Abstract
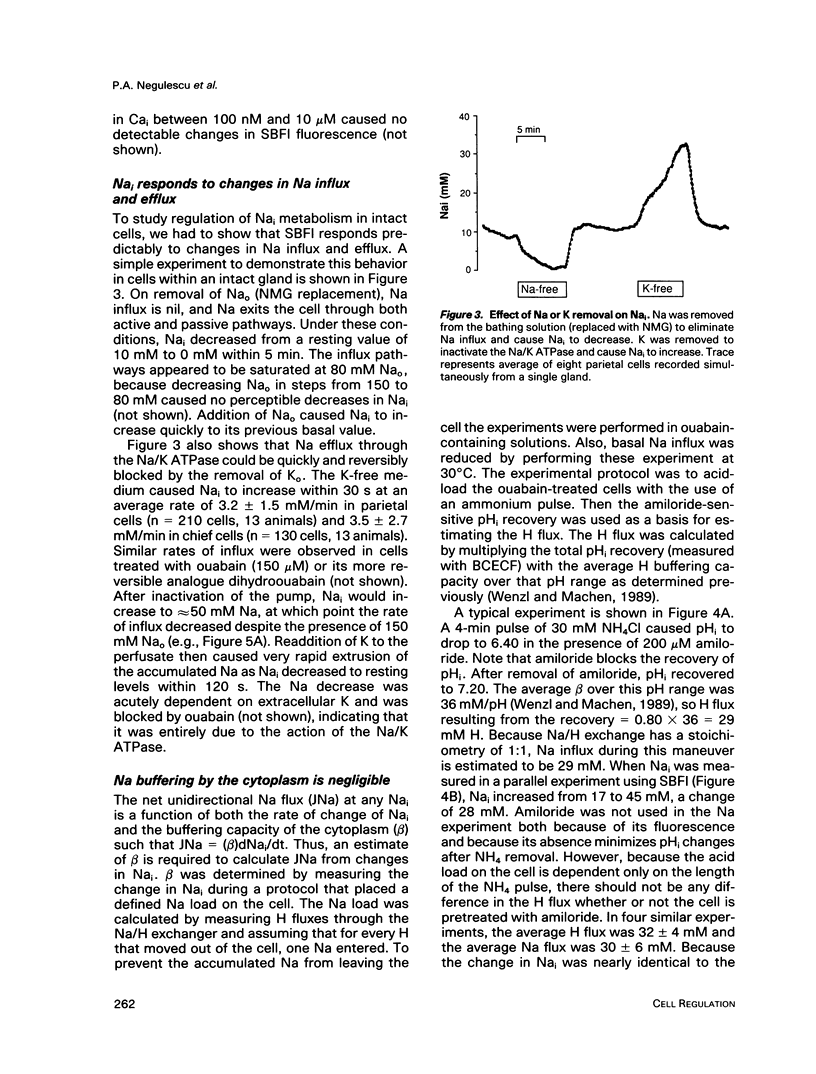
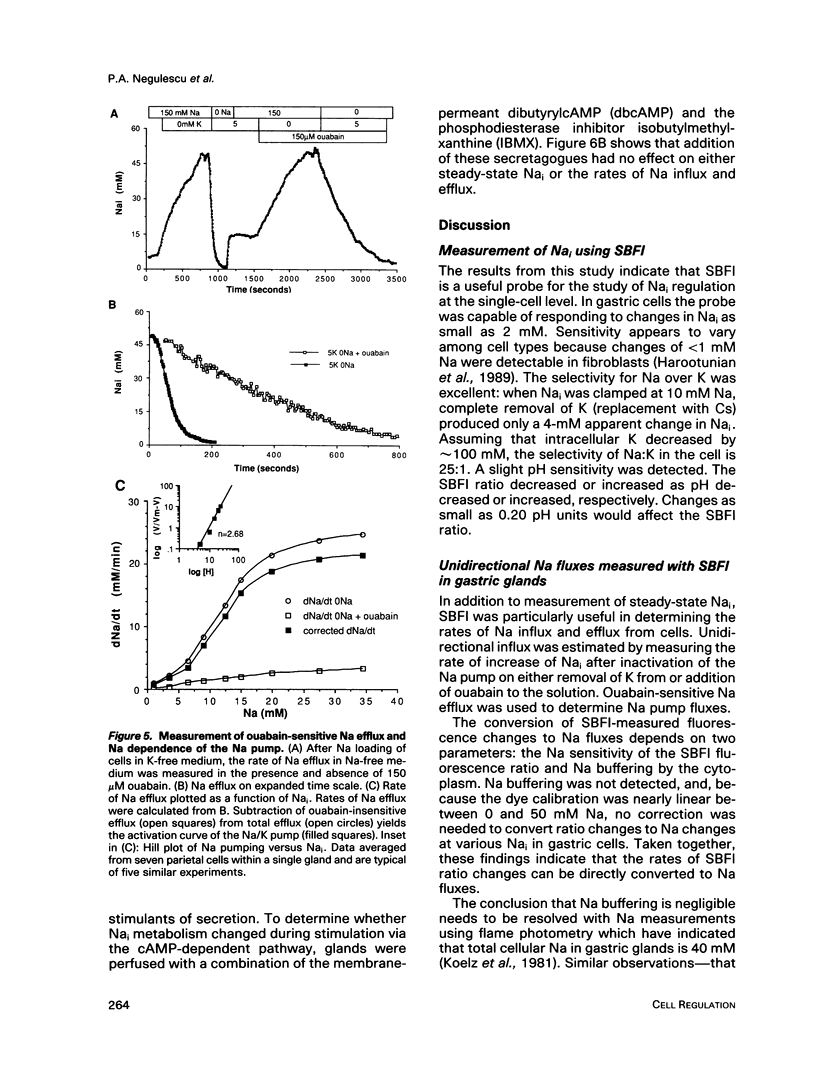
Regulation of cytosolic free Na (Nai) was measured in isolated rabbit gastric glands with the use of a recently developed fluorescent indicator for sodium, SBFI. Intracellular loading of the indicator was achieved by incubation with an acetoxymethyl ester of the dye. Digital imaging of fluorescence was used to monitor Nai in both acid-secreting parietal cells and enzyme-secreting chief cells within intact glands. In situ calibration of Nai with ionophores indicated that SBFI fluorescence (345/385 nm excitation ratio) could resolve 2 mM changes in Nai and was relatively insensitive to changes in K or pH. Measurements on intact glands showed that basal Nai was 8.5 +/- 2.2 mM in parietal cells and 9.2 +/- 3 mM in chief cells. Estimates of Na influx and efflux were made by measuring rates of Nai change after inactivation or reactivation of the Na/K ATPase in a rapid perfusion system. Na/K ATPase inhibition resulting from the removal of extracellular K (Ko) caused Nai to increase at 3.2 +/- 1.5 mM/min and 3.5 +/- 2.7 mM/min in parietal and chief cells, respectively. Na buffering was found to be negligible. Addition of 5 mM Ko and removal of extracellular Na (Nao) caused Nai to decrease rapidly toward 0 mM Na. By subtracting passive Na efflux under these conditions (the rate at which Nai decreased in Na-free solution containing ouabain), an activation curve (dNai/Nai) for the Na/K ATPase was calculated. The pump demonstrated the greatest sensitivity between 5 and 20 mM Nai. At 37 degrees C the pump rate was less than 3 mM/min at 5 mM Nai and 26 mM/min at 25 mM Nai, indicating that the pump has a great ability to respond to changes in Nai in this range. Carbachol, which stimulates secretion from both cell types, was found to stimulate Na influx in both cell types, but did not have detectable effects on Na efflux. dbcAMP+IBMX, potent stimulants of acid secretion, had no effect on Na metabolism.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson P. S. Kinetic properties of the plasma membrane Na+-H+ exchanger. Annu Rev Physiol. 1985;47:545–560. doi: 10.1146/annurev.ph.47.030185.002553. [DOI] [PubMed] [Google Scholar]
- Aronson P. S., Nee J., Suhm M. A. Modifier role of internal H+ in activating the Na+-H+ exchanger in renal microvillus membrane vesicles. Nature. 1982 Sep 9;299(5879):161–163. doi: 10.1038/299161a0. [DOI] [PubMed] [Google Scholar]
- Berglindh T., Obrink K. J. A method for preparing isolated glands from the rabbit gastric mucosa. Acta Physiol Scand. 1976 Feb;96(2):150–159. doi: 10.1111/j.1748-1716.1976.tb10184.x. [DOI] [PubMed] [Google Scholar]
- Berglindh T., Sachs G., Takeguchi N. Ca2+-dependent secretagogue stimulation in isolated rabbit gastric glands. Am J Physiol. 1980 Aug;239(2):G90–G94. doi: 10.1152/ajpgi.1980.239.2.G90. [DOI] [PubMed] [Google Scholar]
- Breitwieser G. E., Altamirano A. A., Russell J. M. Effects of pH changes on sodium pump fluxes in squid giant axon. Am J Physiol. 1987 Oct;253(4 Pt 1):C547–C554. doi: 10.1152/ajpcell.1987.253.4.C547. [DOI] [PubMed] [Google Scholar]
- Chew C. S., Brown M. R. Release of intracellular Ca2+ and elevation of inositol trisphosphate by secretagogues in parietal and chief cells isolated from rabbit gastric mucosa. Biochim Biophys Acta. 1986 Aug 29;888(1):116–125. doi: 10.1016/0167-4889(86)90077-7. [DOI] [PubMed] [Google Scholar]
- Eaton D. C., Hamilton K. L., Johnson K. E. Intracellular acidosis blocks the basolateral Na-K pump in rabbit urinary bladder. Am J Physiol. 1984 Dec;247(6 Pt 2):F946–F954. doi: 10.1152/ajprenal.1984.247.6.F946. [DOI] [PubMed] [Google Scholar]
- Garay R. P., Garrahan P. J. The interaction of sodium and potassium with the sodium pump in red cells. J Physiol. 1973 Jun;231(2):297–325. doi: 10.1113/jphysiol.1973.sp010234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersey S. J., Norris S. H., Gibert A. J. Cellular control of pepsinogen secretion. Annu Rev Physiol. 1984;46:393–402. doi: 10.1146/annurev.ph.46.030184.002141. [DOI] [PubMed] [Google Scholar]
- Horowitz S. B., Paine P. L. Reference phase analysis of free and bound intracellular solutes. II. Isothermal and isotopic studies of cytoplasmic sodium, potassium, and water. Biophys J. 1979 Jan;25(1):45–62. doi: 10.1016/S0006-3495(79)85277-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis S. A., Wills N. K. Apical membrane permeability and kinetic properties of the sodium pump in rabbit urinary bladder. J Physiol. 1983 Aug;341:169–184. doi: 10.1113/jphysiol.1983.sp014799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muallem S., Beeker T., Pandol S. J. Role of Na+/Ca2+ exchange and the plasma membrane Ca2+ pump in hormone-mediated Ca2+ efflux from pancreatic acini. J Membr Biol. 1988 May;102(2):153–162. doi: 10.1007/BF01870453. [DOI] [PubMed] [Google Scholar]
- Negulescu P. A., Reenstra W. W., Machen T. E. Intracellular Ca requirements for stimulus-secretion coupling in parietal cell. Am J Physiol. 1989 Feb;256(2 Pt 1):C241–C251. doi: 10.1152/ajpcell.1989.256.2.C241. [DOI] [PubMed] [Google Scholar]
- Paradiso A. M., Tsien R. Y., Demarest J. R., Machen T. E. Na-H and Cl-HCO3 exchange in rabbit oxyntic cells using fluorescence microscopy. Am J Physiol. 1987 Jul;253(1 Pt 1):C30–C36. doi: 10.1152/ajpcell.1987.253.1.C30. [DOI] [PubMed] [Google Scholar]
- Rink T. J., Montecucco C., Hesketh T. R., Tsien R. Y. Lymphocyte membrane potential assessed with fluorescent probes. Biochim Biophys Acta. 1980;595(1):15–30. doi: 10.1016/0005-2736(80)90243-6. [DOI] [PubMed] [Google Scholar]
- Sejersted O. M., Wasserstrom J. A., Fozzard H. A. Na,K pump stimulation by intracellular Na in isolated, intact sheep cardiac Purkinje fibers. J Gen Physiol. 1988 Mar;91(3):445–466. doi: 10.1085/jgp.91.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. L., Frizzell R. A. Chloride secretion by canine tracheal epithelium: IV. Basolateral membrane K permeability parallels secretion rate. J Membr Biol. 1984;77(3):187–199. doi: 10.1007/BF01870568. [DOI] [PubMed] [Google Scholar]
- Springer C. S., Jr Transmembrane ion pumping: high resolution cation NMR spectroscopy. Ann N Y Acad Sci. 1987;508:130–148. doi: 10.1111/j.1749-6632.1987.tb32900.x. [DOI] [PubMed] [Google Scholar]
- Townsley M. C., Machen T. E. Na-HCO3 cotransport in rabbit parietal cells. Am J Physiol. 1989 Sep;257(3 Pt 1):G350–G356. doi: 10.1152/ajpgi.1989.257.3.G350. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y. Fluorescence measurement and photochemical manipulation of cytosolic free calcium. Trends Neurosci. 1988 Oct;11(10):419–424. doi: 10.1016/0166-2236(88)90192-0. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y. Fluorescent probes of cell signaling. Annu Rev Neurosci. 1989;12:227–253. doi: 10.1146/annurev.ne.12.030189.001303. [DOI] [PubMed] [Google Scholar]
- Ueda S., Loo D. D., Sachs G. Regulation of K+ channels in the basolateral membrane of Necturus oxyntic cells. J Membr Biol. 1987;97(1):31–41. doi: 10.1007/BF01869612. [DOI] [PubMed] [Google Scholar]
- Welsh M. J., Smith P. L., Frizzell R. A. Chloride secretion by canine tracheal epithelium: II. The cellular electrical potential profile. J Membr Biol. 1982;70(3):227–238. doi: 10.1007/BF01870565. [DOI] [PubMed] [Google Scholar]
- Wenzl E., Machen T. E. Intracellular pH dependence of buffer capacity and anion exchange in the parietal cell. Am J Physiol. 1989 Nov;257(5 Pt 1):G741–G747. doi: 10.1152/ajpgi.1989.257.5.G741. [DOI] [PubMed] [Google Scholar]


