Abstract
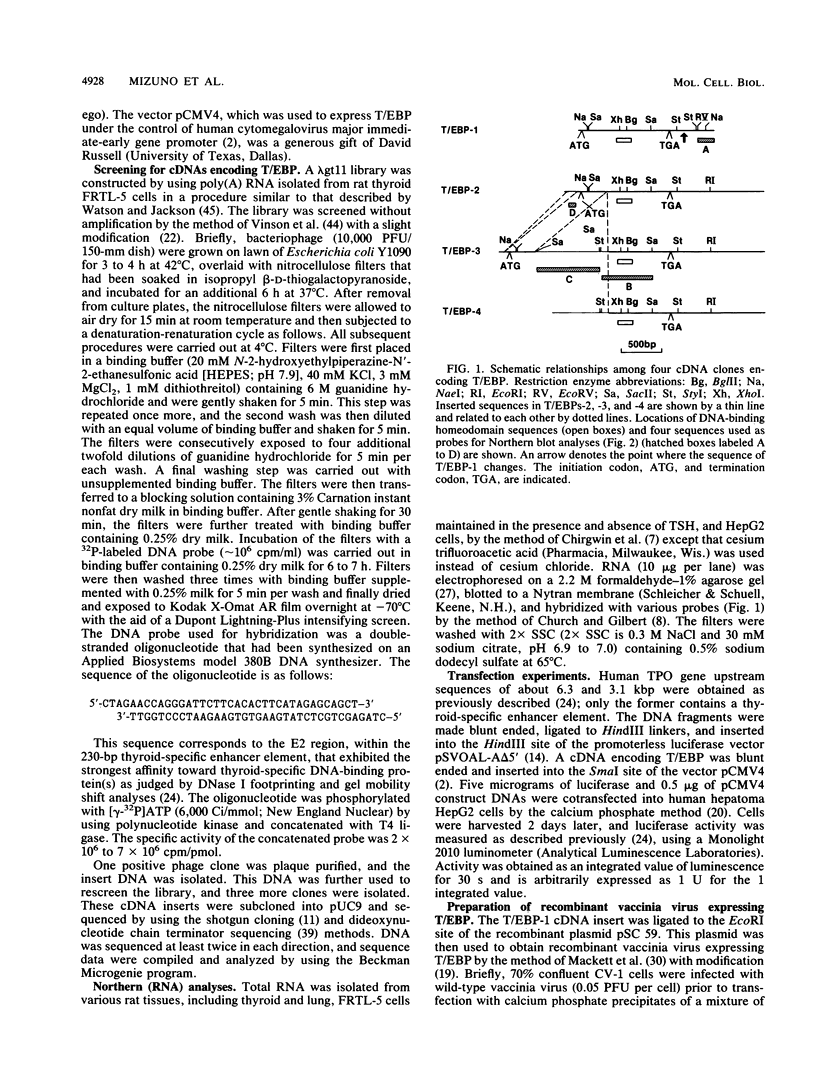
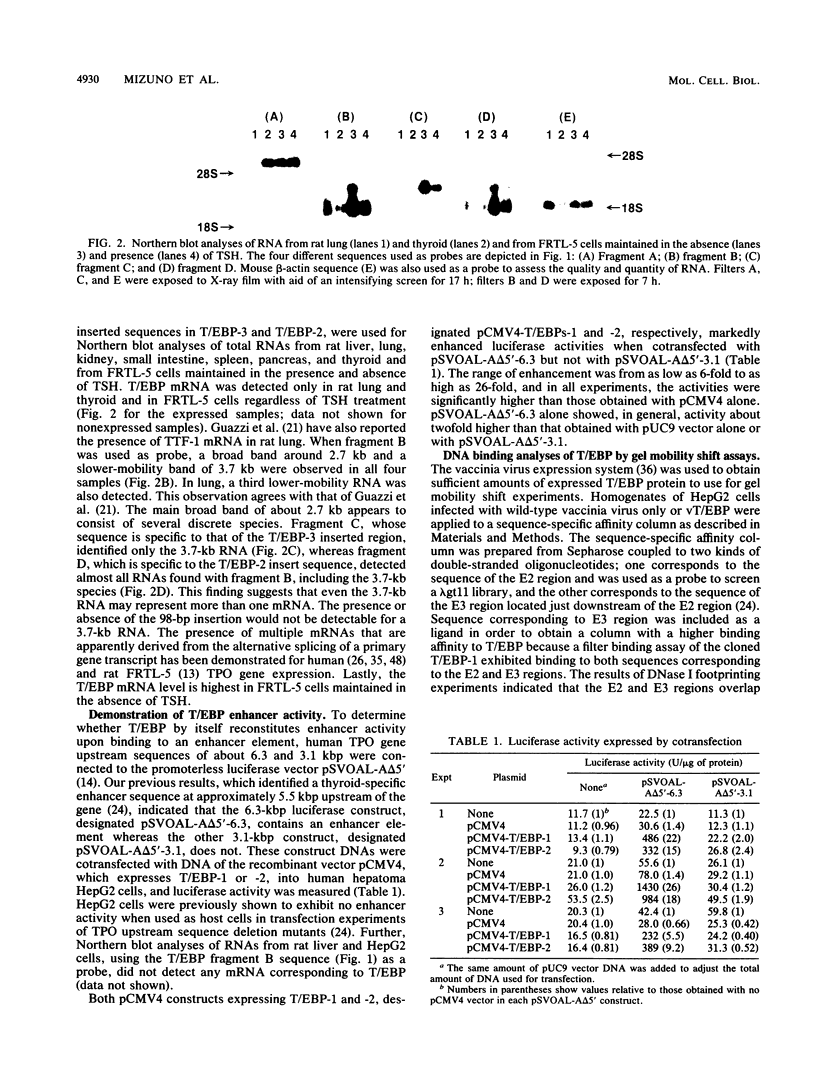
A cDNA clone encoding a thyroid-specific enhancer-binding protein (T/EBP) was isolated from a rat thyroid-derived FRTL-5 cell lambda gt 11 expression library, using a double-stranded oligonucleotide probe. This oligonucleotide was previously demonstrated to have the strongest binding affinity among three cis-acting DNA elements within the thyroid-specific enhancer region located 5.5 kbp upstream of the human thyroid peroxidase gene transcription start site. Nucleotide and deduced amino acid sequences of the cDNA revealed that T/EBP is identical to the previously reported thyroid-specific transcription factor 1 (TTF-1), which binds to the promoter of the rat thyroglobulin gene and controls its thyroid-specific expression. Expression of the T/EBP cDNA under control of the human cytomegalovirus major immediate-early gene promoter conferred thyroid-specific enhancer activity of as high as 26-fold to nonpermissive human hepatoma HepG2 cells when cotransfected with a vector containing 6.3 kbp of upstream sequence of the human thyroid peroxidase gene connected to a luciferase reporter gene. T/EBP was further expressed in HepG2 cells by using the vaccinia virus expression system. The expressed protein was partially purified by using sequence-specific affinity column chromatography and was further shown, by gel mobility shift experiments, to specifically bind to the enhancer-derived double-stranded oligonucleotide. These results clearly indicate that the binding of T/EBP (TTF-1) to the specific cis-acting enhancer element is largely responsible for thyroid-specific enhancer activity.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramowicz M. J., Vassart G., Christophe D. Thyroid peroxidase gene promoter confers TSH responsiveness to heterologous reporter genes in transfection experiments. Biochem Biophys Res Commun. 1990 Feb 14;166(3):1257–1264. doi: 10.1016/0006-291x(90)91001-9. [DOI] [PubMed] [Google Scholar]
- Andersson S., Davis D. L., Dahlbäck H., Jörnvall H., Russell D. W. Cloning, structure, and expression of the mitochondrial cytochrome P-450 sterol 26-hydroxylase, a bile acid biosynthetic enzyme. J Biol Chem. 1989 May 15;264(14):8222–8229. [PubMed] [Google Scholar]
- Avvedimento E. V., Obici S., Sanchez M., Gallo A., Musti A., Gottesman M. E. Reactivation of thyroglobulin gene expression in transformed thyroid cells by 5-azacytidine. Cell. 1989 Sep 22;58(6):1135–1142. doi: 10.1016/0092-8674(89)90511-4. [DOI] [PubMed] [Google Scholar]
- Avvedimento V. E., Musti A. M., Ueffing M., Obici S., Gallo A., Sanchez M., DeBrasi D., Gottesman M. E. Reversible inhibition of a thyroid-specific trans-acting factor by Ras. Genes Dev. 1991 Jan;5(1):22–28. doi: 10.1101/gad.5.1.22. [DOI] [PubMed] [Google Scholar]
- Avvedimento V. E., Musti A., Fusco A., Bonapace M. J., Di Lauro R. Neoplastic transformation inactivates specific trans-acting factor(s) required for the expression of the thyroglobulin gene. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1744–1748. doi: 10.1073/pnas.85.6.1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonapace I. M., Sanchez M., Obici S., Gallo A., Garofalo S., Gentile R., Cocozza S., Avvedimento E. V. Extinction and activation of the thyroglobulin promoter in hybrids of differentiated and transformed thyroid cells. Mol Cell Biol. 1990 Mar;10(3):1033–1040. doi: 10.1128/mcb.10.3.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civitareale D., Lonigro R., Sinclair A. J., Di Lauro R. A thyroid-specific nuclear protein essential for tissue-specific expression of the thyroglobulin promoter. EMBO J. 1989 Sep;8(9):2537–2542. doi: 10.1002/j.1460-2075.1989.tb08391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degroot L. J., Niepomniszcze H. Biosynthesis of thyroid hormone: basic and clinical aspects. Metabolism. 1977 Jun;26(6):665–718. doi: 10.1016/0026-0495(77)90088-9. [DOI] [PubMed] [Google Scholar]
- Deininger P. L. Random subcloning of sonicated DNA: application to shotgun DNA sequence analysis. Anal Biochem. 1983 Feb 15;129(1):216–223. doi: 10.1016/0003-2697(83)90072-6. [DOI] [PubMed] [Google Scholar]
- Derwahl M., Seto P., Rapoport B. An abnormal splice donor site in one allele of the thyroid peroxidase gene in FRTL5 rat thyroid cells introduces a premature stop codon: association with the absence of functional enzymatic activity. Mol Endocrinol. 1990 Jun;4(6):793–799. doi: 10.1210/mend-4-6-793. [DOI] [PubMed] [Google Scholar]
- Derwahl M., Seto P., Rapoport B. Complete nucleotide sequence of the cDNA for thyroid peroxidase in FRTL5 rat thyroid cells. Nucleic Acids Res. 1989 Oct 25;17(20):8380–8380. doi: 10.1093/nar/17.20.8380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foti D., Gestautas J., Rapoport B. Studies on the functional activity of the promoter for the human thyroid peroxidase gene. Biochem Biophys Res Commun. 1990 Apr 16;168(1):281–287. doi: 10.1016/0006-291x(90)91705-w. [DOI] [PubMed] [Google Scholar]
- Foulkes N. S., Borrelli E., Sassone-Corsi P. CREM gene: use of alternative DNA-binding domains generates multiple antagonists of cAMP-induced transcription. Cell. 1991 Feb 22;64(4):739–749. doi: 10.1016/0092-8674(91)90503-q. [DOI] [PubMed] [Google Scholar]
- Fried M., Crothers D. M. Equilibria and kinetics of lac repressor-operator interactions by polyacrylamide gel electrophoresis. Nucleic Acids Res. 1981 Dec 11;9(23):6505–6525. doi: 10.1093/nar/9.23.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Guazzi S., Price M., De Felice M., Damante G., Mattei M. G., Di Lauro R. Thyroid nuclear factor 1 (TTF-1) contains a homeodomain and displays a novel DNA binding specificity. EMBO J. 1990 Nov;9(11):3631–3639. doi: 10.1002/j.1460-2075.1990.tb07574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada K., Gleason S. L., Levi B. Z., Hirschfeld S., Appella E., Ozato K. H-2RIIBP, a member of the nuclear hormone receptor superfamily that binds to both the regulatory element of major histocompatibility class I genes and the estrogen response element. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8289–8293. doi: 10.1073/pnas.86.21.8289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isozaki O., Kohn L. D., Kozak C. A., Kimura S. Thyroid peroxidase: rat cDNA sequence, chromosomal localization in mouse, and regulation of gene expression by comparison to thyroglobulin in rat FRTL-5 cells. Mol Endocrinol. 1989 Nov;3(11):1681–1692. doi: 10.1210/mend-3-11-1681. [DOI] [PubMed] [Google Scholar]
- Kikkawa F., Gonzalez F. J., Kimura S. Characterization of a thyroid-specific enhancer located 5.5 kilobase pairs upstream of the human thyroid peroxidase gene. Mol Cell Biol. 1990 Dec;10(12):6216–6224. doi: 10.1128/mcb.10.12.6216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura S., Hong Y. S., Kotani T., Ohtaki S., Kikkawa F. Structure of the human thyroid peroxidase gene: comparison and relationship to the human myeloperoxidase gene. Biochemistry. 1989 May 16;28(10):4481–4489. doi: 10.1021/bi00436a054. [DOI] [PubMed] [Google Scholar]
- Kimura S., Kotani T., McBride O. W., Umeki K., Hirai K., Nakayama T., Ohtaki S. Human thyroid peroxidase: complete cDNA and protein sequence, chromosome mapping, and identification of two alternately spliced mRNAs. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5555–5559. doi: 10.1073/pnas.84.16.5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Lewin B. Commitment and activation at pol II promoters: a tail of protein-protein interactions. Cell. 1990 Jun 29;61(7):1161–1164. doi: 10.1016/0092-8674(90)90675-5. [DOI] [PubMed] [Google Scholar]
- Libert F., Ruel J., Ludgate M., Swillens S., Alexander N., Vassart G., Dinsart C. Complete nucleotide sequence of the human thyroperoxidase-microsomal antigen cDNA. Nucleic Acids Res. 1987 Aug 25;15(16):6735–6735. doi: 10.1093/nar/15.16.6735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackett M., Smith G. L., Moss B. General method for production and selection of infectious vaccinia virus recombinants expressing foreign genes. J Virol. 1984 Mar;49(3):857–864. doi: 10.1128/jvi.49.3.857-864.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson R. P., Chazenbalk G. D., Gestautas J., Seto P., Filetti S., DeGroot L. J., Rapoport B. Molecular cloning of the complementary deoxyribonucleic acid for human thyroid peroxidase. Mol Endocrinol. 1987 Nov;1(11):856–861. doi: 10.1210/mend-1-11-856. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Musti A. M., Ursini V. M., Avvedimento E. V., Zimarino V., Di Lauro R. A cell type specific factor recognizes the rat thyroglobulin promoter. Nucleic Acids Res. 1987 Oct 26;15(20):8149–8166. doi: 10.1093/nar/15.20.8149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller H. P., Schaffner W. Transcriptional enhancers can act in trans. Trends Genet. 1990 Sep;6(9):300–304. doi: 10.1016/0168-9525(90)90236-y. [DOI] [PubMed] [Google Scholar]
- Nagayama Y., Seto P., Rapoport B. Characterization, by molecular cloning, of smaller forms of thyroid peroxidase messenger ribonucleic acid in human thyroid cells as alternatively spliced transcripts. J Clin Endocrinol Metab. 1990 Aug;71(2):384–390. doi: 10.1210/jcem-71-2-384. [DOI] [PubMed] [Google Scholar]
- Piccini A., Perkus M. E., Paoletti E. Vaccinia virus as an expression vector. Methods Enzymol. 1987;153:545–563. doi: 10.1016/0076-6879(87)53077-4. [DOI] [PubMed] [Google Scholar]
- Rey-Campos J., Chouard T., Yaniv M., Cereghini S. vHNF1 is a homeoprotein that activates transcription and forms heterodimers with HNF1. EMBO J. 1991 Jun;10(6):1445–1457. doi: 10.1002/j.1460-2075.1991.tb07665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santisteban P., Kohn L. D., Di Lauro R. Thyroglobulin gene expression is regulated by insulin and insulin-like growth factor I, as well as thyrotropin, in FRTL-5 thyroid cells. J Biol Chem. 1987 Mar 25;262(9):4048–4052. [PubMed] [Google Scholar]
- Sinclair A. J., Lonigro R., Civitareale D., Ghibelli L., Di Lauro R. The tissue-specific expression of the thyroglobulin gene requires interaction between thyroid-specific and ubiquitous factors. Eur J Biochem. 1990 Oct 24;193(2):311–318. doi: 10.1111/j.1432-1033.1990.tb19339.x. [DOI] [PubMed] [Google Scholar]
- Singh H., LeBowitz J. H., Baldwin A. S., Jr, Sharp P. A. Molecular cloning of an enhancer binding protein: isolation by screening of an expression library with a recognition site DNA. Cell. 1988 Feb 12;52(3):415–423. doi: 10.1016/s0092-8674(88)80034-5. [DOI] [PubMed] [Google Scholar]
- Ursini M. V., Gallo A., Olivetta E., Musti A. M. Protein binding domains of the rat thyroglobulin promoter. Biochem Biophys Res Commun. 1989 Aug 30;163(1):481–488. doi: 10.1016/0006-291x(89)92162-1. [DOI] [PubMed] [Google Scholar]
- Vinson C. R., LaMarco K. L., Johnson P. F., Landschulz W. H., McKnight S. L. In situ detection of sequence-specific DNA binding activity specified by a recombinant bacteriophage. Genes Dev. 1988 Jul;2(7):801–806. doi: 10.1101/gad.2.7.801. [DOI] [PubMed] [Google Scholar]
- Weiss S. J., Philp N. J., Ambesi-Impiombato F. S., Grollman E. F. Thyrotropin-stimulated iodide transport mediated by adenosine 3',5'-monophosphate and dependent on protein synthesis. Endocrinology. 1984 Apr;114(4):1099–1107. doi: 10.1210/endo-114-4-1099. [DOI] [PubMed] [Google Scholar]
- Zanelli E., Henry M., Charvet B., Malthièry Y. Evidence for an alternate splicing in the thyroperoxidase messenger from patients with Graves' disease. Biochem Biophys Res Commun. 1990 Jul 31;170(2):735–741. doi: 10.1016/0006-291x(90)92152-p. [DOI] [PubMed] [Google Scholar]
- de Wet J. R., Wood K. V., DeLuca M., Helinski D. R., Subramani S. Firefly luciferase gene: structure and expression in mammalian cells. Mol Cell Biol. 1987 Feb;7(2):725–737. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]