Abstract
Background
The onset and progression of breast cancer (BC) is influenced by many factors, including the single nucleotide polymorphism (SNP) rs13281615 at 8q24. However, studies of the potential association between rs13281615 at 8q24 and risk of BC have given inconsistent results. We performed a meta-analysis to address this controversy.
Methods
PubMed, EMBASE and the Chinese National Knowledge Infrastructure databases were systematically searched to identify relevant studies. Two curators independently extracted data, and odds ratios (ORs) with 95% confidence intervals (95% CIs) were calculated to assess the strength of the association between rs13281615 at 8q24 and risk of BC.
Results
Fourteen studies are included in the meta-analysis, involving 44,283 cases (5,170 Chinese and 39,113 mixed) and 55,756 controls (5,589 Chinese and 50,167 mixed). The GG and G-allele genotypes of rs13281615 at 8q24 are significantly associated with increased risk of BC (GG vs. AG+AA, OR 1.13, 95% CI 1.08–1.19, P<0.001; G-allele vs. A-allele, OR 1.10, 95% CI 1.06–1.14, P<0.001; GG vs. AA, OR 1.20, 95% CI 1.12–1.29, P<0.001). Conversely, the AA genotype is significantly associated with decreased risk of BC (AA vs. AG+GG, OR 0.89, 95% CI 0.84–0.93, P<0.001).
Conclusion
G-allele genotypes of rs13281615 at 8q24 polymorphism are a risk factor for developing BC, while the AA genotype is a protective factor. Further large and well-designed studies are required to confirm this conclusion.
Introduction
Breast cancer (BC) is one of the most prevalent invasive cancers and the second leading global cause of cancer-related deaths among women [1]. Global BC incidence has been increasing by more than one million new cases annually [2]. Incidence is significantly higher in developed countries than in developing ones; in fact, it was the first malignant disease to pose a significant threat to women [3], [4]. Despite the frequency and severity of BC, the pathogenesis and progression of BC are still not fully understood.
Many researchers have concluded that BC is the cumulative result of multiple environmental factors and genetic alterations [5]. Epidemiological studies have suggested that estrogen stimulation [6], high birth weight [7], obesity [8] and family history of BC [9], [10] may be associated with increased risk of BC in postmenopausal women. However, only some patients with family history of BC develop malignancy; most cases of BC are sporadic. Therefore, genetic polymorphism may contribute to the development of BC. This is supported by numerous meta-analyses that have found certain genetic polymorphisms to correlate strongly with susceptibility to BC [11]–[13]. High-penetrance breast cancer susceptibility genes, such as BRCA1 and BRCA2, have low mutation rates and therefore explain only a small fraction of breast cancers in the general population [14]. This suggests the need to identify additional polymorphisms linked to risk of BC.
Two large-scale genome-wide association studies identified several common polymorphisms that may be linked to susceptibility to BC [15], [16], including single nucleotide polymorphisms (SNPs) at rs 13281615. Located in the non-coding chromosomal region 8q24, the function of rs 13281615 is unclear. The locus lies near the myelocytomatosis oncogene (MYC) at 8q24.12–24.13, which can promote cell proliferation, differentiation and transformation. It plays an important role in the development of many types of cancer, including BC [17]. Sole and coworkers [18] proposed that variations in putative cis-regulators of transcription in 8q24 can significantly alter germline c-MYC expression levels and thereby contribute to cancer susceptibility.
Ever since three studies [15], [19], [20] reported associations between the 8q24 rs13281615 SNP and various cancers, including BC, numerous epidemiological studies have evaluated the association between rs13281615 at 8q24 and BC and have generated sometimes inconsistent results. To provide a clearer picture of the effects of this SNP on risk of BC, a meta-analysis was performed.
Materials and Methods
Publication Search Strategy
PubMed, EMBASE and the Chinese National Knowledge Infrastructure (CNKI) databases were searched through December 2012 for case-control studies about 8q24 rs13281615 SNP and BC risk. The following search terms were used: “8q24,” “rs13281615,” “SNP,” “single nucleotide polymorphism,” “polymorphism,” “mutation,” “variant,” “BC,” “breast neoplasm” and “breast cancer.” Reference lists in each identified article were also searched manually to identify additional eligible studies.
Inclusion Criteria
To be included in the meta-analysis, studies had to (1) assess the association between the 8q24 rs13281615 SNP and risk of BC occurrence, (2) use a case-control design and (3) provide sufficient data for estimating odds ratios (ORs) with 95% confidence intervals (CIs). In the case of multiple studies by the same researchers involving the same or overlapping data sets, we selected the most recent study with the largest number of participants.
Data Extraction
Two curators (W-FG and J-HZ) independently extracted information from included studies. Disagreement was resolved by discussion between the two authors. The following data were extracted: first author’s family name, year of publication, country of origin, source of controls, total numbers of cases and controls, Hardy-Weinberg equilibrium (HWE) of controls and the frequency of rs13281615 genotypes at 8q24 in cases and controls.
Statistical Methods
All statistical tests were performed using Review Manager 5.1.4 software. The strength of association between the 8q24 rs13281615 SNP and BC risk was assessed by calculating ORs with 95% CIs based on the genotype frequencies in cases and controls. Subgroup analysis was performed based on ethnicity, categorized as Chinese or mixed (predominantly Caucasian).
The significance of pooled ORs was determined using the Z-test, with P<0.05 defined as the significance threshold. Meta-analysis was conducted using the random-effects model, except when P>0.10 for the Q-test, indicating lack of heterogeneity among studies; in this case, the fixed-effects model was used. Publication bias was assessed by visual inspection of Begg’s funnel plots. HWE in the control group was assessed using the asymptotic test, with P<0.05 considered significant.
Results
Description of Studies
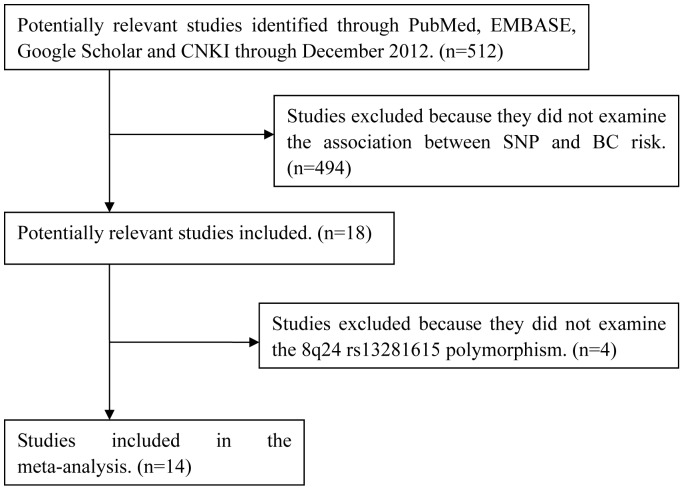
Database searches through December 2012 revealed 512 potentially relevant publications about rs13281615 at 8q24 and risk of BC (Figure 1). Screening titles and abstracts led to exclusion of 494 articles because they were laboratory studies or review articles, they examined other 8q24 polymorphisms, or they were irrelevant to the current study. This left 18 articles, which were read in full. In the end, 14 articles were found to satisfy the inclusion criteria and were included in the meta-analysis [21]–[34]. These articles involved a total of 44,283 cases and 55,756 controls, of which 5,170 cases and 5,589 controls were Chinese [25]–[27], [32], and the remaining 39,113 cases and 50,167 controls were of mixed ethnicity (>95% Caucasian) [21]–[24], [28]–[31], [33], [34]. In one study [22], 95.6% of cases and 96.7% of controls were European, while 4.4% of cases and 3.3% of controls were Asian. The distribution of rs13281615 genotypes at 8q24 in the controls was consistent with HWE (P>0.05) in all but three studies [24], [28], [29]. Table 1 shows principal characteristics of the included studies.
Figure 1. Flow chart of study selection.
(CNKI, Chinese National Knowledge Infrastructure; SNP, single nucleotide polymorphism; BC, breast cancer.).
Table 1. Principal characteristics of studies included in the meta-analysis.
| Study | Country or Ethnicity | Subjects | P HWE | Case genotypes | Control genotypes | |||||
| Cases | Controls | AA | AG | GG | AA | AG | GG | |||
| Fletcher 200821 | Caucasian | Bilateral, family history | PB | 0.37 | 435 | 730 | 305 | 487 | 629 | 225 |
| Garcia-Closas 200822 | Caucasian and Asian | Sporadic | PB | 0.67 | 4879 | 7284 | 2921 | 7650 | 10682 | 3773 |
| Mcinerney 200923 | Caucasian | Sporadic | PB | 0.10 | 272 | 467 | 178 | 355 | 456 | 182 |
| Tamimi 201024 | Caucasian | Sporadic | PB | <0.001 | 175 | 263 | 223 | 161 | 277 | 273 |
| Long 201025 | Chinese | Sporadic | PB | 0.99 | 679 | 1470 | 796 | 745 | 1491 | 745 |
| Li 201126 | Chinese | Sporadic | HB | 0.75 | 111 | 285 | 162 | 149 | 313 | 173 |
| Jiang 201127 | Chinese | Sporadic | PB | 1.00 | 121 | 247 | 125 | 128 | 255 | 127 |
| Harlid 201228 | Caucasian | Sporadic | PB | 0.04 | 1103 | 1723 | 719 | 1766 | 2357 | 884 |
| Teraoka 201129 | Caucasian | Sporadic | PB | 0.04 | 174 | 292 | 140 | 358 | 623 | 213 |
| Antoniou 200930 | Caucasian | Sporadic | PB | 0.10 | 2519 | 3872 | 1396 | 2187 | 3317 | 1158 |
| Gorodnova 201031 | Caucasian | Sporadic | PB | 0.71 | 35 | 63 | 42 | 52 | 84 | 38 |
| Chan 201232 | Chinese | Sporadic | PB | 0.05 | 303 | 554 | 317 | 406 | 693 | 364 |
| Campa 201133 | Caucasian | Sporadic | PB | 0.11 | 2494 | 4044 | 1764 | 3813 | 5609 | 2193 |
| Shan 201234 | Tunisians | Sporadic | PB | 0.50 | 126 | 281 | 194 | 93 | 176 | 96 |
Note: HWE, Hardy-Weinberg equilibrium; PB, population-based; HB, hospital-based.
Test of Heterogeneity
Table 2 shows the association between the 8q24 rs13281615 SNP and BC risk. The heterogeneity of 8q24 rs13281615 A/G allelic contrast, homozygote comparison, and dominant and recessive genetic models was analyzed for all 14 studies. Random-effects models were used to meta-analyze the entire population and the mixed population. The fixed-effects model was used to meta-analyze the Chinese population.
Table 2. Overall and stratified meta-analyses of the association between the 8q24 rs13281615 single nucleotide polymorphism and breast cancer risk.
| Genotype comparison | OR [95% CI] | Z (P value) | Heterogeneity of study design | Model | |||
| ?2 | df (P value) | I2 | |||||
| Total (44,283 cases, 55,756 controls) | |||||||
| G-allele vs. A-allele | 1.10 [1.06, 1.14] | 4.77 (<0.001) | 39.07 | 13 (<0.001) | 67% | Random | |
| GG vs. AA | 1.20 [1.16, 1.24] | 9.80 (<0.001) | 29.50 | 13 (0.006) | 56% | Random | |
| GG vs. AG+AA | 1.13 [1.08, 1.19] | 4.87 (<0.001) | 22.54 | 13 (0.05) | 42% | Random | |
| AG+GG vs. AA | 1.13 [1.07, 1.19] | 4.76 (<0.001) | 26.71 | 13 (0.01) | 51% | Random | |
| AA vs. AG+GG | 0.89 [0.84, 0.93] | 4.76 (<0.001) | 26.71 | 13 (0.01) | 51% | Random | |
| Ethnic subgroups | |||||||
| Chinese Population (5,170 cases, 5,589 controls) | |||||||
| G-allele vs. A-allele | 1.07 [1.02, 1.13] | 2.57 (0.01) | 2.30 | 3 (0.51) | 0% | Fixed | |
| GG vs. AA | 1.17 [1.05, 1.30] | 2.84 (0. 005) | 0.61 | 3 (0.89) | 0% | Fixed | |
| GG vs. AG+AA | 1.10 [1.01, 1.20] | 2.22 (0.03) | 0.30 | 3 (0.96) | 0% | Fixed | |
| AG+GG vs. AA | 1.11 [1.02, 1.22] | 2.39 (0.02) | 0.83 | 3 (0.84) | 0% | Fixed | |
| AA vs. AG+GG | 0.90 [0.82, 0.98] | 2.39(0.02) | 0.83 | 3 (0.84) | 0% | Fixed | |
| Mixed population (39,113 cases, 50,167 controls) | |||||||
| G-allele vs. A-allele | 1.11 [1.06, 1.17] | 4.29 (<0.001) | 36.16 | 9 (<0.001) | 75% | Random | |
| GG vs. AA | 1.22 [1.12, 1.33] | 4.40 (<0.001) | 28.62 | 9 (<0.001) | 69% | Random | |
| GG vs. AG+AA | 1.15 [1.07, 1.22] | 4.04 (<0.001) | 21.59 | 9 (0.01) | 58% | Random | |
| AG+GG vs. AA | 1.13 [1.07, 1.21] | 3.94 (<0.001) | 25.88 | 9 (0.002) | 65% | Random | |
| AA vs. AG+GG | 0.88 [0.83, 0.94] | 3.94 (<0.001) | 25.88 | 9 (0.002) | 65% | Random | |
Quantitative Data Synthesis
Table 2 shows the summary ORs relating the 8q24 rs13281615 SNP to BC risk based on 44,283 cases and 55,756 controls in all 14 studies. We observed an association between rs13281615 genotype at 8q24 and BC risk in the total population, the mixed population and the Chinese population.
Total population
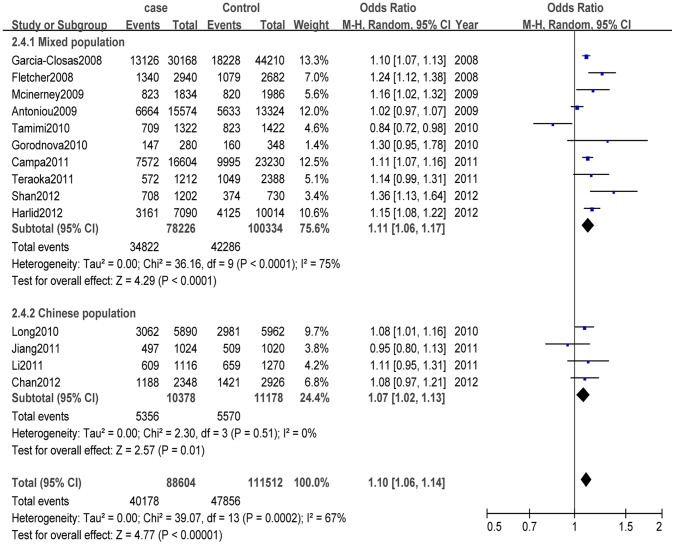
When the dominant genetic comparison model was applied to genotype data for the total population, individuals with the AA genotype of rs13281615 at 8q24 were found to have lower BC risk than individuals with other genotypes based on the random-effects model (OR 0.89, 95% CI 0.84–0.93, P<0.001; I2 = 51%) (Figure S1). In the recessive comparison model, participants with a GG genotype had a higher risk of BC (OR 1.13, 95% CI 1.08–1.19, P<0.001; I2 = 42%) (Figure S2). In the homozygote comparison model, the GG genotype was also associated with higher risk of BC, with a pooled OR of 1.20 (95% CI 1.12–1.29, P<0.001; I2 = 56%) (Figure S3). Most important, by allelic comparison, G-allele genotypes of rs13281615 at 8q24 were associated with higher risk of BC, with a pooled OR of 1.10 (95% CI 1.06–1.14, P<0.001; I2 = 67%) (Figure 2).
Figure 2. Forest plots describing the association of the 8q24 rs13281615 single nucleotide polymorphism with risk of developing breast cancer (G-allele vs. A-allele).
Mixed population
After stratifying the data by ethnicity and applying the recessive comparison model to the mixed population, we found the GG genotype to be associated with higher risk of BC (OR 1.15, 95% CI 1.07–1.22, P<0.001, I2 = 58%). In the homozygote comparison model, the GG genotype was associated with higher risk of BC (OR 1.22, 95% CI 1.12–1.33, P<0.001, I2 = 69%). In the dominant genetic comparison model, the AA genotype was associated with lower risk of BC (OR 0.88, 95% CI 0.83–0.94, P<0.001, I2 = 65%). By allelic comparison, the G-allele was also associated with lower risk of BC (OR 1.11, 95% CI 1.06–1.17, P<0.001, I2 = 75%).
Chinese population
Analysis of the Chinese population in four studies [25]–[27], [32] revealed that the GG genotype was associated with higher risk of BC based on the fixed-effects model (GG vs. AA, OR 1.17, 95% CI 1.05–1.30, P = 0.005, I2 = 0%; GG vs. AG+AA, OR 1.10, 95% CI 1.01–1.20, P = 0.03, I2 = 0%; G-allele vs. A-allele, OR 1.07, 95% CI 1.02–1.13, P = 0.01, I2 = 0%). The AA genotype was associated with lower risk of BC based on the random-effects model (AA vs. AG+GG, OR 0.90, 95% CI 0.82–0.98, P = 0.02, I2 = 0%).
Sensitivity Analysis
Sensitivity analysis was carried out by excluding one study with large heterogeneity [24] or another study with a large weight [22]. In neither case were the pooled ORs significantly affected (data not shown), implying that our results based on all 14 studies were robust. Sensitivity analysis was done by using the random-effect model or the fixed-effect model. Results were not altered (Table S1).
Publication Bias
Begg’s funnel plots were calculated to assess publication bias for reported comparisons of 8q24 rs13281615 SNP and risk of BC. The shape of the funnel plot seemed asymmetrical for GG vs. AG+AA, AA vs. AG+AA, GG vs. AA, and G-allele vs. A-allele, suggesting the presence of publication bias (Figure S4).
Discussion
As with other malignancies, the pathogenesis of BC involves environmental factors, molecular signaling pathways and host genetic factors. Genome-wide association studies conducted since 2007 have identified several genetic loci associated with susceptibility to BC. Some studies have reported an association between 8q24 rs13281615 SNP and BC risk, while others have found no such association. The most likely reason for the inconsistencies among these studies is that they involve relatively small samples. To conduct association studies with larger numbers of participants, we conducted a meta-analysis of published studies. Our results for the total population suggest increased BC risk for subjects carrying the G-allele of rs13281615 at 8q24.
Subgroup analysis by ethnicity allowed us to look for potential ethnic differences in the association. In the Chinese population, the G-allele was associated with increased risk of BC based on allelic contrast, recessive contrast and homozygote comparison. Similarly, for the mixed population (>95% Caucasian), the G-allele was also associated with increased risk of BC. In both the Chinese and mixed populations, the AA genotype of rs13281615 was associated with lower risk of BC. Sensitivity analysis did not alter these results.
Several studies included in the meta-analysis demonstrate that rs13281615 polymorphisms can interact with environmental factors to modulate BC risk [23]–[27], [29], [32], [34]. In fact, rs13281615 polymorphisms were not found to interact with BRCA1 or BRCA2 in BC risk, though only one study [30] examined the possibility of such genetic interactions. These results indicate that the etiology of BC is complex and involves host and environmental factors that may interact synergistically. Indeed, growing epidemiologic evidence suggests that different types of BC have different risk factor profiles and may therefore occur via different pathways.
Incidence of BC and associated mortality differ widely across ethnicities [35]. Caucasians ancestry increases the risk of BC [36]. Incidence of BC among women in most North American and European countries is more than triple that in Asian countries [37]. After analyzing various genetic loci in BC patients in Europe, North America, Australia and Southeast Asia, Easton et al. [15] also suggested that the frequency of the G-allele of rs13281615 at 8q24 was significantly higher among Caucasians than among other populations. Our meta-analysis gave similar results: the G-allele of rs13281615 at 8q24 was a risk factor for BC in the mixed population (>95% Caucasian). At the same time, the G-allele was a risk factor in the Chinese population, which surprisingly showed a higher frequency of the G-allele than did the mixed population. This discrepancy from the work of Easton et al. [15] may reflect the complex multifactorial etiology of BC.
There are several limitations in this meta-analysis. First, the controls were not uniformly defined. Although all controls were healthy populations, it is possible that some of them were community-based, while others were hospital-based. The P value of HWE of three included studies [24], [28], [29] was less than 0.05 (Table 1), suggesting that these study populations were not representative of the broader target population. Second, BC onset and progression are affected by multiple factors, but because of lacking of detailed data, we were unable to conduct stratified analyses based on possible confounders such as age, hormone level, or age at menarche and menopause. Therefore our data may have lacked sufficient statistical power to detect certain associations. Third, again because of a lack of detailed data, we could not examine interactions between the 8q24 rs13281615 SNP and environmental factors or genes known to affect risk of BC. Many gene polymorphisms are associated with risk of BC, and it may be that any single SNP such as rs13281615 at 8q24 is insufficient to cause BC on its own.
Our meta-analysis suggests that the 8q24 rs13281615 SNP affects risk of developing BC: the G-allele increases susceptibility to BC, while the A-allele protects against it. These findings add BC to the list of diseases for which the 8q24 rs13281615 SNP has been implicated as a susceptibility factor, together with prostate cancer [19] and colorectal cancer [11]. Further detailed investigation involving large, multiethnic samples is needed to clarify the role of this SNP in BC, as well as explore gene-gene and gene-environment interactions that may mediate the association between rs13281615 and BC risk.
This meta-analysis is guided by the PRISMA statement (Checklist S1).
Supporting Information
Forest plots describing the association of the 8q24 rs13281615 single nucleotide polymorphism with risk of developing breast cancer (AA vs. AG+GG).
(TIF)
Forest plots describing the association of the 8q24 rs13281615 single nucleotide polymorphism with risk of developing breast cancer (GG vs. AG+AA).
(TIF)
Forest plots describing the association of the 8q24 rs13281615 single nucleotide polymorphism with risk of developing breast cancer (GG vs. AA).
(TIF)
Begg’s funnel plots to examine publication bias for reported comparisons of the 8q24 rs13281615 single nucleotide polymorphism and risk of BC. Data were plotted using pseudo 95% confidence limits. SE, standard error.
(TIF)
Sensitivity analysis results with random-effect model and fixed-effect model.
(DOC)
PRISMA 2009 Checklist.
(DOC)
Acknowledgments
The authors thank Dr Armando Chapin Rodríguez for his language editing, which substantially improved the quality of the manuscript.
Funding Statement
This work was supported by the grants from the National Natural Science Foundation of China (Project No: 81160262/H1602) and Guangxi Natural Science Foundation (Project No: 2011GXNSFD018032) to Le-Qun Li, and the Self-raised Scientific Research Fund of the Ministry of Health of Guangxi Province (Project No: Z2012345) to Jian-Hong Zhong. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Smigal C, Jemal A, Ward E, Cokkinides V, Smith R, et al. (2006) Trends in breast cancer by race and ethnicity: update 2006. CA Cancer J Clin 56: 168–183. [DOI] [PubMed] [Google Scholar]
- 2.NHS breast cancer screening programme. http://www.cancerscreening.nhs.uk/breastscreen/breastcancer.html. Accessed 16 December 2012.
- 3. Sturgeon SR, Schairer C, Grauman D, El Ghormli L, Devesa S (2004) Trends in breast cancer mortality rates by region of the United States, 1950–1999. Cancer Causes Control 15: 987–995. [DOI] [PubMed] [Google Scholar]
- 4. Parkin DM, Bray F, Ferlay J, Pisani P (2005) Global cancer statistics, 2002. CA Cancer J Clin 55: 74–108. [DOI] [PubMed] [Google Scholar]
- 5. Lichtenstein P, Holm NV, Verkasalo PK, Iliadou A, Kaprio J, et al. (2000) Environmental and heritable factors in the causation of cancer–analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med 343: 78–85. [DOI] [PubMed] [Google Scholar]
- 6. Cheung KL (2007) Endocrine therapy for breast cancer: an overview. Breast 16: 327–343. [DOI] [PubMed] [Google Scholar]
- 7. Silva Idos S, De Stavola B, McCormack V (2008) Birth size and breast cancer risk: re-analysis of individual participant data from 32 studies. PLoS Med 5: e193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zaman K, Bodmer A, Pralong F, Castiglione-Gertsch M (2012) [Breast cancer and obesity, a dangerous relation]. Rev Med Suisse 8: 1101–1104. [PubMed] [Google Scholar]
- 9. Berclaz G, Li S, Price KN, Coates AS, Castiglione-Gertsch M, et al. (2004) Body mass index as a prognostic feature in operable breast cancer: the International Breast Cancer Study Group experience. Ann Oncol 15: 875–884. [DOI] [PubMed] [Google Scholar]
- 10. Hankinson SE (2008) Circulating levels of sex steroids and prolactin in premenopausal women and risk of breast cancer. Adv Exp Med Biol 617: 161–169. [DOI] [PubMed] [Google Scholar]
- 11.Pabalan N, Jarjanazi H, Sung LL, Li H, Ozcelik H (2012) Menopausal Status Modifies Breast Cancer Risk Associated with the Myeloperoxidase (MPO) G463A Polymorphism in Caucasian Women: A Meta-Analysis. PLoS One 7. [DOI] [PMC free article] [PubMed]
- 12. Saadat M (2012) Paraoxonase 1 genetic polymorphisms and susceptibility to breast cancer: A meta-analysis. Cancer Epidemiology 36: E101–E103. [DOI] [PubMed] [Google Scholar]
- 13. Zhao EJ, Cui D, Yuan L, Lu WQ (2012) MDM2 SNP309 polymorphism and breast cancer risk: a meta-analysis. Molecular Biology Reports 39: 3471–3477. [DOI] [PubMed] [Google Scholar]
- 14. Walsh T, Casadei S, Coats KH, Swisher E, Stray SM, et al. (2006) Spectrum of mutations in BRCA1, BRCA2, CHEK2, and TP53 in families at high risk of breast cancer. JAMA 295: 1379–1388. [DOI] [PubMed] [Google Scholar]
- 15. Easton DF, Pooley KA, Dunning AM, Pharoah PD, Thompson D, et al. (2007) Genome-wide association study identifies novel breast cancer susceptibility loci. Nature 447: 1087–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hunter DJ, Kraft P, Jacobs KB, Cox DG, Yeager M, et al. (2007) A genome-wide association study identifies alleles in FGFR2 associated with risk of sporadic postmenopausal breast cancer. Nat Genet 39: 870–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Meyer A, Schurmann P, Ghahremani M, Kocak E, Brinkhaus MJ, et al. (2009) Association of chromosomal locus 8q24 and risk of prostate cancer: a hospital-based study of German patients treated with brachytherapy. Urol Oncol 27: 373–376. [DOI] [PubMed] [Google Scholar]
- 18. Sole X, Hernandez P, de Heredia ML, Armengol L, Rodriguez-Santiago B, et al. (2008) Genetic and genomic analysis modeling of germline c-MYC overexpression and cancer susceptibility. BMC Genomics 9: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Haiman CA, Patterson N, Freedman ML, Myers SR, Pike MC, et al. (2007) Multiple regions within 8q24 independently affect risk for prostate cancer. Nat Genet 39: 638–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tomlinson I, Webb E, Carvajal-Carmona L, Broderick P, Kemp Z, et al. (2007) A genome-wide association scan of tag SNPs identifies a susceptibility variant for colorectal cancer at 8q24.21. Nat Genet 39: 984–988. [DOI] [PubMed] [Google Scholar]
- 21. Fletcher O, Johnson N, Gibson L, Coupland B, Fraser A, et al. (2008) Association of genetic variants at 8q24 with breast cancer risk. Cancer Epidemiol Biomarkers Prev 17: 702–705. [DOI] [PubMed] [Google Scholar]
- 22. Garcia-Closas M, Hall P, Nevanlinna H, Pooley K, Morrison J, et al. (2008) Heterogeneity of breast cancer associations with five susceptibility loci by clinical and pathological characteristics. PLoS Genet 4: e1000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McInerney N, Colleran G, Rowan A, Walther A, Barclay E, et al. (2009) Low penetrance breast cancer predisposition SNPs are site specific. Breast Cancer Res Treat 117: 151–159. [DOI] [PubMed] [Google Scholar]
- 24. Tamimi RM, Lagiou P, Czene K, Liu J, Ekbom A, et al. (2010) Birth weight, breast cancer susceptibility loci, and breast cancer risk. Cancer Causes Control 21: 689–696. [DOI] [PubMed] [Google Scholar]
- 25. Long J, Shu XO, Cai Q, Gao YT, Zheng Y, et al. (2010) Evaluation of breast cancer susceptibility loci in Chinese women. Cancer Epidemiol Biomarkers Prev 19: 2357–2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li LH, Guo ZJ, Hua D, He J, Huang CH, et al. (2011) Association of the 8q24 rs13281615 polymorphisms with breast cancer risk and Clinical and Pathological Characteristics in Chinese Han Women. Chinese Journal Of Laboratory Medicine 34: 73–76. [Google Scholar]
- 27. Jiang Y, Han J, Liu J, Zhang G, Wang L, et al. (2011) Risk of genome-wide association study newly identified genetic variants for breast cancer in Chinese women of Heilongjiang Province. Breast Cancer Res Treat 128: 251–257. [DOI] [PubMed] [Google Scholar]
- 28. Harlid S, Ivarsson MI, Butt S, Grzybowska E, Eyfjord JE, et al. (2012) Combined effect of low-penetrant SNPs on breast cancer risk. Br J Cancer 106: 389–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Teraoka SN, Bernstein JL, Reiner AS, Haile RW, Bernstein L, et al. (2011) Single nucleotide polymorphisms associated with risk for contralateral breast cancer in the Women’s Environment, Cancer, and Radiation Epidemiology (WECARE) Study. Breast Cancer Res 13: R114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Antoniou AC, Sinilnikova OM, McGuffog L, Healey S, Nevanlinna H, et al. (2009) Common variants in LSP1, 2q35 and 8q24 and breast cancer risk for BRCA1 and BRCA2 mutation carriers. Hum Mol Genet 18: 4442–4456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gorodnova TV, Kuligina E, Yanus GA, Katanugina AS, Abysheva SN, et al. (2010) Distribution of FGFR2, TNRC9, MAP3K1, LSP1, and 8q24 alleles in genetically enriched breast cancer patients versus elderly tumor-free women. Cancer Genet Cytogenet 199: 69–72. [DOI] [PubMed] [Google Scholar]
- 32. Chan M, Ji SM, Liaw CS, Yap YS, Law HY, et al. (2012) Association of common genetic variants with breast cancer risk and clinicopathological characteristics in a Chinese population. Breast Cancer Res Treat 136: 209–220. [DOI] [PubMed] [Google Scholar]
- 33. Campa D, Kaaks R, Le Marchand L, Haiman CA, Travis RC, et al. (2011) Interactions between genetic variants and breast cancer risk factors in the breast and prostate cancer cohort consortium. J Natl Cancer Inst 103: 1252–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shan J, Mahfoudh W, Dsouza SP, Hassen E, Bouaouina N, et al. (2012) Genome-Wide Association Studies (GWAS) breast cancer susceptibility loci in Arabs: susceptibility and prognostic implications in Tunisians. Breast Cancer Res Treat 135: 715–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McCracken M, Olsen M, Chen MS Jr, Jemal A, Thun M, et al. (2007) Cancer incidence, mortality, and associated risk factors among Asian Americans of Chinese, Filipino, Vietnamese, Korean, and Japanese ethnicities. CA Cancer J Clin 57: 190–205. [DOI] [PubMed] [Google Scholar]
- 36. Ziv E, John EM, Choudhry S, Kho J, Lorizio W, et al. (2006) Genetic ancestry and risk factors for breast cancer among Latinas in the San Francisco Bay Area. Cancer Epidemiology Biomarkers & Prevention 15: 1878–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jemal A, Bray F (2011) Center MM, Ferlay J, Ward E, et al (2011) Global cancer statistics. CA Cancer J Clin 61: 69–90. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Forest plots describing the association of the 8q24 rs13281615 single nucleotide polymorphism with risk of developing breast cancer (AA vs. AG+GG).
(TIF)
Forest plots describing the association of the 8q24 rs13281615 single nucleotide polymorphism with risk of developing breast cancer (GG vs. AG+AA).
(TIF)
Forest plots describing the association of the 8q24 rs13281615 single nucleotide polymorphism with risk of developing breast cancer (GG vs. AA).
(TIF)
Begg’s funnel plots to examine publication bias for reported comparisons of the 8q24 rs13281615 single nucleotide polymorphism and risk of BC. Data were plotted using pseudo 95% confidence limits. SE, standard error.
(TIF)
Sensitivity analysis results with random-effect model and fixed-effect model.
(DOC)
PRISMA 2009 Checklist.
(DOC)