Abstract
Background
Measurement of partial pressure of oxygen (PO2) at high temporal resolution remains a technological challenge. This study introduces a novel PO2 sensing technology based on Multi-Frequency Phase Fluorimetry (MFPF). The aim was to validate MFPF against polarographic Clark-type electrode (CTE) PO2 measurements.
Methodology/Principal Findings
MFPF technology was first investigated in N = 8 anaesthetised pigs at FIO2 of 0.21, 0.4, 0.6, 0.8 and 1.0. At each FIO2 level, blood samples were withdrawn and PO2 was measured in vitro with MFPF using two FOXY-AL300 probes immediately followed by CTE measurement. Secondly, MFPF-PO2 readings were compared to CTE in an artificial circulatory setup (human packed red blood cells, haematocrit of 30%). The impacts of temperature (20, 30, 40°C) and blood flow (0.8, 1.6, 2.4, 3.2, 4.0 L min−1) on MFPF-PO2 measurements were assessed. MFPF response time in the gas- and blood-phase was determined. Porcine MFPF-PO2 ranged from 63 to 749 mmHg; the corresponding CTE samples from 43 to 712 mmHg. Linear regression: CTE = 15.59+1.18*MFPF (R2 = 0.93; P<0.0001). Bland Altman analysis: meandiff 69.2 mmHg, rangediff -50.1/215.6 mmHg, 1.96-SD limits -56.3/194.8 mmHg. In artificial circulatory setup, MFPF-PO2 ranged from 20 to 567 mmHg and CTE samples from 11 to 575 mmHg. Linear regression: CTE = −8.73+1.05*MFPF (R2 = 0.99; P<0.0001). Bland-Altman analysis: meandiff 6.6 mmHg, rangediff -9.7/20.5 mmHg, 1.96-SD limits -12.7/25.8 mmHg. Differences between MFPF and CTE-PO2 due to variations of temperature were less than 6 mmHg (range 0–140 mmHg) and less than 35 mmHg (range 140–750 mmHg); differences due to variations in blood flow were less than 15 mmHg (all P-values>0.05). MFPF response-time (monoexponential) was 1.48±0.26 s for the gas-phase and 1.51±0.20 s for the blood-phase.
Conclusions/Significance
MFPF-derived PO2 readings were reproducible and showed excellent correlation and good agreement with Clark-type electrode-based PO2 measurements. There was no relevant impact of temperature and blood flow upon MFPF-PO2 measurements. The response time of the MFPF FOXY-AL300 probe was adequate for real-time sensing in the blood phase.
Introduction
The polarographic Clark-type electrode remains the gold standard for oxygen partial pressure (PO2) measurement to date, and is the underlying electrochemical principle of conventional blood gas PO2 analysis [1], [2], [3], [4]. Moreover, polarographic PO2 probes, such as the Licox probe, are used for the continuous monitoring of oxygen. These probes provide favourable properties, including high accuracy and stability; however, they lack high temporal resolution [5]. Several publications reveal that oxygen levels may largely fluctuate over time in the systemic (e.g., thoracic aorta) or capillary circulation (e.g. vasomotion, brain resting state) [6], [7], [8], [9], [10] due to oxygen consumption and delivery. Therefore, novel, fast oxygen-sensing technologies are required for the investigation of these physiologic or pathophysiologic phenomena [11], [12].
Recently, a novel technology for fast oxygen sensing based on Multi Frequency Phase Fluorimetry (MFPF), termed NeoFox® (OceanOptics, Dunedin, USA; www.oceanoptics.com), was released. This technology is suitable for 10 Hz oxygen measurements using fluorescent dye sensors such as the FOXY-AL300 (OceanOptics, Dunedin, USA). This indwelling, uncoated ruthenium tipped fibre optic probe allows for rapid measurement of oxygen within blood or tissue. The technology, however, has not yet been compared to conventional polarographic Clark-type electrode (CTE)-based PO2 measurements [13], [14].
This study aimed to validate MFPF-PO2 measurements versus CTE-based measurements of oxygen partial pressure. Firstly, in a porcine lung model, MFPF-PO2 was measured in vitro without the interference of blood flow using two FOXY-AL300 probes (OceanOptics, Dunedin, USA) and was then compared to CTE blood gas PO2 analysis. Secondly, for further validation, we developed an artificial extracorporeal circulation setup primed with a mixture of crystalloid, colloid and human packed red cells to imitate in vivo conditions. In this setup, MFPF-PO2 readings were assessed and then compared to conventionally measured PO2 by CTE. Furthermore, in the artificial circulation setup, temperature and blood flow were randomly varied in order to evaluate their impact on MFPF-PO2 measurements. In addition, MFPF FOXY-AL300 (OceanOptics, Dunedin, USA) probe response times were determined in the gas- and blood-phases to further assess this novel technology. In summary, the overall aim could be achieved.
Materials and Methods
MFPF Technological Principle
The MFPF platform (NeoFox®, OceanOptics, Dundin, USA) was mounted with the indwelling FOXY-AL300 (OceanOptics, Dunedin, USA) aluminium-jacketed optical fibre probe (500 µm outer and 300 µm core diameter) with an uncoated ruthenium complex at the tip to assess luminescence lifetime in the region surrounding the fluorescent dye, which was roughly equal to the fibre-optic tip surface area (about 0.3 mm2). MFPF assesses luminescence lifetime by modulation of light excitation frequency where the oxygen dependent phase shift of the emission is detected. Lifetime is related to phase shift by the equation: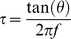 , where τ is calculated lifetime in s, f is LED modulation frequency in Hz, and θ is phase shift measured by the instrument. The signals are transmitted via connected glass fibre cable to the MFPF server (NeoFox®, OceanOptics, Dundin, USA) with an integrated light emitting diode, as well as temperature and atmospheric pressure transducers. An external thermistor probe compensates for temperature changes (once a multi-point temperature calibration is performed). Lifetime data were logged by NeoFox® Software (OceanOptics, Dunedin, USA) for calculation of absolute PO2 values. Absolute PO2 values, luminescence phase shift, luminescence intensity, temperature and ambient pressure were displayed at a digital sampling rate of 10 Hz.
, where τ is calculated lifetime in s, f is LED modulation frequency in Hz, and θ is phase shift measured by the instrument. The signals are transmitted via connected glass fibre cable to the MFPF server (NeoFox®, OceanOptics, Dundin, USA) with an integrated light emitting diode, as well as temperature and atmospheric pressure transducers. An external thermistor probe compensates for temperature changes (once a multi-point temperature calibration is performed). Lifetime data were logged by NeoFox® Software (OceanOptics, Dunedin, USA) for calculation of absolute PO2 values. Absolute PO2 values, luminescence phase shift, luminescence intensity, temperature and ambient pressure were displayed at a digital sampling rate of 10 Hz.
Calibration of the FOXY-AL300 Oxygen Probe
PO2 probes were calibrated at different oxygen levels and temperatures in a closed thermostat controlled gas-tight chamber according to the manufacturer's instructions. The chamber was purged with pure nitrogen (PO2 = 0 mmHg), compressed air (PO2 = 158 mmHg) and pure oxygen (PO2 = 750 mmHg), respectively, in consideration of atmospheric pressure with a fixed laminar flow of 2 L min−1 at 20, 30 and 40°C. After stabilisation, the corresponding lifetimes were used to create a multi-temperature calibration table.
Ethics statement
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institute of Health. The protocol was approved by the Animal State Care and Use Committee of the Rhineland Palatinate, Germany (Permit Number: G07-10-013). All surgery was performed under deep anaesthesia, and all efforts were made to minimise suffering [15]. Animal experiments were performed at the Department of Anesthesiology, Medical Center of the Johannes Gutenberg-University, Mainz, Germany and laboratory experiments at the Department of Anesthesiology and Pain Therapy, Inselspital, Bern University Hospital, and University of Bern, Switzerland.
MFPF Validation in vitro using a porcine lung model
Eight piglets, Sus scrofa domestica (20±2 kg), were investigated. After the induction of anaesthesia with a bolus of fentanyl 5 µg kg−1 i.v., propofol 2 mg kg−1 i.v. and single shot atracurium 0.5 mg kg−1 i.v. via an ear vein, the pigs were orotreachally intubated (ID 8.0 mm cuffed tubing) and ventilated in pressure-controlled mode (AVEA, Viasis Healthcare, Höchberg, Germany). Vascular access was achieved by surgical cut down for the placement of an arterial blood pressure line in the femoral artery. Normocapnia (PaCO2 35–45 mmHg) was maintained with a tidal volume of 6–8 ml kg−1 BW−1. Anaesthesia was maintained by continuous infusion of fentanyl (5 µg−1 kg−1 h−1 i.v.) and propofol (5–10 mg−1 kg−1 h−1 i.v.). During the experiment, FIO2 was adjusted (0.21, 0.4, 0.6, 0.8 and 1.0) to obtain PO2 readings over the whole normobaric measurement range. Core temperature by rectal probe was maintained within the physiologic range (37.0–38.5°C) using a heating blanket.
Blood samples (10 ml each) were withdrawn at each FIO2 level into heparinised gas tight syringes covered with opaque tape. For each sample, the tips of two FOXY-AL300 probes (OceanOptics, Dunedin, USA) were placed into the syringe outlet sealed with a rubber port to avoid air contact. After reaching steady state values, both MFPF-PO2 readings were simultaneously recorded for 2 min using the corresponding animal core temperature for the measurement. Then, the same blood sample was analysed by conventional polarographic Clark-type electrodes (RapidLab 415, BayerHealthcare, Leverkusen) immediately afterwards.
MFPF validation ex vivo using an artificial circulatory setup
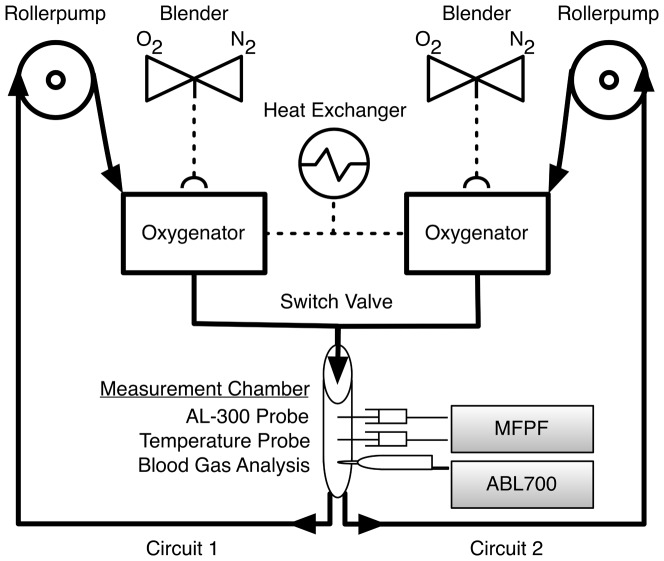
In order to provide standardised conditions and to simulate in vivo conditions, an artificial circulatory system was set up. The apparatus was built of: a) two oxygenators (Jostra Quadrox D, Maquet Cardiopulmonary AG, Hirrlingen, Germany), b) 3/8 inch silicon tubing (Fumedica, Muri, Switzerland) with 3/8–3/8 inch connecting pieces (Luerlock, Fumedica, Muri, Switzerland), c) two multi-flow roller pumps (Stöckert, Munich, Germany), d) a heat exchanger (HCU-30, Maquet Cardiopulmonary AG, Hirrlingen, Germany) and e) an ambient light protected measurement chamber (Figure 1). A switch valve between two independent closed circuits enabled the induction of a step change between highly oxygenated blood (FIO2 1.0, circuit 1) to oxygen free blood (FIO2 0.0, circuit 2) in order to assess the time constants of the FOXY-AL300 probes in blood-phase.
Figure 1. Artificial Circulatory Setup.
Artificial circulatory setup with two independent circuits filled with human packed red blood cells (haematocrit of 30%). A switching valve between the circuits enabled a step-change between highly oxygenated (circuit 1: purged with pure oxygen) and oxygen free blood (circuit 2: purged with nitrogen). Black arrows represent direction of blood flow. Adapting the settings of the rollerpumps and the heating-cooling device (heat exchanger) allowed blood-flow and temperature to be controlled. Via the O2/N2 blenders, oxygen content could be adapted at fixed sweep gas flow over the oxygenators. Measurement chamber contained 1.) ports for insertion of MFPF probes (Foxy-AL 300); 2.) a temperature probe and 3.) a sampling port for Clark-typed based (CTE) PO2 analysis (ABL 700).
The artificial circulatory system was primed with a mixture of crystalloid, colloid and human packed red cells to achieve a haematocrit of 30% and electrolyte values within the physiological limits. This setup allowed for control and variation of blood flow (by variation of rpm at the rollerpumps), temperature (by variation at the membrane equilibrator) and oxygen content (by PO2 variation at the blender).
In order to get measurements over the normobaric measurement range for the validation of MFPF against Clark-type electrode PO2, FIO2 was set at random according to a computer generated list [16] from 0.0 to 1.0. To achieve an FIO2 of less than 0.21, a mixture of oxygen and nitrogen was used. For all measurements, duplicate MFPF PO2 readings (2 FOXY-AL300 probes) were recorded for 1 min duration alongside blood withdrawal for conventional polarographic Clark-type electrode PO2 analysis (ABL 700, Radiometer, Copenhagen, Denmark).
Measurements were performed according to the following protocols:
To assess the agreement of MFPF vs. Clark-type electrode PO2 at physiological body temperature conditions: at a temperature of 37°C, blood flow of 1.6 L min−1 and a sweep gas flow of 1 L min−1; 55 measurements were performed at varying PO2 levels.
To assess the impact of temperature: at a blood flow of 0.5 L min−1 and sweep gas flow 0.5 L min−1, temperature was set at random according to a computer generated list [16] to 20, 30 or 40°C. For each temperature level, 15 measurements were performed at varying PO2 levels.
To assess the impact of blood flow: at a temperature of 37°C and a sweep gas flow of 2 L min−1, blood flow was set at random according to a computer generated list [16] to 0.8, 1.6, 2.4, 3.2 and 4.0 L min−1. For each blood flow level, 10 measurements were performed at varying PO2 levels.
Statistical Analysis
Descriptive and statistical data analysis was performed using SPSS® V.19 (IBM Inc., New York, USA). For metric variables, statistical measures such as mean and standard deviation (mean±SD) were calculated. Linear regression analysis upon PO2 values measured by MFPF and CTE were performed. Bias and precision values were calculated by the Bland-Altman method [17], [18]. The comparability was presented in explorative manner by description of mean differences, range of differences and 1.96-SD limits (lower and upper limit of agreement) [19]. In addition, linear regression analysis of difference versus mean was performed and then tested, if the slope is significantly different from zero. Furthermore, intra-class correlation between the two MFPF probes was assessed. To test the influence of blood flow and temperature variation, a multiple linear regression model was fitted: magnitude of PO2 measurements [(CTE+MFPF)/2], temperature and blood flow as independent variables; differences of MFPF and CTE as dependent variable. The categories of temperature and blood flow were handled with dummy coding. The analyses of data were done in explorative manner and the outcome of a statistical test with a P-value<0.05 was interpreted as statistically significant.
Results
MFPF Validation in vitro using a porcine lung model
Replicated porcine blood sampling revealed 80 duplicate MFPF-PO2 readings (range 63 to 749 mmHg) with the corresponding CTE samples (range 43 to 712 mmHg). The linear regression was described by the equation CTE = 15.59+1.18*MFPF (R2 = 0.93; P<0.0001) (Figure 2A). Agreement by Bland Altman analysis showed a mean difference (meandiff) in PO2 between CTE and MFPF of 69.2±64.1 mmHg (Figure 2B). The differences ranged from −50.1 to 215.6 mmHg within the 1.96-SD limits (lower limit -56.3, upper limit 194.8 mmHg). Linear regression of the difference versus mean showed CTE-MFPF = −0.64+0.20*[(CTE+MFPF)/2] (R2 = 0.36, slope significantly different from zero: P<0.0001). Intra-class correlation between the two MFPF probes showed a reproducibility of R2 = 0.98.
Figure 2. Multi Frequency Phase Fluorimetry PO2 vs. Clark-type Electrode PO2 (porcine blood in vitro, normobaric range).
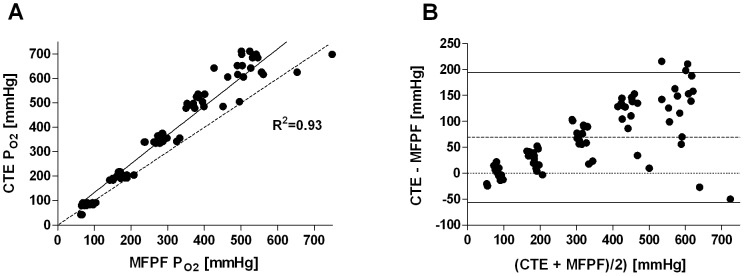
Panel A: Linear regression plot, the solid line displays the line of best fit, the dashed line shows the line of identity; Panel B: Bland-Altman plot showing the differences (CTE-MFPF) versus the means for absolute PO2 values. The dashed line represents the bias, the solid lines the 1.96 standard deviation interval.
MFPF validation ex vivo in artificial circulatory setup
In artificial circulatory setup, MFPF-PO2 measurements ranged from 20 to 567 mmHg and the corresponding CTE samples from 11 to 575 mmHg. Linear regression yielded the equation: CTE = −8.73+1.05*MFPF (R2 = 0.99; P<0.0001) (Figure 3A). Bland-Altman analysis calculated a mean difference (meandiff) between CTE and MFPF PO2 measurements of 6.6±9.8 mmHg. Differences ranged from −9.7 to 20.5 mmHg within the 1.96-SD limits (lower limit -12.7, upper limit 25.8 mmHg) (Figure 3B). Linear regression of the difference versus mean showed CTE-MFPF = −8.62+0.05*[(CTE+MFPF)/2] (R2 = 0.78, slope significantly different from zero: P<0.0001). Linear regression in a sub-set of PO2 between 0 and 140 mmHg yielded CTE = −7.84+1.13*MFPF (R2 = 0.99; P<0.0001) (Figure 4A). Bland-Altman analysis of this sub-analysis showed a mean difference of 1.7±4.9 mmHg (Figure 4B). The differences ranged from −7.8 to 8.1 mmHg within the 1.96-SD limits (lower limit -8.0, upper limit 11.4 mmHg). Linear regression of the difference versus mean yielded CTE-MFPF = −7.47+0.13*[(CTE+MFPF)/2] (R2 = 0.84, slope significantly different from zero: P<0.0001). In total, reproducibility of R2 = 0.99 between the two MFPF probes (intra-class correlation) was depicted.
Figure 3. Multi Frequency Phase Fluorimetry PO2 vs. Clark-type Electrode PO2 (human blood ex vivo, normobaric range).
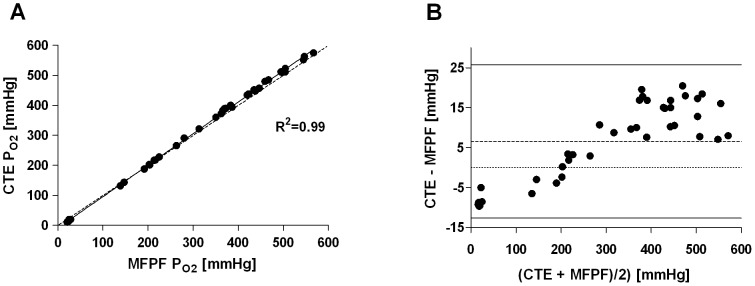
Panel A: Linear regression plot, the solid line displays the line of best fit, the dashed line shows the line of identity; Panel B: Bland-Altman plot showing the differences (CTE-MFPF) versus the means for absolute PO2 values. The dashed line represents the bias, the solid lines the 1.96 standard deviation interval.
Figure 4. Multi Frequency Phase Fluorimetry PO2 vs. Clark-type Electrode PO2 (human blood ex vivo, hypoxic and normoxic range).
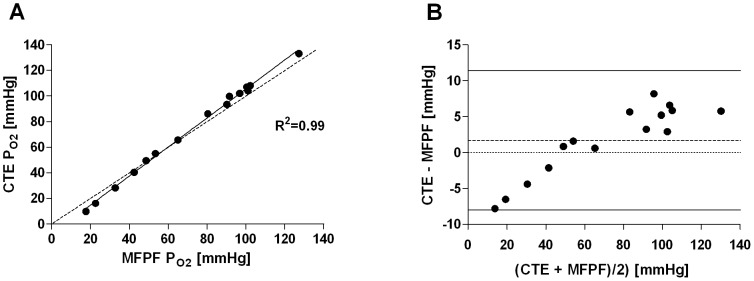
Panel A: Linear regression plot, the solid line displays the line of best fit, the dashed line shows the line of identity; Panel B: Bland-Altman plot showing the differences (CTE-MFPF) versus the means for absolute PO2 values. The dashed line represents the bias, the solid lines the 1.96 standard deviation interval.
Impact of temperature and blood flow on MFPF PO2 measurements
The influence of the temperature upon the differences of CTE and MFPF PO2 measurements reached from −0.6 mmHg (temperature = 20°C) to 31.8 mmHg (temperature = 40°C) (Table 1). The influence of blood flow on difference in PO2 of the two methods reached from 0.6 mmHg (blood flow = 1.6 L min−1) to 14.8 mmHg (blood flow = 0.8 L min−1) (Table 1). The linear regression model with the magnitude of PO2 measurements [(CTE+MFPF)/2], temperature and blood flow as independent variables showed a significant influence upon the magnitude of PO2 measurements and a non-significant influence of temperature or blood flow upon the differences of PO2 measurements (dependent variable) (Table 2).
Table 1. Influence of temperature and blood flow on MFPF PO2 measurements.
| PO2 range [mmHg] | N | MFPF-CTE mean | MFPF-CTE SD | MFPF-CTE median | |
| Temperature [°C] | |||||
| 20.0 | 0–140 | 5 | −0.6 | 4.6 | 0.9 |
| 30.0 | 0–140 | 5 | 0.4 | 5.9 | 0.6 |
| 40.0 | 0–140 | 5 | 5.3 | 2.4 | 5.8 |
| Total | 15 | 1.7 | 5 | 2.9 | |
| 20.0 | 140–600 | 12 | 13.3 | 28.7 | 28.6 |
| 30.0 | 140–600 | 11 | 29.4 | 34.9 | 35.9 |
| 40.0 | 140–600 | 11 | 31.8 | 21.9 | 40.1 |
| Total | 34 | 24.5 | 29.4 | 29.3 | |
| Flow [L min−1] | |||||
| 0.80 | 0–600 | 9 | 14.8 | 4.5 | 17.3 |
| 1.60 | 0–600 | 7 | .6 | 9.7 | −2.4 |
| 2.40 | 0–600 | 8 | 5.4 | 10.5 | 8.6 |
| 3.20 | 0–600 | 7 | 6.5 | 10.1 | 10.1 |
| 4.00 | 0–600 | 9 | 4.1 | 9.4 | 7.1 |
| Total | 40 | 6.6 | 9.8 | 8.4 |
PO2 = oxygen partial pressure [mmHg]; MFPF = Multi Frequency Phase Fluorimetry; CTE = Clark-type electrode; SD = standard deviation.
Table 2. Influence of temperature and blood flow on MFPF PO2 measurements: Linear regression model.
| Non-standardized coefficients | 95.0% CI | ||||
| Independent variable | Regression coefficient | Standard error | P-value | lower limit | upper limit |
| Temperature (base 20 °C) | |||||
| Constant | −2.335 | 10.483 | 0.825 | −23.744 | 19.074 |
| PO2-magnitude [(MFPF + CTE)/2] | 0.062 | 0.027 | 0.031 | 0.006 | 0.118 |
| 30°C | 12.854 | 11.466 | 0.271 | −10.563 | 36.271 |
| 40°C | 19.635 | 11.392 | 0.095 | −3.629 | 42.9 |
| Blood flow (base 0.8 L min−1) | |||||
| Constant | −4.707 | 2.401 | 0.058 | −9.586 | 0.171 |
| PO2-magnitude [(MFPF + CTE)/2] | 0.045 | 0.004 | <0.001 | 0.037 | 0.054 |
| 1.6 L min−1 | −4.461 | 2.441 | 0.076 | −9.422 | 0.5 |
| 2.4 L min−1 | −2.822 | 2.262 | 0.221 | −7.42 | 1.776 |
| 3.2 L min−1 | −2.093 | 2.328 | 0.375 | −6.825 | 2.638 |
| 4.0 L min−1 | −5.188 | 2.171 | 0.023 | −9.601 | −0.776 |
Linear regression model: magnitude of PO2 measurements, temperature and blood flow as independent variables, differences of PO2 measurements as dependent variable. PO2 = oxygen partial pressure; MFPF = Multi Frequency Phase Fluorimetry; CTE = Clark-type electrode; CI = confidence interval.
MFPF FOXY-AL300 Response Time
MFPF in combination with the uncoated FOXY-AL300 probes showed a mean response time of 1.48±0.26 s in gas-phase and 1.51±0.20 s in blood-phase after a step-change from pure oxygen (PO2 = 749 mmHg) to pure nitrogen (PO2 = 0 mmHg) at a digital sampling rate of 10 Hz (Figure 5). The monoexponential response time by Boltzmann fitting represents the time it takes the system to reach 63.2% of its final asymptotic value.
Figure 5. Multi Frequency Phase Fluorimetry/FOXY-AL300 Probe Response Time.
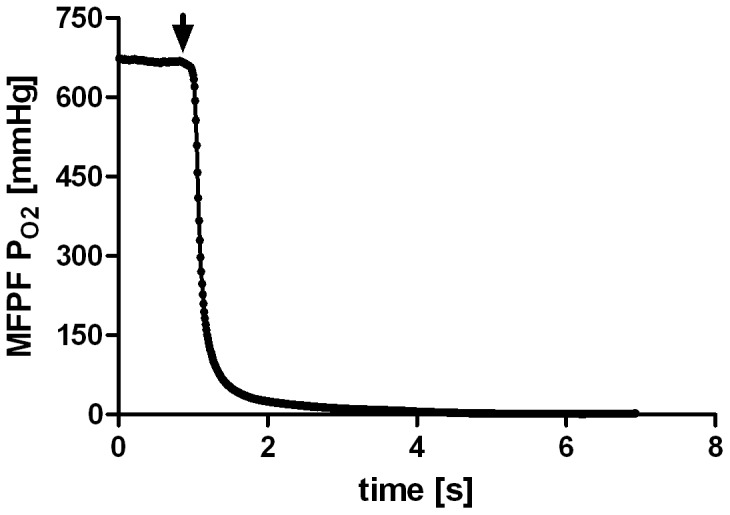
Example of an MFPF step-down manoeuvre in artificial circulatory setup (human blood-phase). The graph displays the absolute MFPF PO2 values over the time course. The arrow marks the time when the switching valve was changed between the oxygenated (750 mmHg) and non-oxygenated blood (0 mmHg) circuit.
Discussion
Findings demonstrate MFPF technologýs capacity for detecting changes in PO2 compared to Clark-type electrode (CTE) based PO2 measurements. For discrete samples, in vitro porcine blood phase measurements (R2 = 0.93) did not give very tight limits of agreement. However, this study was not designed to validate MFPF as replacement for traditional in vitro blood gas analysis. For such a setup, we would have needed a configuration where the sensor and sample are part of a heated block with very tight control of both probe and blood sample temperature. The authors want to point out that the real application of MFPF is for flowing blood when the probe is placed in vivo - which was investigated by ex vivo measurements in an artificial circulatory setup filled with human blood. These findings demonstrate that MFPF technology provides high accuracy for PO2 measurements compared to CTE based PO2 measurements (R2 = 0.99). The ex vivo measurements showed that MFPF technology accurately compensates for variation in temperature and that PO2 measurements are not affected by variation in blood flow. MFPF response time in combination with the indwelling FOXY-AL300 probes was 1.48±0.26 s in the gas-phase and 1.51±0.20 s in the blood-phase (step-change from pure oxygen to pure nitrogen).
In summary, MFPF technology allows for fast, accurate and valid PO2 readings over the normobaric PO2 range (0–749 mmHg) for ex vivo experiments, as MFPF PO2 measurements ranged between 11 and 749 mmHg. This fact is the most important, as novel oxygen sensing technologies should be validated over the whole measurement range.
The in vitro animal model (porcine blood) showed good correlation (R2 = 0.93), although the data was determined in clusters of oxygen concentration (FIO2 0.21, 0.4, 0.6, 0.8, 1.0). The observed high bias with positive drift as well as the enlarged standard deviation can be most likely explained by the systemic experimental error to use the animals' core temperature for the in vitro measurements. The main intention of this first series was to obtain readings without the influence of blood flow. Therefore, we focused on how to avoid air contact to the withdrawn blood and decided not to insert the temperature probe into the syringes. However, we did not expect such an impact of temperature, which presumably seems even more evident at high PO2-levels (Figure 2B). This finding can be explained by the underlying MFPF technology, as in fluorescent quenching, signal intensity increases at lower PO2 values. To fully understand the observed discrepancy of the in vitro (porcine blood) and ex vivo (human blood) results, we evaluated the difference of animal core temperature and blood temperature in the syringes two minutes after withdrawal post hoc in one further animal and found an error range of 0.9 to 1.6°C in ten repeated measures. Nevertheless, in the interesting physiologically hypoxic and normoxic (0–140 mmHg) PO2 range, porcine results show that MFPF technology provides good agreement (Figure 2B) for discrete samples, although in our setup the technique was less accurate for high PO2 values. Due to the underlying technological principle precision of MFPF, PO2 values should increase within this physiologically interesting range (0–140 mmHg), which is supported by the present data. Thus, it seems unlikely that MFPF technology characteristics were responsible for this discrepancy, as intra-class correlation between the MFPF probes was extremely high (R2 = 0.99) in porcine setup and MFPF measurements in the artificial circulatory setup (human blood) showed excellent agreement to CTE, even at high PO2 levels. From our point of view, the porcine results nicely demonstrate the high temperature sensitivity of MFPF measurements and further highlight the importance of correct temperature acquisition. Instead of repeating this series, we decided to develop a new artificial circulatory system for further validation, where we could also investigate the influence of temperature and blood flow by standardised variation of these physiological values.
Under ex vivo conditions in the artificial circulatory setup (human blood), we found excellent correlation (R2 = 0.99) of MFPF and CTE PO2 with measurements equally distributed over the entire normobaric range (Figure 3). In general, MFPF values were slightly lower in comparison to CTE values. These findings were unexpected, as the MFPF technology, unlike polarography, does not consume any oxygen. These results might be explained by the fact that the blood-gas-analysers used allow a potential bias of 5% in calibration drift and it is well known that these are not really linear over the entire range. This underestimation decreases with lowered PO2 values and is therefore of limited clinical importance (Figure 2, 3).
In general and as demonstrated by porcine results, temperature is obviously one major confounder on PO2 measurement [20], [21]. However, the present results of the artificial circulatory setup show that the MFPF technology accurately compensates for alterations in temperature if assessed strictly at the measurement site. This fact highlights the efficiency of multi-temperature compensation performed by MFPF software between 20 and 40°C. In this context, it would be desirable to have sensors available with an integrated temperature thermistor to prevent errors such as temperature probe misplacement. In addition to temperature, blood flow may influence PO2 measurements of an indwelling sensor [22]. The present ex vivo results confirm that MFPF technology correctly measures PO2, even under variations of blood flow within the range of 0.8 to 4.0 L min−1.
One major advantage of MFPF PO2 readings, next to high agreement and reproducibility, is high temporal resolution. We measured the monoexponential time constant for a step change in PO2 at 1.5 s in blood. In contrast, the polagraphic Licox probe showed response times up to several min [5], [23]. Recently, faster PO2 probes, such as the Neurovent PTO probe based on the fluorescent quenching of oxygen, have been presented. Although these probes reported higher response times (30 – 120 s), none of the commercially available technologies come near the temporal resolution of the uncoated ruthenium probe used in the current study [23], [24], [25], [26], [27], [28].
The MFPF system is easy to set up and needs no complex calibration. In this validation study, the FOXY-AL300 probes were individually calibrated to minimise for potential errors, but in less demanding use, a calibration file supplied by the manufacturer for each probe can be used with acceptable accuracy. Furthermore, phase shift is not influenced by the medium the oxygen sensor is placed in, allowing simplified calibration in gas phase. Moreover, phase shift measurement is not influenced by the probe indicator dye concentration, photo bleaching of dye, or excitation source intensity. This results in stable signals with high signal to noise ratio [29].
Unfortunately, we could not obtain readings in a clinical setting in patients, as the indwelling probes available up to now (e.g. the FOXY AL-300) are not licensed for human application. Therefore, we investigated the MFPF technology ex vivo in a self-built artificial circulatory system filled with human blood. Although ruthenium-based dyes are commonly used in biological monitoring applications and are thought to be non-toxic, for future application in humans, the metallic surface of the probe and its tip containing ruthenium need to be coated with silicone or Teflon®. In contrast to a silicone probe coating, Teflon® would allow for more rapid diffusion of oxygen and thereby maintain ultrafast probe response time. The challenge for the sensor engineering community will be to avoid direct contact of the fluorescent dye to blood components. Also, it is necessary to avoid protein absorption and blood clotting to maintain signal stability and accuracy. Therefore, an intensive research effort is still necessary, but if engineering is to advance from laboratory-based research to commercial production levels, the MFPF technique could provide clinicians with an effective tool.
A limitation of the study is that we did not explicitly investigate the measurement stability over time. However, the present findings showed no calibration drift of the probes.
In summary we conclude that ex vivo MFPF-PO2 readings are reproducible, and show excellent correlation and high agreement with the gold standard Clark-type electrode (CTE)-based PO2 analysis. This novel technology adequately compensates for changes in temperature and allows for accurate measurements at various blood flow states. This feasible, accurate and easy to calibrate method has the potential to dynamically follow changes in blood oxygenation under various physiologic and pathophysiologic conditions with a high temporal resolution.
Acknowledgments
O. Aeby and B. Bencivenga for construction of the measurement chamber used in the artificial circulatory system. Department of Cardiovascular Surgery, Swiss Cardiovascular Center, Bern University Hospital, Bern, Switzerland (Director and Chair Prof. Th. Carrel) for collaboration and support for the artificial circulatory setup. B. Röhrig (statistician) for support in data management, reviewing the analysis of the data and proof reading the respective paragraphs of the manuscript.
Funding Statement
The study was supported by research grants of the German Research Council (DFG PAK 415/7-1, Ma 2398-3) and Swiss National Foundation (SNF POIB 117065/1), and by an institutional grant of the Research Department of Anesthesiology and Pain Therapy, Inselspital, Bern, Switzerland. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Clark LC Jr, Wolf R, Granger D, Taylor Z (1953) Continuous recording of blood oxygen tensions by polarography. J Appl Physiol 6: 189–193. [DOI] [PubMed] [Google Scholar]
- 2. Severinghaus JW (2002) The invention and development of blood gas analysis apparatus. Anesthesiology 97: 253–256. [DOI] [PubMed] [Google Scholar]
- 3. Hahn CE (1998) Electrochemical analysis of clinical blood-gases, gases and vapours. Analyst 123: 57R–86R. [DOI] [PubMed] [Google Scholar]
- 4. Kimmich HP, Kreuzer F, Spaan JG, Jank K, de Hemptinne J, et al. (1976) Monitoring of PO2 in human blood. Adv Exp Med Biol 75: 33–40. [DOI] [PubMed] [Google Scholar]
- 5.Hoelper BM, Alessandri B, Heimann A, Behr R, Kempski O (2005) Brain oxygen monitoring: in vitro accuracy, long-term drift and response-time of Licox- and Neurotrend sensors. Acta Neurochir (Wien) 147: 767–774; discussion 774. [DOI] [PubMed]
- 6. Purves MJ (1966) Fluctuations of arterial oxygen tension which have the same period as respiration. Respir Physiol 1: 281–296. [DOI] [PubMed] [Google Scholar]
- 7. Williams EM, Viale JP, Hamilton RM, McPeak H, Sutton L, et al. (2000) Within-breath arterial PO2 oscillations in an experimental model of acute respiratory distress syndrome. Br J Anaesth 85: 456–459. [DOI] [PubMed] [Google Scholar]
- 8. Zaugg M, Lucchinetti E, Zalunardo MP, Zumstein S, Spahn DR, et al. (1998) Substantial changes in arterial blood gases during thoracoscopic surgery can be missed by conventional intermittent laboratory blood gas analyses. Anesth Analg 87: 647–653. [DOI] [PubMed] [Google Scholar]
- 9. Baumgardner JE, Markstaller K, Pfeiffer B, Doebrich M, Otto CM (2002) Effects of respiratory rate, plateau pressure, and positive end-expiratory pressure on PaO2 oscillations after saline lavage. Am J Respir Crit Care Med 166: 1556–1562. [DOI] [PubMed] [Google Scholar]
- 10. Hartmann EK, Boehme S, Bentley A, Duenges B, Klein KU, et al. (2012) Influence of respiratory rate and end-expiratory pressure variation on cyclic alveolar recruitment in an experimental lung injury model. Crit Care 16: R8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ganter M, Zollinger A (2003) Continuous intravascular blood gas monitoring: development, current techniques, and clinical use of a commercial device. Br J Anaesth 91: 397–407. [DOI] [PubMed] [Google Scholar]
- 12. Mahutte CK (1994) Continuous intra-arterial blood gas monitoring. Intensive Care Med 20: 85–86. [DOI] [PubMed] [Google Scholar]
- 13. Klein KU, Boehme S, Hartmann EK, Szczyrba M, David M, et al. (2011) A novel technique for monitoring of fast variations in brain oxygen tension using an uncoated fluorescence quenching probe (Foxy AL-300). J Neurosurg Anesthesiol 23: 341–346. [DOI] [PubMed] [Google Scholar]
- 14. Herweling A, Karmrodt J, Stepniak A, Fein A, Baumgardner JE, et al. (2005) A novel technique to follow fast PaO2 variations during experimental CPR. Resuscitation 65: 71–78. [DOI] [PubMed] [Google Scholar]
- 15. Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG (2010) Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol 8: e1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Urbaniak GC, Plous S (2010) Research Randomizer (Version 3) [Computersoftware]. Research Randomizer website. Available: http://randomizer.org. Accessed 2013 Mar 4.
- 17. Bland JM, Altman DG (1999) Measuring agreement in method comparison studies. Stat Methods Med Res 8: 135–160. [DOI] [PubMed] [Google Scholar]
- 18. Burkhardt H, Weiss C (2008) Evaluating influencing factors in estimation of renal function by extending the Bland-Altman approach. Scand J Clin Lab Invest 68: 171–176. [DOI] [PubMed] [Google Scholar]
- 19. Bland JM, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1: 307–310. [PubMed] [Google Scholar]
- 20. Picandet V, Jeanneret S, Lavoie JP (2007) Effects of syringe type and storage temperature on results of blood gas analysis in arterial blood of horses. J Vet Intern Med 21: 476–481. [DOI] [PubMed] [Google Scholar]
- 21. Madiedo G, Sciacca R, Hause L (1980) Air bubbles and temperature effect on blood gas analysis. J Clin Pathol 33: 864–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Grundmann A, Lubbers DW (1992) Can the flow dependency of the polarographic PO2 electrode be used to measure arterial PO2 and local capillary flow transcutaneously? Adv Exp Med Biol 317: 213–219. [DOI] [PubMed] [Google Scholar]
- 23.Haitsma I, Rosenthal G, Morabito D, Rollins M, Maas AI, et al.. (2008) In vitro comparison of two generations of Licox and Neurotrend catheters. Acta Neurochir Suppl 102: 197–202. [DOI] [PubMed]
- 24. Citerio G, Piper I, Cormio M, Galli D, Cazzaniga S, et al. (2004) Bench test assessment of the new Raumedic Neurovent-P ICP sensor: a technical report by the BrainIT group. Acta Neurochir (Wien) 146: 1221–1226. [DOI] [PubMed] [Google Scholar]
- 25. Huschak G, Hoell T, Hohaus C, Kern C, Minkus Y, et al. (2009) Clinical evaluation of a new multi-parameter neuromonitoring device: measurement of brain tissue oxygen, brain temperature, and intracranial pressure. J Neurosurg Anesthesiol 21: 155–160. [DOI] [PubMed] [Google Scholar]
- 26.Jaeger M, Soehle M, Meixensberger J (2005) Brain tissue oxygen (PtiO2): a clinical comparison of two monitoring devices. Acta Neurochir Suppl 95: 79–81. [DOI] [PubMed]
- 27.Orakcioglu B, Sakowitz OW, Neumann JO, Kentar MM, Unterberg A, et al.. (2011) Evaluation of a novel brain tissue oxygenation probe in an experimental swine model. Neurosurgery 67: 1716–1722; discussion 1722–1713. [DOI] [PubMed]
- 28.Stewart C, Haitsma I, Zador Z, Hemphill JC, 3rd, Morabito D, et al. (2008) The new Licox combined brain tissue oxygen and brain temperature monitor: assessment of in vitro accuracy and clinical experience in severe traumatic brain injury. Neurosurgery 63: 1159–1164; discussion 1164–1155. [DOI] [PubMed]
- 29.Lakowicz JR (2008) Principles of Fluorescence Spectroscopy, Third Edition. J Biomed Opt 13.