Abstract
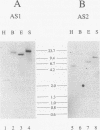
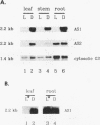
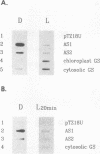
Asparagine synthetase (AS) mRNA in Pisum sativum accumulates preferentially in plants grown in the dark. Nuclear run-on experiments demonstrate that expression of both the AS1 and AS2 genes is negatively regulated by light at the level of transcription. A decrease in the transcriptional rate of the AS1 gene can be detected as early as 20 min after exposure to light. Time course experiments reveal that the levels of AS mRNA fluctuate dramatically during a "normal" light/dark cycle. This is due to a direct effect of light and not to changes associated with circadian rhythm. A novel finding is that the light-repressed expression of the AS1 gene is as dramatic in nonphotosynthetic organs such as roots as it is in leaves. Experiments demonstrate that the small amount of light which passes through the soil is sufficient to repress AS1 expression in roots, indicating that light has a direct effect on AS1 gene expression in roots. The negative regulation of AS gene expression by light was shown to be a general phenomenon in plants which also occurs in nonlegumes such as Nicotiana plumbaginifolia and Nicotiana tabacum. Thus, the AS genes can serve as a model with which to dissect the molecular basis for light-regulated transcriptional repression in plants.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berry-Lowe S. L., Meagher R. B. Transcriptional regulation of a gene encoding the small subunit of ribulose-1,5-bisphosphate carboxylase in soybean tissue is linked to the phytochrome response. Mol Cell Biol. 1985 Aug;5(8):1910–1917. doi: 10.1128/mcb.5.8.1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutry M., Chua N. H. A nuclear gene encoding the beta subunit of the mitochondrial ATP synthase in Nicotiana plumbaginifolia. EMBO J. 1985 Sep;4(9):2159–2165. doi: 10.1002/j.1460-2075.1985.tb03910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce W. B., Christensen A. H., Klein T., Fromm M., Quail P. H. Photoregulation of a phytochrome gene promoter from oat transferred into rice by particle bombardment. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9692–9696. doi: 10.1073/pnas.86.24.9692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael G. G., McMaster G. K. The analysis of nucleic acids in gels using glyoxal and acridine orange. Methods Enzymol. 1980;65(1):380–391. doi: 10.1016/s0076-6879(80)65049-6. [DOI] [PubMed] [Google Scholar]
- Colbert J. T., Hershey H. P., Quail P. H. Autoregulatory control of translatable phytochrome mRNA levels. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2248–2252. doi: 10.1073/pnas.80.8.2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coruzzi G., Broglie R., Edwards C., Chua N. H. Tissue-specific and light-regulated expression of a pea nuclear gene encoding the small subunit of ribulose-1,5-bisphosphate carboxylase. EMBO J. 1984 Aug;3(8):1671–1679. doi: 10.1002/j.1460-2075.1984.tb02031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards J. W., Coruzzi G. M. Photorespiration and light act in concert to regulate the expression of the nuclear gene for chloroplast glutamine synthetase. Plant Cell. 1989 Feb;1(2):241–248. doi: 10.1105/tpc.1.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott R. C., Dickey L. F., White M. J., Thompson W. F. cis-Acting Elements for Light Regulation of Pea Ferredoxin I Gene Expression Are Located within Transcribed Sequences. Plant Cell. 1989 Jul;1(7):691–698. doi: 10.1105/tpc.1.7.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott R. C., Pedersen T. J., Fristensky B., White M. J., Dickey L. F., Thompson W. F. Characterization of a single copy gene encoding ferredoxin I from pea. Plant Cell. 1989 Jul;1(7):681–690. doi: 10.1105/tpc.1.7.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluhr R., Chua N. H. Developmental regulation of two genes encoding ribulose-bisphosphate carboxylase small subunit in pea and transgenic petunia plants: Phytochrome response and blue-light induction. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2358–2362. doi: 10.1073/pnas.83.8.2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluhr R., Kuhlemeier C., Nagy F., Chua N. H. Organ-specific and light-induced expression of plant genes. Science. 1986 May 30;232(4754):1106–1112. doi: 10.1126/science.232.4754.1106. [DOI] [PubMed] [Google Scholar]
- Fluhr Robert, Moses Phyllis, Morelli Giorgio, Coruzzi Gloria, Chua Nam-Hai. Expression dynamics of the pea rbcS multigene family and organ distribution of the transcripts. EMBO J. 1986 Sep;5(9):2063–2071. doi: 10.1002/j.1460-2075.1986.tb04467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galangau F., Daniel-Vedele F., Moureaux T., Dorbe M. F., Leydecker M. T., Caboche M. Expression of leaf nitrate reductase genes from tomato and tobacco in relation to light-dark regimes and nitrate supply. Plant Physiol. 1988 Oct;88(2):383–388. doi: 10.1104/pp.88.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmartin P. M., Chua N. H. Spacing between GT-1 binding sites within a light-responsive element is critical for transcriptional activity. Plant Cell. 1990 May;2(5):447–455. doi: 10.1105/tpc.2.5.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmartin P. M., Sarokin L., Memelink J., Chua N. H. Molecular light switches for plant genes. Plant Cell. 1990 May;2(5):369–378. doi: 10.1105/tpc.2.5.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen G., Guilfoyle T. J. Rapid induction of selective transcription by auxins. Mol Cell Biol. 1985 Jun;5(6):1197–1203. doi: 10.1128/mcb.5.6.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joy K. W., Ireland R. J., Lea P. J. Asparagine synthesis in pea leaves, and the occurrence of an asparagine synthetase inhibitor. Plant Physiol. 1983 Sep;73(1):165–168. doi: 10.1104/pp.73.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaulen Hildegard, Schell Jeff, Kreuzaler Fritz. Light-induced expression of the chimeric chalcone synthase-NPTII gene in tobacco cells. EMBO J. 1986 Jan;5(1):1–8. doi: 10.1002/j.1460-2075.1986.tb04169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay S. A., Keith B., Shinozaki K., Chye M. L., Chua N. H. The rice phytochrome gene: structure, autoregulated expression, and binding of GT-1 to a conserved site in the 5' upstream region. Plant Cell. 1989 Mar;1(3):351–360. doi: 10.1105/tpc.1.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlemeier C., Strittmatter G., Ward K., Chua N. H. The Pea rbcS-3A Promoter Mediates Light Responsiveness but not Organ Specificity. Plant Cell. 1989 Apr;1(4):471–478. doi: 10.1105/tpc.1.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam E., Green P. J., Wong M., Chua N. H. Phytochrome activation of two nuclear genes requires cytoplasmic protein synthesis. EMBO J. 1989 Oct;8(10):2777–2783. doi: 10.1002/j.1460-2075.1989.tb08423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissemore J. L., Quail P. H. Rapid transcriptional regulation by phytochrome of the genes for phytochrome and chlorophyll a/b-binding protein in Avena sativa. Mol Cell Biol. 1988 Nov;8(11):4840–4850. doi: 10.1128/mcb.8.11.4840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandoli D. F., Tepperman J., Huala E., Briggs W. R. Photobiology of diagravitropic maize roots. Plant Physiol. 1984 Jun;75(2):359–363. doi: 10.1104/pp.75.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mösinger E., Batschauer A., Schäfer E., Apel K. Phytochrome control of in vitro transcription of specific genes in isolated nuclei from barley (Hordeum vulgare). Eur J Biochem. 1985 Feb 15;147(1):137–142. doi: 10.1111/j.1432-1033.1985.tb08729.x. [DOI] [PubMed] [Google Scholar]
- Sharrock R. A., Quail P. H. Novel phytochrome sequences in Arabidopsis thaliana: structure, evolution, and differential expression of a plant regulatory photoreceptor family. Genes Dev. 1989 Nov;3(11):1745–1757. doi: 10.1101/gad.3.11.1745. [DOI] [PubMed] [Google Scholar]
- Silverthorne J., Tobin E. M. Demonstration of transcriptional regulation of specific genes by phytochrome action. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1112–1116. doi: 10.1073/pnas.81.4.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson J., Timko M. P., Cashmore A. R., Schell J., Montagu M. V., Herrera-Estrella L. Light-inducible and tissue-specific expression of a chimaeric gene under control of the 5'-flanking sequence of a pea chlorophyll a/b-binding protein gene. EMBO J. 1985 Nov;4(11):2723–2729. doi: 10.1002/j.1460-2075.1985.tb03995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small I. S., Gray J. C. Synthesis of wheat leaf nitrite reductase de novo following induction with nitrate and light. Eur J Biochem. 1984 Dec 3;145(2):291–297. doi: 10.1111/j.1432-1033.1984.tb08551.x. [DOI] [PubMed] [Google Scholar]
- Stockhaus J., Schell J., Willmitzer L. Correlation of the expression of the nuclear photosynthetic gene ST-LS1 with the presence of chloroplasts. EMBO J. 1989 Sep;8(9):2445–2451. doi: 10.1002/j.1460-2075.1989.tb08379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tingey S. V., Coruzzi G. M. Glutamine Synthetase of Nicotiana plumbaginifolia: Cloning and in Vivo Expression. Plant Physiol. 1987 Jun;84(2):366–373. doi: 10.1104/pp.84.2.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tingey S. V., Tsai F. Y., Edwards J. W., Walker E. L., Coruzzi G. M. Chloroplast and cytosolic glutamine synthetase are encoded by homologous nuclear genes which are differentially expressed in vivo. J Biol Chem. 1988 Jul 15;263(20):9651–9657. [PubMed] [Google Scholar]
- Tingey S. V., Walker E. L., Coruzzi G. M. Glutamine synthetase genes of pea encode distinct polypeptides which are differentially expressed in leaves, roots and nodules. EMBO J. 1987 Jan;6(1):1–9. doi: 10.1002/j.1460-2075.1987.tb04710.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai F. Y., Coruzzi G. M. Dark-induced and organ-specific expression of two asparagine synthetase genes in Pisum sativum. EMBO J. 1990 Feb;9(2):323–332. doi: 10.1002/j.1460-2075.1990.tb08114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urquhart A. A., Joy K. W. Transport, metabolism, and redistribution of xylem-borne amino acids in developing pea shoots. Plant Physiol. 1982 May;69(5):1226–1232. doi: 10.1104/pp.69.5.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Tunen A. J., Koes R. E., Spelt C. E., van der Krol A. R., Stuitje A. R., Mol J. N. Cloning of the two chalcone flavanone isomerase genes from Petunia hybrida: coordinate, light-regulated and differential expression of flavonoid genes. EMBO J. 1988 May;7(5):1257–1263. doi: 10.1002/j.1460-2075.1988.tb02939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]