Abstract
Our previous studies showed that mycoplasmas prevented apoptosis and induced the malignant transformation of mammalian cells. Other studies indicate that c-Myc plays an important role in promoting apoptosis and malignant transformation of cells. To understand the role of c-Myc in the mycoplasma induced apoptosis prevention and malignant cell transformation, 32D cells, an IL-3 dependent cell line, were infected and transformed by different species of mycoplasmas. The expression of Myc and ras gene families, apoptosis and the cell cycle during the infection and transformation were examined. Results showed that c-Myc expression was significantly increased in mycoplasma transformed 32D cells. Withdrawal of IL-3 substantially decreased c-Myc expression and led to cell cycle arrest at the G1 phase followed by rapid apoptosis. Infection by M. fermentans or M. penetrans not only alleviated the sharp decrease of c-Myc expression, rescued 32D cells from cell-cycle arrest and prevented apoptosis in IL-3-free culture, but also induced autonomous growth of 32D cells. Although M. hominis and M. salivarium had the ability neither to prevent apoptosis nor to induce malignant transformation, they were still able to rescue the cells from cell cycle arrest. The expression of ras family did not change significantly during the infection and transformation. These results suggest that constitutive expression of c-Myc appears to be associated with the continuous growth and malignant transformation of 32D cells induced by M. fermentans and M. penetrans, but not with rescuing the cell cycle arrest by the mycoplasmas.
Keywords: apoptosis, cell cycle, malignant transformation, Myc, mycoplasmal infection, ras
INTRODUCTION
Mycoplasmas are the smallest microorganisms, capable of self-replication. These microorganisms commonly colonize animals and human bodies as commensals or pathogens (1). Although the mycoplasmal infections could cause certain kinds of diseases (2) and might be cofactors for acquired immune deficient syndrome (AIDS) (3), the majority of people who are infected or colonized by mycoplasmas show no clinically significant signs and symptoms. However, we believe that chronic infections by these low-virulence microorganisms might have a significant impact on host cells. It has been observed in early years that mycoplasmal infections could cause chromosomal change and transformation of mammalian cells in vitro (4, 5). Our recent studies have demonstrated that mycoplasmal infections significantly altered gene expression of human cells (6). Chronic infection with M. fermentans and M. penetrans induced malignant transformation of C3H cells, a mouse embryonic cell line with low inherent spontaneous transformation (7) and 32D cells (8).
32D cells, a mouse multi-potential hematopoietic progenitor cell line, are strictly dependent on interlukin-3 (IL-3) for their growth (9). Withdrawal of IL-3 from 32D cell culture leads to rapid programmed cell death (apoptosis) (10, 11). Infections with M. fermentans or M. penetrans prevent the apoptosis and long-term infections with these mycoplasmas induces malignant transformation of the cells (8). We have successfully established transformed 32D cell lines by chronic infections with M. fermentans and M. penetrans. These cell lines can form tumors after injected into nude mice (8). We believe that the malignant transformation of 32D cells induced by mycoplasmal infections is accomplished through two separate avenues. In the first avenue, mycoplasmas rapidly activate an anti-apoptotic pathway(s) such as NFκB pathway and support continuous cell growth in the IL-3 free condition (8). In the second avenue, mycoplasmas induce malignant transformation that requires infection of live organisms with a latent period and causes the infidelity of genomic transmission in cell division. Despite these interesting findings, the mechanism of the malignant transformation induced by the mycoplasmas is still not clear.
The c-Myc proto-oncogene was first described in 1982 as the cellular homologue to the transforming sequences of the avian myelocytomatosis retrovirus (12). Mutated c-Myc oncoproteins induce malignant cell transformation. Not only did Mutated c-Myc oncoproteins induce cell transformation, augmented expression of normal c-myc was also sufficient for cotransformation of rat embryo cells with a mutant ras gene (13). It was soon found that activated oncogenic c-Myc is a key transforming factor in the etiology of human Burkitt’s lymphoma (14). In fact, over-expression of c-Myc is implicated in many oncogenic processes of both naturally occurred malignant tumors and in vitro induced malignant cells (15, 16) (17, 18). Paradoxically, c-Myc also induces apoptosis (19, 20) and the enforced expression of high level of c-Myc accelerated apoptosis of 32D cells in IL-3-free cultures (10), which is a key cancer-preventing mechanism. Transformation of 32D cells by mycoplasmal infections involves the prevention of cell apoptosis (8). Since overexpression of c-Myc was observed in our previous mycoplasma induced malignant transformation CH3 cell model (21), it will be very interesting to know the role of c-Myc in both apoptotic prevention and malignant transformation of mycoplasma infected 32D cells. In this study, the expression of myc and ras oncogene families in 32D cells was studied during mycoplasmal infection and subsequent transformation. Growth, cell cycle and apoptosis of affected cells were monitored. A linkage between c-Myc expression and prevention of apoptosis as well as induction of malignant transformation by mycoplasmas was explored.
MATERIALS AND METHODS
Mycoplasmal infection of 32D cells
32D cells were cultured in RPMI 1640 medium containing 10% fetal bovine serum and 8% WEHI cell conditioned medium (as IL-3 source). 32D/GTU/c, 32D/MI/c and 32D/PG/c are IL-3 independent 32D cell lines established by transforming 32D cells with M. penetrans GTU-54 strain, M. fermentans incognitus (MI) and PG18 strains, respectively (8). Mycoplasmas in these transformed cell lines have been eradicated by antibiotic treatment. They are able to grow in both IL-3 containing and IL-3 free medium. Cell viability was determined by Trypan blue exclusion assay (22).
M. fermentans (PG 18), M. salivarium and M. hominis PG21T were obtained from National Institute of Health, Bethesda, Maryland; M. penetrans (GTU-54) and M. fermentans MI were isolated from a patient with AIDS in this laboratory (23, 24). The mycoplasmas were cultured in SP4 medium containing 18% fetal bovine serum, 100 units per ml of penicillin and 500 units per ml of polymyxin (23). Mycoplasmas in exponential growth judged by the color change of the culture medium were aliquoted and stored at -70°C. Quantification of mycoplasmas was performed by measuring the color changing unit (CCU) or colony forming unit (CFU) as described in our previous study (6). All mycoplasmal cultures were quantified by at least one of the above methods.
To infect 32D cells, 6 ml of mycoplasmal stock was added into 100 ml of cell culture at 0.5 × 106 to 1 × 106 cells/ml in a 175 cm2 flask with or without IL-3 supplementation. The same volume of SP4 medium was added into control flasks. Titers of the mycoplasmal stocks used in this study were about 107 CCU.
RNase Protection Assay
Total RNA was prepared from the cells using TRIzol reagent (GIBCO BRL, Gaithersburg, MD) according to the manufacturer’s instruction (25). RNAs were dissolved in DEPC (Diethyl pyrocarbonate) treated H2O. The quantity and purity of the RNA were determined by measuring the absorbance at 260 nm and the 260/280 absorbance ratio on a spectrophotometer and by electrophoresis on 1.2% agarose gel using formaldehyde-MOPS (3-[N-Morpholino]propanesulfonic acid) buffer.
mRNAs of myc and ras gene families were analyzed by the RNase protection assay (RPA) using RiboQuant multi-probe kit (PharMingen, San Diego, CA) following instructions of the manufacturer. In brief, 32P-labeled specific anti-sense RNA probes were synthesized by in vitro transcription from multiple DNA template sets (m-Myc and m-Ras, from PharMingen), which include two housekeeping genes, L32 and GAPDH (glyceraldehyde 3-phosphate dehydrogenase) as loading controls. An equal amount of total RNA (20 μl for each sample) was hybridized overnight to the 32P-labeled RNA probes. Unhybridized RNAs and RNA probes were digested with RNases A and T1. The protected mRNAs and probes were precipitated with ethanol and resolved on a 6% denaturing urea polyacrylamide gel. Undigested 32P-labeled probes serve as molecular size markers. The gel was dried and exposed to X-ray film as well as to a phosphoimage screen. The expression of mRNA transcript of a specific gene was quantified on a Storm 860 phosphoimage scanner followed by the analysis with software ImageQuant (Molecular Dynamics, Sunnyvale, CA).
Apoptosis assay
Cell apoptosis was assessed by measuring nucleosome levels in cell lysates using a nucleosome ELISA kit (Oncogene Research Products, Cambridge, MA). Briefly, Cells from 5 to 10 ml of culture were counted, harvested by centrifugation and lysed in 1 ml of lysis buffer. The lysates were diluted with the lysis buffer to equivalent 106 cells/ml and frozen at -20°C for at least 24 h before the assay. To measure nucleosome, cell lysates were added to a 96-well enzyme-linked immunosorbent assay (ELISA) plate that has been coated with anti-nucleosome antibodies. The absorbed nucleosomes on the plate were detected by a biotinylated detector antibody and streptavidin-peroxidase conjugate, and quantified on an ELISA reader.
Determination of cell cycle
Cells were collected and washed once with phosphate buffer saline (PBS) by centrifugation, and then fixed in 50% ethanol. The fixed cells were stored at 4°C until analyzed by flow cytometry. To determine cell cycle distributions, the fixed cells in 50% ethanol were washed with PBS once. To stain cells, about 106 cells were resuspended in 1 ml of 0.1M Tris-HCl, pH7.2 containing 0.07M NaCl, to which equal volume of 10μM 4',6-diamidino-2-phenylindole (DAPI) in 800mM Na2HPO4 was added. The cell suspension was incubated at 4°C overnight. Fluorescence of DAPI stained cells was measured on a flow cytometer. The percentages of cells in G0/G1, S and G2/M phases were determined.
RESULTS
Mycoplasma-transformed 32D cells constitutively express high levels of c-Myc
The expression (mRNA) of myc oncogene family (sin 3, c-Myc, L-Myc, L-Myc, B-myc, Max, mad, mxi, mad3 mad4 and mnt) in mycoplasma-transformed 32D cell lines 32D/GTU/c, 32D/MI/c and 32D/PG/c was examined by using the multiple probe RPA. Parental 32D cells were used as control. In the presence of IL-3, control 32D cells expressed a relatively high level of c-Myc and moderate level of sin3 and Max as well as a low level of mxi, mad4 and mnt. The expression of B-myc, L-Myc, N-Myc, mad, mad3 and mad4 was not detected in these cells (Fig. 1). Thirty-six hours after withdrawal of IL-3 from cell cultures, the expression of c-Myc decreased by 50 to 80%. However, the expression of other members in the myc family was little changed. In the mycoplasma-transformed 32D cells, c-Myc mRNA was also decreased following IL-3 withdrawal though the magnitude of the decrease was smaller than that in the control. However, the expression level of c-Myc mRNA in the mycoplasma-transformed cells was still 1.5-3 times higher than that in control 32D cells. In addition, the levels of sin3, Max and mxi mRNA also decreased in the mycoplasma transformed 32D cells following IL-3 withdrawal (Fig. 1).
Figure 1.
Expression of Myc oncogene family in mycoplasma-transformed and control 32D cells. Mycoplasma-transformed and control 32D cells cultured in RPMI 1640 medium containing 10% FBS and IL-3 at least for 3 weeks were harvested by centrifugation. The cells were transferred to fresh culture medium with (+) or without IL-3 (-) and cultured for 36 hours The expression of myc oncogene family (mRNA) in the cells was determined by the RPA.
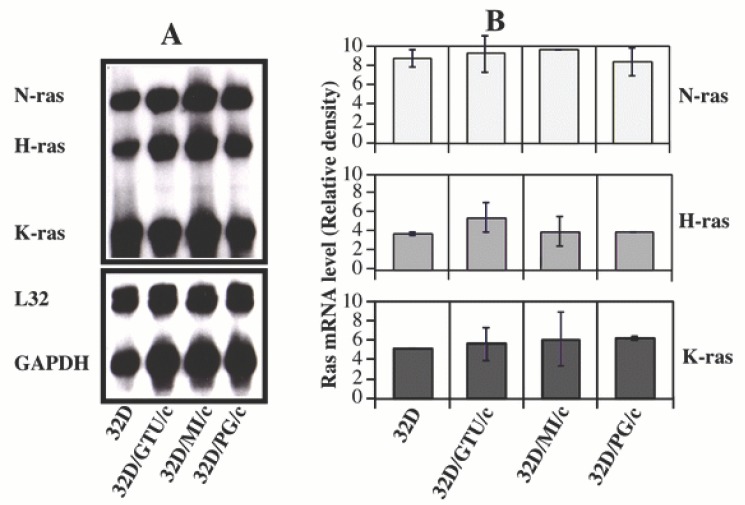
At the same time, we examined the expression of H-ras, K-ras and N-ras oncogenes by the same method (RPA). Results indicated that 32D cells expressed substantial amount of ras mRNAs. However, the expression level was not significantly increased in mycoplasma-transformed 32D cells (Fig. 2). Withdrawal of IL-3 from cell cultures did not significantly affect ras gene expression both in the mycoplasma transformed 32D cells and their control cells.
Figure 2.
Expression of ras oncogene family in mycoplasma-transformed and control 32D cells. The expression of ras genes (Panel A, autoradiograph) was determined as described in Fig. 1 except for using the ras gene template set. Panel B. is the average of 3 independent experiments quantified by the phosphoimage analysis.
Continuous growth of 32D cells following IL-3 withdrawal and mycoplasmal infection was associated with preventing sharp decrease of c-Myc expression in the cells
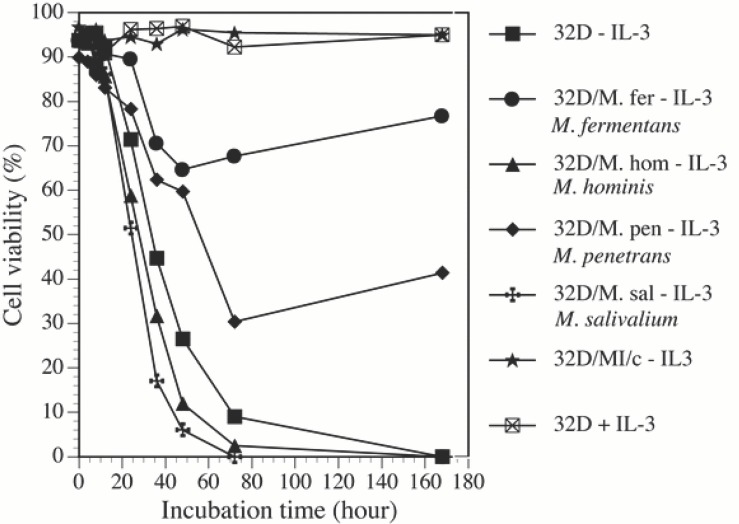
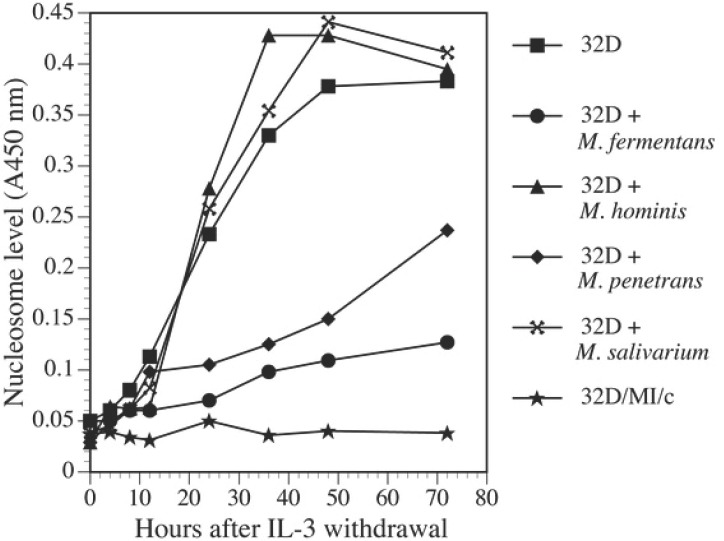
Mycoplasmal infections supported continuous growth of 32D cells in IL-3 free cultures. In this study, we showed that different species of mycoplasmas had different effects on the growth of IL-3 dependent 32D cells in IL-3 deprived cultures (Fig. 3). Withdrawal of IL-3 from cultures caused rapid cell death in noninfected control cell cultures. About 50% of the control cells survived 36 h following IL-3 withdrawal. Only around 10% remained alive on day 3. Almost all control cells died within 7 days. In M. fermentans infected cultures, although some cells died during the first week of infection many cells continued to proliferate. Cell viability in the infected cultures was about 70% and 55% respectively after 36 h and 72 h infection by the mycoplasma. The viability gradually improved 5 days later. M. penetrans could also prevent 32D cell death due to IL-3 withdrawal as M. fermentans did, but it was less effective than M. fermentans. However, M. salivarium and M. hominis did not have any protective effect on 32D cells following IL-3 withdrawal. By using nucleosome ELISA assay, we showed that supporting 32D cell continuous growth by M. fermentans and M. penetrans was accompanied by preventing these cells from apoptosis in IL-3 free cultures (Fig. 4). Similar to the results obtained from the viability assay, M. fermentans was near twice as potent as M. penetrans in preventing 32D cells from the programmed cell death following IL-3 withdrawal. Apoptotic cells in IL-3 free cultures infected with M. fermentans or M. penetrans were about 70% and 50% respectively less than those found in non-infected control cultures 24 h following the infection. However, M. hominis and M. salivarium did not have the ability to protect 32D cells from the apoptosis due to IL-3 deprivation (Fig. 4).
Figure 3.
Effect of mycoplasmas on cell growth. Mycoplasma-transformed and control 32D cells cultured in RPMI 1640 medium containing 10% FBS and IL-3 at least for 3 weeks were harvested by centrifugation and then transferred into fresh medium with (+) or without IL-3 (-) immediately followed by adding mycoplasmal stocks into the cultures. The same amount of SP4 medium was added into non infected control culture. The viability of the cells at set time points was examined by the trypan blue exclusion assay. The results represent 3 similar experiments.
Figure 4.
M. fermentans and M. penetrans prevented apoptosis of 32D cells following IL-3 withdrawal. Cells were harvested and infected with mycoplasmas in IL-3 free cultures as described in Fig. 3. Nucleosome level in cells at different time points was measured by the ELISA assay. The results are representative of 3 separate experiments.
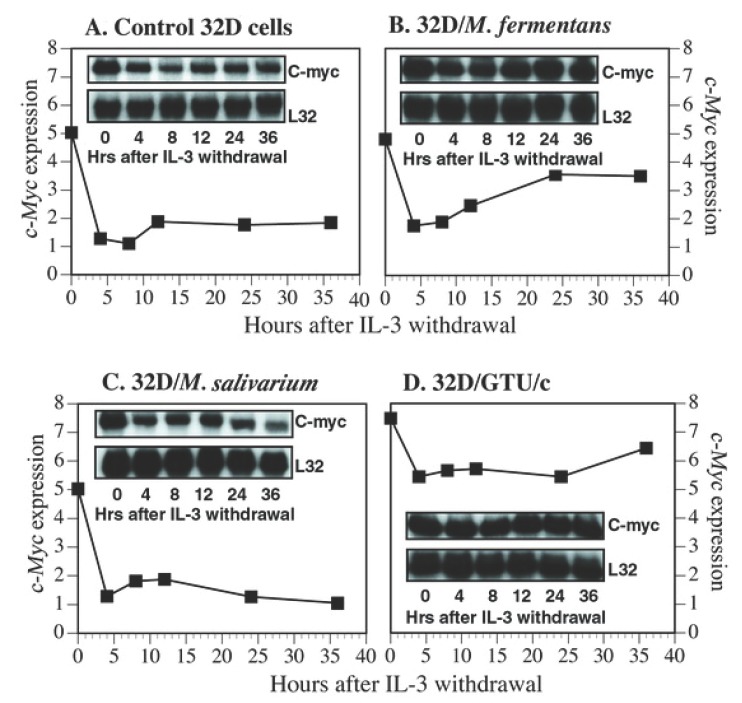
c-Myc promotes apoptosis (20). Enforced c-Myc expression accelerates 32D cell apoptosis process (10). Our results shown above have revealed that the expression of c-Myc in 32D cells is significantly increased following transformation by M. fermentans and M. penetrans. These two mycoplasmas can effectively prevent 32D cells form apoptosis, which, we believe, is an early stage of the mycoplasma induced transformation process. It is not clear if acute mycoplasmal infections also have an impact on the c-Myc expression in this stage. To address this question, short-term mycoplasma infected 32D cells and noninfected control cells were examined for the c-Myc expression. Exponentially growing 32D cells were transferred into IL-3 free medium. At the same time, the cells were infected with 4 different species of mycoplasmas, M. fermentans, M. penetrans, M. salivarium and M. hominis. Total RNA was prepared from the infected and noninfected control cells. The expression of myc and ras gene families in these cells at 0 h, 4 h, 8 h, 12 h, 20 h and 36 h was determined by the multiple-probe RPA. Results showed that the expression of c-Myc mRNA in mycoplasma-infected and control 32D cells rapidly decreased within 4 h following IL-3 withdrawal. In control cells, the c-Myc mRNA level remained low until cells died (Fig. 5A). In M. fermentans or M. penetrans infected 32D cells, after a short period of decrease, the c-Myc expression bounced back to and maintained at a relative high level (Fig. 5B). In contrast, M. salivarium and M. hominis could not support the growth of 32D cells in IL-3 deprived cultures. The expression of c-Myc in these cells was similar to that in control cells (Fig. 5C). Mycoplasma-transformed 32D cells that had grown in IL-3 containing cultures for more than 2 weeks also experienced a temporary slight decrease of c-Myc mRNA short after IL-3 withdrawal. However, the expression level returned to close to its original high level within 36 h (Fig. 5D). The results indicated that the expression of c-Myc was strongly correlated with supporting continuous cell growth by mycoplasmas and IL-3 still had some influence on c-Myc expression in mycoplasma-transformed 32D cells. Infections by the transforming mycoplasmas upregulated c-Myc expression and partially compensated the decrease of c-Myc expression following IL-3 withdrawal. On the other hand, the expression of ras oncogene family was independent of IL-3 regulation and was not significantly affected by the mycoplasmal infection.
Figure 5.
Kinetics of c-Myc expression in mycoplasma infected, transformed and control 32D cells following IL-3 withdrawal. Exponentially growing cells were transferred to IL-3-free cultures and infected with M. fermentans (panel B) or M. salivarium (panel C). Non-infected 32D cells (panel A) and M. penetrans transformed 32D cells (panel D) were also tested. The expression of c-Myc in the cells was determined by the RPA (inlets). Results were quantified by the phosphoimage technique and normalized by the expression of a house-keeping gene L32.
Mycoplasmal infections rescued 32D cells from cell-cycle arrest in the absence of IL-3
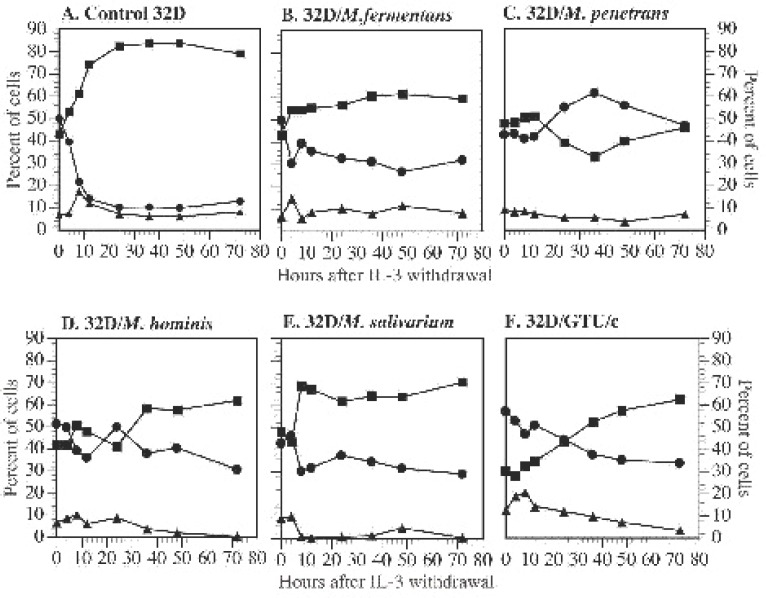
Withdrawal of IL-3 leads to apoptosis of 32D cells within a few days. Within the first 12 h, the cells quickly respond to IL-3 withdrawal by arresting the cell cycle at the G1 phase.(10, 11) Enforced expression of the high level of exogenous c-Myc rescued 32D cells from the cell cycle arrest. However, overexpression of exogenous c-Myc could not change the apoptotic fate of 32D cells due to IL-3 withdrawal, but could enhance the apoptotic process (10). The question is whether mycoplasmal infections and increased expression of endogenous c-Myc has any impact on the cell cycle of 32D cells. To answer this question, we infected 32D cells with mycoplasmas in IL-3 free cultures. Cell cycle distributions of the infected cells, noninfected control 32D cells and three mycoplasma transformed 32D cell lines were examined by the flow cytometry. As expected, withdrawal of IL-3 caused control cells to arrest at the G1 phase. The cells in the G1 phase rapidly accumulated and reached a plateau about 24 h after IL-3 withdrawal (Fig. 6A). These cells died in a few days following this G1 phase arrest. However, 32D cells infected with M. fermentans, M. penetrans, M. hominis and M. salivarium did not stop at the G1 phase following IL-3 withdrawal (Fig.6B, C, D and E). All four species of mycoplasmas tested could rescue 32D cells from cell cycle arrest at the G1 phase in IL-3 deprived cultures and, drove the cells to pass the G1 phase checkpoint and to progress into the S phase. The promotion of this cell cycle progression by mycoplasmas is independent of their ability to prevent apoptosis of 32D cells in IL-3 deprived cultures. However, 32D cells infected with M. hominis and M. salivarium had fewer G2/M phase cells than those infected with M. fermentans and M. penetrans. These results suggest that although M. hominis and M. salivarium could drive the cells to pass the G1 phase checkpoint they were less efficient to drive cells to pass the S phase checkpoint. On the other hand, IL-3 independent, mycoplasma-transformed 32D cells continuously proliferated in the absence of IL-3 or mycoplasmas (Fig. 6F). Results described here and in the above section indicate that there is no close relationship between the expression level of endogenous c-Myc in 32D cells and cell cycle distribution during mycoplasmal infection and cell transformation.
Figure 6.
Mycoplasmal infections rescued 32D cells from cell-cycle arrest at G1 phase in the absence of IL-3. Cells were treated in the same way as described in Fig. 5. These cells included control 32D cells (panel A), 32D cells newly infected M. fermentans (panel B), M. penetrans (panel C), M. hominis (panel D) or M. salivarium (panel E), and M. penetrans transformed cells (panel F). The cells were harvested and prepared at set time points for the cell cycle analysis by flow cytometry. ■G1 phase: ●; S phase: ▲; G2/M phase.
DISCUSSION
Our previous study has revealed that the expression of c-Myc was dramatically increased in mycoplasma transformed C3H cells (21). In this study, we demonstrated that 32D cells expressed relatively high levels of c-Myc and Max transcripts. Withdrawal of IL-3 led to rapid decrease of c-Myc expression, but did not obviously affect Max expression. However, mycoplasma-transformed 32D cells constitutively expressed high levels of c-Myc in the presence or in the absence of IL-3. These results imply that c-Myc has a critical role in mycoplasma induced malignant transformation of 32D cells. These results are consistent with previous studies on the role of c-Myc in cell malignant transformation (26-28). The increased c-Myc oncoproteins in mycoplasma transformed cells can form dimers with its partners, Max or other related proteins, which then bind to the specific DNA sequence (CACA/GTG), activate transcription and regulate cell biological functions. Although early studies indicated that c-Myc and N-myc were equally potent in transforming mammalian cells and L-myc also had weak transformation ability (29) we were unable to detect the expression of N-myc and L-myc in 32D cells before and after the transformation. Apparently, these two oncogenes are not associated with mycoplasma induced malignant transformation.
We have noticed that 32D cells expressed substantial amount of N-ras, H-ras and K-ras. Although there was no significant increase in the expression of the ras oncogenes in mycoplasma transformed 32D cells, the role of the ras gene family in this transformation process could not be completely excluded. .c-Myc collaborates with activated ras oncogenes in transforming primary embryonic rat fibroblasts in vitro (30). Our previous study also demonstrated the increased expression of c-Myc and H-ras in mycoplasma transformed C3H cells (21). Constantly expressed ras oncoproteins in 32D cells might be sufficient to collaborate with increased c-Myc to promote malignant transformation of 32D cells during chronic mycoplasmal infection. The true role of c-Myc in mycoplasma induced malignant transformation might be confirmed by using c-Myc small interfering (si) RNA or c-Myc repressors, such as FUSE-binding protein-interacting repressor (FIR) (31, 32).
Not only can c-Myc promote proliferation and transformation of mammalian cells, but it is also involved in apoptosis. Rat-1 fibroblasts with the high level of c-Myc proteins are more prone to programmed cell death upon serum deprivation (33). Enforced expression of high levels of c-Myc in 32D cells accelerated apoptotic process in IL-3 free culture (10, 34). Abnormal or overexpression of c-Myc alone in cells induced apoptosis by inducing a p53-dependent cell death pathway, hence, protecting the organism from lethal neoplastic changes (35, 36). On the other hand, results of our studies and others (10) have shown that c-Myc in untreated 32D cells rapidly decreased within 4 h following IL-3 withdrawal, followed by rapid cell apoptosis. Furthermore, when 32D cells were infected with mycoplasmas, M. fermentans and M. penetrans not only supported continuous growth of 32D cells in IL-3 free cultures, but also prevented the sharp decrease of c-Myc expression following IL-3 withdrawal. M. hominis and M. salivarium that did not support continuous cell growth in the same IL-3 free culture condition, could not maintain constitutive expression of high levels of c-Myc. These results suggest that c-Myc in 32D cells might act as a survival factor instead of an apoptotic inducer and decrease of endogenous c-Myc expression is associated with apoptosis. The role of endogenous c-Myc seems to be completely different from that of over-expressed exogenous c-Myc in supporting the growth of 32D cells. In fact, co-expression of c-Myc and Bcl-xL, a apoptosis repressor and oncogene, not only suppress c-Myc-induced apoptosis but also expose multiple oncogenic properties of c-Myc and triggers carcinogenic progression (35, 37). M. fermentans and M. penetrans, but not M. hominis nor M. salivarium, increased the expression of Bcl-xL in infected 32D cells (Zhang S, et al. unpublished data). Collaborative actions of c-Myc and Bcl-xL might be the mechanism, through which mycoplasmas prevent apoptosis of 32D cells in IL-3 free condition and induce malignant transformation of the cells.
c-Myc anti-sense transcripts inhibit G1 progression by reducing expression of endogenous c-Myc in Friend murine erythroleukemia cells (F-MEL) (38). Withdrawal of IL-3 causes cell cycle arrest of 32D cells at the G1 phase and over expression of exogenous c-Myc could rescue 32D cells from the cell cycle arrest (10, 39). These evidences indicate that c-Myc plays an important role in cell cycle progression (39, 40). The results from this study showed that mycoplasma mediated G1 progression of 32D cells was independent of the expression of endogenous c-Myc. Only M. fermentans and M. penetrans among four species of mycoplasmas tested can prevent the decrease of c-Myc expression in 32D cells following IL-3 withdrawal. However, all four species of mycoplasmas tested can also prevent the G1 phase arrest of 32D cells caused by IL-3 withdrawal. The results indicate that the cell cycle progression induced by mycoplasmas might go through other pathway(s) rather than the c-Myc pathway. Since M. hominis and M. salivarium failed to rescue the IL-3-free 32D cells from dying of apoptosis, c-Myc could be critical in releasing cell arrests at other check points of the cell cycle.
Generally speaking, withdrawal of growth factors can cause cell cycle arrest at the G1 phase by affecting the G1 phase checkpoint. DNA damage may provent cells from entering the S phase and the G2 phase by affecting DNA replication checkpoint (41). The G1 phase arrest caused by negative growth factors is easily overcome by supplying corresponding growth factors or by adding a growth factor inducer. When the cell cycle is stopped by DNA damage, DNA repair may restore the cell cycle or the cells undergo apoptosis. Our previous studies have revealed that mycoplasmas and mycoplasmal associated membrane proteins (LAMPs) induce the expression of many cytokines (6). Some of these cytokines can support cell growth (42). Therefore, it is not surprising that mycoplasmas can prevent cell cycle arrest at the G1 phase following growth factor withdrawal.
ACKNOWLEDGEMENTS
We like to thank Dr. Robbert L. Becker and Ms. Annette Geissel for their help in the cell cycle assay by flow cytometry. We are grateful to Dr. Shaw-Huey Feng, Ms. Tamara Newsome and Mr. Joe F. Rodriguez for their critical review of this manuscript. This work was supported by grants awarded to S Zhang & S-C Lo (UBUY, UBPA, UBIM and UBZZ) from American Registry of Pathology/Armed Forces Institute of Pathology.
REFERENCES
- 1.Maniloff J, McElhaney RN, Flinch LR, et al. Mycoplasmas, Molecular biology and pathogenesis. Washington DC: American Society for Microbiology; 1992. [Google Scholar]
- 2.Baum S, Taylor-Robison D. Mycoplasma diseases. In: Mandell GL, Bennett JE, Dolin R, editors. Principles and practice of infectious diseases. 4th. New York, Edinburgh, London, Madrid, Melbourne, Milan. Tokyo: Churchill Livingstone; 1995. p. 1701. [Google Scholar]
- 3.Lo SC. Mycoplasmas and AIDS. In: Maniloff J, McElhaney RN, Flinch LR, Baseman JB, editors. Mycoplasmas, Molecular biology and pathogenesis. Washington DC: American Society for Microbiology; 1992. [Google Scholar]
- 4.Paton GR, Jacobs JP, Perkins FT. Chromosome changes in human diploid-cell cultures infected with Mycoplasma. Nature. 1965;207:43. doi: 10.1038/207043a0. [DOI] [PubMed] [Google Scholar]
- 5.Fogh J, Fogh H. Irreversibility of major chromosome changes in a mycoplasma-modified line of FL human amnion cells. Riv. Patol. Nerv. Ment. 1966;87:67. doi: 10.3181/00379727-126-32369. [DOI] [PubMed] [Google Scholar]
- 6.Zhang S, Wear DJ, Lo S. Mycoplasmal infections alter gene expression in cultured human prostatic and cervical epithelial cells. FEMS Immunol. Med. Microbiol. 2000;27:43. doi: 10.1111/j.1574-695X.2000.tb01410.x. [DOI] [PubMed] [Google Scholar]
- 7.Tsai S, Wear DJ, Shih JW, et al. Mycoplasmas and oncogenesis: persistent infection and multistage malignant transformation. Proc. Natl. Acad. Sci. USA. 1995;92:10197. doi: 10.1073/pnas.92.22.10197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feng SH, Tsai S, Rodriguez J, et al. Mycoplasmal infections prevent apoptosis and induce malignant transformation of interleukin-3-dependent 32D hematopoietic cells. Mol. Cell Biol. 1999;19:7995. doi: 10.1128/mcb.19.12.7995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greenberger JS, Sakakeeny MA, Humphries RK, et al. Demonstration of permanent factor-dependent multipotential (erythroid/neutrophil/basophil) hematopoietic progenitor cell lines. Proc. Natl. Acad. Sci. USA. 1983;80:2931. doi: 10.1073/pnas.80.10.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Askew DS, Ashmun RA, Simmons BC, et al. Constitutive c-myc expression in an IL-3-dependent myeloid cell line suppresses cell cycle arrest and accelerates apoptosis. Oncogene. 1991;6:1915. [PubMed] [Google Scholar]
- 11.Zhang S, Tsai S, Geissel A, et al. Mycoplasma-mediated malignant transformation of IL-3 dependent 32D hematopoietic cells is associated with constitutive expression of a high level of c-Myc gene. 99th General Meeting of the the American Society for Microbiology; Atlantic City. 1999. p. 324. [Google Scholar]
- 12.Vennstrom B, Sheiness D, Zabielski J, et al. Isolation and characterization of c-myc, a cellular homolog of the oncogene (v-myc) of avian myelocytomatosis virus strain 29. J. Virol. 1982;42:773. doi: 10.1128/jvi.42.3.773-779.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee WM, Schwab M, Westaway D, et al. Augmented expression of normal c-myc is sufficient for cotransformation of rat embryo cells with a mutant ras gene. Mol. Cell Biol. 1985;5:3345. doi: 10.1128/mcb.5.12.3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spencer CA, Groudine M. Control of c-myc regulation in normal and neoplastic cells. Adv. Cancer Res. 1991;56:1. doi: 10.1016/s0065-230x(08)60476-5. [DOI] [PubMed] [Google Scholar]
- 15.Nesbit CE, Tersak JM, Prochownik EV. MYC oncogenes and human neoplastic disease. Oncogene. 1999;18:3004. doi: 10.1038/sj.onc.1202746. [DOI] [PubMed] [Google Scholar]
- 16.Facchini LM, Penn LZ. The molecular role of Myc in growth and transformation: recent discoveries lead to new insights. Faseb. J. 1998;12:633. [PubMed] [Google Scholar]
- 17.Pelengaris S, Khan M, Evan G. c-MYC: more than just a matter of life and death. Nat. Rev. Cancer. 2002;2:764. doi: 10.1038/nrc904. [DOI] [PubMed] [Google Scholar]
- 18.Luo H, Li Q, O'Neal J, et al. c-Myc rapidly induces acute myeloid leukemia in mice without evidence of lymphoma-associated antiapoptotic mutations. Blood. 2005;106:2452. doi: 10.1182/blood-2005-02-0734. [DOI] [PubMed] [Google Scholar]
- 19.Heikkila R, Schwab G, Wickstrom E, et al. A c-myc antisense oligodeoxynucleotide inhibits entry into S phase but not progress from G0 to G1. Nature. 1987;328:445. doi: 10.1038/328445a0. [DOI] [PubMed] [Google Scholar]
- 20.Prendergast GC. Mechanisms of apoptosis by c-Myc. Oncogene. 1999;18:2967. doi: 10.1038/sj.onc.1202727. [DOI] [PubMed] [Google Scholar]
- 21.Zhang B, Shih JW, Wear DJ, et al. High-level expression of H-ras and c-myc oncogenes in mycoplasma- mediated malignant cell transformation. Proc. Soc. Exp. Biol. Med. 1997;214:359. doi: 10.3181/00379727-214-44104. [DOI] [PubMed] [Google Scholar]
- 22.Cowan JD, Von Hoff DD, Neuenfeldt B, et al. Predictive value of trypan blue exclusion viability measurements for colony formation in a human tumor cloning assay. Cancer Drug Deliv. 1984;1:95. doi: 10.1089/cdd.1984.1.95. [DOI] [PubMed] [Google Scholar]
- 23.Lo SC, Hayes MM, Tully JG, et al. Mycoplasma penetrans sp. nov., from the urogenital tract of patients with AIDS. Int. J. Syst. Bacteriol. 1992;42:357. doi: 10.1099/00207713-42-3-357. [DOI] [PubMed] [Google Scholar]
- 24.Lo SC, Hayes MM, Wang RY, et al. Newly discovered mycoplasma isolated from patients infected with HIV. Lancet. 1991;338:1415. doi: 10.1016/0140-6736(91)92721-d. [DOI] [PubMed] [Google Scholar]
- 25.Simms D, Cizdziel PE, Chomczynski P. TRIzol: A new reagent for optimal single-step isolation of RNA. Focus. 1993;15:532. [Google Scholar]
- 26.Evan GI, Littlewood TD. The role of c-myc in cell growth. Curr. Opin. Genet. Dev. 1993;3:44. doi: 10.1016/s0959-437x(05)80339-9. [DOI] [PubMed] [Google Scholar]
- 27.Amati B, Land H. Myc-Max-Mad: a transcription factor network controlling cell cycle progression, differentiation and death. Curr. Opin. Genet. Dev. 1994;4:102. doi: 10.1016/0959-437x(94)90098-1. [DOI] [PubMed] [Google Scholar]
- 28.Adhikary S, Eilers M. Transcriptional regulation and transformation by Myc proteins. Nat. Rev. Mol. Cell Biol. 2005;6:635. doi: 10.1038/nrm1703. [DOI] [PubMed] [Google Scholar]
- 29.Birrer MJ, Segal S, DeGreve JS, et al. L-myc cooperates with ras to transform primary rat embryo fibroblasts. Mol. Cell Biol. 1988;8:2668. doi: 10.1128/mcb.8.6.2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yancopoulos GD, Nisen PD, Tesfaye A, et al. N-myc can cooperate with ras to transform normal cells in culture. Proc. Natl. Acad. Sci. USA. 1985;82:5455. doi: 10.1073/pnas.82.16.5455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hong J, Zhao Y, Huang W. Blocking c-myc and stat3 by E. coli expressed and enzyme digested siRNA in mouse melanoma. Biochem. Biophys. Res. Commun. 2006;348:600. doi: 10.1016/j.bbrc.2006.07.107. [DOI] [PubMed] [Google Scholar]
- 32.Matsushita K, Tomonaga T, Shimada H, et al. An essential role of alternative splicing of c-myc suppressor FUSE-binding protein-interacting repressor in carcinogenesis. Cancer Res. 2006;66:1409. doi: 10.1158/0008-5472.CAN-04-4459. [DOI] [PubMed] [Google Scholar]
- 33.Evan GI, Wyllie AH, Gilbert CS, et al. Induction of apoptosis in fibroblasts by c-myc protein. Cell. 1992;69:119. doi: 10.1016/0092-8674(92)90123-t. [DOI] [PubMed] [Google Scholar]
- 34.Askew DS, Ihle JN, Cleveland JL. Activation of apoptosis associated with enforced myc expression in myeloid progenitor cells is dominant to the suppression of apoptosis by interleukin-3 or erythropoietin. Blood. 1993;82:2079. [PubMed] [Google Scholar]
- 35.Pelengaris S, Khan M, Evan GI. Suppression of Myc-induced apoptosis in beta cells exposes multiple oncogenic properties of Myc and triggers carcinogenic progression. Cell. 2002;109:321. doi: 10.1016/s0092-8674(02)00738-9. [DOI] [PubMed] [Google Scholar]
- 36.Vafa O, Wade M, Kern S, et al. c-Myc can induce DNA damage, increase reactive oxygen species, and mitigate p53 function: a mechanism for oncogene-induced genetic instability. Mol. Cell. 2002;9:1031. doi: 10.1016/s1097-2765(02)00520-8. [DOI] [PubMed] [Google Scholar]
- 37.Noronha EJ, Sterling KH, Calame KL. Increased expression of Bcl-xL and c-Myc is associated with transformation by Abelson murine leukemia virus. J. Biol. Chem. 2003;278:50915. doi: 10.1074/jbc.M306629200. [DOI] [PubMed] [Google Scholar]
- 38.Prochownik EV, Kukowska J, Rodgers C. c-myc antisense transcripts accelerate differentiation and inhibit G1 progression in murine erythroleukemia cells. Mol. Cell Biol. 1988;8:3683. doi: 10.1128/mcb.8.9.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gartel AL, Shchors K. Mechanisms of c-myc-mediated transcriptional repression of growth arrest genes. Exp. Cell Res. 2003;283:17. doi: 10.1016/s0014-4827(02)00020-4. [DOI] [PubMed] [Google Scholar]
- 40.Nigris FD, Sica V, Herrmann J, et al. c-Myc Oncoprotein: Cell Cycle-Related Events and New Therapeutic Challenges in Cancer and Cardiovascular Diseases. Cell Cycle. 2003;2:325. [PubMed] [Google Scholar]
- 41.Elledge SJ. Cell cycle checkpoints: preventing an identity crisis. Science. 1996;274:1664. doi: 10.1126/science.274.5293.1664. [DOI] [PubMed] [Google Scholar]
- 42.Thomson AW, editor. The cytokine handbook. 3rd. San Diego, London, Boston, New York, Sydney, Tokyo. Toronto: Academic Press; 1998. [Google Scholar]