Abstract
Background
As information is disseminated about best practices, variations in patterns of care should diminish over time.
Objective
To test the hypotheses that differences in rates of a surgical procedure are associated with type of insurance in an era of evolving practice guidelines and that insurance and site differences diminish with time as consensus guidelines disseminate among the medical community.
Methods
We use lymph node dissection among women with ductal carcinoma in situ (DCIS) as an example of a procedure with uncertain benefit. Using a sample of 1051 women diagnosed from 1985 through 2000 at two geographic sites, we collected detailed demographic, clinical, pathologic, and treatment information through abstraction of multiple medical records. We specified multivariate logistic models with flexible functions of time and time interactions with insurance and treatment site to test hypotheses.
Results
Lymph node dissection rates varied significantly according to site of treatment and insurance status after controlling for clinical, pathological, treatment, and demographic characteristics. Rates of lymph node dissection decreased over time, and differences in lymph node dissection rates according to site and generosity of insurance were no longer significant by the end of the study period.
Conclusions
We have demonstrated that rates of a discretionary surgical procedure differ according to non-clinical factors, such as treatment site and type of insurance, and that such unwarranted variation decreases over time with diminishing uncertainty and in an era of diffusion of clinical guidelines.
Keywords: diffusion, insurance, variation, ductal carcinoma in situ
Introduction
Explanations for variation in surgical procedures include medical uncertainty about the merit of a particular treatment,1–3 inappropriate use in regions with high rates of procedures (supplier-induced demand),1 random variation,2 enthusiasm for particular services,4 and defensive medicine.2, 5 Diffusion theory suggests that uncertainty regarding the merits of a treatment leads to variation in the use of that treatment.2, 6 As more knowledge is generated and disseminated about the value and negative consequences of a treatment, practice variations diminish,7 and practice patterns may ultimately converge. In addition, clinical uncertainty about the value of a treatment provides a window of opportunity for economic incentives, particularly reimbursement, to drive physician behavior.5 Even without clinical uncertainty, if treatment strategies have equivalent survival, the choice of treatment may be particularly sensitive to relative payments.8, 9
The purpose of this study was to investigate the impact of evolving practice guidelines on surgical management of the axilla in women with ductal carcinoma in situ (DCIS) and to investigate the role of physician financial incentives in practice patterns. Beginning in 1987, observational studies and expert opinion10 followed by textbooks,11 and then published guidelines12 advised against lymph node dissection in women with DCIS based on lack of benefit.
We hypothesized that lymph node dissection rates have been affected by the generosity of insurance during an era of guideline evolution and dissemination. If financial incentives play a role in use of a medical treatment, higher rates of the treatment would be expected among patients for whom the reimbursements are more favorable. We further hypothesized that differences in rates of lymph node dissection among women with different types of insurance would diminish with time as consensus about the value of a procedure was reached and disseminated among the medical community.
Methods
We conducted a retrospective study to examine patterns of care and outcomes in women with DCIS diagnosed between 1985 and 2000. The institutional review boards at the University of Rochester, the Henry Ford Health System, and the RAND Corporation approved the study.
Conceptual Model
Our conceptual model starts with the Behavioral Model of Health Services Use developed by Andersen and Aday,13, 14 which incorporates patient, physician, and health system factors into an understanding of health care utilization. We thereby account for factors associated with the clinical need for lymph node dissection and other factors that facilitate or impede access to care (referred to as enabling and predisposing factors in the typology of Andersen).
The clinical need for lymph node assessment in women with DCIS is restricted to women with a high likelihood of invasive disease.10, 15, 16 Factors associated with a higher likelihood of invasive disease include a palpable mass,17 extensive disease on mammogram,15 multifocal disease,18 younger age,15 comedo histology,18, 19 and high histologic grade.15 In addition, in patients with a high likelihood of invasive disease who are undergoing mastectomy and autogenous reconstruction, lymph node assessment is particularly appropropriate because lymph node dissection following autogenous breast reconstruction may compromise the blood supply to the reconstructed breast. The above disease, patient, and surgical treatment factors were thus included in our model as need characteristics.
Factors that can facilitate or impede access to care, such as race, income, insurance status, comorbidity, and site of care, have been shown to be associated with receipt of lymph node dissection in women with invasive disease20–25 (in whom lymph node assessment is required for staging and selection of locoregional and systemic treatment). We thus include these enabling and predisposing factors in our model because they may similarly play a role in receipt of lymph node dissection in women with DCIS.
Finally, we expand this framework to include our key hypothesis that diminishing uncertainty over time is associated with diminishing differences in practice by including year of treatment and year of treatment interacted with type of insurance and treatment site in the analytic model.
Study Sample
Study subjects were women diagnosed with DCIS between 1985 and 2000 within two tumor registries – the population-based Monroe County (New York) tumor registry and the tumor registry of the Henry Ford Health System. Exclusion criteria included invasive breast cancer (including microinvasive disease) or a history of a previous breast cancer. The final analytic sample contained 1051 patients.
Monroe County Sample
The Monroe County (New York) Tumor Registry was used to identify women diagnosed with DCIS during our study time frame who received care from 24 surgeons who had 10 or more patients in the Registry. The Registry identified 934 observations. Three observations were duplicate subjects, leaving 931 unique subjects. We were unable to locate charts for 92 subjects. In chart review we confirmed that 153 of the 839 subjects (18.2%) were not eligible because of a previous cancer history or concomitant invasive disease (that is, the patient did not have pure DCIS), leaving 686 subjects. We dropped an additional 21 subjects because of incomplete data, leaving 665 subjects with complete chart abstractions. In analyses following chart abstraction, we identified another 27 women (4.1% of women with complete data) who were not eligible because they had Paget disease or lobular carcinoma in situ, leaving 638 in our final analytic sample. Assuming a similar rate of ineligibility among the subjects not located, we estimate that there were 730 eligible women in our sampling frame. Our final analytic sample consists of 87.4% of the women we estimated to be eligible for inclusion in the study.
Henry Ford Health System Sample
The Henry Ford Health System electronic registry was used to identify women diagnosed with DCIS during our study time period. The registry identified 750 observations, 435 of whom were eligible for inclusion. We were able to local complete data for 421 (96.7%) patients. In analyses following chart abstraction, we dropped an additional 8 patients who were found to be ineligible (because of Paget disease or only lobular carcinoma in situ). As above, we assume a similar ineligibility rate among the women with incomplete records, resulting in a final analytic sample 96.8% of the eligible subjects.
Data Collection
The primary data collection instrument was completed by trained medical record abstractors who obtained data from an exhaustive review of surgical records, hospital records, radiation oncology records, medical oncology records, pathology reports, operative reports, and, when necessary, primary care and gynecology records. The data collection instrument included date of diagnosis, socioeconomic and demographic characteristics (date of birth, self-assigned race and ethnicity, and address for census block group assignment), disease and treatment characteristics (grade, size, width of the surgical margins, presence of a palpable mass at diagnosis, means of detection, mammographic extent of disease, type of surgery, number and type of surgical procedures, and type of lymph node assessment, if any), comorbid conditions included the Charlson comorbidity index,26 menopausal status, and treating physician. Census level socioeconomic status was measured as percentage of people living below poverty and percentage of adults with at least a high school diploma at the census block group level from the 2000 Census. Operative and pathology reports were used to confirm that axillary lymph nodes were not removed unintentionally in patients having mastectomy. Continuous quality checks of the data were performed by a medical oncologist, surgical oncologist, and breast pathologist.
Statistical Analysis
We began by generating univariate and bivariate descriptive statistics. The bivariate analyses assess the relationship between a dichotomous indicator for lymph node dissection and each of the independent measures, including socioeconomic and demographic measures (year of diagnosis, age at diagnosis, race, insurance status), comorbidity, surgical treatment (breast conserving surgery or mastectomy and type of reconstruction), and disease characteristics (mammographic size, histologic subtype, and nuclear grade). We report Pearson’s chi-squared tests of independence for categorical variables. We also report Person’s chi-squared tests of independence for each dichotomous covariate defined for the multivariate model.
We estimated multivariate logistic regression models to describe the relationship between the independent measures and lymph node dissection. Following the discussion above, we included groups of independent variables to control for the characteristics thought to be related to clinical need (receipt of mastectomy, receipt of autogenous reconstruction, extensive or multifocal disease, age, presence of a palpable mass, comedo histology, and high grade histology), predisposing/enabling factors (race, income, insurance, site of care, and comorbidity), and characteristics related to our key hypotheses (calendar time and the interaction of calendar time with insurance and site of care). Medicare plus supplemental private insurance was assumed to be the most generous insurance and Medicaid the least. We included a site indicator and interactions of site and surgical treatment to identify differences in treatment patterns (rates of lymph node dissection) across our two sites by surgical treatment. Because our key hypotheses are related to patterns of lymph node dissection use over time, we specified flexible functions of time and interactions with time. We formulated a standard logistic regression model of the form
where lymph node dissection is indicated as LND, X is a vector of covariates described above, Z includes insurance status and site indicators, t denotes calendar year, and the function ϑ(.) flexibly models time dependence. We specify ϑ(.) as overlapping polynomials, first introduced in the health literature by Garber and MaCurdy in 1993.27 Unlike typical polynomial splines, overlapping polynomials allow functions in different intervals to overlap, turning on or off smoothly. We specified ϑ(.) as
where j indexes the elements of Z, Φ(.) is a standard normal cumulative distribution function, and the α’s are estimated coefficients. The location and smoothness of the overlapping polynomials are determined by μ (a mean shift) and σ (a standard deviation), respectively (see Appendix for illustrations). As σ approaches 0, the overlapping polynomial collapses to an indicator variable that turns on or off at the year given by μ. As σ increases, the overlapping polynomial turns on or off smoothly over time. For example, if we set μ = 1995 and σ = 2, Φ((t−1995)/2) will be approximately zero for observations with calendar year < 1990, it will gradually increase in value reaching 0.50 at 1995 and approximately 1 by 2000. In principle, we could estimate the values of the overlapping smooth-spline parameters (μ, σ). Here, however, we performed a grid search over pairs of μ, σ to maximize model fit. The resulting model estimates allow for insurance status and site to have smooth interactions with time, as determined by the data. The overlapping polynomials illustrated in the Appendix, as described above, show the characteristics of the functions with the optimal parameter values from our estimation.
Appendix.
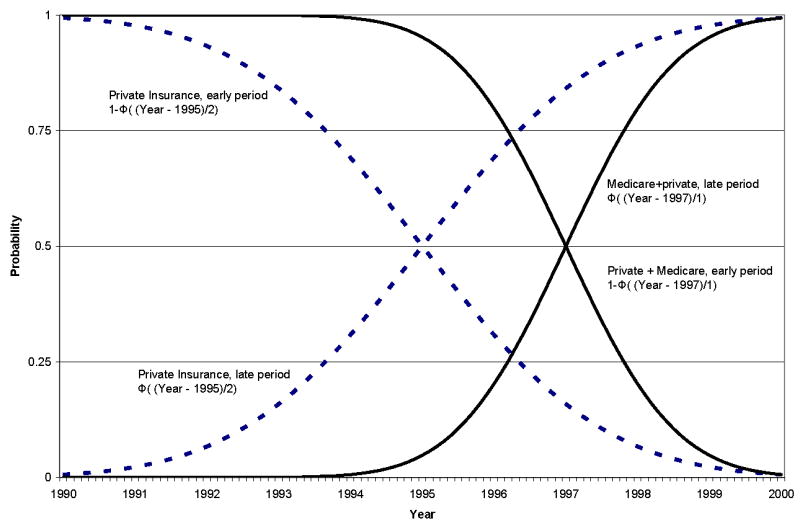
Examples of overlapping polynomials
Our socio-economic measures are based on census tract data. These include various measures of the distribution of race, language spoken at home, travel time to work, education, and income within census tract. Because these measures are highly correlated, we present specifications that include percent below poverty, and percent black in the census tract. In addition to these census tract-level variables, we also have individual-level data on race. By including both the census-based race measure and the individual-level race measure, we can identify the contribution of both neighborhood race effects and individual race effects.
We present results for a parsimonious model in which we drop covariates, one at a time, until all included measures have p-values < 0.20. We do not, however, drop any of the measures that are related to our key hypotheses (treatment measures and insurance status measures). Results from this parsimonious model were not substantively different from results from a model that included all covariates.
After exploratory analyses for best functional form for calendar time, our final models specify calendar time as a second order polynomial (linear and quadratic terms). All analyses are performed using Stata version 9.0 (StataCorp LP, College Station, TX).
Results
Table 1 presents descriptive statistics for the full sample (N = 1051) and the sample for which we were able to obtain census data (N = 1000). Census characteristics are presented in Table 2. The characteristics of the two samples are not substantively different. Most patients were healthy with few comorbid conditions. Approximately 40% of patients had a mastectomy, and 44% of these had reconstruction. The most common histologic subtype was cribriform (36%) followed by comedo (31%). Multifocal disease was present in 34% of the patients.
Table 1.
Sample Characteristics, Individual-Level Measures
| Percent with Characteristic | Census Sample | Census Sample | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Full Sample | Census Sample | With LND | Without LND | ||||||
| N | % | N | % | N | % | N | % | p | |
| Total | 1051 | 1000 | 349 | 34.9 | 651 | 65.1 | |||
|
| |||||||||
| Age (years) | < 0.001 | ||||||||
| Less than 40 | 54 | 5.1 | 49 | 4.9 | 27 | 7.7 | 22 | 3.4 | |
| 40 to 54 | 241 | 22.9 | 230 | 23.0 | 92 | 26.4 | 138 | 21.2 | |
| 55 to 64 | 376 | 35.8 | 357 | 35.7 | 121 | 34.7 | 236 | 36.3 | |
| 65+ | 380 | 36.2 | 364 | 36.4 | 109 | 31.2 | 255 | 39.2 | |
|
| |||||||||
| Calendar Year of Diagnosis | < 0.001 | ||||||||
| Before 1987 | 35 | 3.3 | 34 | 3.4 | 29 | 8.3 | 5 | 0.8 | |
| 1998–1991 | 107 | 10.2 | 104 | 10.4 | 63 | 18.1 | 41 | 6.3 | |
| 1992–1993 | 124 | 11.8 | 117 | 11.7 | 51 | 14.6 | 66 | 10.1 | |
| 1994–1995 | 162 | 15.4 | 152 | 15.2 | 43 | 12.3 | 109 | 16.7 | |
| 1996–1998 | 451 | 42.9 | 426 | 42.6 | 121 | 34.7 | 305 | 46.9 | |
| 1999–2000 | 172 | 16.4 | 167 | 16.7 | 42 | 12.0 | 125 | 19.2 | |
|
| |||||||||
| Insurance | < 0.001 | ||||||||
| Medicare | 324 | 30.8 | 307 | 30.7 | 90 | 25.8 | 217 | 33.3 | |
| Private | 537 | 51.1 | 508 | 50.8 | 194 | 55.6 | 314 | 48.2 | |
| Medicare + Private | 106 | 10.1 | 102 | 10.2 | 37 | 10.6 | 65 | 10.0 | |
| Medicaid/uninsured/other | 18 | 1.7 | 18 | 1.8 | 5 | 1.4 | 13 | 2.0 | |
| Unknown | 66 | 6.3 | 65 | 6.5 | 23 | 6.6 | 42 | 6.5 | |
|
| |||||||||
| Comorbidity† | |||||||||
| Osteoarthritis | 191 | 18.2 | 178 | 17.8 | 59 | 16.9 | 119 | 18.3 | 0.59 |
| Asthma | 67 | 6.4 | 65 | 6.5 | 22 | 6.3 | 43 | 6.6 | 0.85 |
| Depression | 76 | 7.2 | 71 | 7.1 | 20 | 5.7 | 51 | 7.8 | 0.22 |
| Diabetes with insulin | 49 | 4.7 | 49 | 4.9 | 13 | 3.7 | 36 | 5.5 | 0.21 |
| Diabetes no insulin | 24 | 2.3 | 23 | 2.3 | 6 | 1.7 | 17 | 2.6 | 0.37 |
| Congestive heart failure | 29 | 2.8 | 29 | 2.9 | 6 | 1.7 | 23 | 3.5 | 0.10 |
| Hypertension | 380 | 36.2 | 362 | 36.2 | 116 | 33.2 | 246 | 37.8 | 0.15 |
| Smoking | 171 | 16.3 | 162 | 16.2 | 49 | 14.0 | 113 | 17.4 | 0.17 |
| Thyroid | 121 | 11.5 | 119 | 11.9 | 39 | 11.2 | 80 | 12.3 | 0.6 |
|
| |||||||||
| Race | < 0.001 | ||||||||
| White | 835 | 79.4 | 794 | 79.4 | 283 | 81.1 | 511 | 78.5 | |
| Black | 158 | 15.0 | 151 | 15.1 | 47 | 13.5 | 104 | 16.0 | |
| Asian | 10 | 1.0 | 9 | 0.9 | 5 | 1.4 | 4 | 0.6 | |
| Unknown | 47 | 4.5 | 46 | 4.6 | 14 | 4.0 | 32 | 4.9 | |
|
| |||||||||
| Histologic Subtype | |||||||||
| Comedo | 333 | 31.7 | 314 | 31.4 | 138 | 39.5 | 176 | 27.0 | < 0.001 |
| Cribiform | 382 | 36.3 | 364 | 36.4 | 105 | 30.1 | 259 | 39.8 | < 0.001 |
| Apocrine | 10 | 1.0 | 9 | 0.9 | 4 | 1.1 | 5 | 0.8 | 0.55 |
| Other | 23 | 2.2 | 22 | 2.2 | 12 | 3.4 | 10 | 1.5 | 0.05 |
| Unknown | 50 | 4.8 | 50 | 5.0 | 10 | 2.9 | 40 | 6.1 | 0.02 |
| Papillary | 44 | 4.2 | 43 | 4.3 | 9 | 2.6 | 34 | 5.2 | 0.05 |
| Micropapillary | 74 | 7.0 | 70 | 7.0 | 17 | 4.9 | 53 | 8.1 | 0.05 |
|
| |||||||||
| Nuclear Grade | < 0.001 | ||||||||
| Low | 139 | 13.2 | 131 | 13.1 | 19 | 5.4 | 112 | 17.2 | |
| Intermediate | 199 | 18.9 | 196 | 19.6 | 47 | 13.5 | 149 | 22.9 | |
| High | 436 | 41.5 | 409 | 40.9 | 162 | 46.4 | 247 | 37.9 | |
| Unknown | 277 | 26.4 | 264 | 26.4 | 121 | 34.7 | 143 | 22.0 | |
|
| |||||||||
| Multifocal | 358 | 34.1 | 342 | 34.2 | 135 | 38.7 | 207 | 31.8 | 0.03 |
|
| |||||||||
| Surgical Treatment | < 0.001 | ||||||||
| Mastectomy | 414 | 39.4 | 395 | 39.5 | 306 | 87.7 | 89 | 13.7 | |
| Reconstruction | < 0.001 | ||||||||
| Implant | 65 | 6.2 | 62 | 6.2 | 42 | 13.7 | 20 | 22.5 | < 0.001 |
| Autogenous | 97 | 9.2 | 95 | 9.5 | 77 | 22.1 | 18 | 2.8 | < 0.001 |
| None | 233 | 22.2 | 220 | 22.0 | 182 | 59.5 | 38 | 42.7 | < 0.001 |
| Unknown | 7 | 0.7 | 6 | 0.6 | 5 | 1.6 | 1 | 1.1 | 0.01 |
| Breast Conserving Surgery | 637 | 60.6 | 605 | 60.5 | 43 | 12.3 | 562 | 86.3 | |
|
| |||||||||
| Site Dummy | 638 | 60.7 | 604 | 60.4 | 246 | 70.5 | 358 | 55.0 | < 0.001 |
Some patients have more than one comorbid condition
Table 2.
Summary Statistics, Census-Level Measures
| Total Sample | No LND | LND | |||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | p value | |
| Percent black | 17.1% | 30.7% | 18.7% | 32.5% | 14.0% | 27.0% | 0.02 |
| % Not speaking English | 1.7% | 3.1% | 1.7% | 2.9% | 1.9% | 3.5% | 0.38 |
| Education | |||||||
| Percent with less than a HS education | 15.1% | 11.8% | 15.1% | 11.5% | 15.1% | 12.3% | 0.96 |
| Percent with at least a HS diploma | 28.0% | 10.6% | 27.9% | 11.1% | 28.3% | 9.8% | 0.60 |
| Median household income | $53,035 | $24,570 | $52,794 | $25,017 | $53,485 | $23,745 | 0.67 |
| Percent below poverty | 9.6% | 11.2% | 10.0% | 11.3% | 8.9% | 11.0% | 0.14 |
| Percent home owners | 75.4% | 24.1% | 74.5% | 24.6% | 76.9% | 23.1% | 0.13 |
HS = high school
Lymph node dissection was performed in 375 patients (35% of the full sample). Of these, 43 (12%) were sentinel node dissections. Patients who had a mastectomy were more likely to have lymph node assessment performed than were those treated with breast conserving surgery (78% versus 8%, p < 0.001) regardless of whether or not the patient had breast reconstruction. Women who were younger at diagnosis were also more likely to have a lymph node dissection (54% of women under 40 years compared to 30% of women over age 65 years, p = 0.002). Other factors associated with higher likelihood of lymph node assessment were diagnosis earlier in the study period (p < 0.001), higher nuclear grade (p < 0.001), multifocal disease (p = 0.03), and greater mammographic extent of disease (p < 0.001). Race, presence of calcifications (not shown), and comorbidity were not associated with lymph node dissection in bivariate analysis. Woman who had lymph node dissection were more likely to have a mastectomy with autogenous reconstruction in the later years and were more likely to have private insurance in the later years (results not shown).
Table 3 presents results from the multivariate model. We present estimates from the census sample (N = 1000) because of the similarity in the results with those from the full sample (N = 1051). We also found our results to be robust to different specifications in calendar time, census-based SES measures, and the inclusion of insurance by time interactions. In addition to the parameter estimates shown in Table 3, our models included controls for comorbid conditions and disease characteristics (including all measures shown in Table 1), findings of which were substantively consistent with the literature. The model fit well. Calibration (HL = 12.250, p = 0.834) and discrimination (C = 0.942 for both models) were excellent, and the pseudo R-squared statistic was high at 0.555.
Table 3.
Multivariate analyses
| Census Sample (N = 1000) | |||
|---|---|---|---|
|
| |||
| Dependent Variable = lymph node dissection | OR | SE | p |
| Surgical Treatment | |||
| Breast Conserving Surgery | 0.02 | 0.01 | 0.000 |
| Mastectomy/Reconstruction | |||
| Referent | 1.00 | ||
| Implant | 0.58 | 0.21 | 0.13 |
| Autogenous | 3.71 | 2.02 | 0.02 |
| Unknown | 3.67 | 3.85 | 0.22 |
|
| |||
| Census Tract | |||
| Percent below poverty | 0.98 | 0.009 | 0.04 |
|
| |||
| Site * Treatment Interactions | |||
| BCS | 0.20 | 0.12 | 0.007 |
| Mastectomy with autogenous reconstuction | 0.12 | 0.08 | 0.003 |
|
| |||
| Calendar Time | |||
| Year | 0.44 | 0.08 | 0.000 |
| Year^2 | 1.04 | 0.01 | 0.000 |
|
| |||
| Site | |||
| Site * Year < 1995 (early period) | 3.57 | 2.05 | 0.026 |
| Site * Year > 1994 (late period) | 1.19 | 0.45 | 0.64 |
|
| |||
| Insurance Coverage (Referent = Medicare) | |||
| Private*Φ(1− ((t−1995)/2) (early period) | 3.36 | 1.36 | 0.003 |
| Private*Φ((t−1995)/2) (late period) | 1.27 | 0.51 | 0.55 |
| (Private+Medicare)*(1−Φ((t−1997)/1.2) (early period) | 4.25 | 2.43 | 0.01 |
| (Private+Medicare)* Φ((t−1997)/1.2) (late period) | 0.63 | 0.39 | 0.45 |
| Medicaid/uninsured/other | 0.90 | 0.76 | 0.90 |
| Unknown | 1.18 | 0.59 | 0.75 |
|
| |||
| Goodness of Fit Statistics | |||
| Wald test of Significance of Model | 311.480 | 0.000 | |
| Pseudo R2 | 0.555 | ||
| C-Stat | 0.942 | ||
| Hosmer-Lemeshow, 20 groups, chi2(18) | 12.250 | 0.834 | |
Surgical Treatment
Surgical treatment was strongly predictive of lymph node dissection. Women who had breast conserving surgery were far less likely to undergo lymph node dissection than woman who had mastectomy and no reconstruction (OR = 0.018, p < 0.001). Women who had mastectomy with autogenous reconstruction (OR = 3.711, p = 0.016) were far more likely to have lymph node dissection than women who had mastectomy without reconstruction. Mastectomy with unknown reconstruction was strongly associated with higher rates of lymph node dissection, but these estimates were imprecise because of the small sample size (N = 8 women who had unknown reconstruction in the estimation sample). The results were unchanged when we re-estimated the model dropping these 8 observations. Specifications in which we interacted surgical treatment and calendar time revealed no evidence that the relative differences by treatment varied over the study period. Very few cases of mastectomy with autogenous reconstruction were done before 1994, however, limiting the interpretability of these results.
Socioeconomic Factors and Demographics
Neither age nor race was found to be a statistically significant or substantively important predictor of lymph node dissection. We estimated many specifications using a variety of census-based SES measures, with and without census-based race measures and with and without individual-level race measures. In none of these specifications was race a statistically significant predictor, and no other covariate values were substantively affected. Census-based income, however, as measured by Percent Below Poverty, predicted lower rates of lymph node dissection (OR = 0.98, p = 0.04). Exclusion of income did not change the estimates of either the individual-level or census level race variables. The addition of other income and wealth related census-tract variables (such as home ownership and educational attainment) generated similar patterns of results to percent below poverty but were imprecisely estimated because of the high correlation among these variables.
Calendar Time
One of our key hypotheses was that rates of lymph node dissection would decrease over time as best practice information diffused. Our results strongly confirm this. Our specification of a second order polynomial in calendar year (Year and Year squared) uncovered a strong and statistically significant downward trend in lymph node dissection during the study period (as evident in Figures 1 and 2).
Figure 1.
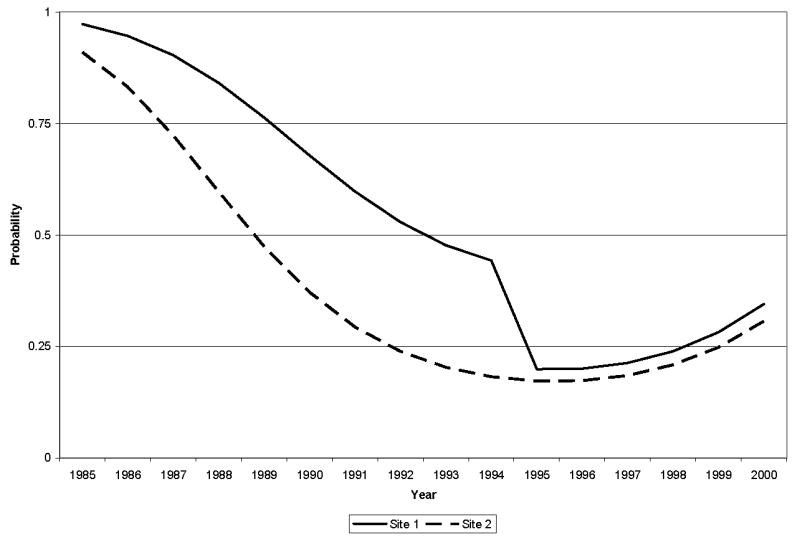
Estimated lymph node dissection rates by site over time, 1985 – 2000.
Figure 2.
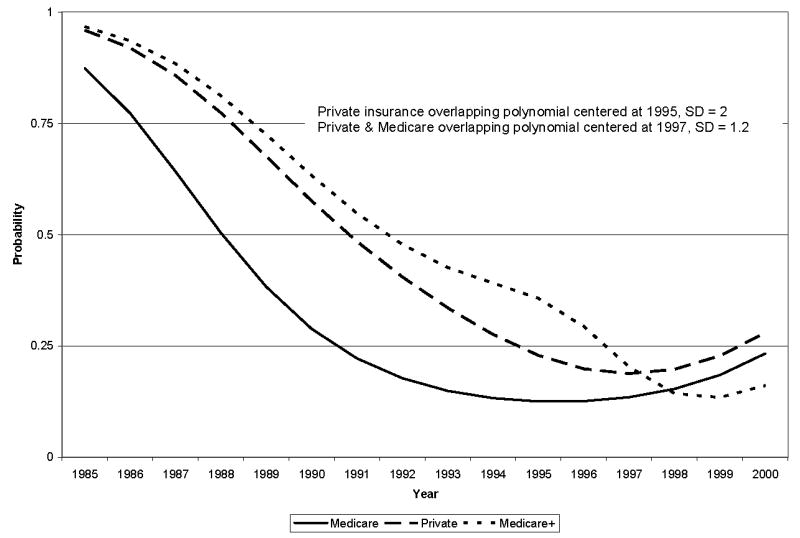
Estimated lymph node dissection rates by insurance type over time, 1985 – 2000.
Calendar Time and Site Interactions
A second key hypothesis was that lymph node dissection rates at different sites would converge over time as best practice information diffused. Again, our results strongly support this finding. Although we found large and statistically significant differences in the rates of lymph node dissection between sites early in the study period (OR = 3.574, p = 0.026), the differences converged to nearly zero in the late 1990’s (OR = 1.192, p = 0.642). Figure 1 presents the results graphically and shows that the rates of lymph node dissection decrease rapidly with time, reaching a low in the late 1990’s and that the differences between sites converged dramatically around 1995. Different specifications of time interactions by site generated substantively similar conclusions, with different rates of decrease by site causing a divergence in lymph node dissection rates that subsequently diminished.
Calendar Time and Insurance Status Interactions
A third key hypothesis was that differences in lymph node dissection rates would be driven by the generosity of insurance but that differences in rates of lymph node dissection among women with different types of insurance would diminish with time. Again, we found evidence that supports the hypothesis. We found no evidence that women with no insurance or Medicaid insurance had rates of lymph node dissection than differed from women with Medicare only (our referent group). More generous insurance, however, was strongly associated with increased rates of lymph node dissection early in the study period. In particular, women who had private insurance (OR = 3.363, p = 0.003) or Medicare with supplemental private insurance (OR = 4.246, p = 0.011) had higher rates of lymph node dissection than women with Medicare only early in the study period. We found that these very large differences began to diminish in the mid 1990’s, and that there were only small and not statistically significant differences across these insurance types by the late 1990’s (OR = 1.272, p = 0.552 and OR = 0.625, p = 0.449 for private and Medicare with supplemental insurance, respectively). Estimates of our overlapping polynomial parameters suggest that the convergence between Medicare (the referent group) and Medicare plus private insurance was centered in 1997 and occurred largely over a 4-year period from 1995 through 1999 (σ = 1.2). Our estimates indicate that the convergence between Medicare and private insurance was centered in 1995 and occurred over a slightly longer period from roughly 1992 through 1998 (σ = 2). The substantive consequence of the overlapping polynomial estimates is illustrated in Figure 2, which presents our estimates graphically. The Figure shows the strong downward trend in lymph node dissection for each of the insurance types during the study period. It also shows the large differences in rates of lymph node dissection by insurance in the late 1980’s and early 1990’s and the paths of convergence of these rates in the late 1990’s. By 1998, the remaining differences in lymph node dissection rates by insurance status were neither large nor statistically significant.
Discussion
In this large sample of women diagnosed with DCIS between 1985 and 2000, lymph node dissection rates varied significantly according to site of treatment and type of insurance after controlling for clinical, pathological, treatment, and demographic characteristics. The decrease in lymph node dissection in women with DCIS in our sample is consistent with that seen in larger samples of patients in Surveillance, Epidemiology, and End Results registries.28, 29 As we had hypothesized, the passage of time was associated with a decrease in the site and insurance variation in lymph node dissection rates, variation that was no longer significant by the end of the study period. This finding may reflect the diffusion of information about best practices facilitated by published practice guidelines12 and expert consensus.30 In addition, provider experience or patient knowledge may drive the divergent treatment rates together over time although it is unlikely that patients have much to do with the changing rates unless their information is coming from an available source that aggregates the information (such as guidelines).
The higher likelihood of lymph node dissection among women with Medicare plus private insurance compared to those with Medicare alone supports our hypothesis that generosity of insurance plays a role in surgeons’ treatment decisions. Although we do not actually know the size of the financial incentives because we do not know the differences in reimbursements and copayments, those with Medicare and supplemental private insurance have more comprehensive coverage than those with Medicare only. Private insurance (under age 65 with no Medicare) is heterogenous, being very generous for some and leaving others with very poor protection. This results in different expected reimbursement for physicians and different out-of-pocket costs for patients. It is also possible that patient demand for lymph node dissection is greater when insurance blunts the cost constraints to patients. While surgeons may be more inclined to perform procedures with higher fees (all things being equal), the choice of procedure is likely to be influenced by how much a patient is willing to pay.
Our findings are consistent with previous research. For example, in a study of 1,787 women with breast cancer, decreasing Medicare mastectomy fees were associated with increasing use of breast conserving surgery.8 Further support for the role of payment incentives in surgical treatment of breast cancer comes from a study demonstrating that area Medicare fees were the strongest predictors of “propensity” (surgical treatment behavior) in scenarios presented to breast surgeons.31
In general, greater generosity of insurance, such as might be the case in patients with Medicare plus supplemental insurance compared with Medicare alone, is considered a favorable condition, increasing access to health care and, in one recent study, access to clinical trials among Medicare patients.32 Little attention has been given to the role of type of insurance and overtreatment. Over 20 years ago, Ware and colleagues advised against assuming that the relationship between generosity of health insurance and health status is always positive: “More generous health insurance may be a two-edged sword.”33 Our findings support this concern. While one would not argue that receipt of lymph node dissection negatively affects survival, unnecessary lymph node surgery adversely affects quality of life34, 35 and contributes to the cost of medical care.36–38
In conclusion, we have demonstrated that rates of a discretionary surgical procedure differ according to non-clinical factors, such as treatment site and type of insurance, and that such unwarranted differences decrease over time with diminishing uncertainty and, perhaps, through diffusion of clinical guidelines. If similar analyses were to be repeated in contemporary patient samples for receipt of sentinel node dissection, a procedure considered less invasive because of removal of fewer lymph nodes, our findings of site and insurance variations may very well be seen. The lack of consensus regarding the need for sentinel node dissection in DCIS19, 39–43 and non-clinical variation in receipt of sentinel node dissection in women with invasive breast cancer44, 45 would be expected to lead to a recapitulation of similar variation that may in time diminish or resolve.
Acknowledgments
Research support: NIH/NCI R01 CA922444-01A1
Contributor Information
Jennifer J. Griggs, Department of Medicine, Hematology/Oncology, University of Michigan, 300 North Ingalls, 3A22, Ann Arbor, MI 48109, Phone: (734) 647-9912, Fax: (734) 763-7672, Email: jengrigg@umich.edu.
Melony E.S. Sorbero, Health Policy Researcher, RAND Corporation, 4570 Fifth Avenue, Suite 600, Pittsburgh, PA 15213, Phone: (412) 683-2300 ext. 4628, Fax: (412) 802-4959, Email: msorbero@rand.org.
Gretchen M. Ahrendt, Associate Professor of Surgery, Magee Womens Hospital of UPMC, 300 Halket Street Suite 2601, Pittsburgh, PA 15213, Phone: (412) 641-1447, Fax: (412) 641-1446, Email: gahrendt@magee.edu.
Azadeh Stark, Senior Staff Investigator, Associate Professor, University of Pennsylvania, School of Medicine, Wayne State School of Medicine, 313-916-2341 (voice), 313-916-8309 (fax), astark1@hfhs.org
Susanne Heininger, Clinical Research Center, #G-5035, University of Rochester Medical Center, 601 Elmwood Avenue, Box MED/CRC, Rochester, New York 14642, Phone: 585-273-1926, Fax: 585-273-1195, Email: susanne_heininger@urmc.rochester.edu.
Heather Taffet Gold, Assistant Professor, Department of Public Health, Weill Medical College of Cornell University, 411 E. 69th Street New York, New York 10021, Phone: (212) 746-1245, Fax: (212) 746-8544, Email: heg2001@med.cornell.edu.
Linda M. Schiffhauer, Assistant Professor, Pathology and Laboratory Medicine, University of Rochester Medical Center, 601 Elmwood Avenue Box 626, Rochester, NY 14642, Phone: (585) 275-3270, Fax: (585) 273-3637, Email: Linda_Schiffhauer@URMC.Rochester.edu
Andrew W. Dick, Senior Economist, RAND Corporation, 4570 Fifth Avenue, Suite 600, Pittsburgh, PA 15213, Phone: (412) 683-2300 × 4474, Fax: (412) 802-4959, Email: Andrew_Dick@RAND.org
References Cited
- 1.Eddy DM. Variations in physician practice: The role of uncertainty. Health Aff (Millwood) 1984;3:74–89. doi: 10.1377/hlthaff.3.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Phelps CE. Diffusion of information in medical care. J Econ Perspect. 1992;6:23–42. doi: 10.1257/jep.6.3.23. [DOI] [PubMed] [Google Scholar]
- 3.Birkmeyer JD, Sharp SM, Finlayson SR, et al. Variation profiles of common surgical procedures. Surgery. 1998;124:917–923. [PubMed] [Google Scholar]
- 4.Chassin MR. Explaining geographic variations. The enthusiasm hypothesis. Med Care. 1993;31:YS37–44. doi: 10.1097/00005650-199305001-00006. [DOI] [PubMed] [Google Scholar]
- 5.Newcomb JL. Persistence of outmoded medical practices (Dissertation) St. Louis: Washington University; 1995. [Google Scholar]
- 6.Wennberg JE. Unwarranted variations in healthcare delivery: Implications for academic medical centres. BMJ. 2002;325:961–964. doi: 10.1136/bmj.325.7370.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Groenewegen PP, Westert GP. Is there a time trend in medical practice variations? : A review of the literature and a critical analysis of theoretical approaches. J Public Health. 2004;12:229–236. [Google Scholar]
- 8.Hadley J, Mandelblatt JS, Mitchell JM, et al. Medicare breast surgery fees and treatment received by older women with localized breast cancer. Health Serv Res. 2003;38:553–573. doi: 10.1111/1475-6773.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shen J, Andersen R, Brook R, et al. The effects of payment method on clinical decision-making: Physician responses to clinical scenarios. Med Care. 2004;42:297–302. doi: 10.1097/01.mlr.0000114918.50088.1c. [DOI] [PubMed] [Google Scholar]
- 10.Silverstein MJ, Rosser RJ, Gierson ED, et al. Axillary lymph node dissection for intraductal breast carcinoma--is it indicated? Cancer. 1987;59:1819–1824. doi: 10.1002/1097-0142(19870515)59:10<1819::aid-cncr2820591023>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 11.Harris LME JR, Morrow M, Hellman S, editors. Diseases of the Breast. Philadelphia: Lippincott-Raven Publishers; 1996. [Google Scholar]
- 12.Carlson RW, Goldstein LJ, Gradishar WJ, et al. NCCN Breast Cancer Practice Guidelines. The National Comprehensive Cancer Network. Oncology (Williston Park) 1996;10:47–75. [PubMed] [Google Scholar]
- 13.Andersen R, Aday LA. Access to medical care in the U.S.: Realized and potential. Med Care. 1978;16:533–546. doi: 10.1097/00005650-197807000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Andersen RM. Revisiting the behavioral model and access to medical care: Does it matter? J Health Sco Behav. 1995;36:1–10. [PubMed] [Google Scholar]
- 15.Yen TW, Hunt KK, Ross MI, et al. Predictors of invasive breast cancer in patients with an initial diagnosis of ductal carcinoma in situ: A guide to selective use of sentinel lymph node biopsy in management of ductal carcinoma in situ. J Am Coll Surg. 2005;200:516–526. doi: 10.1016/j.jamcollsurg.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 16.Zelis JJ, Sickle-Santanello BJ, Liang WC, et al. Do not contemplate invasive surgery for ductal carcinoma in situ. Am J Surg. 2002;184:348–349. doi: 10.1016/s0002-9610(02)00946-7. [DOI] [PubMed] [Google Scholar]
- 17.Goyal A, Douglas-Jones A, Monypenny I, et al. Is there a role of sentinel lymph node biopsy in ductal carcinoma in situ? Analysis of 587 cases. Breast Cancer Res Treat. 2006;98:311–314. doi: 10.1007/s10549-006-9167-2. [DOI] [PubMed] [Google Scholar]
- 18.Veronesi P, Intra M, Vento AR, et al. Sentinel lymph node biopsy for localised ductal carcinoma in situ? Breast. 2005;14:520–522. doi: 10.1016/j.breast.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 19.Tan JC, McCready DR, Easson AM, et al. Role of sentinel lymph node biopsy in ductal carcinoma-in-situ treated by mastectomy. Ann Surg Oncol. 2007;14:638–645. doi: 10.1245/s10434-006-9211-9. [DOI] [PubMed] [Google Scholar]
- 20.Bland KI, Scott-Conner CE, Menck H, et al. Axillary dissection in breast-conserving surgery for stage I and II breast cancer: a National Cancer Data Base study of patterns of omission and implications for survival. J Am Coll Surg. 1999;188:586–595. doi: 10.1016/s1072-7515(99)00056-3. discussion 595-586. [DOI] [PubMed] [Google Scholar]
- 21.Du X, Freeman JL, Goodwin JS. The declining use of axillary dissection in patients with early stage breast cancer. Breast Cancer Res Treat. 1999;53:137–144. doi: 10.1023/a:1006170811237. [DOI] [PubMed] [Google Scholar]
- 22.Truong PT, Bernstein V, Wai E, et al. Age-related variations in the use of axillary dissection: A survival analysis of 8038 women with T1-ST2 breast cancer. 2002;54:794–803. doi: 10.1016/s0360-3016(02)02973-5. [DOI] [PubMed] [Google Scholar]
- 23.Edge SB, Gold K, Berg CD, et al. Patient and provider characteristics that affect the use of axillary dissection in older women with stage I–II breast carcinoma. Cancer. 2002;94:2534–2541. doi: 10.1002/cncr.10540. [DOI] [PubMed] [Google Scholar]
- 24.Enger SM, Thwin SS, Buist DS, et al. Breast cancer treatment of older women in integrated health care settings. J Clin Oncol. 2006;24:4377–4383. doi: 10.1200/JCO.2006.06.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hillner BE, Penberthy L, Desch CE, et al. Variation in staging and treatment of local and regional breast cancer in the elderly. Breast Cancer Res Treat. 1996;40:75–86. doi: 10.1007/BF01806004. [DOI] [PubMed] [Google Scholar]
- 26.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 27.Garber AM, MaCurdy TE. Nursing home discharges and exhaustion of Medicare benefits. J Am Stat Assoc. 1993;88:727–736. [Google Scholar]
- 28.Baxter NN, Virnig BA, Durham SB, et al. Trends in the treatment of ductal carcinoma in situ of the breast. J Natl Cancer Inst. 2004;96:443–448. doi: 10.1093/jnci/djh069. [DOI] [PubMed] [Google Scholar]
- 29.Porembka MR, Abraham RL, Sefko JA, et al. Factors associated with lymph node assessment in ductal carcinoma in situ: Analysis of 1988–2002 SEER data. Ann Surg Oncol. 2008 doi: 10.1245/s10434-008-9947-5. [DOI] [PubMed] [Google Scholar]
- 30.Schwartz GF, Solin LJ, Olivotto IA, et al. Consensus Conference on the Treatment of in situ Ductal Carcinoma of the Breast, 22–25 April 1999. Breast. 2000;9:177–186. doi: 10.1054/brst.1999.0156. [DOI] [PubMed] [Google Scholar]
- 31.Mandelblatt JS, Berg CD, Meropol NJ, et al. Measuring and predicting surgeons’ practice styles for breast cancer treatment in older women. Med Care. 2001;39:228–242. doi: 10.1097/00005650-200103000-00004. [DOI] [PubMed] [Google Scholar]
- 32.Unger JM, Coltman CA, Jr, Crowley JJ, et al. Impact of the year 2000 Medicare policy change on older patient enrollment to cancer clinical trials. J Clin Oncol. 2006;24:141–144. doi: 10.1200/JCO.2005.02.8928. [DOI] [PubMed] [Google Scholar]
- 33.Ware JE, Jr, Brook R, Davies-Avery A, et al. Conceptualization and measurement of health for adults in health insurance study: Vol.1, Model of health and methodology. In: Corporation R, editor. Model for health and methodology. Santa Monica, CA: RAND Corporation; 1980. [Google Scholar]
- 34.Schulze T, Mucke J, Markwardt J, et al. Long-term morbidity of patients with early breast cancer after sentinel lymph node biopsy compared to axillary lymph node dissection. J Surg Oncol. 2006;93:109–119. doi: 10.1002/jso.20406. [DOI] [PubMed] [Google Scholar]
- 35.Mandelblatt JS, Edge SB, Meropol NJ, et al. Sequelae of axillary lymph node dissection in older women with stage 1 and 2 breast carcinoma. Cancer. 2002;95:2445–2454. doi: 10.1002/cncr.10983. [DOI] [PubMed] [Google Scholar]
- 36.Perrier L, Nessah K, Morelle M, et al. Cost comparison of two surgical strategies in the treatment of breast cancer: Sentinel lymph node biopsy versus axillary lymph node dissection. Int J Technol Assess Health Care. 2004;20:449–454. doi: 10.1017/s0266462304001345. [DOI] [PubMed] [Google Scholar]
- 37.Ronka R, Smitten K, Sintonen H, et al. The impact of sentinel node biopsy and axillary staging strategy on hospital costs. Ann Oncol. 2004;15:88–94. doi: 10.1093/annonc/mdh019. [DOI] [PubMed] [Google Scholar]
- 38.Chirikos TN, Berman CG, Luther SL, et al. Cost consequences of sentinel lymph node biopsy in the treatment of breast cancer. A preliminary analysis. Int J Technol Assess Health Care. 2001;17:626–631. doi: 10.1017/s026646230110718x. [DOI] [PubMed] [Google Scholar]
- 39.Lagios MD, Silverstein MJ. Sentinel node biopsy for patients with DCIS: a dangerous and unwarranted direction. Ann Surg Oncol. 2001;8:275–277. doi: 10.1007/s10434-001-0275-2. [DOI] [PubMed] [Google Scholar]
- 40.Lara JF, Young SM, Velilla RE, et al. The relevance of occult axillary micrometastasis in ductal carcinoma in situ: A clinicopathologic study with long-term follow-up. Cancer. 2003;98:2105–2113. doi: 10.1002/cncr.11761. [DOI] [PubMed] [Google Scholar]
- 41.Sakr R, Barranger E, Antoine M, et al. Ductal carcinoma in situ: Value of sentinel lymph node biopsy. J Surg Oncol. 2006;94:426–430. doi: 10.1002/jso.20578. [DOI] [PubMed] [Google Scholar]
- 42.Mabry H, Giuliano AE, Silverstein MJ. What is the value of axillary dissection or sentinel node biopsy in patients with ductal carcinoma in situ? Am J Surg. 2006;192:455–457. doi: 10.1016/j.amjsurg.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 43.van Deurzen CH, Hobbelink MG, van Hillegersberg R, et al. Is there an indication for sentinel node biopsy in patients with ductal carcinoma in situ of the breast? A review. Eur J Cancer. 2007;43:993–1001. doi: 10.1016/j.ejca.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 44.Edge SB. Early adoption and disturbing disparities in sentinel node biopsy in breast cancer. J Natl Cancer Inst. 2008;100:449–450. doi: 10.1093/jnci/djn061. [DOI] [PubMed] [Google Scholar]
- 45.Chen AY, Halpern MT, Schrag NM, et al. Disparities and trends in sentinel lymph node biopsy among early-stage breast cancer patients (1998–2005) J Natl Cancer Inst. 2008;100:462–474. doi: 10.1093/jnci/djn057. [DOI] [PubMed] [Google Scholar]


