Abstract
Fibrillar amyloid plaques are largely composed of amyloid-beta (Aβ) peptides that are metabolized into products, including Aβ1-16, by proteases including matrix metalloproteinase 9 (MMP-9). The balance between production and degradation of Aβ proteins is critical to amyloid accumulation and resulting disease. Regulation of MMP-9 and its endogenous inhibitor TIMP-1 by nitric oxide (NO) has been shown. We hypothesize that NOS2 protects against AD pathology by increasing amyloid clearance through NO regulation of MMP-9/TIMP-1 balance. We show NO-mediated increased MMP-9/TIMP-1 ratios enhanced the degradation of fibrillar Aβ in vitro, which was abolished when silenced for MMP-9 protein translation. The in vivo relationship between MMP-9, NO and Aβ degradation was examined by comparing an AD mouse model that expresses NOS2 with a model lacking NOS2. To quantitate MMP-9 mediated changes, we generated an antibody recognizing the Aβ1-16 fragment, and used mass spectrometry multi-reaction monitoring (MRM) assay for detection of immunoprecipitated Aβ1-16 peptides. Aβ1-16 levels decreased in brain lysates lacking NOS2 when compared to strains that express human APP on the NOS2 background. TIMP-1 increased in the APPSwDI/NOS2−/− mice with decreased MMP activity and increased amyloid burden, thereby supporting roles for NO in the regulation of MMP/TIMP balance and plaque clearance.
Keywords: matrix metalloproteinase-9, tissue inhibitor of metalloproteinase-1, NOS2, nitric oxide, amyloid, mass spectrophotometry, microglia, mouse models, immunity
Introduction
Alzheimer’s disease (AD) is a progressive, neurodegenerative disorder where profound loss of neurons leads to mental decline and ultimately, death of the patient. Beyond progressive cognitive decline during life, confirmation of AD is defined by the post-mortem presence of amyloid plaques largely composed of amyloid-β Aβ) peptides, neurofibrillary tangles composed of fibrillar aggregates of hyper-phosphorylated tau protein, synaptic damage and neuronal loss (Gandy, 2005; Glenner, 1989; Verdile et al., 2004). The abnormal accumulation of Aβ proteins in the brain has been directly related to disease pathology and to cognitive deficits. Removal of Aβ from the brains of mice and, to some degree, from human brain is associated with reduced cognitive loss and/or downstream pathology and supports the centrality of Aβ to Alzheimer’s disease (Aizenstein et al., 2008; Morgan, 2006; Mufson et al., 2012; Vellas et al., 2009). Several hypotheses explaining the etiology of Alzheimer’s disease have been offered including mechanisms associated with the enhanced production, or deficient removal, of Aβ from the brain (Basak et al., 2012; Hawkes et al., 2011; Weller et al., 2008). While enhanced production of Aβ peptides clearly accounts for increased plaque deposition in rare patients with mutated APP genes, impaired Aβ clearance may be the primary factor in the majority of patients with sporadic AD. The major routes of Aβ clearance include Aβ transport across the blood brain barrier into the blood, phagocytosis by microglia and other cells, and extracellular proteolysis (Bell et al., 2012; Huang et al., 2012; Miners et al., 2011; Tanzi et al., 2004; Weller et al., 2008). Proteolytic removal of Aβ can be mediated by several proteases including neprilysin, endothelin converting enzyme, insulin degrading enzyme, as well as the gelatinases, MMP-2 and MMP-9 (Miners JS, 2009).
Our laboratories and others have demonstrated that NO regulates MMP-9 activity and the activity of its endogenous inhibitor, TIMP-1 at both the mRNA and protein levels. At low concentrations, NO increased MMP-9 activity in a guanylyl cyclase-depenent manner through TIMP-1 protein suppression; while at high concentrations, NO directly activated and inactivated the enzyme, which was reactive nitrogen species-dependent (Death et al., 2002; Krishnatry et al., 2011a; Ridnour et al., 2007; Weiss et al., 2010). To better understand the relationship between NO, MMP activity and amyloid degradation, we have used both in vitro and in vivo approaches. The cellular effect of NO on MMP-9 activity and on the MMP/TIMP ratio was studied using well defined NO donors. Our in vivo approach employed mouse models of AD that express mutated human amyloid precursor protein (APP) on a mouse NOS2 knockout background (mNOS2−/−). Thus, Aβ1-40 and Aβ1-42 peptide production and clearance by the brain occur in an environment of lowered NO. Our data support the hypothesis that reduced NO levels leads to the dysregulation of plaque clearance by decreasing the MMP-9/TIMP-1 ratio with concomitant reduction in MMP-9 enzymatic activity and increased Aβ/amyloid plaque deposition.
Materials and Methods
Human samples
De-identified human tissue (IRB number 0182, Duke University Medical Center) was generously supplied by the Kathleen Price Bryan Brain Bank at Duke University Medical Center. Tissue samples were from non-specified regions of the cortex from humans diagnosed with AD and age matched control (non-demented) humans.
Animals
The use of all animals in the study was approved by the Duke University Institutional Animal Care and Use Committee and conformed to the National Institutes of Health Guide for the Care and Use of Animals in Research. The APPSwDI/NOS2−/− mice were produced by crossing APPSwDI (Swedish K760N/M671L, Dutch E693P, and Iowa D694N) (Davis et al., 2004) transgenic mice with NOS2−/− (B6 129PNOS2tau1Lau/J) (Laubach et al., 1995) mice as described previously (Wilcock et al., 2008). All mice were genotyped using standard procedures, kept under barrier conditions on a 12 hour light dark cycle and fed ad libitum with a standard mouse chow. Mice were aged to the appropriate age for the study; either 6, 12, 24, 36, or 52 wks for the mRNA data (6 mice each strain, mixed gender) or 28 to 30 wks for all other data (8 mice each strain, mixed gender) prior for use in the studies. Once aged, all mice were deeply anesthetized and the brains were perfused with phosphate buffered saline (PBS) via cardiac puncture for 15 min. Brains were then quickly removed, split into hemispheres and either flash frozen using liquid nitrogen and stored until used at −80°C or drop fixed with 4% paraformaldehyde, passed through sucrose, and cut into 25 micron sections for immunocytochemistry. In some cases, snap-frozen brains were sectioned using a freezing microtome and frozen sections (10um) were collected following cryoprotection through sucrose. MMP activity was visualized in situ with DQTM gelatin fluorescein conjugate (EnzCheck, Life Technologies, Grand Island) as described by George and Johnson (George and Johnson, 2010).
Gel Zymography and Fluorogenic Peptide Evaluation of MMP Activity
Gelatinase was extracted from brain as described in Zhang and Gottschall (Zhang and Gottschall, 1997) and qualitative analysis of MMP-2 and MMP-9 enzyme activity was performed by gel zymography (Kleiner and Stetler-Stevenson, 1994). Approximately 100 mg frozen brain tissue were homogenized in a two ml Teflon glass homogenizer in ice cold working buffer [50 mM Tris-HCl, pH 7.6, 150 mM NaCl, 5 mM CaCl2, 0.05% Brij-35, 0.02% NaN3] containing 1% Triton X-100. Total protein levels in aliquots (10 μl) of each homogenate sample were quantified using the BCA assay (Thermo Scientific, Rockford, IL). For extraction, homogenates (400 μg) were centrifuged at 12,000 x g for five minutes at 4°C. The recovered supernatants were incubated for sixty minutes with 20 μl gelatin-sepharose 4B with constant shaking at 4°C, and then centrifuged again at 500 x g for two minutes. The pellets were washed, centrifuged at 500 x g for two minutes and then incubated for thirty minutes on ice in working buffer containing 10% DMSO. The samples were centrifuged at 500 x g for two minutes and zymography was then performed on the supernatant from each mouse brain under non-denaturing conditions on 10% gelatin zymogram gels. The gels were washed and incubated in renaturing and developing buffers according to the manufacturer’s recommendation. All zymogram reagents were purchased from Invitrogen (Carlsbad, CA). Positive controls for MMP-9 and MMP-2 latent and active enzyme were extracted as described for mouse brain, from cell lysates of untreated HT10 cell as well as HT10 cells treated with 1 mM 4-aminophenylmercuric acetate (APMA), which activated the MMPs.
Quantitative evaluation of total MMP proteolytic activity in cell conditioned media was measured using the internally quenched synthetic (7-methoxycoumarin-4-acetyl-Pro-Leu-Gly-Leu-β-(2,4-dinitrophenylamino)Ala-Ala-Arg-NH2) MCa peptide assay (Sigma-Aldrich, St. Louis, MO). This peptide fluoresces at (328ex/392em) upon cleavage of the quenching moiety. Equal volumes of samples were added to 200 μl TCNB buffer [50 mM Tris-HCl pH 7.5, 0.2 M NaCl, 10 mM CaCl2, and 0.05% Brij35] containing MCa peptide at a final concentration of 10 μM then incubated for 20 minutes at 37°C. As a measure of cleavage activity, fluorescence was measured at 328ex/392em on a Perkin Elmer (Wellesley, MA) Luminescence LS50B Spectrometer equipped with FL WinLab software.
ELISA Assays
The effects of NO on TIMP-1 levels in the media was measured by TIMP-1 ELISA (R&D Systems, Minneapolis, MN). Media from untreated control and NO treated (24 hr) cells were collected and centrifuged to remove any floating cells. The media were diluted 1:10 and ELISA assay was performed according to the manufacturers recommendation. TIMP-1 levels in control and NO treated cell culture media were determined from a human recombinant TIMP-1 standard curve.
Aβ ELISA was performed using a two-step protein extraction where 150 mg brain powder was first extracted in 250 μl PBS containing 1% complete protease/phosphatase inhibitor (Thermo Scientific, Rockford IL). This homogenate was centrifuged at 16,000 x g at 4°C for 30 minutes and the supernatant was saved as the soluble extract. The pellet was then homogenized in 100 μl 70% formic acid and centrifuged at 16,000 x g at 4°C for 30 minutes. The supernatant was removed, neutralized 1:20 with 1 M Tris-HCl and became the “insoluble” extract. Protein concentration for both the soluble and insoluble extracts was determined using the BCA protein assay according to manufacturer’s instructions. We used the Covance BetaMARK ELISA system (Dedham, MA) to measure soluble and insoluble Aβ40 and Aβ42 according to the manufacturer’s instructions.
Aβ 1-42 Digestion
Soluble and fibrillar Aβ1-42 were prepared as described by Yan et al (Yan et al., 2006). Aβ1-42 (Bachem Bioscience, King of Prussia PA) was dissolved in DMSO (5 mM) and then diluted to 100 μM in TCNB buffer [50 mM Tris-HCl pH 7.5, 0.2 M NaCl, 10 mM CaCl2, and 0.05% Brij35]. Active MMP-2 or MMP-9 was added to a final concentration of 100 nM. The solutions were mixed thoroughly by vortexing, aliquotted into 100 μl volumes, and then incubated at 37°C for 2 to 96 hrs, before addition of 1 μl formic acid to stop the reaction. Digestion fragments were evaluated by liquid chromatography mass spectrometry (LC/MS/MS).
LC/MS/MS Analysis of 1-42 Aβ Digested with MMP-9 or MMP-2
Peptide data were acquired using a QTOF-2 mass spectrometer (Waters, Corporation, Milford, MA) equipped with a New Objective (Woburn, MA) nanospray holder interfaced with a Water’s CapLc high performance liquid chromatograph (Milford, MA). Two μL aliquots of each sample digest were injected onto a New Objective PicoFrit capillary LC column (10 cm by 75 μm) packed with BioBasic C18 (5 micron particle size, 300 angstrom pore size). Peptides were eluted at a flow rate of 500 nL/minute on the following gradient: 0 to 25% B in 22 minutes (linear), 25% B to 50% B in 10 minutes and 50% B to 100% B in 10 minutes. Solvents A and B consisted of 1.0% and 95% acetonitrile in 0.1% formic acid, respectively. The Electrospray voltage was 3.0 kV, and the sample cone voltage was 35 V. The mass spectrometer was operated in the survey mode using the instrument’s automatic switching feature to capture full scan spectra (m/z 400 to 2,000 in 1 second) and product ion spectra (m/z 100 to 2,000 in 1.3 seconds). Product ion spectra were generated from multiple charged precursor ions with variable collision energies ranging from 10 to 60 eV based upon the mass to charge state of the eluting peptide. Argon was used as the collision gas at a nominal pressure of 1 bar. Protein Lynx Global Server (v1.1; Waters Corporation, Milford, MA.) was used to identify Aβ1-42 digestion products using the following parameters: +/− 0.5 dalton peptide tolerance, +/− 0.3 dalton MS/MS tolerance, and the UniProt KB Protein Knowledgebase filtered for Amyloid Beta A4 Protein Precursor (P05067, amino acids 672-713) only.
Antibody Synthesis and Characterization
Antibody 198 was generated at CoCalico Biologicals, Inc. (Reamstown, PA) using their standard protocol, and found to recognize Aβ1-16 digestion fragment of Aβ1-42. Briefly, pre-bled New Zealand White rabbits (5-6 lb) were inoculated a total of five times over ninety days with 500 μg of KLH conjugated peptides. The peptides (CEVHHQKLVFF, AEDVGSNKGC, CEDVGSKNGA, and IIGLMVGGVVC) were designed from MMP-9 cleavage sites of human Aβ 1-42 as described (Yan et al., 2006). All sera was screened by probing western blots of APPSwDI, APPSwDI/NOS2−/−, human control, and human AD brain homogenate for differences in band densities. One antibody, #198 obtained following inoculation with CEVHHQKLVFF peptide, demonstrated a difference in band density patterns between APPSwDI and APPSwDI/NOS2−/−, and control and AD brain homogenates. For characterization, the antibody was incubated with MMP-9 digested human recombinant Aβ1-42 as described below, and eluted peptides were identified by mass spectrometry.
Immunoprecipitation of Aβ 1-16 Digestion Product
Antibody 198 was incubated with degradation products from MMP-9 digested Aβ1-42, purified Aβ1-16 (DAEFRHDSGYEVHHQK), and human and animal brain homogenate (0.5 mg), and then the eluted material was analyzed by mass spectrometry. The recombinant peptides or brain homogenate samples (0.5 mg) were immunoprecipitated in one ml ELB buffer (50 mM HEPES pH 7.2, 250 mM NaCl, 2 mM EDTA, 0.5% NP-40,) containing 10 μg of primary antibody 198 (described above) conjugated to magnetic Protein G Dynabeads (Invitrogen, Grand Island, NY). The samples were incubated overnight at 4°C on a shaker platform. The sample tubes were then placed on a magnet and the solution removed. Samples were washed 3x with 1ml PBS then resuspended in 100 μl PBS. The immunoprecipitated material was eluted from the beads with 2 washes of 100 μl of 0.1 M glycine (pH 2.8), the pooled glycine supernatants were speed vacuumed to near dryness and finally reconstituted in 50 μl of 0.1% formic acid for analysis by mass spectrometry multireaction monitoring assay.
LC/MRM Analysis of DAEFRHDSGYEVHHQK (Aβ 1-16)
LC/MRM data were acquired using a Xevo TQ mass spectrometer equipped with a nanoelectrospray source and a nanoAcquity Ultra Performance LC system (Waters, Corporation, Milford, MA.). Five microliters of each immunoprecipitate Aβ1-16 extract was injected onto a Denali-C18 column (The Nest Group, Inc., Southborough, MA). The column was 10 cm in length with an internal diameter of 75 microns. The particle size and porosity of the packing material were 5 microns and 120 angstroms, respectively. The peptide was eluted using a flow rate of 300 nL/minute with the following gradient: 1% B to 80% B in 15 minutes. Solvents A and B consisted of 0.1% formic acid and 99.9% acetonitrile in 0.1% formic acid, respectively. The column effluent entering the mass spectrometer was subjected to an electrospray ionization voltage of 2.75kV and a cone voltage of 30V. The 4+ precursor ion (m/z 489.9) was subjected to a collision gas of argon using 18V of collision energy. The transitions monitored were (m/z 489.9> 110.3 (immonium ion of histidine)) and (m/z 489.9>159.2 (A2 ion)). The dwell time was 0.928 seconds. Quantification of Aβ1-16 (DAEFRHDSGYEVHHQK) was based on the peak area of the two LC-MRM transitions combined. MassLynx (version 4.1, SCN174) was used for data acquisition and peak integration. The limit of detection was defined when the MRM peak signal was greater than 4 times the baseline noise level. MRM results are reported as an average of 3 analyses per sample +/− SEM.
Cell Culture
Non-immune activated murine BV2 microglia and human U373 astrocyte cells were grown in DMEM supplemented with 10% FBS and penicillin-streptomycin. Cellular effects of NO were evaluated following exposure to the long lasting NO donor DETA/NO. The donor was prepared as a 100 mM stock concentration in 10 mM NaOH to prevent its decomposition. Release of NO from DETA/NO occurred in a pH and temperature dependent manner upon the addition of donor to cell culture media pH 7.4-7.5 (DETA/NO T1/2 ~ 20 Hr at 37°C) (Davies et al., 2001). For experiments, the cells were plated and grown overnight (80-90% confluence unless otherwise specified). On the following day, the cells were treated with DETA/NO for 24 hr at 37°C in serum-free, phenol red-free RPMI. Cell conditioned media of NO-treated cells was collected for gel zymography for MMP-2/MMP-9 and ELISA assay for TIMP-1 levels. Results were obtained from cell cultures within 20 passages.
Suppression of MMP-9 Translation
Silencing of MMP-9 protein translation was accomplished using a translation blocking antisense 22-mer oligo (Gene Tools, Philomath OR) designed specifically for the AUG translational start site of mouse MMP-9 (GenBank # NM_013599: seq 5′-GCTGCCAGGGACTCATGGTGAG). This oligonucleotide complements the sequence from −6 thru +16 relative to the initiation codon of MMP-9 mRNA. Briefly, BV2 cells (~50% confluence) were incubated with 10 μM antisense oligo and 6 μl Endo-porter delivery system (Gene Tools, Philomath OR) per ml of growth media for 24 hrs. Suppression of secreted MMP-9 protein levels were then verified using gel zymography.
Quantitative RT-PCR
Whole brain lysates from APPSwDI, APPSwDI/NOS2−/−, NOS2−/− and WT mice at 6, 12, 24, 36, and 52 wks of age (minimum of 5 individual mice per age) were used to prepare RNA for analysis of MMP-9, TIMP-1, MMP-2 and TIMP-2 gene expression. mRNA was extracted from approximately 40 mg frozen pulverized tissue using the RNeasy tissue kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions. RNA was quantified using the nanodrop spectrophotometer (Thermo Scientific, Rockford IL) and cDNA produced using the cDNA High Capacity kit (Applied Biosystems, Foster City CA) according to the manufacturer’s instructions. Real-time PCR was performed using the TaqMan Gene Expression assay kit (Applied Biosystems, Foster City CA) also according to the manufacturer’s instructions and as previously described (Wilcock et al., 2008). All PCR probes were from Applied Biosystems and all data were normalized to ß-actin using wt mice of the same age as the comparator. Fold change in gene expression was calculated using the 2 (-delta delta C(T) method (Livak and Schmittgen, 2001).
Statistics
Statistical comparisons were made using Student’s t-test or one-way ANOVA and Dunnet’s multiple comparison post-test. Results are reported as mean +/− SEM and were considered statistically significant at p < 0.05.
Results
To better understand the ability of NO to regulate gelatinase, we first examined the dose response effects of NO on expression and/or activity of MMP-9, MMP-2 and TIMP-1 in cultured BV2 microglia and U373 astrocytes. Matrix metalloproteinases such as MMP-2 and MMP-9 are expressed in astrocytes, microglia, and neurons and are known to be up-regulated under inflammatory conditions that may be associated with neurodegenerative diseases such as AD (Colton et al., 1993; Gottschall and Yu, 1995; Roher et al., 1994). The effect of NO on MMP-9 and MMP-2 activity was studied using gel zymography that both separates the proteins based on molecular weight and detects the ability of these gelatinases to degrade a gelatin-based substrate. For these experiments, non-immune activated BV2 microglial cells were exposed for 24 hr in culture to varying levels of NO using DETA/NO, a well described NO donor and the levels of MMP-9 and MMP-2 secreted into the media were measured (Ridnour et al., 2007). DETA/NO was employed because its prolonged NO release rate (T1/2 ~ 20 hr at 37°C) may be more representative of a chronic disease state such as that found within the AD brain. A typical zymogram is shown in Fig. 1A and depicts a NO-induced dose dependent increase in the levels of secreted MMP-9 activity from BV2 microglia cells. The NO-induced level peaked at 500 μM DETA/NO, which, as shown previously is associated with > 400 nM steady state NO (Wei et al., 2009). MMP-2 activity was not detected in the BV2 cells. Analysis of TIMP-1 levels by ELISA in the same conditioned media samples showed a dose dependent decrease in TIMP-1 with increasing doses of DETA/NO (Fig 1B). These results indicate that the relative ratio of total MMP-9 proteolytic activity to total TIMP-1 anti-proteolytic activity (MMP-9/TIMP-1 ratio) increases as NO increases (Fig 1C). In contrast, NO had little effect on gelatinase activities secreted from astrocytes (Fig. 1D). Our results demonstrated NO modulation of MMP-9/TIMP-1 balance, and suggests that the overall level of MMP-9 activity may be altered under local conditions that dictate microglial functional state and the integrated level of NO produced in this microenvironment.
Figure 1.
Effect of NO using the slow releasing NO donor, DETA/NO (24 hr exposures), on secreted levels of MMP-9 and TIMP-1 from cultured BV2 microglia and U373 astrocytes. All results were obtained from cell cultures within 20 passages. A) The effect of increasing concentrations of NO on secreted levels of MMP-2 and MMP-9 were evaluated using gel zymography on equal volumes of media. B) Secreted TIMP-1 protein levels were measured using an ELISA assay on the same NO treated samples as in A. TIMP-1 results are plotted as the average of duplicate measurements and are representative of two independent experiments. C) MMP-9/TIMP-1 balance at each NO concentration was assessed by calculating the ratio of densitometric measurements of MMP-9 bands shown in A divided by TIMP-1 levels shown in B. The results are presented as % Control. D) NO effects on MMP-2 and MMP-9 secreted from human U373 astrocytes. The cells were plated and treated as described for BV2 cells (see methods section) and equal volumes’ of media were assayed using gel zymography.
To the best of our knowledge, MMP-9 is the only brain protease that is capable of digesting fibrillar forms of Aβ (fAβ), with the possible exception of MT1-MMP (Liao and Van Nostrand, 2010; Yan et al., 2006). To examine a role for NO in MMP-9 digestion of fibrillar Aβ (fAβ) BV2 cells were allowed to condition serum-free, phenol red-free media for 5 hr. The cells were then treated for 30 min with varying concentrations of DEA/NO, a NO donor from the same family as DETA/NO but which demonstrates more rapid NO production kinetics (T1/2 ~ 3 min) (Thomas et al., 2002). The media were collected and concentrated, and the levels of MMP-9 (Fig 2A) and total MMP activity (Fig 2B) were measured using gel zymography and the MCa peptide assay, respectively. After verification of NO-induced MMP activity, the same conditioned media was spiked with fAβ (1 nmol) and incubated 5 days at 37°C as described by Yan et al (Yan et al., 2006). At the end of this incubation period, thioflavin-T fluorescence was measured to access degradation of the spiked fAβ by NO-induced MMP activity (LeVine, 1999). We found a significant reduction in spiked fAβ levels in a DEA/NO concentration-dependent manner compared to untreated controls (Fig 2C). Moreover, the NO-induced fAβ degradation was similar to that of cell free media containing 100 nM activated human recombinant MMP-9 shown in Fig 2D and previously reported by Yan et al (Yan et al., 2006). In our hands, fAβ was reduced by approximately 25% in the presence of human recombinant MMP-9. A similar percent change was found in the NO-treated BV2 cells supporting that active MMP-9 was present. In contrast, when MMP-9 protein translation was blocked in the BV2 cells (Fig 2E), the NO-induced effect on fAβ digestion was not observed (Fig 2F).
Figure 2.
Changes in MMP activity and (fAβ) degradation with increasing NO. A) Gel zymography demonstrates increased MMP-9 in the conditioned media of BV2 cells treated with the rapid releasing (T½ ~ 2 min) NO donor DEA/NO for 30 min. B) MCa peptide fluorescence demonstrates enhanced total MMP activity in the conditioned media of BV2 cells treated with DEA/NO. C) fAβ spiked into conditioned media of DEA/NO treated cells shows enhanced degradation with increasing NO levels, and is similar to a control experiment using activate recombinant human MMP-9 (100 nM, panel D) rather than cell conditioned media. E) Gel zymography showing reduced MMP-9 in BV2 cells treated with a MMP-9 morpholino, which blocks MMP-9 protein translation. F) Enhanced degradation of fAβ by BV2 conditioned media from NO donor treated cells requires MMP-9. Graphed results are presented as mean +/− SEM (n = 3), with the exception of D, which shows single point measurements.
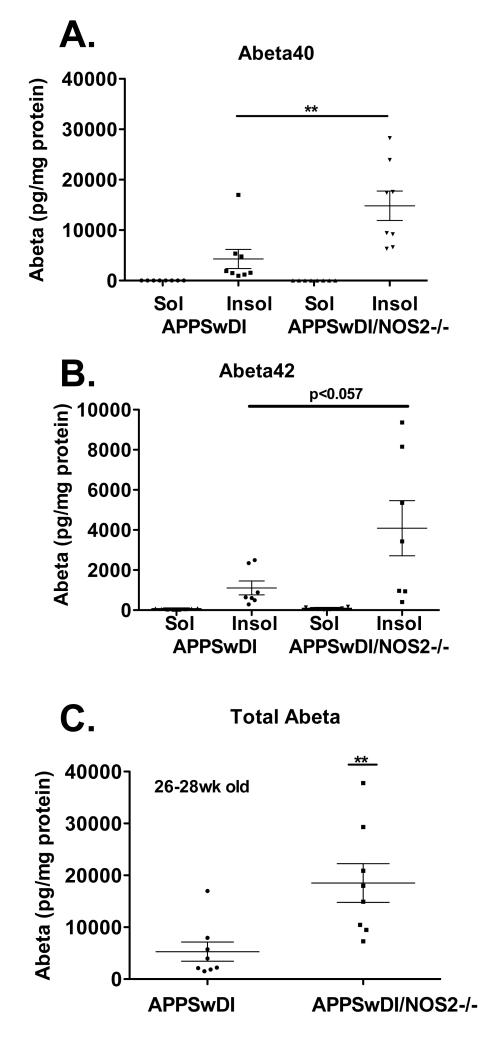
The above results indicate that NO can modulate MMP-9 activity and subsequent degradation of fAβ by MMP-9 in vitro. To explore a role for NOS2 and NO in regulation of in vivo amyloid plaque clearance, we first examined differences in total Aβ levels between APPSwDI and APPSwDI/NOS2−/− mice. The only genetic difference between these two strains is the functional deletion of the NOS2 gene and corresponding loss of NOS2-derived NO in all cell types that express NOS2 (Laubach et al., 1998; Wilcock et al., 2008). Both mice strains show robust Aβ production and amyloid accumulation. Although the levels are not significantly different with advanced pathology as found at 54 weeks of age or greater (Wilcock et al., 2008), the rate of Aβ accumulation appears to be different in younger mice. As shown in Fig 3, the average total brain Aβ level in 28-30 week old APPSwDI mice is significantly lower than the level found in APPSwDI/NOS2−/− mice at the same age with insoluble Aβ accounting for most of the differences. Additionally, trends for both Aβ1-40 and Aβ1-42 were identical. To explore the possibility that the difference in Aβ levels shown in Fig 3 could be a result of enhanced proteolysis and higher clearance of Aβ in the APPSwDI mice compared to APPSwDI/NOS2−/− mice, we examined the expression and activity levels of gelatinases in mice from both strains using quantitative RT-PCR, in situ immunohistochemistry, and gelatinase zymography.
Figure 3.
Aβ levels in APPSwDI and APPSwDI/NOS2−/− mice. Levels were measured using an ELISA for human Aβ with each point representing an individual mouse assayed; A) Soluble and insoluble levels of Aβ40 in APPSwDI compared to APPSwDI/NOS2−/− mice at 26-28 wks of age. B) Soluble and insoluble levels of Aβ42 for the same mice. C) Soluble and insoluble levels for Aβ40 and Aβ42 were combined to generate total Aβ for each mouse strain.** p < 0.01 using the unpaired Student’s t test.
Expression levels for MMP-9, MMP-2, TIMP-1 and TIMP-2 mRNAs for APPSwDI and APPSwDI/NOS2−/− mice are shown in Fig 4. To better understand differences between strains, we determined the fold-change in mRNA in APP mutant and control mice at 6, 12, 24, 36, and 52 weeks of age. MMP-9 mRNA was highest in the APPSwDI mice compared to either NOS2−/− or WT control mice with a significant increase observed at 36 and 52 wks of age compared to APPSwDI/NOS2−/− mice (Fig 4A). TIMP-1 expression however was significantly lower at each age in APPSwDI mice compared to the APP mice lacking NOS2 (Fig 4B). Highest values for TIMP-1 mRNA expression were found in 52 wk old APPSwDI/NOS2−/−. The data indicate that the MMP/TIMP1 ratios were highest in APPSwDI and lowest in the mice that expressed APP on a NOS2 knockout background, predicting that MMP-9 proteolytic activity is likely to be higher in APPSwDI mice. MMP-2 or TIMP-2 did not show similar expression patterns for mRNA over the same time period and, in fact, appeared repressed compared to WT mice.
Figure 4.

Comparison of the fold changes in mRNA for MMP-9, MMP-2, TIMP-1 and TIMP-2 between APPSwDI, APPSwDI/NOS2−/− and control mice (NOS2−/− or WT). Data points represent the average (±SEM) from whole brain lysates for each strain at 6, 12, 24, 36 and 52 wks of age.A) MMP9 mRNA levels were significantly higher in APPSwDI mice than either WT or NOS2−/− control mice at each age, except at 12 wks. * = p<0.05 using unpaired students t test. A comparison between APPSwDI and APPSwDI/NOS2−/− mice showed significantly increased APPSwDI MMP9 mRNA levels at 36 and 52 wks. No significant changes were found at 6,12, or 24 wks. #= p<0.05 (unpaired students t test) B) mRNA levels for TIMP1 for APPSwDI mice were not significantly different that either NOS2−/− or WT mice. TIMP1 mRNA levels in APPSwDI mice were significantly lower than the corresponding values at each age in APPSwDI/NOS2−/− mice. # = p < 0.05, ## = p < 0.01 and ### = p < 0.001 (unpaired students t test). APPSwDI/NOS2−/− mice showed an increase within strain with age*** = p < 0.001 (1-way ANOVA). C, D) Comparisions of APPSwDI and APPSwDI/NOS2−/− mice to each other or to control mice showed no significant changes in mRNA levels for MMP2 and TIMP2.
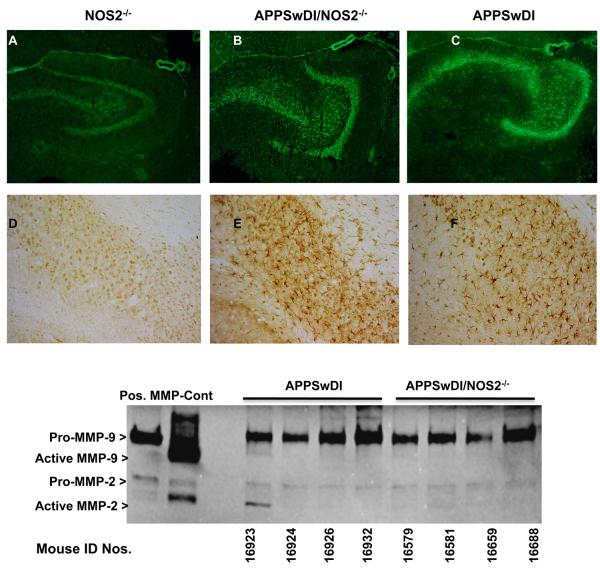
MMP activities in a specific brain region were detected using in situ gelatinase zymography. In this case MMP activity is shown by an increase in fluorescence generated from the MMP-mediated cleavage of matrix proteins conjugated to quenched fluorescein. Figure 5 shows representative views of the hippocampus in fresh frozen brain slices from NOS2−/− (Fig 5A), APPSwDI/NOS2−/− (Fig 5B), or APPSwDI (Fig 5C) mice. Robust MMP activity, as indicated by increased fluorescence, is observed in the hippocampus of the APPSwDI mouse compared to either NOS2−/− or APPSwDI/NOS2−/− mice. The presence of MMP-9 in the brain was further confirmed using immunostaining on corresponding sections from the above mice (Fig 5 D-F). We also used gel zymography to measure the level of pro-MMP-9 (upper migrating band) and active MMP-9 (lower migrating band) as shown in Fig 5G. Bands corresponding to pro-MMP-9 were abundant, while bands corresponding to pro-MMP-2 were faint. Lower migrating bands representative of active MMP-9 and 2 were faint. Gel zymography is mainly qualitative and analysis of band density of the pro-forms did not show a significant difference between APPSwDI and APPSwDI/NOS2−/− mice.
Figure 5.
Analysis of in situ gelatinase activity. A-C) DQTM gelatin fluorescein conjugate was used to detect active MMP in the hippocampus of 26-28 wk old (A) NOS2−/−, (B) APPSwDI/NOS2−/− and (C) APPSwDI mice brain. D-F) Immunostain for MMP in the subiculum of similar NOS2−/−, APPSwDI/NOS2−/− and APPSwDI mice brains. G) gel zymography from whole brain lysates show abundant pro-MMP9 activity with little Pro-MMP2 activity in 26-28 wk old APPSwDI and APPSwDI/NOS2−/− mice. Little to no active MMPs were observed.
Although the above data support a potential role for NO regulation of gelatinase activity in the increased proteolysis and clearance of Aβ in APPSwDI mice brain (+NOS2/NO) vs a reduced proteolysis and clearance in the APPSwDI/NOS2−/− mice (-NOS2/NO), MMPs are labile enzymes and their activities can be difficult to quantify. Thus, to further establish the impact of NOS2/NO on MMP activity and Aβ degradation in our AD models, we developed an antibody recognizing Aβ1-16, which is a stable and non-toxic digestion product of Aβ1-42 in the presence of active gelatinase enzymes MMP-2 and MMP-9 (Supp Fig 1 and Table I) (Roher et al., 1994; Yan et al., 2006). To verify antibody specificity, excess amounts of this antibody were incubated with a mixture of peptides generated from MMP-9 degraded Aβ1-42 summarized in Supp Fig.1 and Table I. The bound peptides were eluted and examined by LC/ESI/MRM and data dependent LC/ESI/MS/MS. Figures 6A and 6B show an MRM spectra of Aβ1-16 immunoprecipitated from the MMP-9 digested Aβ1-42 mixture and immunoprecipitated APPSwDI brain homogenate, respectively. The LC/ESI/MRM chromatographic peak at 20.86 minutes in Fig 6D contains all 4 of the transition product ions shown in Fig 5A and 5B. The relative abundance of these transition product ions were similar to those identified in the MRM spectra of purified Aβ1-16 standard shown in Fig 6C. The LC/ESI/MS/MS spectrum (Fig 6E) of the Aβ1-16 immunoprecipitate shown in Fig 6D shows all ions and confirms the presence of Aβ1-16. LC/ESI/MS/MS also confirmed the presence of Aβ1-14 and Aβ1-13 but in much less abundance. Observed B and Y ion (m/z) values were within +/− 0.5 daltons of the theoretical calculations obtained from UCSF’s Protein Prospector (prospector.ucsf.edu). Together, these results confirm 1) the presence of Aβ1-16 product in MMP-9 degraded Aβ1-42, 2) Aβ1-16 specificity of antibody 198, and 3) MRM detection of Aβ1-16 product immunoprecipitated from brain homogenate.
Table 1.
MMP-2 Quadrupole Time of Flight (QTOF) Digestion Fragments of Aβ1-42 (DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVVIA) by MMP-2 and MMP-9.
| Aβ Residue | Peptide Sequence | Observed MH+ |
|---|---|---|
| 1-30 | DAEFRHDSGYEVHHQKLVFFAEDVGSNKGA | 3389.6 |
| 1-17 | DAEFRHDSGYEVHHQKL | 2068.0 |
| 1-16 | DAEFRHDSGYEVHHQK | 1954.9 |
| 1-14 | DAEFRHDSGYEVHH | 1698.7 |
| 1-13 | DAEFRHDSGYEVH | 1561.7 |
| 1-11 | DAEFRHDSGYE | 1325.5 |
| 1-9 | DAEFRHDSG | 1033.4 |
| 4-14 | FRHDSGYEVHH | 1383.6 |
| 4-13 | FRHDSGYEVH | 1246.6 |
| 4-11 | FRHDSGYE | 1109.5 |
| 17-34 | LVFFAEDVGSNKGAIIGL | 1850.0 |
| 18-34 | VFFAEDVGSNKGAIIGL | 1736.9 |
| 17-33 | LVFFAEDVGSNKGAIIG | 1736.9 |
| 17-30 | LVFFAEDVGSNKGA | 1453.7 |
| 18-30 | VFFAEDVGSNKGA | 1340.6 |
| 20-33 | FAEDVGSNKGA | 1377.7 |
| 21-33 | AEDVGSNKGA | 1230.6 |
| 19-30 | FFAEDVGSNKGA | 1241.6 |
| 20-30 | FAEDVGSNKGA | 1094.5 |
| 20-28 | FAEDVGSNK | 966.5 |
| 22-28 | EDVGSNK | 748.4 |
| 20-26 | FAEDVGS | 724.3 |
| 18-26 | VFFAEDVGS | 970.4 |
| 19-26 | FFAEDVGS | 871.4 |
| 35-42 | MVGGVVIA | 745.5 |
| 36-42 | VGGVVIA | 614.4 |
| 37-42 | GGVVIA | 515.3 |
| MMP-9 | ||
| 1-30 | DAEFRHDSGYEVHHQKLVFFAEDVGSNKGA | 3389.6 |
| 1-16 | DAEFRHDSGYEVHHQK | 1954.9 |
| 1-14 | DAEFRHDSGYEVHH | 1698.7 |
| 1-13 | DAEFRHDSGYEVH | 1561.7 |
| 1-11 | DAEFRHDSGYE | 1325.5 |
| 14-30 | HQKLVFFAEDVGSNKGA | 1846.9 |
| 17-34 | LVFFAEDVGSNKGAIIGL | 1850.0 |
| 17-30 | LVFFAEDVGSNKGA | 1453.7 |
| 17-26 | LVFFAEDVGS | 1083.5 |
| 19-30 | FFAEDVGSNKGA | 1241.6 |
| 20-34 | FAEDVGSNKGAIIGL | 1490.8 |
| 20-30 | FAEDVGSNKGA | 1094.5 |
| 20-28 | FAEDVGSNK | 966.5 |
| 35-42 | MVGGVVIA | 745.5 |
Figure 6.
Mass spectrometry confirmation of antibody recognition of Aβ1–16 product in a digestion mixture of human recombinant Aβ1–42 and active MMP-9 enzyme. Panel A-C) MRM spectra showing four selected product ions from Aβ1–16 peak eluting at 20.86 min in a mixture of antibody 198 and MMP-9 degraded human recombinant Aβ1-42 shown in panel D. Panel E) Data dependent LC/ESI/MS/MS analysis of Aβ1-16 peak shown in panel D confirms amino acid sequence by theoretical calculations of the b- and y- ions using UCSF ProteinProspector software.
Next, we utilized MRM to quantify levels of Aβ1-16 as an indicator of gelatinase activity in brain lysates from the 28-30 wk old APPSwDI and APPSwDI/NOS2−/− mice. This method provided a direct and quantifiable index of gelatinase activity that includes MMP-2 and MMP-9 in our models. Levels of Aβ1-16 were significantly elevated in APPSwDI mice compared to APPSwDI/NOS2−/− mice (Fig 7A), supporting a direct role of gelatinases in proteolysis of Aβ peptides in the AD mouse models. Further, the significant difference between the mouse strains implies a role for NOS2-derived NO in promoting the degradation and clearance of Aβ. Finally, Aβ1-16 levels in brain samples from humans diagnosed with AD and age matched control brains were also quantified using MRM. As shown in Fig 7B, human AD brain demonstrated a similar trend of less Aβ1-16 when compared to age-matched controls. These results suggest reduced gelatinase activity and thus support the concept of dysregulation of plaque clearance in AD vs non-AD brain.
Figure 7.
MRM analysis of Aβ1-16 product in A) APPSwDI and APPSwDI/NOS2−/− brain and B) human control and human AD brain. Results are presented as mean +/− SEM (n = 3 AD and n = 3 control). * = p < 0.05 using the student’s t test.
Discussion
The levels of Aβ peptides in the CSF of normal humans are known to fluctuate as part of daily sleep wake cycles (Kang et al., 2009), strongly suggesting that a dynamic balance between Aβ production and clearance is a normal physiological event in the brain. Amyloid accumulation that leads to disease, then, is due to a disruption of this balance caused by abnormally increased production or abnormally decreased clearance of the peptides. While some changes in production have been documented, a large number of studies suggest that defective clearance is a primary event associated with deposition of amyloid that results in the ensuing pathology characteristic of AD (see reviews by Miners (Miners et al., 2008), DeStrooper (De Strooper, 2010) or Tanzi (Tanzi et al., 2004)). Proteolytic cleavage of Aβ is critical to clearance and at least 5 different proteases are known to cleave Aβ1-40 and Aβ1-42, which constitute amyloid deposits in the parenchyma and in the cerebral vasculature. These include Insulin degrading enzyme (IDE), neprilysin (NEP), endothelin converting enzyme (ECE1,2), plasmin, cathespin b and the matrix metalloproteinase family including MMP-2, -3, -9 and MT1-MMP (De Strooper, 2010; Liao and Van Nostrand, 2010; Miners et al., 2009). A variety of smaller Aβ peptides are produced by the action of these proteases which generally are less toxic, do not aggregate easily to form deposits and can be more readily cleared from the brain (Miners et al., 2008).
In this study we have examined the role of gelatinases, and in particular MMP-9, in the clearance of amyloid. Identification of peptide fragments in brain lysates can be used as a method to determine which proteases are active under pathological conditions. Accordingly we have used the degradation product Aβ1-16 as an index of proteolytic activity of MMPs. MMP-2 and MMP-9 are well known to degrade soluble Aβ in vitro (Backstrom et al., 1996; Hartell and Suh, 2000; Qiu et al., 1997; Roher et al., 1994; Yan et al., 2006) and studies using MMP knockout mice or gene silencing have further indicated that amyloid deposition is increased when MMP activity is decreased (Van Vickle et al., 2008; Yin et al., 2006). We have used both in vitro and in vivo models to delineate a role for NO in the regulation of MMP-9 and hence, in the regulation of amyloid deposition/clearance. Our data using a unique mouse model of AD that expresses mutated human APP genes on a mouse NOS2 knockout background, confirm the ability of MMP-9 to degrade fAβ. But, importantly, we now show that MMP-9 activity and its inhibitor, TIMP-1, are regulated by NO, thus connecting amyloid clearance, at least in part, to levels of NO generated during the immune response and to the MMP-9/TIMP-1 balance in the local environment.
The matrix metalloproteinase family and in particular, MMP-9, are tightly linked to immune activation and thus contribute to matrix restructuring and wound healing during acute and chronic inflammatory events in the brain and throughout the body (Candelario-Jalil et al., 2009; del Zoppo, 2010). While MMP-9 is found in neurons of the hippocampus and cortex and in blood vessel endothelial cells (Backstrom et al., 1996), it is primarily produced by immune activated microglia and astrocytes, which are the equivalent of the brain’s immune cells (Colton et al., 1993; Gottschall et al., 1995). For example, treatment of cultured BV2 cells or primary microglia with lipopolysaccharide (LPS), cytokines such as IL-1β or Aβ peptides increases expression and activity of MMP-9 (Colton et al., 1993; Crocker et al., 2008; Gottschall, 1996). In AD brain, MMP-9 expression is found in astrocytes and microglia around amyloid plaques and in the blood vessel walls associated with cerebrovascular amyloid deposits (Backstrom et al., 1996; Miners et al., 2008). Interestingly, the protein levels of MMP between AD and normal control brain are not significantly different, but rather activity differences in their levels of activity can be found (Backstrom et al., 1996).
NO is likely to be a critical regulatory factor for MMP activity during immune responses. NO-mediated changes in MMP expression and activity have been shown in multiple studies with both increased and decreased proteolytic activities observed (Bove et al., 2007; Liu et al., 2006; Shin et al., 2007). While some of these differences may be tissue and immune stimulation specific, Ridnour et al (Ridnour et al., 2007) showed that different levels of NO may have different actions on MMP-9 expression and activity. In our experiments on ANA-1 cells, NO’s primary effect at low concentrations was indirect, via an action on expression of the MMP-9 inhibitor known as TIMP-1. Higher NO flux (>500 nM), however, directly activated MMP-9 via the production of reactive nitrogen species (RNS) (Ridnour et al., 2007). Multiple sites on both pro-MMP-9 and MMP-9 are altered by RNS. In astrocytes, nitration of tyrosine residues on MMP-9 resulted in activation of the enzyme and stimulated MMP-mediated functions such as cell migration (Van Vickle et al., 2008; Wang et al., 2011). As shown by mass spectrometry analysis, other RNS mediated modifications such as thionitrate, sulfinic acid and sulfonic acid modification of the “cysteine-switch” of pro-MMP-9 have been found. In addition, exposure to nitroglycerin induced modifications in the fibronectin domain of the pro-MMP-9 protein that influenced substrate recognition and binding. These same authors also showed that the NO donor DETA/NO promoted sulfonic acid modifications of the cysteine switch of pro-MMP-9 (Krishnatry et al., 2011a; Krishnatry et al., 2011b). Collectively, these reports support a primary role for NO in regulation of MMP-9 proteolytic activity.
In a manner similar to MMP regulation, immune cells release the TIMP family of inhibitors as part of the matrix restructuring system that occurs during the repair and resolution phase of an immune response. This phase of immunity is commonly associated with low NO levels (Colton and Wilcock, 2009; Gordon and Taylor, 2005). In the brain, TIMP-1 is released primarily from microglia or astrocytes into the local environment where it acts to reduce proteolysis by binding to the MMP catalytic site and thus decreasing MMP proteolytic activity (Candelario-Jalil et al., 2009; Gardner and Ghorpade, 2003; Van den Steen et al., 2002). Nitration of TIMP-1 has recently been shown to reduce its ability to bind and inhibit active MMP-9 (Patruno et al., 2012). In support of this observation, we have recently employed mass spectrometry to identify tyrosine nitration of TIMP-1 at Y95 and Y143 where these tyrosine residues reside near disulfide bonds that are critical for TIMP-1 binding and inhibition of active MMPs (Ridnour et al., 2012, PLoS ONE, in press). High levels of NO, thus, would be likely to reduce TIMP-1’s anti-proteolytic activity. In addition, our in vitro data from NO treated BV2 microglia showed decreased TIMP-1 levels by ELISA as NO levels increased. The resulting increased ratio of MMP-9/TIMP-1 favors MMP-9-mediated proteolysis and is consistent with the increased fAβ degradation observed in the culture supernatants as well as the increased Aβ1-16 in APPSwDI (High NOS2) mice when compared to APPSwDI/NOS2−/− (NOS2 null) animals. Thus, the MMP-9/TIMP-1 balance reflects the true extent of proteolytic activity regulated by NO.
We have examined the in vivo relationship between MMP-9, NO and Aβ degradation by comparing an AD mouse model that expresses NOS2 with a mouse model of AD that lacks NOS2. Both the APPSwDI parent strain and the bigenic APPSwDI/NOS2−/− strain show abundant Aβ in aged animals. However, the level of Aβ in younger (28-30wk) APPSwDI mice is less than that observed in APPSwDI/NOS2−/− of the same age. We predicted that the lower Aβ level may be due to increased clearance caused by increased MMP-9 proteolytic activity in the APPSwDI strain. In situ zymography suggested that hippocampal gelatinase activity was indeed greater in APPSwDI mice when compared to either APPSwDI/NOS2−/− or NOS2−/− mice. However, we were unable to quantitate significant differences using gel zymography on gelatinase-extracted whole brain lysates. Quantitative RT-PCR also showed that there was no significant differences in MMP-9 mRNA expression levels at 12 wks between the 2 mice strains. However, TIMP-1 mRNA expression was significantly higher in the APPSwDI/NOS2−/− mice that do not have NOS2 or immune activated NO production compared to APPSwDI mice that have a normal NOS2. These data are consistent with a decreased MMP-9/TIMP-1 ratio that may lead to decreased proteolytic activity and decreased Aβ clearance in the mice lacking NOS2.
The use of the antibody recognizing Aβ1-16 has provided a more direct analysis of the role of MMP-9 activity in Aβ degradation by allowing us to quantitate this specific cleavage product of Aβ by mass spectrometry analysis. A significantly higher level of Aβ1-16 was found in the parent mice expressing NOS2 compared to the bigenic mice lacking NOS2. Of the enzymes that degrade Aβ, MMP-2 and MMP-9 are the most likely candidates to generate Aβ1-16 in our mouse models of AD (Yan et al., 2006). However, of these only MMP-9 has shown NO-mediated regulation in our study and thus would be affected by NOS2 knockout. Also, insoluble Aβ was increased in the APPSwDI/NOS2−/− animal brains and MMP-9, but not MMP-2, can degrade insoluble Aβ. Thus, these results suggest that the increased production of Aβ1-16 in APPSwDI mice may be due to enhanced MMP-9 activity and Aβ clearance in the presence of NOS2-derived NO. Lower Aβ1-16 levels in the APPSwDI/NOS2−/− mice, then, may reflect a lower MMP/TIMP ratio and lower MMP activity. Although the sample size is too low to obtain statistical significance, there is a strong trend that shows Aβ1-16 is lower in cortical lysates from humans with AD compared to their age matched controls. This difference may also reflect a relatively lower level of NOS2 activation and NO production in humans with AD compared to normal age matched individuals.
Interestingly, Aβ1-16 may have independent effects in the brain that influence amyloid-mediated pathology. Van Muiswinkel et al (Van Muiswinkel et al., 1999) have shown that Aβ1-16 can block Aβ1-42 induced production of superoxide anion by NOX2 (NADPH oxidase) in human monocyte derived macrophages. Aβ1-16, however, can also bind copper ions and may alter brain functions through this action (Ma et al., 2006). Nonetheless, our results show reduced Aβ1-16 in APPSwDI/NOS2−/− animals and these animals progressively exhibit exacerbated AD pathology when compared to the APPSwDI mouse containing functional NOS2 enzyme.
In summary, changes in NO and NO’s regulation of MMP-9 and TIMP-1 are likely to play pivotal roles in immune responses in the brain. In humans with AD, the predisposition for inherently lowered NOS2 activation compared to mice (Colton et al., 1996; Ganster et al., 2001; Guo et al., 2012; Weinberg et al., 1995), coupled with increased TIMP-1 due to the M2 activation found in chronic immune responses tips the MMP-9/TIMP-1 balance in favor of restricted proteolytic activity and hence amyloid accumulation. Increasing MMP activity by antibody based immunization against Aβ (Wilcock et al., 2011) or by the accumulation of peripheral polymorphonuclear phagocytes as seen in MOG45D immunization of Aβ transgenic mice (Koronyo-Hamaoui et al., 2009) can reduce amyloid. However, increased proteolytic activity at the blood brain barrier may also lead to neurovascular unit changes that are not beneficial (Bell et al., 2012; Wilcock et al., 2011).
Supplementary Material
Acknowledgements
This work was funded in part by an NIH grant (AG031845) awarded to CAC. The authors would also like to thank the Intramural Research Program of the National Institutes of Health, National Cancer Institute, and Center for Cancer Research who have supported this work.
Abbreviations
- NOS2
nitric oxide synthase
- NO
nitric oxide
- MMP
matrix metalloproteinase
- TIMP-1
Tissue inhibitor of metalloproteinase
- MRM
mass spectrometry multi-reaction monitoring
Footnotes
Conflict of Interest: Michael P. Vitek is a principal in Cognosci Inc, Research Triangle Park NC and Carol A. Colton is his spouse. All others have no conflict of interest to report.
Supporting Information: Supplement Figure 1: A) Summary of Aβ1-42 digestion sites by MMP-2 and MMP-9 recombinant enzymes. B) Time course degradation of soluble Aβ 1-42 by MMP-2 and MMP-9. C) Aβ 1-16 product formation during Aβ 1-42 degradation by MMP-2 and MMP-9.
References
- Aizenstein HJ, Nebes RD, Saxton JA, Price JC, Mathis CA, Tsopelas ND, Ziolko SK, James JA, Snitz BE, Houck PR, Bi W, Cohen AD, Lopresti BJ, DeKosky ST, Halligan EM, Klunk WE. Frequent amyloid deposition without significant cognitive impairment among the elderly. Archives of neurology. 2008;65:1509–1517. doi: 10.1001/archneur.65.11.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backstrom JR, Lim GP, Cullen MJ, Tokes ZA. Matrix metalloproteinase-9 (MMP-9) is synthesized in neurons of the human hippocampus and is capable of degrading the amyloid-beta peptide (1-40) J Neurosci. 1996;16:7910–7919. doi: 10.1523/JNEUROSCI.16-24-07910.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basak JM, Kim J, Pyatkivskyy Y, Wildsmith KR, Jiang H, Parsadanian M, Patterson BW, Bateman RJ, Holtzman DM. Measurement of apolipoprotein E and amyloid beta clearance rates in the mouse brain using bolus stable isotope labeling. Molecular neurodegeneration. 2012;7:14. doi: 10.1186/1750-1326-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RD, Winkler EA, Singh I, Sagare AP, Deane R, Wu Z, Holtzman DM, Betsholtz C, Armulik A, Sallstrom J, Berk BC, Zlokovic BV. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature. 2012;485:512–516. doi: 10.1038/nature11087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bove PF, Wesley UV, Greul AK, Hristova M, Dostmann WR, van der Vliet A. Nitric oxide promotes airway epithelial wound repair through enhanced activation of MMP-9. American journal of respiratory cell and molecular biology. 2007;36:138–146. doi: 10.1165/rcmb.2006-0253SM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candelario-Jalil E, Yang Y, Rosenberg GA. Diverse roles of matrix metalloproteinases and tissue inhibitors of metalloproteinases in neuroinflammation and cerebral ischemia. Neuroscience. 2009;158:983–994. doi: 10.1016/j.neuroscience.2008.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colton C, Wilt S, Gilbert D, Chernyshev O, Snell J, Dubois-Dalcq M. Species differences in the generation of reactive oxygen species by microglia. Mol Chem Neuropathol. 1996;28:15–20. doi: 10.1007/BF02815200. [DOI] [PubMed] [Google Scholar]
- Colton CA, Keri JE, Chen WT, Monsky WL. Protease production by cultured microglia: substrate gel analysis and immobilized matrix degradation. Journal of neuroscience research. 1993;35:297–304. doi: 10.1002/jnr.490350309. [DOI] [PubMed] [Google Scholar]
- Colton CA, Wilcock DM. Assessing Activation States in Microglia. CNS Neurol Disord Drug Targets. 2009 doi: 10.2174/187152710791012053. [DOI] [PubMed] [Google Scholar]
- Crocker SJ, Frausto RF, Whitton JL, Milner R. A novel method to establish microglia-free astrocyte cultures: comparison of matrix metalloproteinase expression profiles in pure cultures of astrocytes and microglia. Glia. 2008;56:1187–1198. doi: 10.1002/glia.20689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies KM, Wink DA, Saavedra JE, Keefer LK. Chemistry of the diazeniumdiolates. 2. Kinetics and mechanism of dissociation to nitric oxide in aqueous solution. Journal of the American Chemical Society. 2001;123:5473–5481. doi: 10.1021/ja002899q. [DOI] [PubMed] [Google Scholar]
- De Strooper B. Proteases and proteolysis in Alzheimer disease: a multifactorial view on the disease process. Physiological reviews. 2010;90:465–494. doi: 10.1152/physrev.00023.2009. [DOI] [PubMed] [Google Scholar]
- Death AK, Nakhla S, McGrath KC, Martell S, Yue DK, Jessup W, Celermajer DS. Nitroglycerin upregulates matrix metalloproteinase expression by human macrophages. Journal of the American College of Cardiology. 2002;39:1943–1950. doi: 10.1016/s0735-1097(02)01907-1. [DOI] [PubMed] [Google Scholar]
- del Zoppo GJ. The neurovascular unit, matrix proteases, and innate inflammation. Annals of the New York Academy of Sciences. 2010;1207:46–49. doi: 10.1111/j.1749-6632.2010.05760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandy S. The role of cerebral amyloid beta accumulation in common forms of Alzheimer disease. The Journal of clinical investigation. 2005;115:1121–1129. doi: 10.1172/JCI25100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganster RW, Taylor BS, Shao L, Geller DA. Complex regulation of human inducible nitric oxide synthase gene transcription by Stat 1 and NF-kappa B. Proc Natl Acad Sci U S A. 2001;98:8638–8643. doi: 10.1073/pnas.151239498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner J, Ghorpade A. Tissue inhibitor of metalloproteinase (TIMP)-1: the TIMPed balance of matrix metalloproteinases in the central nervous system. Journal of neuroscience research. 2003;74:801–806. doi: 10.1002/jnr.10835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George SJ, Johnson JL. In situ zymography. Methods Mol Biol. 2010;622:271–277. doi: 10.1007/978-1-60327-299-5_17. [DOI] [PubMed] [Google Scholar]
- Glenner GG. The pathobiology of Alzheimer’s disease. Annual review of medicine. 1989;40:45–51. doi: 10.1146/annurev.me.40.020189.000401. [DOI] [PubMed] [Google Scholar]
- Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- Gottschall PE. beta-Amyloid induction of gelatinase B secretion in cultured microglia: inhibition by dexamethasone and indomethacin. Neuroreport. 1996;7:3077–3080. doi: 10.1097/00001756-199611250-00057. [DOI] [PubMed] [Google Scholar]
- Gottschall PE, Yu X. Cytokines regulate gelatinase A and B (matrix metalloproteinase 2 and 9) activity in cultured rat astrocytes. Journal of neurochemistry. 1995;64:1513–1520. doi: 10.1046/j.1471-4159.1995.64041513.x. [DOI] [PubMed] [Google Scholar]
- Gottschall PE, Yu X, Bing B. Increased production of gelatinase B (matrix metalloproteinase-9) and interleukin-6 by activated rat microglia in culture. Journal of neuroscience research. 1995;42:335–342. doi: 10.1002/jnr.490420307. [DOI] [PubMed] [Google Scholar]
- Guo Z, Shao L, Zheng L, Du Q, Li P, John B, Geller DA. miRNA-939 regulates human inducible nitric oxide synthase posttranscriptional gene expression in human hepatocytes. Proc Natl Acad Sci U S A. 2012;109:5826–5831. doi: 10.1073/pnas.1118118109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartell NA, Suh YH. Peptide fragments of beta-amyloid precursor protein: effects on parallel fiber-Purkinje cell synaptic transmission in rat cerebellum. Journal of neurochemistry. 2000;74:1112–1121. doi: 10.1046/j.1471-4159.2000.741112.x. [DOI] [PubMed] [Google Scholar]
- Hawkes CA, Hartig W, Kacza J, Schliebs R, Weller RO, Nicoll JA, Carare RO. Perivascular drainage of solutes is impaired in the ageing mouse brain and in the presence of cerebral amyloid angiopathy. Acta neuropathologica. 2011;121:431–443. doi: 10.1007/s00401-011-0801-7. [DOI] [PubMed] [Google Scholar]
- Huang Y, Potter R, Sigurdson W, Santacruz A, Shih S, Ju YE, Kasten T, Morris JC, Mintun M, Duntley S, Bateman RJ. Effects of age and amyloid deposition on Abeta dynamics in the human central nervous system. Archives of neurology. 2012;69:51–58. doi: 10.1001/archneurol.2011.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JE, Lim MM, Bateman RJ, Lee JJ, Smyth LP, Cirrito JR, Fujiki N, Nishino S, Holtzman DM. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science. 2009;326:1005–1007. doi: 10.1126/science.1180962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiner DE, Stetler-Stevenson WG. Quantitative zymography: detection of picogram quantities of gelatinases. Analytical biochemistry. 1994;218:325–329. doi: 10.1006/abio.1994.1186. [DOI] [PubMed] [Google Scholar]
- Koronyo-Hamaoui M, Ko MK, Koronyo Y, Azoulay D, Seksenyan A, Kunis G, Pham M, Bakhsheshian J, Rogeri P, Black KL, Farkas DL, Schwartz M. Attenuation of AD-like neuropathology by harnessing peripheral immune cells: local elevation of IL-10 and MMP-9. Journal of neurochemistry. 2009;111:1409–1424. doi: 10.1111/j.1471-4159.2009.06402.x. [DOI] [PubMed] [Google Scholar]
- Krishnatry AS, Fung SM, Brazeau DA, Soda D, Fung HL. Nitroglycerin alters matrix remodeling proteins in THP-1 human macrophages and plasma metalloproteinase activity in rats. Nitric oxide : biology and chemistry / official journal of the Nitric Oxide Society. 2011a;24:66–76. doi: 10.1016/j.niox.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnatry AS, Kamei T, Wang H, Qu J, Fung HL. Identification of nitroglycerin-induced cysteine modifications of pro-matrix metalloproteinase-9. Rapid communications in mass spectrometry : RCM. 2011b;25:2291–2298. doi: 10.1002/rcm.5118. [DOI] [PubMed] [Google Scholar]
- Laubach VE, Foley PL, Shockey KS, Tribble CG, Kron IL. Protective roles of nitric oxide and testosterone in endotoxemia: evidence from NOS-2-deficient mice. Am J Physiol. 1998;275:H2211–2218. doi: 10.1152/ajpheart.1998.275.6.H2211. [DOI] [PubMed] [Google Scholar]
- LeVine H., 3rd Quantification of beta-sheet amyloid fibril structures with thioflavin T. Methods in enzymology. 1999;309:274–284. doi: 10.1016/s0076-6879(99)09020-5. [DOI] [PubMed] [Google Scholar]
- Liao MC, Van Nostrand WE. Degradation of soluble and fibrillar amyloid beta-protein by matrix metalloproteinase (MT1-MMP) in vitro. Biochemistry. 2010;49:1127–1136. doi: 10.1021/bi901994d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Rosenberg GA, Liu KJ. AUF-1 mediates inhibition by nitric oxide of lipopolysaccharide-induced matrix metalloproteinase-9 expression in cultured astrocytes. Journal of neuroscience research. 2006;84:360–369. doi: 10.1002/jnr.20895. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Ma QF, Hu J, Wu WH, Liu HD, Du JT, Fu Y, Wu YW, Lei P, Zhao YF, Li YM. Characterization of copper binding to the peptide amyloid-beta(1-16) associated with Alzheimer’s disease. Biopolymers. 2006;83:20–31. doi: 10.1002/bip.20523. [DOI] [PubMed] [Google Scholar]
- Miners JS, Baig S, Palmer J, Palmer LE, Kehoe PG, Love S. Abeta-degrading enzymes in Alzheimer’s disease. Brain Pathol. 2008;18:240–252. doi: 10.1111/j.1750-3639.2008.00132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miners JS, Baig S, Tayler H, Kehoe PG, Love S. Neprilysin and insulin-degrading enzyme levels are increased in Alzheimer disease in relation to disease severity. Journal of neuropathology and experimental neurology. 2009;68:902–914. doi: 10.1097/NEN.0b013e3181afe475. [DOI] [PubMed] [Google Scholar]
- Miners JS, Barua N, Kehoe PG, Gill S, Love S. Abeta-degrading enzymes: potential for treatment of Alzheimer disease. Journal of neuropathology and experimental neurology. 2011;70:944–959. doi: 10.1097/NEN.0b013e3182345e46. [DOI] [PubMed] [Google Scholar]
- Morgan D. Immunotherapy for Alzheimer’s disease. Journal of Alzheimer’s disease : JAD. 2006;9:425–432. doi: 10.3233/jad-2006-9s348. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Binder L, Counts SE, DeKosky ST, de Toledo-Morrell L, Ginsberg SD, Ikonomovic MD, Perez SE, Scheff SW. Mild cognitive impairment: pathology and mechanisms. Acta neuropathologica. 2012;123:13–30. doi: 10.1007/s00401-011-0884-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patruno A, Pesce M, Marrone A, Speranza L, Grilli A, De Lutiis MA, Felaco M, Reale M. Activity of matrix metallo proteinases (MMPs) and the tissue inhibitor of MMP (TIMP)-1 in electromagnetic field-exposed THP-1 cells. Journal of cellular physiology. 2012;227:2767–2774. doi: 10.1002/jcp.23024. [DOI] [PubMed] [Google Scholar]
- Qiu WQ, Ye Z, Kholodenko D, Seubert P, Selkoe DJ. Degradation of amyloid beta-protein by a metalloprotease secreted by microglia and other neural and non-neural cells. The Journal of biological chemistry. 1997;272:6641–6646. doi: 10.1074/jbc.272.10.6641. [DOI] [PubMed] [Google Scholar]
- Ridnour LA, Windhausen AN, Isenberg JS, Yeung N, Thomas DD, Vitek MP, Roberts DD, Wink DA. Nitric oxide regulates matrix metalloproteinase-9 activity by guanylyl-cyclase-dependent and -independent pathways. Proc Natl Acad Sci U S A. 2007;104:16898–16903. doi: 10.1073/pnas.0702761104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roher AE, Kasunic TC, Woods AS, Cotter RJ, Ball MJ, Fridman R. Proteolysis of A beta peptide from Alzheimer disease brain by gelatinase A. Biochemical and biophysical research communications. 1994;205:1755–1761. doi: 10.1006/bbrc.1994.2872. [DOI] [PubMed] [Google Scholar]
- Shin CY, Lee WJ, Choi JW, Choi MS, Ryu JR, Oh SJ, Cheong JH, Choi EY, Ko KH. Down-regulation of matrix metalloproteinase-9 expression by nitric oxide in lipopolysaccharide-stimulated rat primary astrocytes. Nitric oxide : biology and chemistry / official journal of the Nitric Oxide Society. 2007;16:425–432. doi: 10.1016/j.niox.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Tanzi RE, Moir RD, Wagner SL. Clearance of Alzheimer’s Abeta peptide: the many roads to perdition. Neuron. 2004;43:605–608. doi: 10.1016/j.neuron.2004.08.024. [DOI] [PubMed] [Google Scholar]
- Thomas DD, Miranda KM, Espey MG, Citrin D, Jourd’heuil D, Paolocci N, Hewett SJ, Colton CA, Grisham MB, Feelisch M, Wink DA. Guide for the use of nitric oxide (NO) donors as probes of the chemistry of NO and related redox species in biological systems. Methods in enzymology. 2002;359:84–105. doi: 10.1016/s0076-6879(02)59174-6. [DOI] [PubMed] [Google Scholar]
- Van den Steen PE, Dubois B, Nelissen I, Rudd PM, Dwek RA, Opdenakker G. Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9) Critical reviews in biochemistry and molecular biology. 2002;37:375–536. doi: 10.1080/10409230290771546. [DOI] [PubMed] [Google Scholar]
- Van Muiswinkel FL, Raupp SF, de Vos NM, Smits HA, Verhoef J, Eikelenboom P, Nottet HS. The amino-terminus of the amyloid-beta protein is critical for the cellular binding and consequent activation of the respiratory burst of human macrophages. Journal of neuroimmunology. 1999;96:121–130. doi: 10.1016/s0165-5728(99)00019-3. [DOI] [PubMed] [Google Scholar]
- Van Vickle GD, Esh CL, Daugs ID, Kokjohn TA, Kalback WM, Patton RL, Luehrs DC, Walker DG, Lue LF, Beach TG, Davis J, Van Nostrand WE, Castano EM, Roher AE. Tg-SwDI transgenic mice exhibit novel alterations in AbetaPP processing, Abeta degradation, and resilient amyloid angiopathy. The American journal of pathology. 2008;173:483–493. doi: 10.2353/ajpath.2008.071191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellas B, Black R, Thal LJ, Fox NC, Daniels M, McLennan G, Tompkins C, Leibman C, Pomfret M, Grundman M. Long-term follow-up of patients immunized with AN1792: reduced functional decline in antibody responders. Current Alzheimer research. 2009;6:144–151. doi: 10.2174/156720509787602852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdile G, Fuller S, Atwood CS, Laws SM, Gandy SE, Martins RN. The role of beta amyloid in Alzheimer’s disease: still a cause of everything or the only one who got caught? Pharmacological research : the official journal of the Italian Pharmacological Society. 2004;50:397–409. doi: 10.1016/j.phrs.2003.12.028. [DOI] [PubMed] [Google Scholar]
- Wang HH, Hsieh HL, Yang CM. Nitric oxide production by endothelin-1 enhances astrocytic migration via the tyrosine nitration of matrix metalloproteinase-9. Journal of cellular physiology. 2011;226:2244–2256. doi: 10.1002/jcp.22560. [DOI] [PubMed] [Google Scholar]
- Wei L, Gravitt PE, Song H, Maldonado AM, Ozbun MA. Nitric oxide induces early viral transcription coincident with increased DNA damage and mutation rates in human papillomavirus-infected cells. Cancer research. 2009;69:4878–4884. doi: 10.1158/0008-5472.CAN-08-4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg JB, Misukonis MA, Shami PJ, Mason SN, Sauls DL, Dittman WA, Wood ER, Smith GK, McDonald B, Bachus KE, et al. Human mononuclear phagocyte inducible nitric oxide synthase (iNOS): analysis of iNOS mRNA, iNOS protein, biopterin, and nitric oxide production by blood monocytes and peritoneal macrophages. Blood. 1995;86:1184–1195. [PubMed] [Google Scholar]
- Weiss JM, Ridnour LA, Back T, Hussain SP, He P, Maciag AE, Keefer LK, Murphy WJ, Harris CC, Wink DA, Wiltrout RH. Macrophage-dependent nitric oxide expression regulates tumor cell detachment and metastasis after IL-2/anti-CD40 immunotherapy. The Journal of experimental medicine. 2010;207:2455–2467. doi: 10.1084/jem.20100670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller RO, Subash M, Preston SD, Mazanti I, Carare RO. Perivascular drainage of amyloid-beta peptides from the brain and its failure in cerebral amyloid angiopathy and Alzheimer’s disease. Brain Pathol. 2008;18:253–266. doi: 10.1111/j.1750-3639.2008.00133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcock DM, Lewis MR, Van Nostrand WE, Davis J, Previti ML, Gharkholonarehe N, Vitek MP, Colton CA. Progression of amyloid pathology to Alzheimer’s disease pathology in an amyloid precursor protein transgenic mouse model by removal of nitric oxide synthase 2. J Neurosci. 2008;28:1537–1545. doi: 10.1523/JNEUROSCI.5066-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcock DM, Morgan D, Gordon MN, Taylor TL, Ridnour LA, Wink DA, Colton CA. Activation of matrix metalloproteinases following anti-Abeta immunotherapy; implications for microhemorrhage occurrence. J Neuroinflammation. 2011;8:115. doi: 10.1186/1742-2094-8-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan P, Hu X, Song H, Yin K, Bateman RJ, Cirrito JR, Xiao Q, Hsu FF, Turk JW, Xu J, Hsu CY, Holtzman DM, Lee JM. Matrix metalloproteinase-9 degrades amyloid-beta fibrils in vitro and compact plaques in situ. The Journal of biological chemistry. 2006;281:24566–24574. doi: 10.1074/jbc.M602440200. [DOI] [PubMed] [Google Scholar]
- Yin KJ, Cirrito JR, Yan P, Hu X, Xiao Q, Pan X, Bateman R, Song H, Hsu FF, Turk J, Xu J, Hsu CY, Mills JC, Holtzman DM, Lee JM. Matrix metalloproteinases expressed by astrocytes mediate extracellular amyloid-beta peptide catabolism. J Neurosci. 2006;26:10939–10948. doi: 10.1523/JNEUROSCI.2085-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JW, Gottschall PE. Zymographic measurement of gelatinase activity in brain tissue after detergent extraction and affinity-support purification. Journal of neuroscience methods. 1997;76:15–20. doi: 10.1016/s0165-0270(97)00065-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.