Abstract
Controversy has arisen regarding the effectiveness of neuraminidase inhibitors (NIs), especially against influenza-related complications. A literature search was performed to critically assess the evidence collected by the available systematic reviews (SRs) regarding the benefits and disadvantages of NIs (oseltamivir, zanamivir) compared to placebos in healthy and at-risk individuals of all ages for prophylaxis and treatment of seasonal influenza. A SR was done using the Cochrane Database of Systematic Reviews, Health Technology Assessment Database, Database of Abstracts of Reviews of Effects, and Medline (January 2006–July 2012). Two reviewers selected SRs based on randomized clinical trials, which were restricted to intention-to-treat results, and they assessed review (AMSTAR) and study quality indicators (GRADE). The SRs included (N = 9) were of high quality. The efficacy of NIs in prophylaxis ranged from 64% (16–85) to 92% (37–99); the absolute risk reduction ranged from 1.2% to 12.1% (GRADE moderate to low). Clinically relevant treatment benefits of NIs were small in healthy adults and children suffering from influenza-like illness (GRADE high to moderate). Oseltamivir reduced antibiotic usage in healthy adults according to one SR, but this was not confirmed by other reviews (GRADE low). Zanamivir showed a preventive effect on antibiotic usage in children (95% (77–99);GRADE moderate) and on the occurrence of bronchitis in at-risk individuals (59% (30–76);GRADE moderate). No evidence was available on the treatment benefits of NIs in elderly and at-risk groups and their effects on hospitalization and mortality. In oseltamivir trials, nausea, vomiting and diarrhea were significant side-effects. For zanamivir trials, no adverse effects have been reported. The combination of diagnostic uncertainty, the risk for virus strain resistance, possible side effects and financial cost outweigh the small benefits of oseltamivir or zanamivir for the prophylaxis and treatment of healthy individuals. No relevant benefits of these NIs on complications in at-risk individuals have been established.
Introduction
In non-high-risk individuals, seasonal influenza is a self-limiting disease. Some people, such as the elderly, young children and people with concomitant morbidities, are at a higher risk for developing serious flu complications. Influenza vaccination is the best prevention method and first choice of physicians for prophylaxis [1]. Sometimes, vaccination is not available, when the vaccine is not tolerated or a mismatch between the vaccine strain and the circulating strain occurs, such as during emerging pandemics. Even vaccination is not 100% efficacious. Efficacy reaches only 40% in the elderly and there is limited good-quality evidence of the vaccine effectiveness on complications, such as pneumonia, hospitalization and influenza specific and overall mortality [2], [3], [4], [5]. Specific antiviral agents against influenza could be useful [1] for the treatment of or pre−/post-exposure prophylaxis for seasonal or pandemic influenza. The alleviation of symptoms, the reduction of antibiotic usage and the reduction of influenza-related complications such as bronchitis, otitis media, pneumonia, hospitalization and mortality are clinically relevant targets of their effect.
Among the currently available neuraminidase inhibitors (NIs), oseltamivir and zanamivir are the most widely used and tested. In Europe, a striking variation in the use of NIs is observed among different countries [6]. Viral neuraminidase enzyme activity is essential for the release of recently formed virus particles from infected cells and is thus required for the further spread of an infectious influenza virus in the body [1]. Compared with the M2 proton channel inhibitors (amantadine and rimantadine), which currently are not recommended for the prevention or treatment of seasonal influenza, the NIs are also effective against influenza B viruses, although to a lesser extent than against influenza A [7]. Zanamivir is only available for inhalation in adults and children older than five years (because the systemic absorption is limited). Oseltamivir can be taken orally (tablets or suspension) by adults and children older than one year [1]. The effect size of the NIs is inversely correlated with the time-gap between the onset of the symptoms and the start of the medication intake [8].
Recently, controversy has arisen regarding the effect of NIs against influenza-related complications [9], [10]. In several publications [9], [11], Jefferson et al. explained the difficulties that they encountered in retrieving the full reports of unpublished trials from Roche, especially those included in the review from Kaiser et al. [12], which raised a concern of reliability. As a result, the conclusions of the updated Cochrane review were changed to reflect the gap in the knowledge caused by excluding unpublished material [10].
To help clinicians and policymakers make sense of these controversies, the focus of this review was to see how the different systematic reviews (SRs) dealt with these evidence issues and to determine how these SRs represented the existing evidence. Concurrently, we aimed to synthesize the current evidence to enable clinicians to derive a management strategy.
Therefore, an extensive literature search was performed to summarize and critically evaluate the evidence collected by the existing SRs regarding the benefits and disadvantages of the use of NIs (oseltamivir, zanamivir) compared to placebos in healthy and at-risk individuals of all ages for the prophylaxis and treatment of seasonal influenza.
Methods
Search Strategy
Inclusion and exclusion criteria
Only SRs mainly based on randomized clinical trials (RCTs) that discussed the use of NIs (oseltamivir and zanamivir) for the prophylaxis and treatment of seasonal influenza and that evaluated NIs versus placebos in healthy adults, children, elderly and at-risk individuals were considered. No search was performed before 2006 because the most recently updated SRs were the focus of this review. SRs that included observational studies besides RCTs could be included, but only the results of the RCTs are shown. To respect randomization and to allow for extrapolation to current clinical practices, only the intention-to-treat (ITT) results are discussed. Narrative reviews and meta-analyses that did not systematically search the literature and did not critically assess the quality of the included trials were excluded. SRs published in languages other than English, French, Dutch or German were not eligible.
For the prophylaxis results, a distinction was made between seasonal prophylaxis, outbreak control and post-exposure prophylaxis, for which NIs were given up to 42 days, 14 days and 10 days, respectively. In prophylaxis for adults, no dosages other than those that were recommended are shown (oseltamivir, 75 mg orally once daily and zanamivir, 2×5 mg inhaled once daily). In children, dosages were adjusted according to their body weight.
In the treatment trials, only trials that used orally administered oseltamivir at 2×75 mg/day (according to weight in children) or the recommended dose of 2×10 mg/day inhaled zanamivir are shown.
Outcomes
The efficacy (against laboratory-proven influenza) of prophylaxis, the effectiveness in reducing the time to symptom alleviation and to a return to normal activity (as defined by the original trial protocol), the effectiveness against complications in treatment and the potential risks (adverse events) of the NIs versus placebos are the main outcomes measured. They are expressed as relative risk (RR), efficacy E = (1−RR)×100 or odds ratio (OR), unless stated otherwise in the SRs (e.g., random risk difference, mean or median difference). The most robust and reliable pooled results are presented. Absolute risk reduction was calculated where appropriate. No new pooling of results was performed.
Search details
First, the Cochrane Database of Systematic Reviews, the Health Technology Assessment Database (HTA) and the Database of Abstracts of Reviews of Effects were consulted using the keywords ‘influenza AND oseltamivir OR zanamivir OR neuraminidase (all fields)’ from 2006 to 2012. After checking the inclusion dates for the SRs retrieved, a PubMed search was conducted using the following search strategy: (“influenza, human”[MeSH Terms] OR (“influenza”[All Fields] AND “human”[All Fields]) OR “human influenza”[All Fields] OR “influenza”[All Fields]) AND (“neuraminidase”[MeSH Terms] OR “neuraminidase”[All Fields]) OR “oseltamivir”[MeSH Terms] OR “oseltamivir”[All Fields] OR “zanamivir”[MeSH Terms] OR “zanamivir”[All Fields] AND (Meta-Analysis[ptyp] OR Review[ptyp]) AND (English[lang] OR French[lang] OR German[lang] OR Dutch[lang]) AND (“2006/01/01”[PDAT] : “2012/08/01”[PDAT]).
Study Selection and Data Extraction
BM and VPK selected the appropriate publications firstly on the basis of the title/abstract and secondly on the full text, applying the inclusion and exclusion criteria. The reasons for exclusion were recorded. Data were extracted by BM regarding the outcomes of the studies including the number of trials and the number of participants. In cases of disagreement, EV’s evaluation was used.
Quality Appraisal
BM and KVP assessed the quality of the SRs using the AMSTAR tool [13]. In cases of disagreement, EV’s evaluation was used. The quality of the evidence for the individual outcomes was graded using the GRADE classification method [14] and presented according to the GRADE profiler 3.6© format (http://ims.cochrane.org/revman/gradepro). The risk of bias, inconsistency, indirectness and imprecision were considered by BM and KVP while reviewing all of the sources contributing to the evidence of the same outcome. The ‘risk of bias’ assessment of the RCTs that was focused on sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, selective reporting, incomplete outcome data and other biases with a possible impact on the final estimate of the outcome was considered [15]. The ‘risk of bias’ assessment was based on the quality of the assessments made by the selected SRs. In the case of incongruence, the original study was consulted and reassessed. The quality of the evidence (GRADE) was labeled as follows: high (no or only one problem), moderate (2 problems) or low (3 or more problems).
No formal protocol was published in English. Registration was not conducted.
Results
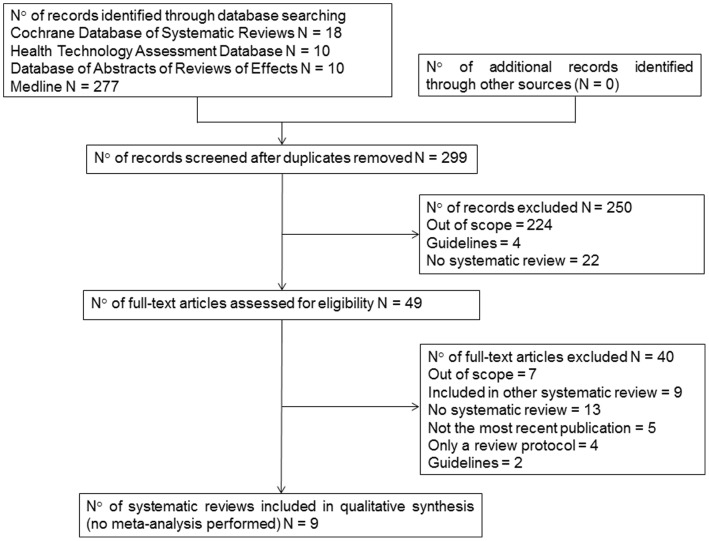
The search results are described in Figure 1. Three Cochrane reviews [10], [16], [17], two HTA clinical appraisals [8], [18] from the UK, one HTA from Canada [19] and three additional meta-analyses [20], [21], [22] were withheld. Tappenden [18], Jackson [21], Khazeni [22], Burch [8], Deonandan [19] and Falagas et al. [20] handled all ages and risk groups. Jefferson et al. [10] restricted his SR to healthy adults, children and mixed populations.
Figure 1. Flow of information for the search (PRISMA).
Six systematic reviews [10], [17], [18], [19], [21], [22] described the results of prophylaxis for influenza using oseltamivir and zanamivir. Khazeni et al. [22] restricted their review to the extended-duration chemoprophylaxis. Treatment results were discussed by four SRs [8], [10], [16], [17]. Falagas et al. [20] restricted his SR to the effect of NIs on influenza-related complications (Table 1).
Table 1. General characteristics of the included systematic reviews.
| First Author/Publication year | Source | Search date up to | Interventiona | Prophy-laxisb | Treatmentb | Effect on flu related compli-cationsb | Adverse eventsb | Target groups | Unpublish-ed trials | Lan-guage restric-tions |
| Burch, 2009 [8] | HTA UK | Nov 2007 | A/O/Z | N = 34 | N = 19 | N = 19 | All ages and at-risk groups | yes | yes | |
| Deonandan, 2007 [19] | HTA Canada | Aug 2006 | O/Z | N = 11 | N = 11 | All ages and at-risk groups | no | no | ||
| Falagas, 2010 [20] | J Antimicrob Chemother | Sept 2009 | O/Z | N = 11 | N = 9 | All ages and at-risk groups | no | yes | ||
| Jackson, 2011 [21] | J Infect | August 2009 | A/O/Z | N = 12 | N = 3 | All ages and at-risk groups | no | yes | ||
| Jagannath, 2010 [16] | Cochrane SR | August 2009 | O/Z | N = 0 | N = 0 | N = 0 | Persons with cystic fibrosis | yes | no | |
| Jefferson, 2012 [10] | Cochrane SR | April 2011 | O/Z/P | N = 6 | N = 19 | N = 8 | N = 18 | Healthy adults/mixed populations/children | yes | no |
| Khazeni, 2009 [22] | Ann Intern Med | June 2009 | O/Z | N = 6 | N = 6 | All ages and at-risk groups | no | no | ||
| Wang, 2012 [17] | Cochrane SR | Jan 2012 | O/Z/L | N = 3 | N = 6 | N = 6 | N = 9 | Children healthy and at risk | no | no |
| Tappenden, 2009 [18] | HTA UK | July 2007 | A/O/Z | N = 15 | N = 0 | All ages and at-risk groups | yes | yes |
A = amantadine; O = oseltamivir; Z = zanamivir; P = peramivir; L = laninamivir.
N = number of trials included in SR.
The review of Jagannath et al. [16] could not retrieve any trials describing the benefits or disadvantages of NIs among persons suffering from cystic fibrosis.
The extensive HTA report of Burch et al. [8] is also summarized in The Lancet Infectious Diseases [23]. The Jackson et al. [21] SRs updated the Tappenden et al. [18] SR using the same methods and rigor.
In total, 35 reviews were excluded because of a lack of an exhaustive, systematic literature search and frequently because of a lack of critical quality appraisals for the included RCTs (Table 2). Three Cochrane reviews only showed a protocol version.
Table 2. List of excluded reviews with reasons.
| Reference (A–Z) | Reason for exclusion |
| Beigel J et al. Antiviral Res. 2008 [49] | no systematic literature search, narrative review |
| Bettis R et al. Clin Drug Investig. 2006 [50] | no systematic literature search, narrative review |
| Bijl D. Int J Risk Saf Med. 2011 [51] | no systematic literature search, narrative review |
| Burch J et al. Lancet Infect Dis. 2009 [23] | Journal publication of Health Technology Appraisal of Burch et al. [8] |
| Chidiac C. Rev Prat. 2008 [52] | no systematic literature search, narrative review |
| Clark NM et al. Semin Respir Crit Care Med. 2011 [53] | no systematic literature search, narrative review |
| Dutkowski R. J Antimicrob Chemother. 2010 [54] | no systematic literature search, narrative review |
| Ferraris O et al. Pathol Biol (Paris). 2010 [55] | no systematic literature search, narrative review |
| Freemantle N et al. BMJ. 2009 [56] | no systematic literature search, narrative review; no RCTs included, evidence based on observational studies |
| Health Technology Assessment, 2010; HTA-32010000424 [57] | older version of Turner et al., replaced by Burch et al. [8] and Tappenden et al. [18] |
| Heneghan CJ. Health Technology Assessment programme, 2011, HTA-32011001126 [58] | only a protocol version, final version not available |
| Hernán MA et al. Clin Infect Dis. 2011 [34] | no systematic literature search, no critical quality appraisal for the included RCTs |
| Holzgrabe U. Pharm Unserer Zeit. 2011 [59] | no systematic literature search, narrative review |
| Jamieson B et al. Can Fam Physician. 2009 [60] | no systematic literature search, narrative review |
| Jefferson T et al. Cochrane Syst Rev. 2006 [61] | not the most recent publication of the same research group (Jefferson et al. 2012 [10]) |
| Jefferson T et al. Lancet. 2006 [62] | journal publication of Cochrane Syst. Rev. Jefferson et al. 2006 [61] |
| Jefferson T et al. BMJ. 2009 [63] | evidence included in Cochrane Syst. Rev. Jefferson et al. 2010 [64] |
| Jefferson T et al. Cochrane Syst Rev. 2010 [64] | not the most recent publication of the same research group (Jefferson et al. 2012) (withdrawn) |
| Jefferson T et al. Health Technol Assess. 2010 [65] | same evidence included in the Cochrane Syst. Rev. of Jefferson et al. 2010 [64] |
| Jefferson T et al. Cochrane Syst Rev: 2011 [66] | only a protocol version, final version not available |
| Jones M et al. Expert Opin Drug Saf. 2006 [67] | Evidence included in the Cochrane syst. Rev. of Jefferson et al. 2006 [61] |
| Klebe G et al. Pharm Unserer Zeit. 2011 [68] | no systematic literature search, narrative review |
| Lee N et al. Antivir Ther. 2012 [69] | no systematic literature search, narrative review |
| Lynch JP et al. Semin Respir Crit Care Med. 2007 [70] | no systematic literature search, narrative review |
| Mallia P et al. Int J Chron Obstruct Pulmon Dis. 2007 [71] | no systematic literature search, narrative review |
| Matheson NJ et al. Cochrane Syst Rev. 2007 [72] | not the most recent publication of the same research group (Wang et al. [17], Jefferson et al. 2012 [10]) |
| McCullers JA. Antivir Ther. 2011 [73] | no systematic literature search, narrative review |
| Moscona A. Annu Rev Med. 2008 [74] | no systematic literature search, narrative review |
| National Institute for Health and Clinical Excellence (NICE), 2009; HTA-32011000098 (TA-168) [40] | NICE Technology appraisal guidance based on the systematic review of Burch et al. [8] |
| National Institute for Health and Clinical Excellence (NICE), 2008; HTA-32011000382 (TA-67) [75] | NICE Technology appraisal guidance based on the systematic review of Tappenden P et al. [18] |
| Nayak JL et al. Pediatr Ann. 2009 [76] | no systematic literature search, narrative review |
| No author. Med Lett Drugs Ther.2006 [77] | no systematic literature search, narrative review |
| No author. Med Lett Drugs Ther. 2009 [78] | no systematic literature search, narrative review |
| No author. Med Lett Drugs Ther. 2012 [79] | no systematic literature search, narrative review |
| Nüesch R. Ther Umsch. 2007 [80] | no systematic literature search, narrative review |
| Oxford JS. Influenza Other Respi Viruses. 2007 [81] | no systematic literature search, narrative review |
| Preziosi P. Expert Opin Pharmacother. 2011 [82] | no systematic literature search, narrative review |
| Ruf BR et al. Dtsch Med Wochenschr. 2008 [83] | no systematic literature search, narrative review |
| Ruf BR et al. Infection Control & Hospital Epidemiology. 2009 [84] | no systematic literature search, narrative review |
| Salzberger B. Internist (Berl). 2006 [85] | no systematic literature search, narrative review |
| Schirmer P et al. Expert Opin Drug Saf. 2009 [86] | no systematic literature search, narrative review |
| Shun-Shin M et al. BMJ. 2009 [87] | not the most recent publication of the same research group (Wang et al. [17], Jefferson et al, 2012 [10]) |
| Smith JR et al. Adv Ther. 2011 [88] | no systematic literature search, narrative review |
| Tambyah PA. Respirology. 2008 [89] | no systematic literature search, narrative review |
| Tappenden P et al. Health Technology Assessment, 2009; HTA-32008100360 [90] | replaced by the systematic review of Tappenden P et al. [18] |
| Toovey S et al. Drug Saf. 2008 [91] | no systematic literature search, narrative review |
| Townsend KA et al. Pharmacotherapy. 2006 [92] | no systematic literature search, narrative review |
| Tullu MS. J Postgrad Med. 2009 [93] | no systematic literature search, narrative review |
| Wang K et al. Cochrane Database Syst Rev. 2012 [94] | replaced by the systematic review of Wang et al. [17] |
| Wesseling G. Int J Chron Obstruct Pulmon Dis. 2007 [95] | no systematic literature search, narrative review |
| Whitley RJ. Expert Opin Drug Metab Toxicol. 2007 [96] | no systematic literature search, narrative review |
| Yang Ming et al. Cochrane Syst Rev: Protocols 2010 [97] | only a protocol version, final version not available |
| Yang Ming et al. Cochrane Syst Rev: Protocols 2010 [98] | only a protocol version, final version not available |
Quality Appraisal
Systematic reviews
In general, the SRs of Burch et al. [8], Tappenden et al. [18], Jefferson et al. [10] and Wang et al. [17] were of excellent quality according to the AMSTAR checklist [13]. Although differences were noted in their search methods, database sources, inclusion/exclusion criteria, data extraction, quality appraisals and statistical analyses, they provided an extensive description of the methods used, the quality and the general characteristics of the included and excluded trials. The latest Cochrane SR by Jefferson et al. [10] based the inclusion/exclusion criteria, the quality appraisal and the data extraction only on extensive clinical data reports, which contrasted with the other SRs that reported results based on published or short reported trials.
The SRs of Jackson [21] and Khazeni et al. [22] were also well performed, but they were only summarized in a concise publication. Although a thorough search procedure was performed in all of these SRs to unravel unpublished studies, funnel plots to assess publication bias were seldom used, and only the SR of Jefferson et al. [10] worked with a full trial list. For some outcomes, a considerable variability in the included and excluded trials exists between the different SRs. The SR of Deonandan et al. [19] was completed by one reviewer, included case-control and observational studies and did not provide useful outcome measures. The SR of Falagas et al. [20] combined different dose regimens of the NIs and only presented effectiveness results among the participants with confirmed influenza (no intention-to-treat analysis). Finally, mistakes were made in extracting the correct numbers from the original papers. The SRs from Deonandan [19] and Falagas et al. [20] did not include recent RCTs that were not yet included in other SRs. For all of these reasons, these SRs will not be discussed further (Table 3).
Table 3. AMSTAR quality appraisal of the included SRs.
| AMSTAR questions | Burch, 2009 [8] | Deonandan, 2007 [19] | Falagas, 2010 [20] | Jackson et al, 2011 [21] | Jagannath, 2010 [16] | Jefferson, 2012 [10] | Khazeni, 2009l [22] | Wang, 2012 [17] | Tappenden, 2009 [18] |
| 1. Was an “a priori” design provided? | yes | not specified | not specified | yes | yes | yes | no | yes | yes |
| 2. Was there duplicate study selection and data extraction? | yes | no | yes | partially | yes | yes | yes | yes | yes |
| 3. Was a comprehensive literature search performed? | yes | yes | yes | yes | yes | yes | yes | yes | yes |
| 4. Was the status of publication (i.e., grey literature) used as an inclusion criterion? | yes | yes | no | yes | yes | yes | yes | yes | no |
| 5. Was a list of studies (included and excluded) provided? | yes | partially | partially | partially | no | yes | partially | yes | yes |
| 6. Were the characteristics of the included studies provided? | yes, | yes | yes | yes | NA | yes | yes | yes | yes |
| 7. Was the scientific quality of the included studies assessed and documented? | yes | partially | yes | yes | NA | yes | yes | yes | yes |
| 8. Was the scientific quality of the included studies used appropriately in formulating conclusions? | yes | no | yes | no | NA | yes | yes | yes | no |
| 9. Were the methods used to combine the findings of studies appropriate? | yes | NA | yes | yes | yes | yes | yes | yes | yes |
| 10. Was the likelihood of publication bias assessed? | no | no | partially, no specific tests were performed | no | NA | yes | no | partially, no specific tests were performed | no |
| 11. Was the conflict of interest included? | yes for the reviewers, no for the included RCTs | no | unclear for the reviewers, no for the included RCTs | no | yes for the reviewers, no for the included RCTs | yes | yes for the reviewers, no for the included RCTs | yes for the reviewers, no for the included RCTs | yes for the reviewers, no for the included RCTs |
SR = systematic review; NA = not applicable.
Original publications
Very few prophylaxis and treatment trials showed a well-reported methodology and had a minor risk of bias [8], [10]. Other prophylaxis and treatment trials were at risk of bias because of the poor description of the allocation concealment, the number of withdrawals (losses to follow-up), blinding, randomization methods and power calculations [8], although the randomization and allocation concealment of the trials were regarded as adequate in most studies by Jefferson [10]. Very few original studies published results regarding the ITT population (which indicates all of the participants with influenza-like illness (ILI)), and at least two studies were open-label [24], [25]. Complications and adverse events were poorly and possibly selectively reported or misclassified in most of the trials [10]. Adverse events similar to influenza symptoms were generally excluded from the trials [10]. Important baseline characteristics such as vaccination status and antibiotic usage were not always reported [10]. The quality of the zanamivir publications was graded better than that of the oseltamivir reports [8], [10].
Clinical Effects
Prophylaxis
In healthy adults, the seasonal prophylaxis against influenza showed a significant efficacy of 76% (95% CI 42–90) for oseltamivir (GRADE moderate) corresponding with an absolute risk reduction (ARR) of 3.6% (95% CI 2.0–4.3) and 68% (95% CI 37–83) for zanamivir (GRADE moderate) – ARR = 4.1% (95% CI 2.3–5.1). For post-exposure prophylaxis, 81% (95% CI 55–92) efficacy for oseltamivir (GRADE moderate) – ARR = 7.0% (95% CI 4.8–8.0) and 79% (95% CI 67–87) for zanamivir (GRADE moderate) – ARR = 6.9% (95% CI 5.8–7.6) were shown (Table 4).
Table 4. Efficacy of the use of oseltamivir and zanamivir in prophylaxis against symptomatic, laboratory-confirmed influenza in healthy adults, children, elderly and at-risk individuals according to different systematic reviews (ITT – pooled results).
| Outcome | NIa,b | Number of days | First author/review | Number of included studies | Number of participants | Percentage of influenza in placebo group | Estimate (95% CI)c | Quality GRADEe | References of included studies |
| Healthy adults | |||||||||
| Seasonal prophylaxis | O | 42 | Tappenden [18] | 1 | 1039 | 4.8% | 0.27 (0.09 to 0.83) | moderate | Hayden 1999 [99] |
| Jackson [21] | 1 | 1039 | 4.8% | 0.24 (0.09 to 0.54) | Hayden 1999 [99] | ||||
| Khazeni [22] | 2 | 1039/308 | 4.8%/13.7% | 0.24 (0.10 to 0.58)/(0.24 (0.09 to 0.61)d | Hayden 1999 [99]/Kashiwaghi 2000 [47] | ||||
| Z | 28 | Tappenden [18] | 2 | 1107/316 | 6.1%/3.8% | 0.32 (0.17 to 0.63)/(0.49 (0.12 to 1.92)d | moderate | Monto 1999 [30]/GSK study 167/101 | |
| Jackson [21] | 1 | 1107 | 6.1% | 0.32 (0.17 to 0.63) | Monto 1999 [30] | ||||
| Khazeni [22] | 1 | 1107 | 7.8% | 0.33 (0.18 to 0.59) | Monto 1999 [30] | ||||
| Post-exposure prophylaxis | O | 7 to 10 | Tappenden [18] /Jackson [21] | 2 | 1747 | 8.7% | 0.19 (0.08 to 0.45) | moderate | Welliver 2001 [42]/Hayden 2004 [26] |
| Z | 5 to 10 | Tappenden [18] /Jackson [21] | 3 | 2416 | 8.7% | 0.21 (0.13 to 0.33) | moderate | Hayden 2000 [100]/Monto 2002 [101]/Kaiser 2000 [102] | |
| Children | |||||||||
| Post-exposure prophylaxis | O | 10 | Tappenden [18] /Jackson [21] / Wang [17] | 1 | 215 | 18.9% | 0.36 (0.15 to 0.84) | low | Hayden 2004 [26] |
| O&Z | 10 | Wang [17] | 3 | 863 | 12.8% | −0.08 (−0.12 to −0.05) | moderate | Hayden 2000 [100]/Monto 2002 [101]/WV16193 | |
| At-risk elderly | |||||||||
| Seasonal prophylaxis | O | 42 | Tappenden [18] /Jackson [21] | 1 | 548 | 4.4% | 0.08 (0.01 to 0.63) | low | Peters 2001 [103] |
| Khazeni [22] | 1 | 548 | 4.4% | 0.08 (0.01 to 0.63) | Peters 2001 [103] | ||||
| Z | 28 | Tappenden [18] /Jackson [21] | 1 | 1896 | 0.5% | 0.20 (0.02 to 1.72) | moderate | LaForce 2007 [27] | |
| At-risk adults and adolescents (67–68% vaccinated) | |||||||||
| Seasonal prophylaxis | Z | 28 | Tappenden [18] /Jackson [21] | 1 | 3363 | 1.4% | 0.17 (0.07 to 0.44) | moderate | LaForce 2007 [27] |
| Elderly subjects in long-term care (10% vaccinated) | |||||||||
| Outbreak control | Z | 14 | Tappenden [18] /Jackson [21] | 1 | 489 | 9.2% | 0.68 (0.36 to 1.27) | low | Ambrozaitis 2005 [28] |
O = Oral oseltamivir 75 mg 1×/day; dosage adjusted to weight in children.
Z = Inhaled zanamivir 10 mg 1×/day; dosage adjusted to weight in children.
relative risk or random risk difference (Wang).
no pooling of results.
GRADE quality of evidence: high; moderate; low.
ITT = intention-to-treat; NI = neuraminidase inhibitor; CI = confidence interval.
In children, only post-exposure prophylaxis studies were performed. One study with oseltamivir [26] found 64% (95% CI 16–85) efficacy (GRADE low quality) – ARR = 12.1% (95% CI 3.0–16.1). Oseltamivir and zanamivir studies combined showed an ARR of 8% (95% CI 5–12) (pooled results [17] – GRADE moderate quality).
In at-risk adults and adolescents, seasonal prophylaxis with zanamivir was determined by one study [27] to have 83% (95% CI 56–93) efficacy (GRADE moderate quality) – ARR = 4.0% (95% CI 1.6–4.4). In the at-risk elderly population of the same study, no significant efficacy was found (GRADE moderate quality). In at-risk elderly individuals, one study with oseltamivir during an influenza epidemic found 92% (95% CI 37–99) efficacy (GRADE low quality) – ARR = 1.2% (95% CI 0.8–1.3).
In the long-term care elderly, an outbreak control study [28] with zanamivir found no evidence of efficacy (GRADE low quality).
Treatment
Jefferson et al. [10]only published results for the effect of oseltamivir on the alleviation of symptoms and selected different studies compared to Burch et al. [8]. Pooled results showed that oseltamivir and zanamivir treatment alleviated the symptoms of influenza less than one day sooner. The time to return to normal activity could be reduced by one and half a days by oseltamivir and by less than half a day by zanamivir according to Burch et al. [8](GRADE high to moderate) (Table 5).
Table 5. Treatment effect of oseltamivir and zanamivir versus placebo in healthy adults, children, elderly and at-risk populations (ITT – pooled results).
| Outcomes | NIa,b | First author/review | Number of included studies | Number of participants | Estimate (95%CI)c | Quality GRADEe | References of included studies |
| Healthy adults | |||||||
| Time to alleviation of symptoms | O | Burch [8] | 4 | 1410 | −13.29 (−25.15 to −3.43) | moderate | Li 2003 [104]/Nicholson 2000 [105]/Treanor 2000 [106]/Roche WVI5730 |
| Jefferson [10] | 5 | 3713 | −21.3 (−29.59 to −12.98) | Whitley 2001 [107]/Nicholson 2000 [105]/Treanor 2000 [106]/M76001/WV15819 | |||
| Z | Burch [8] | 6 | 2701 | −0.57 (−1.07 to −0.08) | high | GSK NAIA3002/GSK NAI 3001/Hayden 1997 [108]/Mäkelä 2000 [109]/MIST 1998 [110]/Puhakka 2003 [111] | |
| Time to return to normal activity | O | Burch [8] | 3 | 951 | −31.94 (−46.95 to −16.93) | moderate | Li 2003 [104]/Nicholson 2000 [105]/Treanor 2000 [106] |
| Z | Burch [8] | 4 | 3025 | −0.37 (−0.84 to 0.09) | high | Hayden 1997 [108]/Mäkelä 2000 [109]/MIST 1998 [110]/Puhakka 2003 [111] | |
| Children | |||||||
| Time to alleviation of symptoms | O | Burch [8] | 2 | 1029 | −21.05 (–33.81 to −8.29) | moderate | Johnston 2005 [112]/Whitley 2001 [107] |
| Z | Burch [8] | 2 | 737 | −0.94 (−1.43 to −0.46) | moderate | Hedrick 2000 [29]/GSK NAI30028 | |
| Wang [17] | 2 | 471/266 | −0.5 (p = 0.001)/−0.5 (p = 0.04)d | Hedrick 2000 [29]/GSK NAI30028 | |||
| Time to return to normal activity | O | Burch [8] | 1 | 695 | −30.08 (−43.35 to −16.81) | moderate | Whitley 2001 [107] |
| Z | Burch [8] | 1 | 471 | −0.5 (−1.25 to 0.25) | moderate | Hedrick 2000 [29] | |
| Elderly | |||||||
| Time to alleviation of symptoms | O | Burch [8] | 1 | 736 | −10.00 (−45.05 to 25.05) | low | Martin 2001 [113] |
| Z | Burch [8] | 5 | 475 | −1.13 (−2.90 to 0.63) | low | Boivin 2000 [114]/GSK NAI30012/Mäkelä 2000 [109]/MIST 1998 [110]/Murphy 2000 [115] | |
| Time to return to normal activity | O | Burch [8] | 3 | 734 | −98.07 (−170.98 to −25.16) | low | Roche WVI15819/Roche WVI15876/Roche WVI15878 |
| At-risk individuals | |||||||
| Time to alleviation of symptoms | O | Burch [8] | 2 | 1472 | −17.84 (−36.20 to 0.52) | moderate | Martin 2001 [113]/Johnston 2005 [112] |
| Z | Burch [8] | 7 | 1252 | −0.98 (−1.84 to −0.11) | moderate | Mäkelä 2000 [109]/Monto 1999 [30]/Murphy 2000 [115]/Boivin 2000 [114]/MIST 1998 [110]/Hedrick 2000 [29]/GSKNAI30012 | |
| Time to return to normal activity | O | Burch [8] | 5 | 1134 | −58.84 (−116.58 to −1.11) | low | Roche WVI15812/Roche WVI15872/Roche WVI15819/Roche WVI15876/Roche WVI15878 |
| Z | Burch [8] | 6 | 613 | −0.96 (−2.32 to 0.41) | low | Murphy 2000 [115]/GSK NAIB2007/Mäkelä 2000 [109]/MIST 1998 [110]/Hedrick 2000 [29]/Boivin 2000 [114] | |
O = 150 mg oseltamivir daily during 5 days in adults, elderly; dosage adjusted to weight in children.
Z = 2×10 mg inhaled zanamivir daily during 5 days in adults, elderly; dosage adjusted to weight in children.
difference in median hours in oseltamivir trials and difference in median days in zanamivir trials.
no pooling of results.
GRADE quality of evidence: high; moderate; low.
ITT = intention-to-treat; NI = neuraminidase inhibitor; CI = confidence interval.
In children, treatment with oseltamivir was only described in two published studies [8]. Oseltamivir treatment alleviated symptoms less than one day sooner (GRADE moderate) and allowed a return to normal activity more than one day sooner (GRADE moderate). For treatment with zanamivir, less than one day was awarded in the alleviation of symptoms (GRADE moderate) [8]. No significant result was reached for the return to normal activity according to Burch et al. [8] (GRADE moderate).
Burch et al. [8] presented treatment results for NIs in elderly and at-risk individuals by extracting the subgroup from a mixed population out of the original studies. In the elderly, no evidence of an effect of oseltamivir (GRADE low) or zanamivir (GRADE low) on the alleviation of symptoms could be found by pooling these results. For the time to return to normal activity, only pooled results of three unpublished studies gave a significant reduction of four days for oseltamivir (GRADE low).
By pooling five unpublished study results, oseltamivir treatment showed more than a two day reduction in at-risk adults in the time to return to normal activity, but this conclusion had a low quality of evidence. No significant effect was found for the alleviation of symptoms (GRADE moderate). In the at-risk adults treated with zanamivir, a significant benefit of a one day reduction could be found for the alleviation of symptoms. No significant benefit could be shown for the reduction in the time needed to return to normal activity (GRADE low).
Complications
Drawing conclusions based on complications remains difficult and unreliable because of a lack of sound published data (Table 6). In healthy adults, oseltamivir treatment showed no significant effects on complications, except for a significant effect on antibiotic use by 63% (95% CI 52–71) found by Burch et al. [8], but this was not confirmed by Jefferson et al. [10]. Jefferson et al. [10] showed a significant preventive effect of zanamivir on asthma exacerbations: OR 0.54 (0.34–0.86) (pooled results – GRADE high).
Table 6. The rate of complications in healthy adults, children, elderly and at-risk individuals treated with oseltamivir and zanamivir versus placebo according to different systematic reviews (ITT – pooled results).
| Outcome | NI a, b | First author/review | Number of included studies | Number of participants | Estimate (95% CI)c | Quality GRADEe | References of included studies |
| Healthy adults | |||||||
| All types | O | Burch [8] | 1 | 419 | 0.61 (0.32 to 1.13) | low | Treanor 2000 [106] |
| Pneumonia | O | Burch [8] | 2 | 784 | 0.33 (0.03 to 3.16) | moderate | Nicholson 2000 [105]/Kashiwagi 2000 [47] |
| Z | Burch [8] | 1 | 588 | 1.36 (0.63 to 2.93) | moderate | Puhakka 2003 [111] | |
| Bronchitis | O | Burch [8] | 1 | 476 | 1.38 (0.43 to 4.40) | low | Nicholson 2000 [105] |
| Z | Burch [8] | 2 | 1054 | 1.08 (0.54 to 2.17) | moderate | Puhakka 2003 [111]/GSK NAI30011 | |
| Antibiotic usage | O | Burch [8] | 2 | 1652 | 0.37 (0.29 to 0.48) | low | Deng 2004 [24]/Nicholson 2000 [105] |
| Z | Burch [8] | 1 | 276 | 0.68 (0.31 to 1.51) | low | Hayden 1997 [108] | |
| Hospitalization | O | Burch [8] | 3 | 2071 | 0.97 (0.33 to 2.90) | high | Deng 2004 [24]/Nicholson 2000 [105]/Treanor 2000 [106] |
| Jefferson [10] | 8 | 4696 | 0.95 (0.57 to 1.61) | Nicholson 2000 [105]/Treanor 2000 [106]/Whitley 2001 [107]/M76001/WV15707/WV15730/WV15812–15872/WV15819–15876–15978 | |||
| Z | Burch [8] | 1 | 588 | 1.37 (0.86 to 2.17) | moderate | Puhakka 2003 [111] | |
| GP consultation | Z | Burch [8] | 1 | 588 | 1.05 (0.75 to 1.46) | moderate | Puhakka 2003 [111] |
| Astma exacerbation | Z | Jefferson [10] | 9 | 5269 | 0.54 (0.34 to 0.86) | high | Hedrick 2000 [29]/Hayden 2000 [100]/Hayden 1997 [108]/Monto 1999 [30]/Boivin 2000 [114]/Mäkelä 2000 [109]/MIST 1998 [110]/NAIB2007 |
| Children | |||||||
| All types | Z | Burch [8] | 2 | 732 | 0.88 (0.62 to 1.24) | moderate | GSK NAI30028/Hedrick 2000 [29] |
| Pneumonia | O | Burch [8] | 2 | 1029 | 0.58 (0.26 to 1.28) | low | Johnston 2005 [112]/Whitley 2001 [107] |
| Z | Burch [8] | 1 | 266 | 0.51 (0.07 to 3.65) | low | GSK NAI30028 | |
| Bronchitis | O | Burch [8] | 1 | 334 | 4.94 (0.57 to 42.74) | low | Johnston 2005 [112] |
| Z | Burch [8] | 2 | 732 | 1.05 (0.28 to 3.89) | moderate | GSK NAI30028/Hedrick 2000 [29] | |
| Antibiotic usage | O | Burch [8] | 1 | 695 | 0.96 (0.46 to 1.99) | low | Whitley 2001 [107] |
| Z | Burch [8] | 1 | 471 | 0.05 (0.01 to 0.23) | moderate | Hedrick 2000 [29] | |
| Hospitalization | O | Burch [8] | 1 | 695 | 0.20 (0.01 to 4.24) | low | Whitley 2001 [107] |
| Z | Burch [8] | 1 | 266 | 1.55 (0.06 to 38.36) | low | GSK NAI30028 | |
| GP consultation | Z | Burch [8] | 1 | 266 | 0.85 (0.44 to 1.64) | low | GSK NAI30028 |
| Otitis media | O | Wang [17] | 1 | 334 | −0.01 (−0.05 to 0.03) | low | Johnston 2005 [112] |
| Burch [8] | 1 | 695 | 0.82 (0.27 to 2.50) | Whitley 2001 [107] | |||
| Z | Burch [8] | 1 | 266 | 0.63 (0.16 to 2.40) | low | GSK NAI30028 | |
| Astma exacerbation | O | Wang [17] | 1 | 177 | −0.05 (−0.15 to 0.05) | low | Johnston 2005 [112] |
| Elderly | |||||||
| All types | Z | Burch [8] | 1 | 358 | 0.84 (0.54 to 1.32) | low | GSK NAI20012 |
| Pneumonia | Z | Burch [8] | 1 | 358 | 0.87 (0.17 to 4.38) | low | GSK NAI20012 |
| Bronchitis | Z | Burch [8] | 1 | 358 | 0.46 (0.20 to 1.02) | low | GSK NAI20012 |
| Antibiotic usage | Z | Burch [8] | 1 | 358 | 0.73 (0.43 to 1.24) | low | GSK NAI20012 |
| At-risk individuals | |||||||
| All types | Z | Burch [8] | 4 | 575 | 0.73 (0.51 to 1.04) | moderate | GSK NAI30012/Boivin 2000 [114]/Mäkelä 2000 [109]/MIST 1998 [110] |
| Pneumonia | O | Burch [8] | 1 | 334 | 0.48 (0.04 to 5.34) | low | Johnston 2005 [112] |
| Z | Burch [8] | 2 | 881 | 0.57 (0.15 to 2.23) | moderate | Murphy 2000 [115]/GSK NAI30012 | |
| Bronchitis | O | Burch [8] | 1 | 334 | 4.94 (0.57 to 42.74) | low | Johnston 2005 [112] |
| Z | Burch [8] | 3 | 1210 | 0.41 (0.24 to 0.70) | moderate | Murphy 2000 [115]/GSK NAI30012/GSK NAI30020 | |
| Antibiotic usage | O | Burch [8] | 1 | 334 | 0.96 (0.46 to 1.99) | low | Johnston 2005 [112] |
| Z | Burch [8] | 4 | 575 | 0.71 (0.47 to 1.07) | moderate | GSK NAI30012/Boivin 2000 [114]/Mäkelä 2000 [109]/MIST 1998 [110] | |
| Hospitalization | O | Burch [8] | 1 | 329 | 0.33 (0.01 to 8.14) | low | Roche NV16871 |
| Z | Burch [8] | 1 | 524 | 0.50 (0.12 to 2.01) | moderate | Murphy 2000 [115] | |
O = 150 mg oseltamivir daily during 5 days in adults, elderly; dosage adjusted to weight in children.
Z = 2×10 mg inhaled zanamivir daily during 5 days in adults, elderly; dosage adjusted to weight in children.
Estimate: odds ratio (Burch, Jefferson); risk difference (Wang).
no pooling of results.
GRADE quality of evidence: high; moderate; low.
ITT = intention-to-treat; NI = neuraminidase inhibitor; CI = confidence interval; GP = general practitioner.
In children, oseltamivir treatment did not show a significant effect on complications. In children treated with zanamivir, only one study [29] showed a reduction of 95% (95% CI 77–99) on antibiotic usage [8] (GRADE moderate).
In the elderly, no studies provided ITT results for the effect of oseltamivir on complications. No evidence of a benefit could be shown for zanamivir, but the studies on this topic are scarce (GRADE low).
In at-risk individuals, no significant effect could be found on the complications from influenza following oseltamivir treatment (GRADE low). Burch et al. [8] showed a significant effectiveness of 59% (95% CI 30–76) for zanamivir on bronchitis in at-risk individuals (GRADE moderate).
Adverse events
In healthy adults, nausea and vomiting were the most prominent adverse effects in the oseltamivir trials (OR 1.79 (95% CI 1.1–2.93)) (GRADE high) (Table 7).
Table 7. Adverse events of oseltamivir and zanamivir versus placebo in prophylaxis and treatment trials (ITT – pooled results).
| Outcome | Trial | NIa,b | First author/review | Number of included studies | Number of participants | Estimate (95% CI)c | Quality GRADEe | References of included studies |
| Healthy adults | ||||||||
| Overall | Treatment | O | Burch [8] | 4 | 1623 | 0.81 (0.59 to 1.12) | low | Deng 2004 [62]/Tan2002 [116]/Kashiwaghi 2000 [47]/Roche WV15730 |
| Z | Burch [8] | 2 | 1054 | 1.03 (0.79 to 1.34) | moderate | Puhakka 2003 [111]/GSK NAI30011 | ||
| Nausea | Prophylaxis | O | Khazeni [22] | 3 | 1039/308/548 | 1.70 (1.15 to 2.50)/1.97 (0.61 to 6.42)/1.08 (0.48 to 2.40)d | low | Hayden 1999 [99]/Kashiwaghi 2000 [47]/Peters 2001 [103] |
| Prophylaxis/Treatment | O | Jefferson [10] | 9 | 5651 | 1.62 (1.17 to 2.26) | high | Treanor 2000 [106]/Nicholson 2000 [105]/Whitley 2001 [107]/Welliver 2001 [42]/M76001/WV15707/WV15730/WV15812–15872/WV15819–15876–15978 | |
| Vomiting | Prophylaxis | O | Khazeni [22] | 3 | 1039/308/548 | 3.24 (1.07 to 9.88)/1.73 (0.52 to 5.78)/1.23 (0.33 to 4.54)d | low | Hayden 1999 [99]/Kashiwaghi 2000 [47]/Peters 2001 [103] |
| Prophylaxis/Treatment | O | Jefferson [10] | 9 | 5651 | 2.32 (1.62 to 3.31) | high | Treanor 2000 [106]/Nicholson 2000 [105]/Whitley 2001 [107]/Welliver 2001 [42]/M76001/WV15707/WV15730/WV15812–15872/WV15819–15876–15978 | |
| Diarrhoea | Prophylaxis | O | Khazeni [22] | 2 | 308/548 | 0.69 (0.38 to 1.25)/0.81 (0.34 to 1.92)d | low | Kashiwaghi 2000 [47]/Peters 2001 [103] |
| Treatment | O | Jefferson [10] | 9 | 5651 | 0.72 (0.53 to 0.97) | high | Treanor 2000 [106]/Nicholson 2000 [105]/Whitley 2001 [107]/Welliver 2001 [42]/M76001/WV15707/WV15730/WV15812–15872/WV15819–15876–15978 | |
| Drug related | Treatment | O | Burch [8] | 2 | 509 | 1.45 (0.83 to 2.53) | moderate | Li 2003 [104]/Roche WVI5730 |
| Z | Burch [8] | 4 | 1406 | 1.11 (0.76 to 1.62) | moderate | Hayden 1997 [108]/Matsumoto 1999 [117]/Puhakka 2003 [111]/GSK NAI30011 | ||
| Withdrawel from trial due to adverse events | Prophylaxis/Treatment | O | Jefferson [10] | 9 | 5651 | 1.08 (0.66 to 1.76) | high | Treanor 2000 [106]/Nicholson 2000 [105]/Whitley 2001 [107]/Welliver 2001 [42]/M76001/WV15707/WV15730/WV15812–15872/WV15819–15876–15978 |
| Serious | Prophylaxis | O | Khazeni [22] | 1 | 308 | 0.33 (0.01 to 8.02) | low | Kashiwaghi 2000 [47] |
| Z | Khazeni [22] | 3 | 1107/3363/138 | 1.00 (0.06 to 15.98)/1.07 (0.54 to 2.11)/0.58 (0.15 to 2.35)d | low | Monto 1999 [30]/LaForce 2007 [27]/Webster1999 [118] | ||
| Treatment | O | Burch [8] | 3 | 985 | 0.32 (0.03 to 1.17) | moderate | Li 2003 [104]/Nicholson 2000 [105]/Roche WVI5730 | |
| Z | Burch [8] | 3 | 1130 | 1.44 (0.28 to 7.35) | moderate | Matsumoto 1999 [117]/Puhakka 2003 [111]/GSK NAI30011 | ||
| Children | ||||||||
| Overall | Prophylaxis/Treatment | O&Z | Wang [17] | 4 | 1766 | −0.03 (−0.07 to 0.01) | moderate | Johnston 2005 [112]/Hedrick 2000 [29]/Whitley 2001 [107]/NAI30028 |
| Treatment | O | Burch [8] | 1 | 334 | 0.91 (0.59 to 1.40) | low | Johnston 2005 [112] | |
| Z | Burch [8] | 2 | 737 | 0.88 (0.62 to 1.24) | moderate | Hedrick 2000 [29]/NAI30028 | ||
| Nausea | Prophylaxis/Ttreatment | O&Z | Wang [17] | 4 | 1766 | −0.01 (−0.03 to 0.00) | moderate | Johnston 2005 [112]/Hedrick 2000 [29]/Whitley 2001 [107]/NAI30028 |
| Vomiting | Treatment | O | Wang [17] | 3 | 1435 | 0.06 (0.03 to 0.10) | high | Heinonen 2010 [119]/Johnston 2005 [112]/Whitley 2001 [107] |
| Z | Wang [17] | 2 | 737 | 0.00 (−0.02 to 0.02) | moderate | Hedrick 2000 [29]/NAI30028 | ||
| Diarrhoea | Prophylaxis/Treatment | O&Z | Wang [17] | 5 | 2172 | −0.01 (−0.03 to 0.00) | moderate | Heinonen 2010 [119]/Johnston 2005 [112]/Hedrick 2000 [29]/Whitley 2001 [107]/NAI30028 |
| Drug related | Treatment | Z | Burch [8] | 2 | 737 | 1.32 (0.59 to 2.92) | moderate | Hedrick 2000 [29]/NAI30028 |
| Serious | Prophylaxis/Treatment | O&Z | Wang [17] | 5 | 2172 | 0.0 (0.0 to 0.01) | moderate | Heinonen 2010 [119]/Johnston 2005 [112]/Hedrick 2000 [29]/Whitley 2001 [107]/NAI30028 |
| Treatment | O | Burch [8] | 1 | 695 | 1.54 (0.25 to 9.24) | low | Whitley 2001 [107] | |
| Z | Burch [8] | 2 | 737 | 2.29 (−0.24 to 22.09) | low | Hedrick 2000 [29]/GSK NAI30028 | ||
| Withdrawel from trial due to ad-verse events | Prophylaxis/Treatment | O&Z | Wang [17] | 3 | 1143 | 0.01 (−0.02 to 0.03) | moderate | Heinonen 2010 [119]/Hedrick 2000 [29]/NAI30028 |
| At-risk individuals | ||||||||
| Overall | Treatment | O | Burch [8] | 2 | 452 | 0.96 (0.63 to 1.46) | moderate | Lin 2004 [25]/Johnston 2005 [112] |
| Z | Burch [8] | 4 | 1286 | 1.24 (0.96 to 1.60) | high | Murphy 2000 [115]/MIST 1998 [110]/GSK NAI30020/GSK NAI30012 | ||
| Drug related | Treatment | Z | Burch [8] | 1 | 524 | 1.01 (0.55 to 1.85) | moderate | Murphy 2000 [115] |
| Serious | Treatment | Z | Burch [8] | 3 | 1210 | 0.72 (0.32 to 1.62) | low | Murphy 2000 [115]/GSK NAI30020/GSK NAI30012 |
O = treatmet trial 150 mg oseltamivir daily during 5 days in adults, elderly; dosage adjusted to weight in children; prophylaxis trial = treatment dosage/2.
Z = treatmet trial 2×10 mg inhaled zanamivir daily during 5 days in adults, elderly; dosage adjusted to weight in children; prophylaxis trial = treatment dosage/2.
odds ratio and risk difference (Wang).
no pooling of results.
GRADE quality of evidence: high; moderate; low.
ITT = intention-to-treat; NI = neuraminidase inhibitor; CI = confidence interval.
In healthy adults and children, no significant adverse effects were recorded in the treatment trials with zanamivir.
In children and at-risk individuals treated with oseltamivir or zanamivir, no significant overall drug-related or serious adverse effects could be found (pooled results) [8].
Discussion
Summary
The nine systematic reviews retrieved were of high quality, but they differed in their inclusion/exclusion criteria, in their quality assessment, in their data handling and finally in their conclusions. Many quality shortcomings about the included published and unpublished trials were reported.
In seasonal prophylaxis of laboratory-proven influenza, oseltamivir and zanamivir showed more than 50% effective in healthy adults and at-risk individuals (moderate to low quality). Post-exposure prophylaxis with both NIs proved to be more than 50% effective in healthy adults and children (moderate to low quality).
In healthy adults and children with ILI, both NIs showed a small treatment benefit of half a day and less than one day in the alleviation of symptoms (high to moderate quality). In elderly individuals with ILI, no significant reduction of illness days could be shown for both NIs (low quality). In at-risk individuals, no significant effect could be found for oseltamivir (moderate quality), while zanamivir showed a benefit of almost one day (moderate).
Zanamivir exclusively showed a preventive effect on antibiotic usage in children. In the prevention of influenza complications in the elderly, no benefit could be found for oseltamivir or zanamivir, but studies are scarce and of low quality in that area. In an at-risk population, an effect could be shown for zanamivir on the occurrence of bronchitis (moderate quality).
The different trials poorly reported adverse effects. In the prophylaxis and treatment studies among healthy adults and children, nausea and vomiting were prominent for oseltamivir. In at-risk individuals, no adverse effects were significant in the limited number of treatment trials, although one reviewer found more vomiting among children treated with oseltamivir. Zanamivir treatment showed no adverse effects.
Results in Perspective
It is disappointing to find that the different NI trials focused on healthy adults rather than on the elderly and individuals at risk of developing serious influenza complications. Additionally, the choice of a primary outcome such as alleviation of symptoms or return to normal activity with a corresponding small benefit has limited clinical importance [8]. On the other hand, the effect on complications was only estimated as a secondary outcome, and trial results were often unpublished. This makes the evidence of this clinically relevant outcome a source for discussion. The trials were not designed or powered to give results regarding serious complications, hospitalization and mortality. The meta-analyses, performed by the pharmaceutical companies (Monto 1999 [30], Lalezari 2001 [31], Kaiser 2003 [12]), were of limited quality and partly based on unpublished material that was not submitted for peer-review. The methodological shortcomings of the Kaiser review [12] triggered the Cochrane review group [10] to rely only on clinical trial reports containing published and unpublished trial results, which were retrieved from the regulatory authorities and the pharmaceutical companies that produce oseltamivir and zanamivir (Roche and GSK) [32]. This collection of trial reports is on-going for zanamivir because no prophylaxis or treatment results were given for zanamivir by the latest Cochrane review by Jefferson et al. [10]. For oseltamivir, this review only considered the treatment effect on the alleviation of symptoms and on hospitalization. Other outcomes were not analyzed because of a high risk of bias. After the inclusion date of our review, Ebell et al. reported an independent meta-analysis about the effectiveness of oseltamivir treatment in adults including published and unpublished results. They concluded that no evidence of an effect could be found on hospitalization, pneumonia or the combined outcome of pneumonia, otitis media and sinusitis in the ITT population [33]. Additionally, the underreporting of side-effects was a second reason for the Cochrane reviewers to reconsider their conclusions [10]. Oseltamivir might provoke undesired neuro-psychiatric reactions such as hallucinations, suicidal tendencies and sudden death [10]. Interesting new hypotheses were tested and confirmed (post-protocol analysis) such as the difference in adverse event rates between the placebo groups of the oseltamivir and zanamivir trials and the lower antibody response in the oseltamivir groups with consequential bias (underreporting of confirmed influenza cases in the active treatment groups) [10]. On the request of Roche, Hernán et al. [34] reanalyzed the Kaiser review and added one new RCT without performing an independent, systematic literature search or quality appraisal of the included trials. No characteristics about the participants were provided. The reviewers tried to avoid the analytical problems that occurred in the Kaiser review and concluded that oseltamivir reduced the risk of lower respiratory tract complications requiring antibiotic treatment by 28% (95% CI 11 to 42%) [34]. The Cochrane Neuraminidase Inhibitors Review Team [10], [35] made critical comments on this re-analysis, which elicited a reply by Hernán et al. [36] and thereby illustrates the ongoing discussion.
The recent meta-analysis of Falagas et al. [20] of intermediate quality stated that NIs are generally effective in preventing influenza-related complications in healthy and at-risk persons, but data were only given for the subgroup with proven influenza infections. Data on individual complications were scarce and statistically insignificant.
Notwithstanding all of these shortcomings and the limited evidence of benefits that exist, many guidelines advise the use of NIs in people at risk for influenza-related complications, including individuals with chronic respiratory, cardiac, liver and renal disorders, diabetes and immunosuppression or for elderly living in nursing homes [37], [38], [39], [40], [41]. For prophylaxis, the first choice is influenza vaccination, but NIs could be considered in cases of non-vaccination or following a mismatch between the vaccine and circulating strains in at-risk groups according to the international guidelines [37], [38], [39], [40], [41]. Cost-effectiveness seems favorable for the use of NIs to treat influenza in at-risk populations, although cost-effectiveness studies are based on many assumptions, especially regarding the exact estimates of the risk and effect size of NIs on secondary complications and mortality [8].
An extra argument to use NIs might be the favorable effect on eliminating the transmission of the virus. Although virus production and excretion are slightly reduced in treated individuals, they are never completely blocked, and this claim by Roche [42] has never been proven [10]. The combination of other preventive measures such as influenza vaccination and non-pharmaceutical measures such as social distancing, case isolation, hand washing and the use of masks, is more appropriate and effective [43], [44].
In addition to the limited usefulness of NIs, a growing number of resistant influenza strains [45], especially those resistant to oseltamivir (up to 98% in the 2008–2009 season according to the WHO and ECDC), might make NIs unusable in the future [8].
Limitations of This Review
This search focused exclusively on SRs dealing with the use of the NIs oseltamivir and zanamivir against seasonal influenza. Very few included SRs actually gave results for the newer NIs, such as peramivir and laninamivir. Only the SR of Wang et al. reported the study results of one trial on the treatment effect of laninamivir in children [46]. Guidelines discussed the prophylaxis and treatment of pandemic influenza based on the existing evidence on seasonal influenza and by extrapolating the same evidence. To avoid bias and stay close to the clinical and diagnostic uncertainty, only ITT studies were shown in this review. Publications in other languages than English, French, Dutch and German were excluded. However, by rerunning the search without language restrictions, we had no indication that we were missing any relevant reviews.
Some limitations and difficulties were met in the comparison of the different SRs
The different inclusion/exclusion criteria for trials that were used in the different reviews influenced the pooled outcomes. Wang [17], Jackson [21] and Khazeni et al. [22] did not use unpublished trial results compared to the other included SRs that did. Some trial results remained unpublished as extensively stated by Jefferson et al. [10], [32]. Tappenden [18] and Burch et al. [8] did not include trials that were published in Chinese or Japanese, which gave rise to translation problems. Jefferson et al. [10] did not make subgroups that the original researchers did not predefine, while others such as Burch et al. [8] defined subgroups consequently out of a mixed population by diminishing nominators and denominators accordingly. The methods used by Burch et al. [8] are prone to bias by eliminating randomization.
Jefferson et al. [10] pooled the data for both adults and children together, which makes separate conclusions for each population difficult. From the same editorial group (Cochrane Acute Respiratory Infections Group), the review of Wang et al. [17] on the effect of NIs among children only showed pooled results for both oseltamivir and zanamivir treatments together. Therefore, no distinct conclusions can be made for the NIs separately. In trials where more than one treatment group was compared with the placebo group, each reviewer handled the numbers differently. Jefferson [10] added the numbers of all of the different treatment groups, which made the intervention heterogeneous. Khazeni [22] also added results from two treatment groups and doubled the placebo numbers, which inflates the relevance of this study in the pooled results. It is unclear why Khazeni et al. [22] gave different event numbers for the Kashiwaghi [47] and Monto et al. [30] studies. Comparison with the originally published results and between the different reviews required some effort, especially where significant differences occurred between the reviews.
Most trials were designed and sponsored by Roche or GlaxoSmithKline, and independent studies are scarce. In addition to the differences in reporting quality, graded as moderate by most of the reviewers, the published trials showed differences in the number of participants, vaccinated participants, and participants with laboratory-proven influenza and treatment days, and the trials showed a different day for the assessment of the outcomes. They included different age categories and mixed healthy and at-risk people, rarely mentioning results for subgroups separately. The inclusion of participants was restricted to those suffering from influenza-like symptoms for less than 36 to 48 hours after the onset of illness. All of the treatment studies had high percentages of laboratory-proven influenza (up to 80%) [10] because they performed the studies only during influenza epidemics and excluded atypical cases. Therefore, any extrapolation of the results to the real clinical situation is limited. By consequence, their results in a subgroup of participants with laboratory-proven influenza (not shown) were only slightly better than the ITT results. The participants assessed the outcomes such as ‘alleviation of symptoms’ and ‘return to normal activity’ themselves, which introduced variability among the different trials. These outcomes were then represented in different ways: according to ITT or per protocol; or according to ILI or laboratory-proven influenza ( = subgroup). Complications such as pneumonia, bronchitis, sinusitis and otitis media were diagnosed in different ways, mostly without a clear definition and without measuring severity. No clear distinction was made between adverse events and complications. All of this heterogeneity is a source for different conclusions and recommendations.
Recommendations for the Future
New RCTs need to focus on at-risk participants and measure severe influenza complications as an outcome, which must be powered accordingly. This also applies to the more recently developed NIs, peramivir and laninamivir, which were not discussed in this review. Head-to-head studies between oseltamivir and zanamivir and with the newer NIs might be valuable. Overall, the use of NIs has to be established among other prevention and treatment options for influenza.
The effect size of NIs is positively correlated with the accuracy and speed of the clinical diagnosis of influenza. Rapid point of care tests are promising for optimizing accuracy, but their place in the clinical diagnosis still has to be established [48].
In the future, a new policy should be established regarding the ownership of trial results. All of the stakeholders should acquire full access to clinical data reports and individual study results to avoid publication bias and selective reporting afterwards.
Conclusion
In healthy adults and children, prophylaxis or treatment of ILI is not recommended, although effectiveness has been shown. The combination of diagnostic uncertainty, risk for virus strain resistance, side-effects and financial cost outweighs the small benefits. Prophylaxis of at-risk and elderly groups might be considered in individual cases when influenza vaccination did not take place, when it is not appropriate or is ineffective because of virus strain mismatch, when influenza is circulating in the community and when contact with an infected person could not be avoided by other measures. No evidence is available that shows a benefit for treatment in elderly and at-risk individuals, vaccinated or not, on relevant outcomes such as hospitalization and mortality.
Supporting Information
PRISMA 2009 checklist.
(DOC)
Funding Statement
This systematic literature search was funded by the governmental organization NIHDI (National Institute for Health and Disability Insurance) in Belgium. The sponsor played a role in the study design but had no influence on the collection, analysis, and interpretation of data, on the writing of the report or on the decision to submit the paper for publication. The researchers were independent from the funders.
References
- 1. Moscona A (2005) Neuraminidase inhibitors for influenza. N Engl J Med 353: 1363–1373. [DOI] [PubMed] [Google Scholar]
- 2.Jefferson T, Di Pietrantonj C, Al-Ansary LA, Ferroni E, Thorning S, et al. (2010) Vaccines for preventing influenza in the elderly. Cochrane Database of Systematic Reviews: CD004876. [DOI] [PubMed]
- 3.Jefferson T, Di Pietrantonj C, Rivetti A, Bawazeer GA, Al-Ansary LA, et al. (2010) Vaccines for preventing influenza in healthy adults. Cochrane Database of Systematic Reviews: CD001269. [DOI] [PubMed]
- 4.Jefferson T, Rivetti A, Harnden A, Di Pietrantonj C, Demicheli V (2008) Vaccines for preventing influenza in healthy children. Cochrane Database of Systematic Reviews: CD004879. [DOI] [PubMed]
- 5. Michiels B, Govaerts F, Remmen R, Vermeire E, Coenen S (2011) A systematic review of the evidence on the effectiveness and risks of inactivated influenza vaccines in different target groups. Vaccine 29: 9159–9170. [DOI] [PubMed] [Google Scholar]
- 6. Adriaenssens N, Coenen S, Kroes AC, Versporten A, Vankerckhoven V, et al. (2011) European Surveillance of Antimicrobial Consumption (ESAC): systemic antiviral use in Europe. J Antimicrob Chemother 66: 1897–1905. [DOI] [PubMed] [Google Scholar]
- 7. Kawai N, Ikematsu H, Tanaka O, Matsuura S, Maeda T, et al. (2011) Comparison of the clinical symptoms and the effectiveness of neuraminidase inhibitors for patients with pandemic influenza H1N1 2009 or seasonal H1N1 influenza in the 2007–2008 and 2008–2009 seasons. J Infect Chemother 17: 375–381. [DOI] [PubMed] [Google Scholar]
- 8. Burch J, Paulden M, Conti S, Stock C, Corbett M, et al. (2009) Antiviral drugs for the treatment of influenza: a systematic review and economic evaluation. Health Technol Assess 13: 1–265. [DOI] [PubMed] [Google Scholar]
- 9. Cohen D (2009) Complications: tracking down the data on oseltamivir. BMJ 339: b5387. [DOI] [PubMed] [Google Scholar]
- 10.Jefferson T, Jones MA, Doshi P, Del Mar CB, Heneghan CJ, et al. (2012) Neuraminidase inhibitors for preventing and treating influenza in healthy adults and children. Cochrane Database of Systematic Reviews: CD008965. [DOI] [PubMed]
- 11. Doshi P (2009) Neuraminidase inhibitors–the story behind the Cochrane review. BMJ 339: b5164. [DOI] [PubMed] [Google Scholar]
- 12. Kaiser L, Wat C, Mills T, Mahoney P, Ward P, et al. (2003) Impact of oseltamivir treatment on influenza-related lower respiratory tract complications and hospitalizations. Arch Intern Med 163: 1667–1672. [DOI] [PubMed] [Google Scholar]
- 13. Shea BJ, Hamel C, Wells GA, Bouter LM, Kristjansson E, et al. (2009) AMSTAR is a reliable and valid measurement tool to assess the methodological quality of systematic reviews. J Clin Epidemiol 62: 1013–1020. [DOI] [PubMed] [Google Scholar]
- 14. Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, et al. (2011) GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 64: 383–394. [DOI] [PubMed] [Google Scholar]
- 15.Higgins JPT, Green S (editors) (2009) Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated September 2009]. The Cochrane Collaboration. Available: www.cochrane-handbook.org.
- 16. Jagannath VA, Asokan GV, Fedorowicz Z, Singaram JS, Lee TW (2010) Neuraminidase inhibitors for the treatment of influenza infection in people with cystic fibrosis. Cochrane Database of Systematic Reviews 3: CD008139. [DOI] [PubMed] [Google Scholar]
- 17.Wang K, Shun-Shin M, Gill P, Perera R, Harnden A (2012) Neuraminidase inhibitors for preventing and treating influenza in children (published trials only). Cochrane Database of Systematic Reviews: CD002744. [DOI] [PMC free article] [PubMed]
- 18.Tappenden P, Jackson R, Cooper K, Rees A, Simpson E, et al. (2009) Amantadine, oseltamivir and zanamivir for the prophylaxis of influenza (including a review of existing guidance no. 67): a systematic review and economic evaluation. Health Technol Assess 13: iii, ix–xii, 1–246. [DOI] [PubMed]
- 19.Deonandan R (2006) Oseltamivir and Zanamivir for the Prevention of influenza. Canadian Agency for Drugs and Technologies in Health. [PubMed]
- 20. Falagas ME, Koletsi PK, Vouloumanou EK, Rafailidis PI, Kapaskelis AM, et al. (2010) Effectiveness and safety of neuraminidase inhibitors in reducing influenza complications: a meta-analysis of randomized controlled trials J Antimicrob Chemother. 65: 1330–1346. [DOI] [PubMed] [Google Scholar]
- 21. Jackson RJ, Cooper KL, Tappenden P, Rees A, Simpson EL, et al. (2011) Oseltamivir, zanamivir and amantadine in the prevention of influenza: a systematic review. J Infect 62: 14–25. [DOI] [PubMed] [Google Scholar]
- 22. Khazeni N, Bravata DM, Holty JE, Uyeki TM, Stave CD, et al. (2009) Systematic review: safety and efficacy of extended-duration antiviral chemoprophylaxis against pandemic and seasonal influenza. Ann Intern Med 151: 464–473. [DOI] [PubMed] [Google Scholar]
- 23. Burch J, Corbett M, Stock C, Nicholson K, Elliot AJ, et al. (2009) Prescription of anti-influenza drugs for healthy adults: a systematic review and meta-analysis. Lancet Infect Dis 9: 537–545. [DOI] [PubMed] [Google Scholar]
- 24. Deng WW, Li QY, Zhong NS (2004) [A multicenter study of efficacy and safety of oseltamivir in the treatment of suspected influenza patients] Zhonghua Yi Xue Za Zhi. 84: 2132–2136. [PubMed] [Google Scholar]
- 25. Lin JT, Yu XZ, Cui DJ, Chen XY, Zhu JH, et al. (2004) [A multicenter randomized controlled study of the efficacy and safety of oseltamivir in the treatment of influenza in a high risk population]. Zhonghua Jie He He Hu Xi Za Zhi 27: 455–459. [PubMed] [Google Scholar]
- 26. Hayden FG, Belshe R, Villanueva C, Lanno R, Hughes C, et al. (2004) Management of influenza in households: a prospective, randomized comparison of oseltamivir treatment with or without postexposure prophylaxis. J Infect Dis 189: 440–449. [DOI] [PubMed] [Google Scholar]
- 27. LaForce C, Man CY, Henderson FW, McElhaney JE, Hampel FC Jr, et al. (2007) Efficacy and safety of inhaled zanamivir in the prevention of influenza in community-dwelling, high-risk adult and adolescent subjects: a 28-day, multicenter, randomized, double-blind, placebo-controlled trial. Clin Ther 29: 1579–1590. [DOI] [PubMed] [Google Scholar]
- 28. Ambrozaitis A, Gravenstein S, van Essen GA, Rubinstein E, Balciuniene L, et al. (2005) Inhaled Zanamivir Versus Placebo for the Prevention of Influenza Outbreaks in an Unvaccinated Long-term Care Population. J Am Med Dir Assoc 6: 367–374. [DOI] [PubMed] [Google Scholar]
- 29. Hedrick JA, Barzilai A, Behre U, Henderson FW, Hammond J, et al. (2000) Zanamivir for treatment of symptomatic influenza A and B infection in children five to twelve years of age: a randomized controlled trial. Pediatr Infect Dis J 19: 410–417. [DOI] [PubMed] [Google Scholar]
- 30. Monto AS, Robinson DP, Herlocher ML, Hinson JM, et al. (1999) Zanamivir in the prevention of influenza among healthy adults - a randomized controlled trial. JAMA 282: 31–35. [DOI] [PubMed] [Google Scholar]
- 31. Lalezari J, Campion K, Keene O, Silagy C (2001) Zanamivir for the treatment of influenza A and B infection in high-risk patients: a pooled analysis of randomized controlled trials. Arch Intern Med 16: 212–217. [DOI] [PubMed] [Google Scholar]
- 32. Doshi P, Jones M, Jefferson T (2012) Rethinking credible evidence synthesis. BMJ 344: d7898. [DOI] [PubMed] [Google Scholar]
- 33.Ebell MH, Call M, Shinholser J (2012) Effectiveness of oseltamivir in adults: a meta-analysis of published and unpublished clinical trials. Fam Pract Sep 20. [DOI] [PubMed]
- 34. Hernán MA, Lipsitch M (2011) Oseltamivir and risk of lower respiratory tract complications in patients with flu symptoms: a meta-analysis of eleven randomized clinical trials. Clin Infect Dis 53: 277–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cochrane Neuraminidase Inhibitors Review Team (2011) Does oseltamivir really reduce complications of influenza? Clin Infect Dis 53: 1302–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hernán MA, Lipsitch M (2011) Reply to Cochrane Neuraminidase Inhibitors Review Team. Clin Infect Dis 53: 1303–1304. [Google Scholar]
- 37. Harper SA, Englund JA, File TM, Gravenstein S, Hayden FG, et al. (2009) Seasonal influenza in adults and children–diagnosis, treatment, chemoprophylaxis, and institutional outbreak management: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis 48: 1003–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.NHG Standaard [Practice guideline] (2008) Influenza en influenzavaccinatie [Influenza and influenza vaccination] M35 NHG - Nederlands Huisartsen Genootschap [The Dutch College of General Practitioners].
- 39.NICE (2008) NICE technology appraisal guidance 158: Oseltamivir, amantadine (review) and zanamivir for the prophylaxis of influenza (Includes a review of NICE technology appraisal guidance 67) London.
- 40.NICE (2009) Amantadine, oseltamivir and zanamivir for the treatment of influenza TA-168(Structured abstract). National Institute for Health and Clinical Excellence: HTA-32011000098.
- 41.Van de Vyver N, Janssens W, De Sutter A, Michiels B, Govaerts F, et al. (2006) Antivirale middelen bij seizoensgriep en grieppandemie. Literatuurstudie en ontwikkeling van praktijkrichtlijnen. [Antiviral agents in seasonal and pandemic influenza. Literature study and development of practice guidelines]. Brussel: Federaal Kenniscentrum voor de gezondheidszorg [The Belgian Health Care Knowledge Centre] (KCE). KCE reports 49 A (D/2006/2010.2273/2065) p.
- 42. Welliver R, Monto AS, Carewicz O, Schatteman E, Hassman M, et al. (2001) Effectiveness of oseltamivir in preventing influenza in household contacts: a randomised controlled trial. JAMA 285: 748–754. [DOI] [PubMed] [Google Scholar]
- 43.Jefferson T, Del Mar CB, Dooley L, Ferroni E, Al-Ansary LA, et al. (2011) Physical interventions to interrupt or reduce the spread of respiratory viruses. Cochrane Database of Systematic Reviews: CD006207. [DOI] [PMC free article] [PubMed]
- 44. Lee VJ, Lye DC, Wilder-Smith A (2009) Combination strategies for pandemic influenza response - a systematic review of mathematical modeling studies. BMC Med 7: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Thorlund K, Awad T, Boivin G, Thabane L (2011) Systematic review of influenza resistance to the neuraminidase inhibitors. BMC Infect Dis 11: 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sugaya N, Ohashi Y (2010) Long-acting neuraminidase inhibitor laninamivir octanoate (CS-8958) versus oseltamivir as treatment for children with influenza virus infection. Antimicrob Agents Chemother 54: 2575–2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kashiwagi S, Kudoh S, Watanabe A, Yoshimura I (2000) [Efficacy and safety of the selective oral neuraminidase inhibitor oseltamivir for prophylaxis against influenza–placebo-controlled double-blind multicenter phase III trial]. Kansenshogaku Zasshi 74: 1062–1076. [DOI] [PubMed] [Google Scholar]
- 48. Michiels B, Thomas I, Van Royen P, Coenen S (2011) Clinical prediction rules combining signs, symptoms and epidemiological context to distinguish influenza from influenza-like illnesses in primary care: a cross sectional study. BMC Family Practice 12: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Beigel J, Bray M (2008) Current and future antiviral therapy of severe seasonal and avian influenza. Antiviral Res 78: 91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bettis R, Iacuzio D, Jung T, Fuchs R, Aultman R, et al. (2006) Impact of influenza treatment with oseltamivir on health, sleep and daily activities of otherwise healthy adults and adolescents. Clin Drug Investig 26: 329–340. [DOI] [PubMed] [Google Scholar]
- 51. Bijl D (2011) Pandemic influenza vaccines and neuraminidase inhibitors: efficacy and side effects. Int J Risk Saf Med 23: 65–71. [DOI] [PubMed] [Google Scholar]
- 52. Chidiac C (2008) [Influenza]. Rev Prat 58: 445–453. [PubMed] [Google Scholar]
- 53. Clark NM, Lynch JP (2011) Influenza: epidemiology, clinical features, therapy, and prevention. Semin Respir Crit Care Med 32: 373–392. [DOI] [PubMed] [Google Scholar]
- 54. Dutkowski R (2010) Oseltamivir in seasonal influenza: cumulative experience in low-and high-risk patients. J Antimicrob Chemother 65: ii11–ii24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ferraris O, Escuret V, Bouscambert-Duchamp M, Lina B, Morfin F (2010) [Role of neuraminidase inhibitors for the treatment of influenza A virus infections]. Pathol Biol (Paris) 58: e69–78. [DOI] [PubMed] [Google Scholar]
- 56. Freemantle N, Calvert M (2009) What can we learn from observational studies of oseltamivir to treat influenza in healthy adults? BMJ 339: b5248. [DOI] [PubMed] [Google Scholar]
- 57.Health Technology Assessment (2010) Systematic review and economic modelling of the use of neuraminidase inhibitors for the prevention and treatment of influenza A and B (Structured abstract). Health Technology Assessment: HTA-32010000424.
- 58.Heneghan CJ (2011) Update and amalgamation of two Cochrane Reviews: neuraminidase inhibitors for preventing and treating influenza in healthy adults and children (project record). NIHR Health Technology Assessment programme: HTA-32011001126.
- 59. Holzgrabe U (2011) [How safe was oseltamivir care in the flu pandemic?]. Pharm Unserer Zeit 40: 151–154. [DOI] [PubMed] [Google Scholar]
- 60. Jamieson B, Jain R, Carleton B, Goldman RD (2009) Use of oseltamivir in children. Can Fam Physician 55: 1199–1201. [PMC free article] [PubMed] [Google Scholar]
- 61.Jefferson T, Demicheli V, Di Pietrantonj C, Jones M, Rivetti D (2006) Neuraminidase inhibitors for preventing and treating influenza in healthy adults. Cochrane Database of Systematic Reviews: CD001265. [DOI] [PubMed]
- 62. Jefferson T, Demicheli V, Rivetti D, Jones M, Di Pietrantonj C, et al. (2006) Antivirals for influenza in healthy adults: systematic review. Lancet 367: 303–313. [DOI] [PubMed] [Google Scholar]
- 63. Jefferson T, Jones M, Doshi P, Del Mar C (2009) Neuraminidase inhibitors for preventing and treating influenza in healthy adults: systematic review and meta-analysis. BMJ 339: b5106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jefferson T, Jones M, Doshi P, Del Mar C, Dooley L, et al. (2010) Neuraminidase inhibitors for preventing and treating influenza in healthy adults. Cochrane Database of Systematic Reviews: CD001265. [DOI] [PMC free article] [PubMed]
- 65. Jefferson T, Jones M, Doshi P, Del Mar C, Dooley L, et al. (2010) Neuraminidase inhibitors for preventing and treating influenza in healthy adults: a Cochrane review. Health Technol Assess 14: 355–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jefferson T, Jones MA, Doshi P, Del Mar CB, Heneghan CJ, et al. (2011) Neuraminidase inhibitors for preventing and treating influenza in healthy adults and children - a review of clinical study reports (protocol). Cochrane Database of Systematic Reviews: 1.
- 67. Jones M, Del Mar C (2006) Safety of neuraminidase inhibitors for influenza. Expert Opin Drug Saf 5: 603–608. [DOI] [PubMed] [Google Scholar]
- 68. Klebe G, Schlitzer M (2011) [M2 inhibitors and neuraminidase inhibitors]. Pharm Unserer Zeit 40: 144–150. [DOI] [PubMed] [Google Scholar]
- 69. Lee N, Ison MG (2012) Diagnosis, management and outcomes of adults hospitalized with influenza. Antivir Ther 17: 143–157. [DOI] [PubMed] [Google Scholar]
- 70. Lynch JP, Walsh EE (2007) Influenza: evolving strategies in treatment and prevention. Semin Respir Crit Care Med 28: 144–158. [DOI] [PubMed] [Google Scholar]
- 71. Mallia P, Johnston SL (2007) Influenza infection and COPD. Int J Chron Obstruct Pulmon Dis 2: 55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Matheson NJ, Harnden AR, Perera R, Sheikh A, Symmonds-Abrahams M (2007) Neuraminidase inhibitors for preventing and treating influenza in children. Cochrane Database of Systematic Reviews: CD002744. [DOI] [PubMed]
- 73. McCullers JA (2011) Preventing and treating secondary bacterial infections with antiviral agents. Antivir Ther 16: 123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Moscona A (2008) Medical management of influenza infection. Annu Rev Med 59: 397–413. [DOI] [PubMed] [Google Scholar]
- 75.NICE (2008) Oseltamivir, amantadine and zanamivir for the prophylaxis of influenza (including a review of TA67) (Structured abstract). National Institute for Health and Clinical Excellence: HTA-32011000382.
- 76. Nayak JL, Treanor JJ (2009) Antiviral treatment and prophylaxis of influenza virus in children. Pediatr Ann 38: 667–674. [DOI] [PubMed] [Google Scholar]
- 77. Antiviral drugs prophylaxis and treatment of influenza. Review. Med Lett Drugs Ther 48: 87–88. [PubMed] [Google Scholar]
- 78. Antiviral drugs for influenza. Review. Med Lett Drugs Ther 51: 89–92. [PubMed] [Google Scholar]
- 79. Antiviral drugs for influenza. Review. Med Lett Drugs Ther 54: 1–3. [PubMed] [Google Scholar]
- 80. Nüesch R (2007) [Antiviral treatment of influenza in humans]. Ther Umsch 64: 635–641. [DOI] [PubMed] [Google Scholar]
- 81. Oxford JS (2007) Antivirals for the treatment and prevention of epidemic and pandemic influenza. Influenza Other Respi Viruses 1: 27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Preziosi P (2011) Influenza pharmacotherapy: present situation, strategies and hopes. Expert Opin Pharmacother 12: 1523–1549. [DOI] [PubMed] [Google Scholar]
- 83. Ruf BR, Sandow P, Lehmann P, Del Piero L, Skopnik H (2008) [Significance of the neuraminidase inhibitor oseltamivir for the management of seasonal influenza]. Dtsch Med Wochenschr 133: 1259–1264. [DOI] [PubMed] [Google Scholar]
- 84. Ruf BR, Szucs T (2009) Reducing the burden of influenza-associated complications with antiviral therapy. Infection Control & Hospital Epidemiology 37: 186–196. [DOI] [PubMed] [Google Scholar]
- 85. Salzberger B (2006) [Antiviral therapy: from influenza to Pfeiffer’s disease]. Internist (Berl) 47: 1245–1250. [DOI] [PubMed] [Google Scholar]
- 86. Schirmer P (2009) Holodniy M. Oseltamivir for treatment and prophylaxis of influenza infection. Expert Opin Drug Saf 8: 357–371. [DOI] [PubMed] [Google Scholar]
- 87. Shun-Shin M, Thompson M, Heneghan C, Perera R, Harnden A, et al. (2009) Neuraminidase inhibitors for treatment and prophylaxis of influenza in children: systematic review and meta-analysis of randomised controlled trials. BMJ 339: b3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Smith JR, Rayner CR, Donner B, Wollenhaupt M, Klumpp K, et al. (2011) Oseltamivir in seasonal, pandemic, and avian influenza: a comprehensive review of 10-years clinical experience. Adv Ther 28: 927–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Tambyah PA (2008) Update on influenza anti-virals. Respirology 13 Suppl 1: S19–21. [DOI] [PubMed] [Google Scholar]
- 90.Tappenden P, Jackson R, Cooper K, Rees A, Simpson E, et al. (2009) Zanamivir, oseltamivir and amantadine for the treatment of influenza (an update review of existing guidance) (Structured abstract). Health Technology Assessment: HTA-32008100360. [DOI] [PubMed]
- 91. Toovey S, Rayner C, Prinssen E, Chu T, Donner B, et al. (2008) Assessment of neuropsychiatric adverse events in influenza patients treated with oseltamivir: a comprehensive review. Drug Saf 31: 1097–1114. [DOI] [PubMed] [Google Scholar]
- 92. Townsend KA, Eiland LS (2006) Combating influenza with antiviral therapy in the pediatric population. Pharmacotherapy 26: 95–103. [DOI] [PubMed] [Google Scholar]
- 93. Tullu MS (2009) Oseltamivir. J Postgrad Med 55: 225–230. [DOI] [PubMed] [Google Scholar]
- 94.Wang K, Shun-Shin M, Gill P, Perera R, Harnden A (2012) Neuraminidase inhibitors for preventing and treating influenza in children. Cochrane Database of Systematic Reviews: CD002744. [DOI] [PubMed]
- 95. Wesseling G (2007) Occasional review: influenza in COPD: pathogenesis, prevention, and treatment. Int J Chron Obstruct Pulmon Dis 2: 5–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Whitley RJ (2007) The role of oseltamivir in the treatment and prevention of influenza in children. Expert Opin Drug Metab Toxicol 3: 755–767. [DOI] [PubMed] [Google Scholar]
- 97.Yang Ming, Dong Bi Rong, Wu Hong Mei, Li Ting, Liu Guan J (2010) Interventions for treating influenza: an overview of Cochrane systematic reviews. Cochrane Database of Systematic Reviews: Protocols : CD008799.
- 98.Yang Ming, Wu Hong Mei, Li Ting, Dong Bi Rong, Liu Guan J (2010) Interventions for preventing influenza: an overview of Cochrane systematic reviews. Cochrane Database of Systematic Reviews: Protocols: CD008501.
- 99. Hayden FG, Atmar RL, SchillingM, Johnson C, Poretz D, et al. (1999) Use of the selective oral neuraminidase inhibitor oseltamivir to prevent influenza. N Engl J Med 341: 1336–1343. [DOI] [PubMed] [Google Scholar]
- 100. Hayden FG, Gubareva LV, Monto AS, Klein TC, Elliott MJ, et al. (2000) Inhaled zanamivir for the prevention of influenza in families. N Engl J Med 343: 1282–1289. [DOI] [PubMed] [Google Scholar]
- 101. Monto AS, Pichichero ME, Blanckenberg SJ, Ruuskanen O, Cooper C, et al. (2002) Zanamivir prophylaxis: an effective strategy for the prevention of influenza types A and B within households. J Infect Dis 186: 1582–1588. [DOI] [PubMed] [Google Scholar]
- 102. Kaiser L, Henry D, Flack NP, Keene O, Hayden FG (2000) Short-term treatment with zanamivir to prevent influenza: results of a placebo-controlled study. Clin Infect Dis 30: 587–589. [DOI] [PubMed] [Google Scholar]
- 103. Peters PH, Gravenstein S, Norwood P, De Bock V, Van Couter A, et al. (2001) Long-term use of oseltamivir for the prophylaxis of influenza in a vaccinated frail older population. J Am Geriatr Soc 49: 1025–1031. [DOI] [PubMed] [Google Scholar]
- 104. Li L, Cai B, Wang M, Zhu Y (2003) A double-blind, randomized, placebo-controlled multicenter study of oseltamivir phosphate for treatment of influenza infection in China. Chin Med J (Engl) 116: 44–48. [DOI] [PubMed] [Google Scholar]
- 105. Nicholson KG, Aoki FY, Osterhaus AD, Trottier S, Carewicz O, et al. (2000) Efficacy and safety of oseltamivir in treatment of acute influenza: a randomised controlled trial. Neuraminidase Inhibitor Flu Treatment Investigator Group. Lancet 355: 1845–1850. [DOI] [PubMed] [Google Scholar]
- 106. Treanor JJ, Hayden FG, Vrooman PS, Barbarash R, Bettis R, et al. (2000) Efficacy and safety of the oral neuraminidase inhibitor oseltamivir in treating acute influenza: a randomized controlled trial. US Oral Neuraminidase Study Group. JAMA 283: 1016–1024. [DOI] [PubMed] [Google Scholar]
- 107. Whitley RJ, Hayden FG, Reisinger KS, Young N, Dutkowski R, et al. (2001) Oral oseltamivir treatment of influenza in children. Pediatr Infect Dis J 20: 127–133. [DOI] [PubMed] [Google Scholar]
- 108. Hayden FG, Osterhaus AD, Treanor JJ, Fleming DM, Aoki FY, et al. (1997) Efficacy and safety of the neuraminidase inhibitor zanamivir in the treatment of influenzavirus infections. GG167 Influenza Study Group. N Engl J Med 337: 874–880. [DOI] [PubMed] [Google Scholar]
- 109. Makela MJ, Pauksens K, Rostila T, DM F, Man CY, et al. (2000) Clinical efficacy and safety of the orally inhaled neuraminidase inhibitor zanamivir in the treatment of influenza: a randomized, double-blind, placebo-controlled European study. J Infect 40: 42–48. [DOI] [PubMed] [Google Scholar]
- 110. The MIST (Management of Influenza in the Southern Hemisphere Trialists) Study Group (1998) Randomised trial of efficacy and safety of inhaled zanamivir in treatment of influenza A and B virus infections. Lancet 352: 1877–1881. [PubMed] [Google Scholar]
- 111. Puhakka T, Lehti H, Vainionpaa R, Jormanainen V, Pulkkinen M, et al. (2003) Zanamivir: a significant reduction in viral load during treatment in military conscripts with influenza. Scand J Infect Dis 35: 52–58. [DOI] [PubMed] [Google Scholar]
- 112. Johnston SL, Ferrero F, Garcia ML, Dutkowski R (2005) Oral oseltamivir improves pulmonary function and reduces exacerbation frequency for influenza-infected children with asthma. Pediatr Infect Dis J 24: 225–232. [DOI] [PubMed] [Google Scholar]
- 113. Martin C, Mahoney P, Ward P (2001) Oral oseltamivir reduces febrile illness in patients considered at high risk of influenza complications. Int Congr Ser 1219: 807–811. [Google Scholar]
- 114. Boivin G, Goyette N, Hardy I, Aoki F, Wagner A, et al. (2000) Rapid antiviral effect of inhaled zanamivir in the treatment of naturally occurring influenza in otherwise healthy adults. J Infect Dis 181: 1471–1474. [DOI] [PubMed] [Google Scholar]
- 115. Murphy KR, Eivindson A, Pauksens K, Stein WJ, Tellier G, et al. (2000) Efficacy and safety of inhaled zanamivir for the treatment of influenza in patients with asthma or chronic obstructive pulmonary disease: A double-blind, randomised, placebo-controlled, multicentre study. Clinical Drug Investigation 20: 337–349. [Google Scholar]
- 116. Tan W, Hou J, Chen X, Xiong H, Li XQ, et al. (2002) [A randomized, double-blinded and controlled clinical evaluation of oseltamivir in the treatment of influenza]. Clin Med J China 9: 528–531. [Google Scholar]
- 117. Matsumoto K, Ogawa N, Nerome K, Numazaki Y, Kawakami Y, et al. (1999) Safety and efficacy of the neuraminidase inhibitor zanamivir in treating influenza virus infection in adults: results from Japan. GG167 Group. Antivir Ther 4: 61–68. [PubMed] [Google Scholar]
- 118. Webster A, Boyce M, Edmundson S, Miller I (1999) Coadministration of orally inhaled zanamivir with inactivated trivalent influenza vaccine does not adversely affect the production of antihaemagglutinin antibodies in the serum of healthy volunteers. Clin Pharmacokinet 36 Suppl 1: 51–58. [DOI] [PubMed] [Google Scholar]
- 119. Heinonen S, Silvennoinen H, Lehtinen P, Vainionpää R, Vahlberg T, et al. (2010) Early oseltamivir treatment of influenza in children 1–3 years of age: a randomized controlled trial. Clin Infect Dis 51: 887–894. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA 2009 checklist.
(DOC)