Abstract
Background
Hospitalizations with community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA) infection have increased in New York City, with substantial geographic variation across neighborhoods. While individual-level risk factors, such as age, sex, HIV infection, and diabetes have been described, the role of neighborhood-level factors (e.g., neighborhood HIV prevalence or income) has not been examined.
Methods
To explore plausible neighborhood-level factors associated with CA-MRSA-related hospitalizations, a retrospective analysis was conducted using New York City hospital discharges from 2006 and New York City-specific survey and health department surveillance data. CA-MRSA-related hospitalizations were identified using diagnosis codes and admission information. Associations were determined by using sex-specific multilevel logistic regression.
Results
The CA-MRSA hospitalization rate varied by more than six-fold across New York City neighborhoods. Females hospitalized with CA-MRSA had more than twice the odds of residing in neighborhoods in the highest quintile of HIV prevalence (adjusted odds ratio [AOR]Q5 vs. Q1 2.3, 95% CI: 1.2, 2.7). Both males and females hospitalized with CA-MRSA had nearly twice the odds of residing in neighborhoods with moderately high proportion of men who have sex with men (MSM) residing in the neighborhood (males: AORQ4 vs. Q1 1.7, 95% CI: 1.1, 2.7; females: AORQ4 vs. Q1 2.0, 95% CI: 1.1, 3.6); but this association did not hold for neighborhoods in the highest quintile (males: AORQ5 vs. Q1 1.2, 95% CI: 0.76, 1.8; females: AORQ5 vs. Q1 1.5, 95% CI: 0.82, 2.7).
Conclusions
Neighborhood-level characteristics were associated with CA-MRSA hospitalization odds, independent of individual-level risk factors, and may contribute to the population-level burden of CA-MRSA infection.
Keywords: Antibiotic resistance, Hospitalizations, Multilevel analysis
Background
Community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA) has become a common cause of morbidity in the United States and around the world [1-3]. In 2006, the rate of hospitalization with CA-MRSA in New York City was estimated to be 10.7 per 100,000 people [4], more than twice that of the US population [5], and varied greatly across neighborhoods [4], raising the possibility of neighborhood-level risk factors for CA-MRSA.
Several individual-level factors have been found to be associated with an increased risk of CA-MRSA infection, including male sex [4,5], drug use [6], participation in contact sports [7], sharing of personal items [8,9], and homelessness [6,10]. Neighborhood-level or geographic risk factors are correlated with individual-level factors, but their role in CA-MRSA hospitalization has not been systematically examined. Plausible neighborhood-level risk factors for CA-MRSA include neighborhood HIV prevalence and the proportion of men in the neighborhood who are men who have sex with men (MSM), as rates of CA-MRSA are higher among HIV positive persons [11,12] as well as MSM [13]; neighborhood income distribution, as CA-MRSA risk factors including crowding [14] and limited access to medical care [10] may be more common among persons living in poverty; and levels of emergency department (ED) usage [15], because persons lacking health insurance may rely on the ED for medical treatment for worsening CA-MRSA infections.
In order to evaluate the influence of neighborhood factors on CA-MRSA hospitalizations among New York City residents, we used a combination of data sources and examined the multilevel associations between neighborhood-level factors and individual odds of CA-MRSA hospitalization relative to all other non-Staphylococcus aureus hospitalizations, controlling for measured individual-level risk factors. Understanding neighborhood-level risk factors for CA-MRSA, as has been done for a number of other health outcomes [16-18], is important to help to target public health surveillance and interventions for what has become an increasing public health concern.
Methods
Study population
We examined data on all hospitalizations except those related to births and those of children under 1 year of age (assuming most were hospitalized for birth in the past year) among New York City residents in 2006. Neighborhood designations were based on aggregations of zip codes into 42 New York City United Hospital Fund (UHF) neighborhoods, ranging in population from approximately 35,000 to 499,000 residents [19].
Definitions
Data on hospitalizations were drawn from the New York Statewide Planning and Research Cooperative System (SPARCS), an event-based dataset of hospital discharges containing patient demographics, 15 diagnosis fields, billing and address information [20]. Although data were considered identified, they did not contain names, raw birthdates, or street addresses. Patient zip code and UHF neighborhood of residence were used in this analysis.
To identify MRSA hospitalizations, diagnosis fields (principal and other) for each hospitalization in 2006 were evaluated using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes. Hospitalizations with diagnosis codes for Staphylococcus aureus pneumonia (482.41), Staphylococcus aureus septicemia (038.11), or Staphylococcus aureus infection specified elsewhere or unspecified (041.11), and an additional ICD-9-CM (V09.0) indicating resistance to drugs (in this case, methicillin) were considered MRSA-related hospitalizations [4]. MRSA hospitalizations were classified as CA-MRSA if discharge records indicated the patient (1) had MRSA diagnosis on admission based on a present-on-admission indicator; (2) had not been transferred from another health care facility, including nursing homes, long term care facilities and hospitals or was admitted for complications from a surgical procedure; (3) had not been hospitalized earlier in 2006; and (4) was not diagnosed with a chronic condition that results in frequent hospitalization including chronic renal failure, cancer, chronic obstructive pulmonary disease, or congestive heart failure [4,21]. Other MRSA hospitalizations were considered to be healthcare-associated (HA-MRSA).
Individual-level covariates
Age, sex, recorded diabetes diagnosis, and HIV-related diagnoses were previously found to be associated with CA-MRSA hospitalization in New York City [4] and therefore were included in this analysis. Age was divided into 4 categories: under 18, 18 to 44, 45 to 64, and 65 and greater years of age. Principal or secondary diagnosis codes, based on ICD-9-CM codes, were used to classify hospitalizations related to diabetes and a pre-existing indicator based on ICD-9-CM codes and disease-related groupings (DRGs) was used to identify HIV.
Neighborhood-level variables
HIV prevalence, the percentage of UHF neighborhood residents living with HIV/AIDS, was estimated using the number of persons diagnosed and living with HIV/AIDS by UHF neighborhood provided by the Bureau of HIV/AIDS at the New York City Department of Health and Mental Hygiene (NYCDOHMH) [22] and New York City UHF neighborhood population estimates [23]. MSM proportion, defined as the proportion of men who report having sex with at least one man in the past year, was based on survey data collected by the New York City Community Health Survey, an annual random-digit-dial survey of 10,000 residents [24]. Aggregate estimates combining survey data from 2003 to 2006 were created by the Bureau of Epidemiology Services at NYCDOHMH. Data on neighborhood income came from the 2000 US Census. Median household income for each zip code was averaged to create UHF neighborhood estimates. The number of emergency department visits by neighborhood of residence [25] was provided by the Syndromic Surveillance Unit at the NYCDOHMH and were converted to UHF neighborhood rates (ED visits per 100,000 population) based on New York City population estimates [23]. UHF neighborhood-level variables were divided into quintiles for the purposes of statistical analyses.
Analysis
To examine differences in CA-MRSA rates across neighborhoods, we mapped the 2006 CA-MRSA hospitalization rates by UHF neighborhood population. Mapping was performed with ArcGIS 9. To determine the associations between neighborhood-level characteristics and hospitaliza-tion with CA-MRSA at the individual-level, we employed multilevel modeling to compare characteristics of persons hospitalized with CA-MRA were compared to those of non-Staphylococcus hospitalizations. Multilevel modeling, also known as hierarchical modeling, was necessary because the data were organized at the neighborhood- and individual-levels; the individuals were nested within neighborhoods. We hypothesized neighborhood-level factors would be associated with CA-MRSA hospitalization independent of measured individual-level risk factors. To identify individual-level factors associated with CA-MRSA hospitalization, we constructed sex-specific logistic regression models with only individual-level factors. We then constructed similar models including a single neighborhood level factor. Finally, multilevel models that contained both individual-level factors and a neighborhood-level factor were constructed to explore sex-specific associations between neighborhood-level factors and CA-MRSA hospitalizations, adjusting for individual-level covariates. We report crude and adjusted odds ratios with 95% confidence intervals. To reduce the impact of possible misclassification, HA-MRSA hospitalizations and hospitalizations with any other Staphylococcus diagnoses were excluded from the analysis. Analyses were performed with SAS 9.1.
SPARCS hospitalization data was obtained through a data use agreement between the NYCDOHMH and SPARCS Data Protection Review Board. This project was approved by the NYCDOHMH Institutional Review Board as research involving materials that have already been collected.
Results
Descriptive and crude analyses
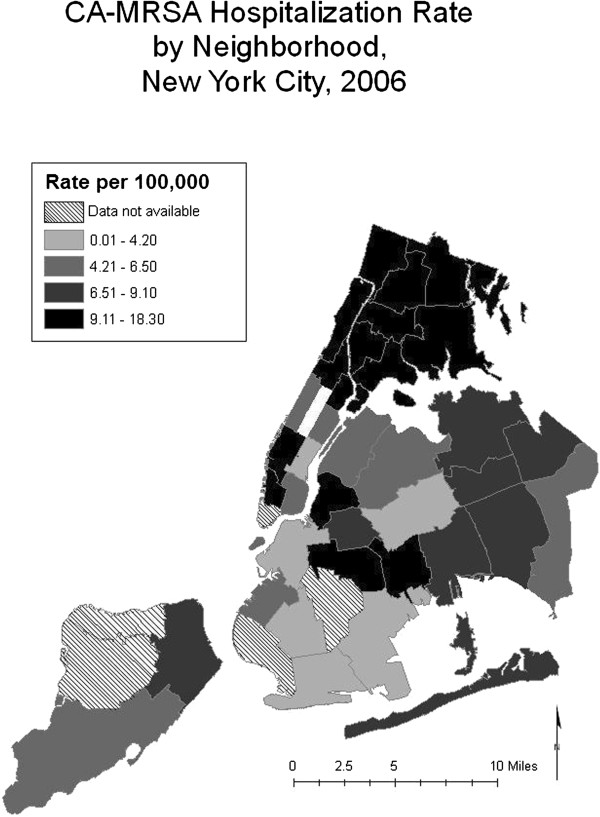
Among New York City residents in 2006, there were 645 CA-MRSA hospitalizations and 908,184 non-Staphylococcus hospitalizations. The CA-MRSA hospitalization rate varied by more than six-fold across neighborhoods, ranging from 3.0 to 18.3 per 100,000. Higher rates clustered in certain areas, including the Bronx and some UHF neighborhoods in Manhattan (Figure 1).
Figure 1.
CA-MRSA hospitalization rate per 100,000 people, New York City neighborhoods, 2006.
Sex-specific bivariate analysis of crude associations between individual-level age, diabetes diagnosis, and HIV diagnosis are shown in Table 1. Among males, those hospitalized with CA-MRSA tended to be younger, with an odds ratio (OR) of 3.5 (95% CI 2.3, 5.2) for those under 18, 4.0 (95% CI 3.0, 5.4) for those aged 18 to 44, and 2.4 (95% CI 1.8, 3.3) for those aged 45 to 64, each compared to 65 and older. Among females, CA-MRSA hospitalizations were as likely to be 18 to 44 years old as they were to be 65 and older (OR 1.1, 95% CI 0.75, 5.5). CA-MRSA hospitalizations had more than 3 times the odds of having an HIV diagnosis (males: OR 3.3, 95% CI 2.5, 4.3; females: OR 3.1, 95% CI: 1.9, 5.1). Having a diabetes diagnosis was significantly associated with CA-MRSA hospitalization only among females (OR 1.4, 95% CI 1.1, 1.9).
Table 1.
Crude odds ratios for associations between individual-level and neighborhood-level characteristics and hospitalizations with CA-MRSA, New York City, 2006
| |
Males |
Females |
||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
|
Individual |
||||
|
Age (years) |
||||
| < 18 |
3.5 |
2.3, 5.2 |
4.7 |
3.1, 6.9 |
| 18-44 |
4.0 |
3.0, 5.4 |
1.1 |
0.75, 1.5 |
| 45-64 |
2.4 |
1.8, 3.3 |
1.9 |
1.4, 2.6 |
| ≥ 65 |
1.0 |
- |
1.0 |
- |
|
Diabetes |
||||
| Yes |
0.91 |
0.72, 1.2 |
1.4 |
1.1, 1.9 |
| No |
1.0 |
- |
1.0 |
- |
|
HIV |
||||
| Yes |
3.3 |
2.5, 4.3 |
3.1 |
1.9, 5.1 |
| No |
1.0 |
- |
1.0 |
- |
|
Neighborhood |
||||
|
HIV Prevalence |
||||
| ≤ 0.37% |
1.0 |
- |
1.0 |
- |
| 0.38-0.73% |
1.2 |
0.75, 2.0 |
1.6 |
0.90, 2.9 |
| 0.74%-1.3% |
1.6 |
0.97, 2.7 |
1.9 |
1.0, 3.4 |
| 1.3-1.65% |
1.6 |
0.98, 2.7 |
1.2 |
0.65, 2.4 |
| > 1.65% |
1.7 |
1.0, 2.7 |
2.7 |
1.5, 5.0 |
|
MSM Proportion |
||||
| ≤ 5.7% |
1.0 |
- |
1.0 |
- |
| 5.8-7.0% |
1.4 |
0.81, 2.3 |
2.0 |
1.1, 3.8 |
| 7.1-8.0% |
1.6 |
1.0, 2.4 |
1.6 |
0.94, 2.8 |
| 8.1-9.8% |
1.9 |
1.2, 3.1 |
2.1 |
1.1, 3.9 |
| > 9.8% |
1.4 |
0.90, 2.2 |
1.5 |
0.84, 2.8 |
|
Income per year ($) |
||||
| ≤ 26,000 |
1.2 |
0.82, 1.9 |
1.2 |
0.70, 2.1 |
| 26,001-35,000 |
0.81 |
0.51, 1.3 |
0.64 |
0.35, 1.2 |
| 35,000-41,000 |
1.30 |
0.85, 2.1 |
0.97 |
0.52, 1.8 |
| 41,000-48,000 |
0.69 |
0.44, 1.1 |
0.77 |
0.44, 1.4 |
| > 48,000 |
1.0 |
- |
1.0 |
- |
|
ED Usage Rate* |
||||
| ≤ 24,000 |
1.0 |
- |
1.0 |
- |
| 24,001-32,600 |
0.68 |
0.42, 1.1 |
1.0 |
0.54, 2.0 |
| 32,601-44,200 |
1.1 |
0.71, 1.6 |
1.4 |
0.76, 2.4 |
| 44,201-56,000 |
1.3 |
0.86, 2.0 |
1.4 |
0.77, 2.6 |
| > 56,000 | 1.2 | 0.78, 1.8 | 1.8 | 0.99, 3.3 |
NOTE. CI, confidence interval; ED, emergency department; HIV, human immunodeficiency virus; MSM, men who have sex with men; OR, odds ratio.
* Emergency department visits per 100,000 people.
In crude sex-specific analyses including only neighborhood HIV prevalence, those hospitalized with CA-MRSA were significantly more likely to come from the highest HIV prevalence neighborhoods in NYC compared with those from the lowest prevalence neighborhoods for both males (ORQ5 vs. Q1 1.7, 95% CI 1.0, 2.7) and females (OR Q5 vs. Q1 2.7, 95% CI 1.5-5.0). Males and females hospitalized with CA-MRSA were also more likely to be from neighborhoods with moderately high versus lower MSM proportion (males: OR Q4 vs. Q1 1.9, 95% CI 1.2, 3.1; females: OR Q4 vs. Q1 2.1, 95% CI 1.1, 3.9), but the association did not hold at higher levels of neighborhood MSM proportion. There was no significant crude association with neighborhood income distribution or ED usage (Table 1).
Multivariable analysis
In multivariable logistic regression models, all individual-level risk factors examined were statistically significantly associated with CA-MRSA hospitalization. Individual-level risk factors remained significant and of similar magnitude with the inclusion of each neighborhood-level characteristic in separate sex-specific multilevel models except diabetes, which was not significant in the crude model for hospitalized males but was significant in all adjusted models (adjusted odds ratio (AOR) 1.3, 95% CI 1.0, 1.7) that included a neighborhood factor.
After adjusting for individual-level risk factors, females hospitalized with CA-MRSA had more than twice the odds of residing in UHF neighborhoods with the highest HIV prevalence compared to the lowest (AORQ4 vs. Q1 2.3, 95% CI 1.2, 2.7). Though adjusted odds ratios at other levels of HIV prevalence were not statistically significant, the odds of hospitalization with CA-MRSA increased with increasing HIV prevalence. There were no significant differences by neighborhood HIV prevalence among males. In separate models, both males and females hospitalized with CA-MRSA had nearly twice the odds of residing in neighborhoods with moderately high proportion of men who have sex with men residing in the neighborhood (males: AORQ4 vs. Q1 1.7, 95% CI: 1.1, 2.7; females: AORQ4 vs. Q1 2.0, 95% CI: 1.1-3.6); but this association did not hold for neighborhoods in the highest quintile proportion of MSM (males: AORQ5 vs. Q1 1.2, 95% CI: 0.76, 1.8; females: AORQ5 vs. Q1 1.5, 95% CI: 0.82-2.7). UHF neighborhood income and ED usage rate were not significant predictors of CA-MRSA hospitalization in multilevel analyses for males or females (Table 2).
Table 2.
Adjusted odds ratios for associations between neighborhood-level characteristics and hospitalizations with CA-MRSA, controlling for individual-level characteristics, New York City, 2006
| |
Males |
Females |
||
|---|---|---|---|---|
| AOR | 95% CI | AOR | 95% CI | |
|
HIV Prevalence |
||||
| ≤ 0.37% |
1.0 |
- |
1.0 |
- |
| 0.38-0.73% |
1.1 |
0.67, 1.7 |
1.5 |
0.84, 2.7 |
| 0.74%-1.3% |
1.3 |
0.81, 2.2 |
1.7 |
0.90, 3.1 |
| 1.3-1.65% |
1.2 |
0.73, 2.0 |
1.1 |
0.56, 2.1 |
| > 1.65% |
1.1 |
0.71, 1.9 |
2.3 |
1.2, 2.7 |
|
MSM Proportion |
||||
| ≤ 5.7% |
1.0 |
- |
1.0 |
- |
| 5.8-7.0% |
1.2 |
0.72, 1.9 |
1.9 |
1.0, 3.5 |
| 7.1-8.0% |
1.3 |
0.86, 1.9 |
1.4 |
0.84, 2.5 |
| 8.1-9.8% |
1.7 |
1.1, 2.7 |
2.0 |
1.1, 3.6 |
| > 9.8% |
1.2 |
0.76, 1.8 |
1.5 |
0.82, 2.7 |
|
Income per year ($) |
||||
| ≤ 26,000 |
0.94 |
0.64, 1.4 |
0.95 |
0.55, 1.6 |
| 26,001-35,000 |
0.76 |
0.49, 1.2 |
0.58 |
0.32, 1.1 |
| 35,000-41,000 |
1.3 |
0.85, 1.9 |
0.89 |
0.49, 1.6 |
| 41,000-48,000 |
0.69 |
0.45, 1.0 |
0.72 |
0.41, 1.3 |
| > 48,000 |
1.0 |
- |
1.0 |
- |
|
ED Usage Rate* |
||||
| ≤ 24,000 |
1.0 |
- |
1.0 |
- |
| 24,001-32,600 |
0.63 |
0.40, 1.0 |
0.98 |
0.51, 1.9 |
| 32,601-44,200 |
0.92 |
0.62, 1.4 |
1.2 |
0.69, 2.2 |
| 44,201-56,000 |
1.0 |
0.70, 1.6 |
1.2 |
0.64, 2.2 |
| > 56,000 | 0.83 | 0.55, 1.3 | 1.4 | 0.76, 2.6 |
NOTE. AOR, adjusted odds ratio; CI, confidence interval; ED, emergency department; HIV, human immunodeficiency virus; MSM, men who have sex with men.
*Emergency department visits per 100,000 people.
Discussion
Our analyses suggest important, biologically plausible neighborhood-level factors that may, in part, drive CA-MRSA hospitalization rates at the population-level in New York City. In sex-specific multilevel analyses, strong positive associations between higher UHF neighborhood HIV prevalence and MSM proportion with higher odds of CA-MRSA hospitalization persisted, even after controlling for individual factors. Additionally, important differences in individual and neighborhood-level risk factors for CA-MRSA hospitalization between males and females were observed.
Our analysis showed that neighborhood HIV prevalence was associated with increased odds of CA-MRSA hospitalization among females even after controlling for individual HIV-status. While the associations between HIV prevalence and odds of hospitalization with CA-MRSA were not statistically significant at lower HIV prevalence levels, a dose–response relationship is suggested. Studies, including ours, have found HIV positive persons to be at an increased risk for CA-MRSA infection and hospitalization with CA-MRSA, possible due to increased viral load, weakened immune systems, lack of antiretroviral therapy, or problems with skin integrity [4,11,26,27]. This raises the possibility that neighborhood HIV prevalence may play a role in CA-MRSA transmission even among HIV-negative persons, and that the attributable fraction of CA-MRSA hospitalizations associated with HIV may be greater than previously thought.
We also found higher neighborhood MSM proportion to be associated with CA-MRSA hospitalization for both males and females. While we did observe differences by neighborhood MSM proportion at the extreme ends of the distribution, we did not observe a dose–response relationship and the association did not extend to neighborhoods in the highest quintile of neighborhood MSM proportion. A population-based study in San Francisco found that zip codes with higher percentage of partnerships being same-sex male also had higher rates of CA-MRSA infection [28]. In New York City-based analyses, high rates of CA-MRSA observed among MSM were associated with HIV infection, crystal methamphetamine use, physical contact with someone with a skin infection, sex at private parties, and perhaps membership in social networks that include others engaged in these behaviors [13]. Though we were unable to control for MSM status at the individual-level in our analysis of males, the finding that neighborhood proportion of MSM was associated with the risk of CA-MRSA hospitalization among females is intriguing, and suggests the possibility of a neighborhood-level effect. Epidemiologic data suggest that HIV prevalence among MSM in New York City is high (e.g., 8.8% overall, and 17.7% among MSM in Manhattan) [29], making it difficult to distinguish between the potentially independent role of each factor. The lack of a dose–response relationship among males or females, however, make these results difficult to interpret, and the association between neighborhood MSM proportion and CA-MRSA needs to be evaluated in future research before stronger conclusions can be drawn.
Epidemiologic studies have shown that neighborhood factors have independent associations with a number of health outcomes, including neighborhood socioeconomic status with diabetes [18] and heart disease [16], and neighborhood poverty with relapse into injecting drug use [17]. Although neighborhood characteristics have commonly been explored with regard to chronic diseases and health outcomes, they likely also affect infectious disease risk. The extent of transmission or acquisition in a given geographic area is dependent upon a number of relevant factors that may in fact vary by neighborhood, including the number of hosts susceptible to infection and the number of hosts who are currently infected or are carriers of the microbial agent. Contacts between infectious and susceptible persons will occur in many settings, including the neighborhood in which infectious persons reside [30].
Our analysis has limitations that merit discussion. At the individual level, V-code 09.0 and ICD-9 CM codes indicating Staphylococcus infection have been used in published literature to identify MRSA cases in administrative data [4,31-34] though some analyses have shown conflicting results as to the accuracy of these definitions [35,36]. Schweizer, et al. found that use of administrative coding lacked sensitivity and have low positive predictive value for hospital-associated infections [35]. A second study by Schaefer, et al. found low sensitivity, leading to an underestimation of the number of cases, but high positive predictive value particularly for community-associated infections [36]. In addition, lack of laboratory data and healthcare history may have led to misclassification of susceptible versus resistant infection and of community- versus hospital-associated infection.
It was not possible to apply the Centers for Disease Control and Prevention’s definition of CA-MRSA to our dataset [37]; however, study inclusion and exclusion criteria were used as a proxy. Patients could not be linked across calendar years and therefore examining the full 12 months prior to hospitalization was not possible. This particularly affects those patients hospitalized early in 2006. Additionally, the definition used in this analysis likely resulted in exclusion of some CA-MRSA cases (those with certain chronic diseases also associated with HA-MRSA) but was necessary to improve the specificity of our outcome definition. Staphylococcus infections which were not recorded on the hospital discharge record or incorrect diagnosis coding could have resulted in some misclassification. Specifically, Staphylococcus infections other than CA-MRSA may have been incorrectly classified as CA-MRSA rather than excluded from analysis. Finally, the primary cause of hospitalization need not have been CA-MRSA, which may lead to an overestimation of hospitalizations primarily due to CA-MRSA.
At the UHF neighborhood level, HIV prevalence, MSM proportion, and ED usage values may be underestimates due to lack of diagnosis, reporting, or inaccurate survey responses and 2000 Census data may not accurately represent neighborhood income in 2006. Misclassification of these neighborhood-level covariates was non-differential by outcome status at the individual-level. Additionally, neighborhood measures are aggregations of individual-level information. Variation will exist among individuals living in a single neighborhood. Finally, there are many ways to designate “neighborhoods” in geographic analyses, including UHF, zip code, cities, et cetera and the specific designations used in our analysis may have affected our results. Due to small numbers of neighborhoods, we were unable to control for multiple neighborhood-level factors in the same model, 7which could result in uncontrolled confounding at the neighborhood level. We were also unable to measure individual socioeconomic status and MSM status and therefore could not control for them in modeling.
Conclusions
CA-MRSA-associated hospitalizations are relatively common in New York City and elsewhere, with substantial geographic variability. Using hospital discharge data, we were able to examine nearly 1 million hospitalizations in a population-based analysis, providing an extensive view of the New York City population. Our combination of this data with other population-level sources available gave us a unique opportunity to examine possible neighborhood-level risk factors for CA-MRSA hospitalizations while controlling for important individual risk factors. This analysis suggests that, over and above individual-level risk factors, CA-MRSA hospitalization may be linked to neighborhood-level characteristics such as HIV prevalence and MSM proportion. It is important to further understand the epidemiology of this infectious disease in order to initiate prevention strategies targeted to appropriate populations and risk factors. As many public health interventions are undertaken at the neighborhood-level, it is essential to elucidate possible neighborhood-level risk factors for morbidity. Future research should focus on examining the impact of neighborhood-level factors such as HIV prevalence and MSM proportion on the incidence of CA-MRSA and Staphylococcus aureus infections, as well as hospitalizations in other cities and nationally (i.e., over wider geographic areas) and to further determine the independent role of each of these factors. Additionally, more focused research studies are needed to understand the complex nature of the high CA-MRSA hospitalization rates found in this study in certain neighborhoods of New York City, such as the Bronx.
Abbreviations
AIDS: Autoimmune deficiency syndrome; AOR: Adjusted odds ratio; CA-MRSA: Community-associated methicillin-resistant Staphylocccus aureus; ED: Emergency department; HA-MRSA: Healthcare-associated methicillin-resistant Staphylococcus aureus; HIV: Human immunodeficiency virus; ICD-9-CM: International classification of diseases: ninth edition: clinical modification; MRSA: Methicillin-resistant Staphylococcus aureus; MSM: Men who have sex with men; NYCDOHMH: New York City Department of Health and Mental Hygiene; OR: Odds ratio; SPARCS: Statewide planning and research cooperative system; UHF: United hospital fund
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
AMF, MAM, DW, and DN conceived and designed the study. MAM and DW aided in acquiring study datasets. AMF performed statistical analyses. AMF and DN interpreted results. AMF wrote the report. MAM, DW, and DN commented and revised report. All authors read and improved final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
Amanda M Farr, Email: Amanda.Farr@truvenhealth.com.
Melissa A Marx, Email: MarxM@zm.cdc.gov.
Don Weiss, Email: DWeiss@health.nyc.gov.
Denis Nash, Email: DNash@hunter.cuny.edu.
Acknowledgements
None. The authors received no external funding for this work.
References
- Mera RM, Suaya JA, Amrine-Madsen H, Hogea CS, Miller LA, Lu EP, Sahm DF, O'Hara P, Acosta CJ. Increasing role of Staphylococcus aureus and community-acquired methicillin-resistant Staphylococcus aureus infections in the United States: a 10-year trend of replacement and expansion. Microb Drug Resist. 2011;17:321–328. doi: 10.1089/mdr.2010.0193. [DOI] [PubMed] [Google Scholar]
- Song JH, Hsueh PR, Chung DR, Ko KS, Kang CI, Peck KR, Yeom JS, Kim SW, Chang HH, Kim YS, Jung SI, Son JS, So TM, Lalitha MK, Yang Y, Huang SG, Wang H, Lu Q, Carlos CC, Perera JA, Chiu CH, Liu JW, Chongthaleong A, Thamlikitkul V, Van PH. Spread of methicillin-resistant Staphylococcus aureus between the community and the hospitals in Asian countries: an ANSORP study. J Antimicrob Chemother. 2011;66:1061–1069. doi: 10.1093/jac/dkr024. [DOI] [PubMed] [Google Scholar]
- Nichol KA, Adam HJ, Hussain Z, Mulvey MR, McCracken M, Mataseje LF, Thompson K, Kost S, Lagace-Wiens PR, Hoban DJ, Zhanel GG. Comparison of community-associated and health care-associated methicillin-resistant Staphylococcus aureus in Canada: results of the CANWARD 2007–2009 study. Diagn Microbiol Infect Dis. 2011;69:320–325. doi: 10.1016/j.diagmicrobio.2010.10.028. [DOI] [PubMed] [Google Scholar]
- Farr AM, Aden B, Weiss D, Nash D, Marx MA. Trends in hospitalization for community-associated methicillin-resistant staphylococcus aureus in New York city, 1997–2006: data from New York State’s statewide planning and research cooperative system. Infect Control Hosp Epidemiol. 2012;33:725–731. doi: 10.1086/666329. [DOI] [PubMed] [Google Scholar]
- Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, Ray S, Harrison LH, Lynfield R, Dumyati G, Townes JM, Craig AS, Zell ER, Fosheim GE, McDougal LK, Carey RB, Fridkin SK. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298:1763–1771. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- Gilbert M, MacDonald J, Gregson D, Siushansian J, Zhang K, Elsayed S, Laupland K, Louie T, Hope K, Mulvey M, Gillespie J, Nielsen D, Wheeler V, Louie M, Honish A, Keays G, Conly J. Outbreak in Alberta of community-acquired (USA300) methicillin-resistant Staphylococcus aureus in people with a history of drug use, homelessness or incarceration. CMAJ. 2006;175:149–154. doi: 10.1503/cmaj.051565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begier EM, Frenette K, Barrett NL, Mshar P, Petit S, Boxrud DJ, Watkins-Colwell K, Wheeler S, Cebelinski EA, Glennen A, Nguyen D, Hadler JL. A high-morbidity outbreak of methicillin-resistant Staphylococcus aureus among players on a college football team, facilitated by cosmetic body shaving and turf burns. Clin Infect Dis. 2004;39:1446–1453. doi: 10.1086/425313. [DOI] [PubMed] [Google Scholar]
- Nguyen DM, Mascola L, Brancoft E. Recurring methicillin-resistant Staphylococcus aureus infections in a football team. Emerg Infect Dis. 2005;11:526–532. doi: 10.3201/eid1104.041094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turabelidze G, Lin M, Wolkoff B, Dodson D, Gladbach S, Zhu BP. Personal hygiene and methicillin-resistant Staphylococcus aureus infection. Emerg Infect Dis. 2006;12:422–427. doi: 10.3201/eid1203.050625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young DM, Harris HW, Charlebois ED, Chambers H, Campbell A, Perdreau-Remington F, Lee C, Mankani M, Mackersie R, Schecter WP. An epidemic of methicillin-resistant Staphylococcus aureus soft tissue infections among medically underserved patients. Arch Surg. 2004;139:947–951. doi: 10.1001/archsurg.139.9.947. discussion 951–94. [DOI] [PubMed] [Google Scholar]
- Crum-Cianflone NF, Burgi AA, Hale BR. Increasing rates of community-acquired methicillin-resistant Staphylococcus aureus infections among HIV-infected persons. Int J STD AIDS. 2007;18:521–526. doi: 10.1258/095646207781439702. [DOI] [PubMed] [Google Scholar]
- Popovich KJ, Weinstein RA, Aroutcheva A, Rice T, Hota B. Community-associated methicillin-resistant Staphylococcus aureus and HIV: intersecting epidemics. Clin Infect Dis. 2010;50:979–987. doi: 10.1086/651076. [DOI] [PubMed] [Google Scholar]
- Marx MA, Cook H, Rehana Z, Krieger D, Yeung A, Lue Y, John A, Duquaine D, Kapell D, Ip D, Mediavilla J, Kornblum J, Kreiswirth B, Weiss D. Final Program and Abstract Book of 2008 Annual Conference on Antimicrobial Resistance: 23–25 June 2008. Bethesda: National Foundation for Infectious Diseases; 2008. Results of a Case–control Investigation of Risk Factors for CA-MRSA in New York City, 2005–2007; p. 44. [Google Scholar]
- Fridkin SK, Hageman JC, Morrison M, Sanza LT, Como-Sabetti K, Jernigan JA, Harriman K, Harrison LH, Lynfield R, Farley MM. Methicillin-resistant Staphylococcus aureus disease in three communities. NEJM. 2005;352:1436–1444. doi: 10.1056/NEJMoa043252. [DOI] [PubMed] [Google Scholar]
- Begley CE, Vojvodic RW, Seo M, Burau K. Emergency room use and access to primary care: evidence from Houston, Texas. J Health Care Poor Underserved. 2006;17:610–624. doi: 10.1353/hpu.2006.0098. [DOI] [PubMed] [Google Scholar]
- Petersen KL, Bleil ME, McCaffery J, Mackey RH, Sutton-Tyrrell K, Muldoon MF, Manuck SB. Community socioeconomic status is associated with carotid artery atherosclerosis in untreated, hypertensive men. Am J Hypertens. 2006;19:560–566. doi: 10.1016/j.amjhyper.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Nandi A, Glass TA, Cole SR, Chu H, Galea S, Celentano DD, Kirk GD, Vlahov D, Latimer WW, Mehta SH. Neighborhood poverty and injection cessation in a sample of injection drug users. Am J Epidemiol. 2010;171:391–398. doi: 10.1093/aje/kwp416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan S, Cozier YC, Rosenberg L, Palmer JR. Socioeconomic status and incidence of type 2 diabetes: results from the Black Women's Health Study. Am J Epidemiol. 2010;171:564–570. doi: 10.1093/aje/kwp443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- New York City United Hospital Fund (UHF) Neighborhoods and NYC Zip Code Areas 2006. http://www.nyc.gov/html/doh/downloads/pdf/data/appb.pdf.
- SPARCS Overview. http://www.health.state.ny.us/statistics/sparcs/operations/overview.htm.
- Kozak LJ, DeFrances CJ, Hall MJ. National hospital discharge survey: 2004 annual summary with detailed diagnosis and procedure data. Vital Health Stat 13. 2006;162:1–209. [PubMed] [Google Scholar]
- New York City HIV/AIDS Annual Surveillance Statistics. http://www.nyc.gov/html/doh/html/ah/hivtables.shtml.
- Neighborhood Population Estimates. http://www.nyc.gov/html/doh/html/episrv/popest_methods.shtml-5.
- Community Health Survey: Methodology. http://www.nyc.gov/html/doh/html/survey/chs-methods.shtml.
- Heffernan R, Mostashari F, Das D, Karpati A, Kulldorff M, Weiss D. Syndromic surveillance in public health practice, New York City. Emerg Infect Dis. 2004;10:858–864. doi: 10.3201/eid1005.030646. [DOI] [PubMed] [Google Scholar]
- Mathews WC, Caperna JC, Barber RE, Torriani FJ, Miller LG, May S, McCutchan JA. Incidence of and risk factors for clinically significant methicillin-resistant Staphylococcus aureus infection in a cohort of HIV-infected adults. J Acquir Immune Defic Syndr. 2005;40:155–160. doi: 10.1097/01.qai.0000179464.40948.b9. [DOI] [PubMed] [Google Scholar]
- Coopman SA, Johnson RA, Platt R, Stern RS. Cutaneous disease and drug reactions in HIV infection. NEJM. 1993;328:1670–1674. doi: 10.1056/NEJM199306103282304. [DOI] [PubMed] [Google Scholar]
- Diep BA, Chambers HF, Graber CJ, Szumowski JD, Miller LG, Han LL, Chen JH, Lin F, Lin J, Phan TH, Carleton HA, McDougal LK, Tenover FC, Cohen DE, Mayer KH, Sensabaugh GF, Perdreau-Remington F. Emergence of multidrug-resistant, community-associated, methicillin-resistant Staphylococcus aureus clone USA300 in men who have sex with men. Ann Intern Med. 2008;148:249–257. doi: 10.7326/0003-4819-148-4-200802190-00204. [DOI] [PubMed] [Google Scholar]
- Manning SE, Thorpe LE, Ramaswamy C, Hajat A, Marx MA, Karpati AM, Mostashari F, Pfeiffer MR, Nash D. Estimation of HIV prevalence, risk factors, and testing frequency among sexually active men who have sex with men, aged 18–64 years–New York City, 2002. J Urban Health. 2007;84:212–225. doi: 10.1007/s11524-006-9135-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halloran ME. In: Epidemiologic Methods for the Study of Infectious Diseases. Thomas JC, Weber DJ, editor. New York: Oxford University Press; 2001. Concepts of transmission and dynamics; pp. 56–85. [Google Scholar]
- Gerber JS, Coffin SE, Smathers SA, Zaoutis TE. Trends in the incidence of methicillin-resistant Staphylococcus aureus infection in children's hospitals in the United States. Clin Infect Dis. 2009;49:65–71. doi: 10.1086/599348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frei CR, Makos BR, Daniels KR, Oramasionwu CU. Emergence of community-acquired methicillin-resistant Staphylococcus aureus skin and soft tissue infections as a common cause of hospitalization in United States children. J Pediatric Surg. 2010;45:1967–1974. doi: 10.1016/j.jpedsurg.2010.05.009. [DOI] [PubMed] [Google Scholar]
- Byrd KK, Holman RC, Bruce MG, Hennessy TW, Wenger JD, Bruden DL, Haberling DL, Steiner C, Cheek JE. Methicillin-resistant Staphylococcus aureus-associated hospitalizations among the American Indian and Alaska native population. Clin Infect Dis. 2009;49:1009–1015. doi: 10.1086/605560. [DOI] [PubMed] [Google Scholar]
- Herigon JC, Hersh AL, Gerber JS, Zaoutis TE, Newland JG. Antibiotic management of Staphylococcus aureus infections in US children's hospitals, 1999–2008. Pediatrics. 2010;125:e1294–e1300. doi: 10.1542/peds.2009-2867. [DOI] [PubMed] [Google Scholar]
- Schweizer ML, Eber MR, Laxminarayan R, Furuno JP, Popovich KJ, Hota B, Rubin MA, Perencevich EN. Validity of ICD-9-CM coding for identifying incident methicillin-resistant Staphylococcus aureus (MRSA) infections: is MRSA infection coded as a chronic disease? Infect Control Hosp Epidemiol. 2011;32:148–154. doi: 10.1086/657936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer MK, Ellingson K, Conover C, Genisca AE, Currie D, Esposito T, Panttila L, Ruestow P, Martin K, Cronin D, Costello M, Sokalski S, Fridkin S, Srinivasan A. Evaluation of international classification of diseases, ninth revision, clinical modification codes for reporting methicillin-resistant staphylococcus aureus infections at a hospital in illinois. Infect Control Hosp Epidemiol. 2010;31:463–468. doi: 10.1086/651665. [DOI] [PubMed] [Google Scholar]
- Diagnosis and Testing of MRSA Infections. http://www.cdc.gov/mrsa/diagnosis/index.html#vs.