Abstract
Chemotherapy is the main treatment modality for certain types of cancer. It is important to monitor and ensure that these chemotherapeutic drugs are potent and effective prior to patient administration. The objective of the study is to evaluate the cytotoxic activity and potency of selected commercially available generic anticancer drugs in comparison with originator using various human cancer cell lines in an in vitro cell-based assay. Half-maximal inhibitory concentration (IC50) of the different chemotherapeutic agents was obtained from an experimentally derived dose-response curve. Relative potency of the drugs was estimated according to Parallel Line assay. This study demonstrated that the selected generic oncology products tested had similar efficacy compared with the originator. Both products showed comparable results as shown both in vitro cytotoxicity assay and statistical analysis. In vitro cell-based cytotoxicity assay promises to be a useful, reliable and rapid method for demonstrating chemotherapeutic drug activity.
Keywords: IC50, in vitro cytotoxicity, dose-response, relative potency
INTRODUCTION
Chemotherapy with cytotoxic drugs is the main treatment modality for certain types of cancer (1). It is important to monitor and ensure that these chemotherapeutic drugs are potent and effective prior to patient administration. In vitro cell-based assays have been developed to rapidly determine the cytotoxic activity of several compounds. Cell-based assays are also useful in identifying variations in susceptibility of different target cells to several chemotherapeutic agents (2, 3).
There are several chemotherapeutic drugs available in the market. Potency of these products has been tested at the site of production and has passed quality control and quality assurance requirements. However, these products may be exposed to different environmental stress conditions during transport and storage. Hence, it may be necessary to test randomly selected lots for their activity to ensure efficacy.
The objective of the study is to evaluate the cytotoxic activity and potency of selected commercially available generic anticancer drugs in comparison with originator using various human cancer cell lines in an in vitro cell-based assay.
MATERIALS AND METHODS
Cell lines
HT-29 and HeLa cells were grown on E-MEM Minimum Essential Medium (MEM) with Earle’s salt and nonessential amino acids, supplemented with 5% heat-inactivated fetal calf serum (FCS), 1 mM sodium pyruvate, and 2 mM L-glutamate. NCI-H2126 cells were grown on HITES medium supplemented with 5% fetal bovine, 0.005 mg/mL insulin, 0.01 mg/mL transferrin, 30 nM sodium selenite, 10 nM hydrocortisone, 10 nM beta-estradiol, 10 mM HEPES, and 2 mM L-glutamine. SKOV-3 cells were grown on McCoy’s 5a medium supplemented with 10% fetal bovine serum. PC-3 cells were grown on F12-K medium supplemented with 10% fetal bovine serum. All cells were grown at 37°C in 95% air with the addition of 5% CO2.
Cytotoxicity Assay
MTT Assay. The details of this assay have been described previously (3, 4). Briefly, cells were seeded at 1 × 105 cells/mL in 96 well microtiter plates in Minimum Essential Medium with fetal bovine serum. The cells were incubated overnight for attachment. Drug concentrations in serial three-fold dilutions were added in triplicates and incubated for 48h at 5% CO2 at 37°C (see list of drugs and corresponding cell line used in Table 1). Thereafter, the cells were treated with 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltratrazolium bromide (MTT) (Sigma Chemical Co., St. Louis, MO). Four hours later, all of the medium including MTT solution (5 mg/mL) was aspirated from the wells. The remaining formazan crystals were dissolved in DMSO and the absorbance was measured at 570 nm using a 96 well microplate reader (SynergyTM HT, Bio-Tek Instruments, Inc.). The cytotoxicity index was determined using the untreated cells as negative control. The percentage of cytotoxicity was calculated using the background-corrected absorbance follows (3, 4):
Table 1.
List of Generic Oncology Products, the innovator products and the corresponding tumor cell line used
| Generic-oncology Products | Originator | Tumor cell line used | Origin |
|---|---|---|---|
| Paclitaxel | Brand X | MCF-7 | Breast carcinoma |
| NCI-H2126 | non-small cell lung carcinoma | ||
| Docetaxel | Brand X | MCF-7 | Breast carcinoma |
| SKOV-3 | ovarian carcinoma | ||
| PC-3 | prostate carcinoma | ||
| NCI-H2126 | non-small cell lung carcinoma | ||
| Oxaliplatin | Brand X | HT-29 | colorectal carcinoma |
| Bicalutamide | Brand X | PC-3 | prostate carcinoma |
| Anastrozole | Brand X | MCF-7 | breast carcinoma |
IC50 determination
The IC50 was extrapolated from the dose-response graph. The drug concentration that reduced the viability of cells by 50% (IC50) was determined by plotting triplicate data points over a concentration range and calculating values using regression analysis of PRISM program.
Statistical Analysis
Analysis of the dose-response curve was done using the Software GraphPad PRISM. Relative potency of the drugs was estimated according to Parallel Line Assay (PLA version 1.2.06) (5, 6). Calculation of confidence limits and significance testing were made at the level of p=0.05.
RESULTS
Evaluation of Cytotoxic Effect Using MTT Test
Metabolic activity can be evaluated by measuring the activity of a mitochondrial enzyme succinate dehydrogenase using MTT test. MTT is designed for the quantification of cytotoxic index in cell population using 96 well plate format. This test is widely used in the in vitro evaluation of the cytotoxic potency of drugs. In the present study we applied the MTT test to evaluate the potency of selected commercially available generic anticancer drugs in comparison with originator using various human cancer cell lines in an in vitro cell-based assay.
Dose-response curve
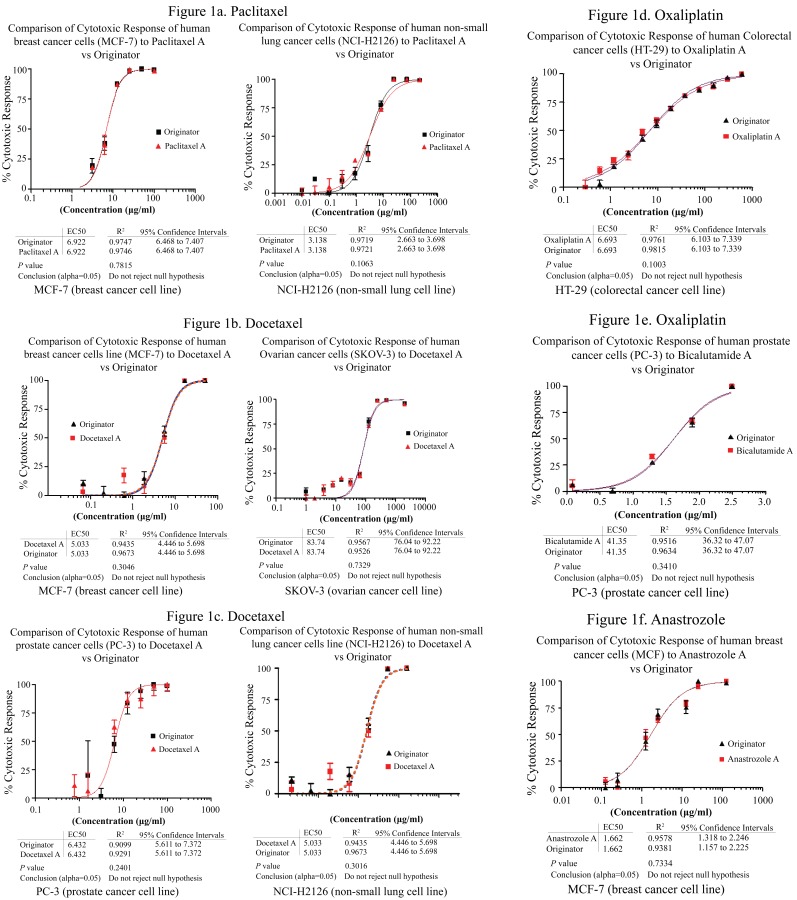
The cytotoxic response of the different cell lines to different generic oncology products versus the originator is shown in Figure 1 (a-f). The dose response curve exhibited by the generic oncology products is comparable with the originator using the indicated cancer cell lines.
Figure 1.
Comparison of the dose-response curve of selected generic oncology products with originator. A similar dose-response was observed in increasing dose concentration of the drugs added to the cells in culture. The dose-response is similar and statistical analysis proved that the difference is not significant (p>0.05). The IC50 was estimated from the curve generated. The lower the IC50, the more cytotoxic the drug is to that specific cancer cell type.
IC50 and Relative Potency
Table 2 shows the half maximal effective dose deduced from the generated dose-response curve and the relative potency of the selected generic oncology products. Statistical analysis was done using parallel line assay (PLA version 1.2.06) and both were found to be comparably cytotoxic and potent. A value of 1 (± 0.2) means that the relative potency of the tested product is almost the same as that of the competitor product (6). The generic oncology products in comparison with the competitor passed the test for linearity, test of slope and test of parallelism.
Table 2.
The half maximal effective dose (IC50) and relative potency of the selected generic oncology products
| CELL LINES | IC50 (m/mL) 95% Confidence Interval | Relative Potency 95% Confidence Interval |
|---|---|---|
| PACLITAXEL | ||
| MCF-7 (breast cancer cells) | 6.9 (6.19-7.58) | 0.9 (0.72-1.15) |
| NCI-H2126 (non-small cell lung cells) | 3.1 (2.66-3.69) | 0.95 (0.45-1.94) |
| DOCETAXEL | ||
| MCF-7 (breast cancer cells) | 5 (4.44-5.69) | 1.2 (0.69-2.15) |
| SKOV-3 (ovarian cancer cells) | 83.7 (76.04-92.2) | 1.08 (0.65-1.78) |
| PC-3 (prostate cancer cells) | 6.4 (5.61-7.37) | 0.9 (0.48-1.53) |
| NCI-H2126 (non-small cell lung cells) | 5 (4.44-5.69) | 1.1 (0.72-1.79) |
| OXALIPLATIN | ||
| HT-29 (colorectal cancer cells) | 6.7 (6.10-7.33) | 0.9 (0.71-1.01) |
| BICALUTAMIDE | ||
| PC-3 (prostate cancer cells) | 41.3 (36.3-47.07) | 1.1 (0.97-1.3) |
| ANASTROZOLE | ||
| MCF-7 (breast cancer cells) | 1.6 (1.31-2.24) | 0.9 (0.45-1.96) |
DISCUSSION
We used a cell-based assay to demonstrate that the potency of the generic oncology products is comparable with innovator drugs. Drug chemosensitivity assays were developed to evaluate anti-neoplastic drugs using cell cultures. Incorporation of cell culture studies offers good possibility as gold standard to assess the drugs due to the controlled conditions and easy procedures. A cytotoxicity test based on mitochondrial activity is then used to evaluate in vitro drug efficacy (4).
One of the major goals of oncology is to predict the response of patients with cancer to chemotherapeutic agents by employing laboratory methods variously called “tumor chemosensitivity assays”, “drug response assays”, or “drug sensitivity assays”, in vitro (4). The MTT assay is one of the methods used to predict the drug response in malignancies (4). In vitro cytotoxicity assays were applied to evaluate anti-neoplastic drugs on target cell lines. Incorporation of cell culture studies offers good possibility as gold standard to assess the drugs due to the controlled conditions and automated procedures (6).
The cytotoxic response of different cell lines to different oncology products is evaluated using high-throughput cell-based assay, the MTT assay. MTT assay is a laboratory test and a standard colorimetric assay (an assay which measures changes in colour) for measuring cellular proliferation (cell growth) (7, 8). The MTT assay reported by Mosmann is a rapid and convenient colorimetric assay for cellular growth and survival in vitro. In this paper, the MTT assay was modified as a chemosensitivity test, and its potential was investigated. This method also has several advantages with respect to rapidity, quantitation, management of many samples, and cell number required for the assay. Application of this assay to chemosensitivity testing seems to be valuable and useful.
MTT measures cell respiration and the amount of formazan produced is proportional to the number of living cells present in culture. An increase or decrease in cell number results in a concomitant change in the amount of formazan formed, indicating the degree of cytotoxicity caused by the drug. IC50 is the concentration of the tested drug able to cause the death of 50% of the cells and can be predictive of the degree of cytotoxic effect. The lower the value, the more cytotoxic is the substance. Figure 1 (a-f) shows the comparison of the IC50 of some chemotherapeutic drugs against human cancer cell lines.
The MTT-based assay relies upon the cellular reduction of tetrazolium salts to their intensely colored formazans. The test is easy to perform in hematological malignancies and is adaptable for high throughput of samples, although there are some minor limitations in its application resulting from metabolic interference. This class of assay is highly accurate for predicting drug resistance, whereas its predictive value for drug sensitivity depends on the type of disease and drug or drug combination used (9). They have been found to predict clinical response to fluradabine FLD in B-CLL and were useful for predetermining clinical potential of a single drug or drug combination in AML patients (4). The premise of in vitro drug response testing is that it can provide the knowledge of the relative efficacy of the various agents used in standard therapy before an empiric in vivo trial. Cell-based assays may help in the selection of chemotherapeutic drugs with the greatest likelihood for clinical effectiveness, and in the exclusion of ineffective therapy. This can lead to improved disease management, response, survival and use of financial resources. This study demonstrated that selected generic oncology products tested has similar efficacy compared with originator. Both products showed comparable results as proven by both in vitro cytotoxicity assay and statistical analysis.
ACKNOWLEDGMENTS
The authors would like to thank Bio-Onco Division of United Laboratories, Inc. for the support and supply of the materials used in the study.
REFERENCES
- 1.Koch S, Mayer F, Honecker F, Schittenhelm M, et al. Efficacy of cytotoxic agents used in the treatment of testicular germ cell tumours under normoxic and hypoxic conditions in vitro. Br. J. Cancer. 2003;89:2133–2139. doi: 10.1038/sj.bjc.6601375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alami N, Paterson J, Belanger S, Juste S, et al. Comparative analysis of xanafide cytotoxicity in breast cancer cell lines. Br. J. Cancer. 2007;97:58–64. doi: 10.1038/sj.bjc.6603829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carmichael J, DeGraff WG, Gazdar AF, Minna JD, et al. Evaluation of a tetrazolium based semiautomated colorimetric assay: assessment of chemosensitivity testing. Cancer Res. 1987;47:936–942. [PubMed] [Google Scholar]
- 4.Hayon T, Dvilansky A, Sphilberg O, Nathan I. Appraisal of the MTT-based Assay as a Useful Tool for Predicting Drug Chemosensitivity in Leukemia. Leukemia and Lymphoma. 2003 Sep;44(11):1957–1962. doi: 10.1080/1042819031000116607. [DOI] [PubMed] [Google Scholar]
- 5.PLA 1.2. Analysis of Parallel-Line Assays User Manual. Stegmann Systemberatung. 2000.
- 6.Brown F, Mire-Sluis A. Developments in Biologicals. The Design and Analysis of Potency Assays for Biotechnology Products. Geneve: Karger AG Medecine et Hygiene; 2002. p. 107. ISBN. 3-8055-7425-8. [Google Scholar]
- 7.Ulukaya E, Colakogullari M, Wood E. Interference by Anti-Cancer Chemotherapeutic Agents in the MTT-Tumor Assay. Chemotherapy. 2004;50:43–50. doi: 10.1159/000077285. [DOI] [PubMed] [Google Scholar]
- 8.Mosmann T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Meth. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 9.Wilson AP. In: Cytotoxicity and Viability Assays in Animal Cell Culture: A Practical Approach. 3rd. Masters JRW, editor. Vol. 1. Oxford: Oxford University Press; 2000. pp. 175–219. [Google Scholar]