involve discontinuous associated gene conversion suggest that these recombination events involve a heteroduplex DNA intermediate.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bollag R. J., Liskay R. M. Conservative intrachromosomal recombination between inverted repeats in mouse cells: association between reciprocal exchange and gene conversion. Genetics. 1988 May;119(1):161–169. doi: 10.1093/genetics/119.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollag R. J., Waldman A. S., Liskay R. M. Homologous recombination in mammalian cells. Annu Rev Genet. 1989;23:199–225. doi: 10.1146/annurev.ge.23.120189.001215. [DOI] [PubMed] [Google Scholar]
- Capecchi M. R. High efficiency transformation by direct microinjection of DNA into cultured mammalian cells. Cell. 1980 Nov;22(2 Pt 2):479–488. doi: 10.1016/0092-8674(80)90358-x. [DOI] [PubMed] [Google Scholar]
- Esposito M. S. Evidence that spontaneous mitotic recombination occurs at the two-strand stage. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4436–4440. doi: 10.1073/pnas.75.9.4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabre F. Induced intragenic recombination in yeast can occur during the G1 mitotic phase. Nature. 1978 Apr 27;272(5656):795–798. doi: 10.1038/272795a0. [DOI] [PubMed] [Google Scholar]
- Hellgren D., Sahlén S., Lambert B. Unequal SCE is a rare event in homologous recombination between duplicated neo gene fragments in CHO cells. Mutat Res. 1990 Jan;243(1):75–80. doi: 10.1016/0165-7992(90)90126-5. [DOI] [PubMed] [Google Scholar]
- Jackson J. A., Fink G. R. Gene conversion between duplicated genetic elements in yeast. Nature. 1981 Jul 23;292(5821):306–311. doi: 10.1038/292306a0. [DOI] [PubMed] [Google Scholar]
- Katzenberg D. R., Tilley S. A., Birshtein B. K. Nucleotide sequence of an unequal sister chromatid exchange site in a mouse myeloma cell line. Mol Cell Biol. 1989 Mar;9(3):1324–1326. doi: 10.1128/mcb.9.3.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein H. L. Different types of recombination events are controlled by the RAD1 and RAD52 genes of Saccharomyces cerevisiae. Genetics. 1988 Oct;120(2):367–377. doi: 10.1093/genetics/120.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latt S. A. Sister chromatid exchange formation. Annu Rev Genet. 1981;15:11–55. doi: 10.1146/annurev.ge.15.120181.000303. [DOI] [PubMed] [Google Scholar]
- Letsou A., Liskay R. M. Effect of the molecular nature of mutation on the efficiency of intrachromosomal gene conversion in mouse cells. Genetics. 1987 Dec;117(4):759–769. doi: 10.1093/genetics/117.4.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F. L., Sternberg N. Homologous recombination between overlapping thymidine kinase gene fragments stably inserted into a mouse cell genome. Mol Cell Biol. 1984 May;4(5):852–861. doi: 10.1128/mcb.4.5.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liskay R. M., Stachelek J. L., Letsou A. Homologous recombination between repeated chromosomal sequences in mouse cells. Cold Spring Harb Symp Quant Biol. 1984;49:183–189. doi: 10.1101/sqb.1984.049.01.021. [DOI] [PubMed] [Google Scholar]
- Maloney D. H., Fogel S. Gene conversion, unequal crossing-over and mispairing at a non-tandem duplication during meiosis of Saccharomyces cerevisiae. Curr Genet. 1987;12(1):1–7. doi: 10.1007/BF00420720. [DOI] [PubMed] [Google Scholar]
- Ray A., Machin N., Stahl F. W. A DNA double chain break stimulates triparental recombination in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6225–6229. doi: 10.1073/pnas.86.16.6225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman H., Fabre F. Gene conversion and associated reciprocal recombination are separable events in vegetative cells of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6912–6916. doi: 10.1073/pnas.80.22.6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein R., Helms C., Rosenberg N. Concerted deletions and inversions are caused by mitotic recombination between delta sequences in Saccharomyces cerevisiae. Mol Cell Biol. 1987 Mar;7(3):1198–1207. doi: 10.1128/mcb.7.3.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITHIES O., CONNELL G. E., DIXON G. H. Chromosomal rearrangements and the evolution of haptoglobin genes. Nature. 1962 Oct 20;196:232–236. doi: 10.1038/196232a0. [DOI] [PubMed] [Google Scholar]
- Sang H., Whitehouse H. L. Genetic Recombination at the Buff Spore Color Locus in SORDARIA BREVICOLLIS. II. Analysis of Flanking Marker Behavior in Crosses between Buff Mutants. Genetics. 1983 Feb;103(2):161–178. doi: 10.1093/genetics/103.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiestl R. H., Igarashi S., Hastings P. J. Analysis of the mechanism for reversion of a disrupted gene. Genetics. 1988 Jun;119(2):237–247. doi: 10.1093/genetics/119.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimke R. T. Gene amplification in cultured cells. J Biol Chem. 1988 May 5;263(13):5989–5992. [PubMed] [Google Scholar]
- Smith A. J., Berg P. Homologous recombination between defective neo genes in mouse 3T6 cells. Cold Spring Harb Symp Quant Biol. 1984;49:171–181. doi: 10.1101/sqb.1984.049.01.020. [DOI] [PubMed] [Google Scholar]
- Stark G. R., Debatisse M., Giulotto E., Wahl G. M. Recent progress in understanding mechanisms of mammalian DNA amplification. Cell. 1989 Jun 16;57(6):901–908. doi: 10.1016/0092-8674(89)90328-0. [DOI] [PubMed] [Google Scholar]
- Stringer J. R., Kuhn R. M., Newman J. L., Meade J. C. Unequal homologous recombination between tandemly arranged sequences stably incorporated into cultured rat cells. Mol Cell Biol. 1985 Oct;5(10):2613–2622. doi: 10.1128/mcb.5.10.2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramani S., Rubnitz J. Recombination events after transient infection and stable integration of DNA into mouse cells. Mol Cell Biol. 1985 Apr;5(4):659–666. doi: 10.1128/mcb.5.4.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theivendirarajah K., Whitehouse H. L. Further evidence that aberrant segregation and crossing over in Sordaria brevicollis may be discrete, though associated, events. Mol Gen Genet. 1983;190(3):432–437. doi: 10.1007/BF00331073. [DOI] [PubMed] [Google Scholar]
- Wagner M. J., Sharp J. A., Summers W. C. Nucleotide sequence of the thymidine kinase gene of herpes simplex virus type 1. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1441–1445. doi: 10.1073/pnas.78.3.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. Y., Maher V. M., Liskay R. M., McCormick J. J. Carcinogens can induce homologous recombination between duplicated chromosomal sequences in mouse L cells. Mol Cell Biol. 1988 Jan;8(1):196–202. doi: 10.1128/mcb.8.1.196. [DOI] [PMC free article] [PubMed] [Google Scholar]