Abstract
Background
Electroencephalogram (EEG) acquisition is routinely performed to support an epileptic origin of paroxysmal events in patients referred with a possible diagnosis of epilepsy. However, in children with partial epilepsies the interictal EEGs are often normal. We aimed to develop a multivariable diagnostic prediction model based on electroencephalogram functional network characteristics.
Methodology/Principal Findings
Routinely performed interictal EEG recordings at first presentation of 35 children diagnosed with partial epilepsies, and of 35 children in whom the diagnosis epilepsy was excluded (control group), were used to develop the prediction model. Children with partial epilepsy were individually matched on age and gender with children from the control group. Periods of resting-state EEG, free of abnormal slowing or epileptiform activity, were selected to construct functional networks of correlated activity. We calculated multiple network characteristics previously used in functional network epilepsy studies and used these measures to build a robust, decision tree based, prediction model. Based on epileptiform EEG activity only, EEG results supported the diagnosis of with a sensitivity and specificity of 0.77 and 0.91 respectively. In contrast, the prediction model had a sensitivity of 0.96 [95% confidence interval: 0.78–1.00] and specificity of 0.95 [95% confidence interval: 0.76–1.00] in correctly differentiating patients from controls. The overall discriminative power, quantified as the area under the receiver operating characteristic curve, was 0.89, defined as an excellent model performance. The need of a multivariable network analysis to improve diagnostic accuracy was emphasized by the lack of discriminatory power using single network characteristics or EEG's power spectral density.
Conclusions/Significance
Diagnostic accuracy in children with partial epilepsy is substantially improved with a model combining functional network characteristics derived from multi-channel electroencephalogram recordings. Early and accurate diagnosis is important to start necessary treatment as soon as possible and inform patients and parents on possible risks and psychosocial aspects in relation to the diagnosis.
Introduction
Epilepsy is a common neurological disorder, yet, accurate diagnosis and classification at an early stage still poses a challenge to the clinician. The diagnosis of epilepsy is primarily based on the clinical history and may be supported by information provided by interictal EEG recording. For proper classification of the epilepsy syndrome the EEG is indispensable. Additional neuroimaging or sleep deprivation EEG is often used when the initial clinical diagnosis is not conclusive or when more information is needed for classification of the epilepsy syndrome and assessment of prognosis. Clinical diagnosis has a high interobserver variation. One study found that 25 percent of patients were incorrectly diagnosed as having had a seizure at the initial presentation [1]. In addition, evaluation of EEG abnormalities may be subjective [2] and is not very sensitive. For example, epileptiform activity is demonstrated only in 29 to 55 percent of patients on routinely performed EEG recordings [3]. To a lesser extent, EEG abnormalities may also be found in healthy controls, especially in children [4]. Together, interictal EEG recordings are supportive but often not conclusive in the initial clinical diagnosis of epilepsy. Developing EEG measurements with increased sensitivity and specificity would be highly valuable in the early clinical diagnosis of epilepsy. Accurate diagnosis at an early stage may allow more rapid optimization of treatment and improve counselling with regard to lowering risks with necessary life rules and restrictions. Particularly children with partial epilepsy could benefit from early and accurate diagnosis, since interictal epileptiform EEG activity is often absent [4], possibly causing a delay in diagnosis and decision-making.
In this study we used the expanding knowledge on functional neural network organization in normal and diseased brain [5]. Brain network organization is typically summarized using multiple network characteristics such as global efficiency, local clustering, power-law degree distribution and centrality measures [6]. Recently, it has been shown that interictal functional network characteristics differ between controls and patients with chronic partial epilepsy [7], [8], [9], [10], [11].
We aimed to explore the clinical value of functional network characteristics by investigating network characteristics separately and combined in a multivariable diagnostic prediction model. We hypothesized that functional network characteristics enhance sensitivity and specificity of diagnosis of partial epilepsy in children at initial assessment.
Materials and Methods
Patients
Children referred, between January 2006 and December 2010, to the outpatient department of pediatric neurology, University Medical Center Utrecht, The Netherlands after one or more suspected epileptic event(s) were eligible for our study. We included children who were eventually diagnosed with new onset partial epilepsy. Children with neurological or psychiatric co-morbidities, including developmental delay, were excluded. The clinical diagnosis of epilepsy was defined by at least two unprovoked seizures within one year, judged by two neurologists to be of epileptic origin. The clinical diagnosis was supported in a subset of patients by epileptiform abnormalities (interictal epileptiform discharges (IEDs) such as sharp waves, (poly) spikes or (poly) spike-wave complexes or abnormal slowing), on routinely performed EEG. In patients clinically diagnosed with epilepsy but with a normal routine EEG recording, the diagnosis was confirmed by subsequent sleep deprivation EEG recordings, neuroimaging or clinical follow-up with history of more highly suspected events. An MRI was performed in all children diagnosed with epilepsy, not classified as idiopathic focal epilepsy. Epilepsy was excluded in the control group, based on clinical history, EEG results, and at least one year of uneventful follow up. This control group was individually matched with the patient group on gender and age. Neither patients nor controls had a history of febrile seizures, generalized epilepsy, or were on (chronic) anticonvulsive medication.
The institutional ethical committee approved the study and concluded that the Dutch Medical Research Involving Human Subjects Act did not apply, and written informed consent was not required.
Data acquisition and selection
Routinely performed interictal EEG recording was available for each child. Interictal EEGs were recorded according the international 10–20 system (SystemPlus Evolution, Micromed) in awake and eyes closed (resting-state) condition against an average reference electrode. Impedance of each electrode was kept below 5 kΩ. Data was high- and low-pass filtered at 0.16 and 70 Hz, respectively. Sampling frequency was 512 Hz.
All EEG recordings contained 21 scalp electrodes (F8, F4, Fz, F3, F7, T8, C4, Cz, C3, T7, P8, P4, Pz, P3, P7, O1, O2, Fp1, Fp2, A1, and A2) and were visually inspected (EvD). To assure stable EEG brain dynamics for the calculation of network characteristics, for each subject we selected four epochs (eight seconds each) at the beginning of the interictal EEG recording [12], [13]. All epochs were free of IEDs, abnormal slowing, and electrocardiographic or motion-induced artifacts. Two frontoparietal and basal temporal electrodes (Fp1, Fp2, A1, and A2) were excluded to minimize eye-movement artifacts. The epochs were independently re-inspected by a clinical epileptologist (FEJ) on artifacts and IEDs. Finally, all selected EEG epochs were converted to ASCII files to enable functional network analysis. All data were additionally filtered to obtain standard broadband frequencies in the range of 0.5 to 45 Hz.
To check whether subjects with partial epilepsy could be distinguished from controls by means of spectral analysis, we computed the relative and absolute power spectral densities averages over epochs and subjects [14].
Computation of functional network characteristics
Individual functional EEG networks were constructed for each subject using their broadband filtered data. Functional network organization is based on the relatively new concept of functional connectivity. The statistical interdependencies for each pair of EEG electrode time series are considered as functional connectivity and used to construct a functional network per subject for each of the four epochs and were averaged per subject. Multiple complementary methods exist to estimate the statistical interdependency between two time series [15]. We based our functional network construction on a functional connectivity index that was previously applied in epilepsy studies, namely the synchronization likelihood (SL) [13], [16], [17], [18]. SL detects both linear and nonlinear dependencies between the time series and is considered to be a measure of generalized synchronization [19]. The SL expresses the functional connectivity as a value between 0 and 1, and allows the construction of weighted functional networks (i.e., the connectivity strength between two electrodes is preserved) [20]. Weighted SL networks were constructed using the freely available BrainWave software (http://home.kpn.nl/stam7883/brainwave.html).
We have chosen to include network characteristics used to study functional networks in epilepsy and published recently in neuroscience papers (for review see [6]). For each weighted network, we calculated the following characteristics: weighted degree centrality (strength), weighted shortest path length, weighted clustering coefficient, weighted betweenness centrality, weighted closeness centrality and weighted eigenvector centrality. An additional characteristic was the powerlaw distribution index [21], [22]. Apart from mean values of each network characteristic across all network nodes, we calculated both minimal and maximal values to increase the information used for building a multivariable diagnostic prediction model. The mathematical forms of the network characteristics are provided in the next paragraph.
Mathematical forms of the network characteristics
Network construction
For each of the 70 subjects we constructed a weighted undirected network, described by the graph G = (N, W), where N is the set all 17 EEG electrodes and 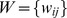 is the N×N symmetric weight matrix, where wii = 0 and wij the synchronization likelihood index determined between electrode i and j
[19].
is the N×N symmetric weight matrix, where wii = 0 and wij the synchronization likelihood index determined between electrode i and j
[19].
Weighted degree centrality (strength)
Four network characteristics of edge centrality were included in the model, which determined the relative importance of a node within the graph [23]. The weighted degree centrality, or strength for node i was defined as
The minimal, mean and maximal strength were defined as
Weighted shortest path length
For a given node i in the graph, the shortest path algorithm finds the path with lowest cost (i.e. the shortest path length) between that node and every other node. For the weighted shortest path length, the path between two nodes i and j is find by minimizing the sum of weights assigned to the edges on their path. The average shortest path length for node i to all other nodes is defined as
Here, 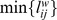 is the weighted shortest path length between node i and j. We considered high values of the synchronization index as close functional distance and low values of the synchronization index as large functional distance (i.e.
is the weighted shortest path length between node i and j. We considered high values of the synchronization index as close functional distance and low values of the synchronization index as large functional distance (i.e. 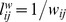 ). In our dataset no disconnected nodes were present. The minimal, mean and maximal weighted shortest path were defined as
). In our dataset no disconnected nodes were present. The minimal, mean and maximal weighted shortest path were defined as
Weighted closeness centrality
Edges that have short distances to other edges have high closeness; this principle is used in the calculation of the weighted closeness centrality. Formally
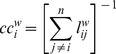 |
The minimal, mean and maximal weighted closeness centrality were defined as
Weighted betweenness centrality
The weighted betweenness centrality relies on the identification of the number of weighted shortest paths that pass through a node. The more passages the higher the betweenness centrality. The weighted betweenness centrality is defined as
where 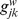 is the shortest path between two nodes and
is the shortest path between two nodes and 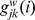 is the number of those nodes that pass through node i. The mean and maximal weighted betweenness centrality were defined as
is the number of those nodes that pass through node i. The mean and maximal weighted betweenness centrality were defined as
Eigenvector centrality
Eigenvector centrality is based on the greatest eigenvector of the weight matrix W
[24]. The eigenvectors of the weight matrix indicate the nodes that show high connections with most other nodes. In contrast to the weighted degree centrality, it specifically favors nodes that are connected to nodes that are themselves central within the network [25]. If 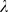 is the largest eigenvalue and ecw the corresponding eigenvector, then
is the largest eigenvalue and ecw the corresponding eigenvector, then 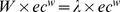 or similar
or similar 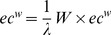 and
and 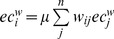 , where
, where 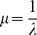 is the proportionality factor so that
is the proportionality factor so that 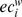 is proportional to the sum of connectivity scores of all nodes connected to it. We used the minimal, mean and maximal values of the eigenvector in our model, defined as
is proportional to the sum of connectivity scores of all nodes connected to it. We used the minimal, mean and maximal values of the eigenvector in our model, defined as
Weighted clustering coefficient
The clustering coefficient is a measure of degree to which nodes in a graph tend to cluster together. We used the weighted clustering coefficient [26], [27]. The weighted clustering coefficient for each node i was defined as
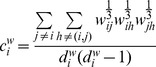 |
and takes into account the weights of all edges in a triangle, excluding weights not participating in any triangle. The minimal, mean and maximal weighted clustering coefficient were defined as
Power law scaling index
The collection of (weighted) degree centrality measures 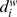 in a functional brain network often forms a distribution, p(d), that decays as a power law [28]. This power law decay ranges from a minimal value
in a functional brain network often forms a distribution, p(d), that decays as a power law [28]. This power law decay ranges from a minimal value 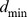 to the maximal value, and scales with the index α as
to the maximal value, and scales with the index α as 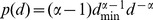 . The power law scaling index α was one of the network characteristics included in the prediction model. The calculation of
. The power law scaling index α was one of the network characteristics included in the prediction model. The calculation of 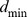 and α was done by a robust maximum likelihood method [21].
and α was done by a robust maximum likelihood method [21].
Model development
The diagnostic prediction model was built with available software packages in the open-source R environment [29], [30]. The predictive diagnostic model has been based on a robust ensemble algorithm, namely the random forest classifier [31] using a free implementation provided as the random forest package [32] The core of the random forest classifier is the binary decision tree, a data type that stores elements hierarchically in nodes. Each decision tree is grown on different bootstrapped sample collections (i.e., randomly drawn instances with replacement from the original dataset) on a randomly selected subset of all available predictors. The random selection of predictors increases the generalizability of the individual decision trees, whereas the collection of multiple decision trees in one forest increases model performance [31].
For our data, a subset of 5 random predictors for each decision tree was found to give highest accuracy. After building the random forest diagnostic prediction model, we assessed the ability to differentiate between subjects with and without partial epilepsy using the area under the Receiver Operating Characteristic (ROC) curve (AUC) [33]. An AUC higher than 0.8 reflects excellent discrimination [34]. However, the AUC is typically considered to be too optimistic when the diagnostic model is tested on the same data that is used to build the model. Internal validation methods correct for this. We used the regular bootstrapping approach for internal validation, which is the preferred method when dealing with relative small datasets [35], [36]. With this method, the model is rebuild on multiple random samples drawn with replacement from the full dataset. In this study we only report the bootstrap corrected results.
Statistical analysis
Group differences on all network characteristics were individually assessed with the independent Student T-test. Each diagnostic prediction model was fit on 1000-bootstrapped realization of the original dataset. We calculated a bootstrap corrected average ROC curve and corresponding average AUC, sensitivity, specificity, positive predictive and negative predictive values. All statistical analyses were performed in R using the pROC [37] package [29].
Results
Patient characteristics
A total of 419 children visited the outpatient department of pediatric neurology, between January 2006 and December 2010, after a recent event of possible epileptic origin. In total, 75 children were diagnosed with generalized epilepsies, 69 with partial epilepsy and 38 with febrile seizures. In 52 cases, diagnosis remained undetermined but clinical follow-up revealed no event(s). Of the 69 patients who were eventually diagnosed with partial epilepsy, only 35 patients both met our strict inclusion criteria and had an EEG recording of sufficient quality for functional network analysis (11 girls and 24 boys, mean age 10.1±3.4 years). In 185 children, epilepsy was excluded and an alternative clinical diagnosis was made for the paroxysmal event(s). From this group, 35 controls were selected; individually match on age and gender with the patient group (mean age 9.9±3.1 years). Detailed clinical characteristics are provided as supplemental information (for patients Table S1 and for controls Table S2). None of the children had paroxysmal event in the days prior or post EEG recording, thereby excluding pre- or postictal changes of the EEG signal [16]. Epileptiform interictal EEG activity was present in 77% (27 out of 35) of patients and supported clinical diagnosis. Subsequent sleep deprivation EEG was performed in 5 patients showing epileptiform activity in all cases. In the remaining 3 patients, the diagnosis of epilepsy was reconfirmed by clinical follow-up. The average number of seizures prior to presentation at the outpatient department was 5 (range: 1–36). The events of the children in the control group were eventually diagnosed as syncope (11 cases), behavioural/psychogenic events, including tic and stereotypy (11 cases), staring/non-attentiveness without epileptic origin (7 cases), pavor nocturnus (3 cases), arrhythmia (1 case), segmental non-epileptic myoclonic jerks (1 case) and breath holding spells (1 case). In the control group, aspecific EEG abnormalities were present in 9% (3 out of 35).
Power spectrum and network characteristics
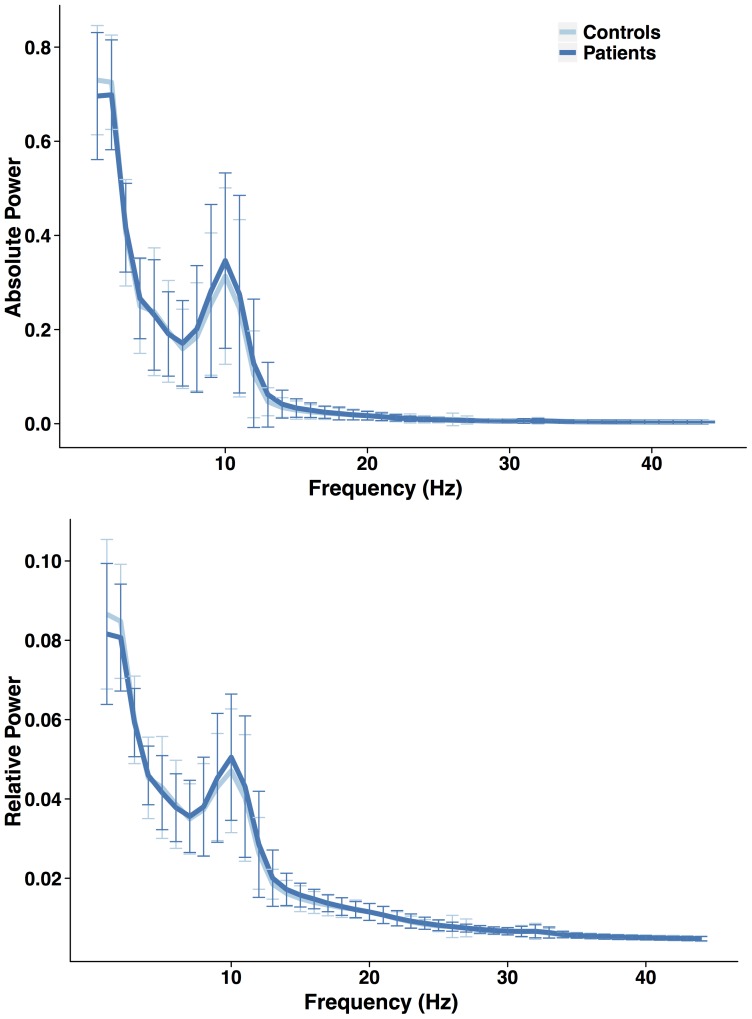
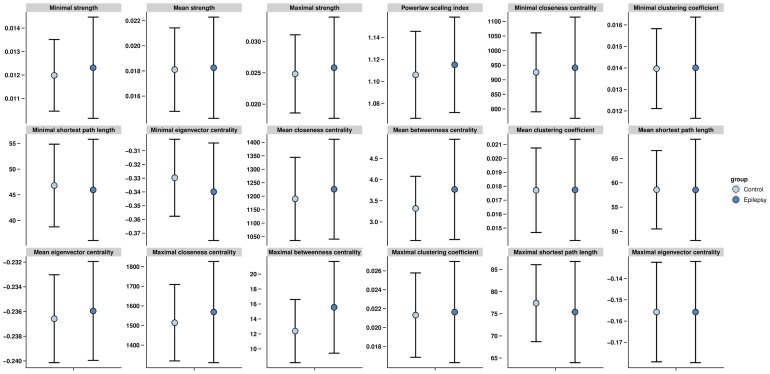
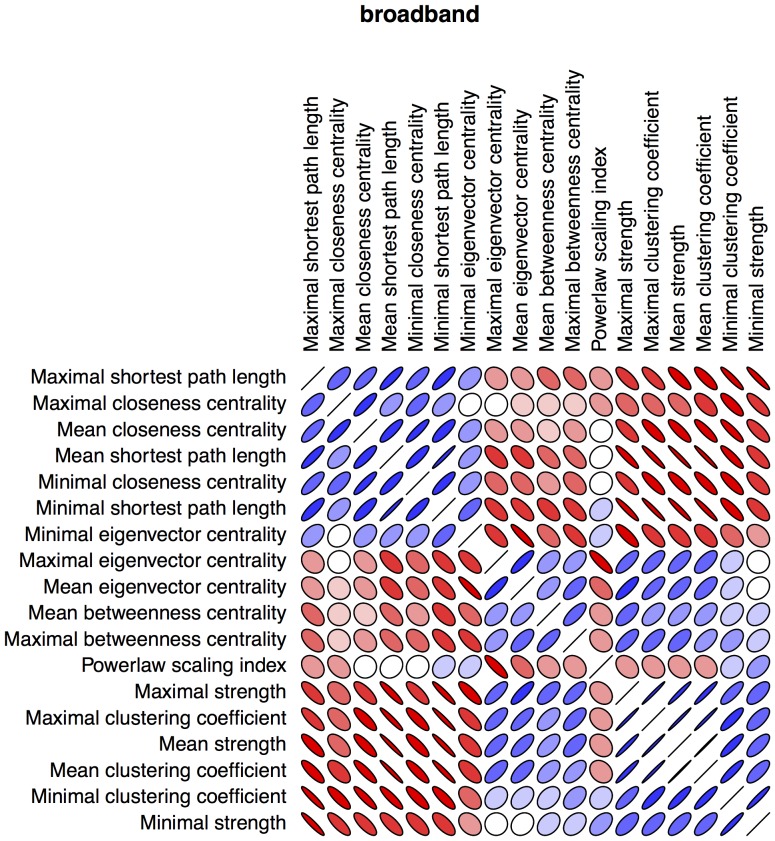
Power spectral density revealed no differences between patients and controls ( Figure 1 ). None of the individual network characteristics was significantly different between groups ( Figure 2 ). The lack of discriminatory power in the frequency analysis and individual network characteristics emphasized the need for a multivariable model. The network characteristics used in the multivariable model contained unique information, as shown in our correlation matrix ( Figure 3 ).
Figure 1. (power spectral density plots of patients and controls).
Mean absolute and relative power spectral densities between 0 and 45 Hz averaged over epochs and subjects per group. Variation, defined as the standard deviation, is indicated by cross bars. Spectral densities between groups largely overlap.
Figure 2. (individual network characteristics of patients and controls).
Mean and standard deviation of all network characteristics, used in the predictive model, calculated for the broadband frequency. No significant differences were found for each characteristic between groups.
Figure 3. (correlation matrix included network characteristics).
Pearson correlation matrix of all network characteristics for the broadband frequency. Red color indicates positive correlation, blue color indicates negative correlation. Tensor anisotropy indicates strength of correlation (0 correlation, circular; full correlation, single line).
Diagnostic model
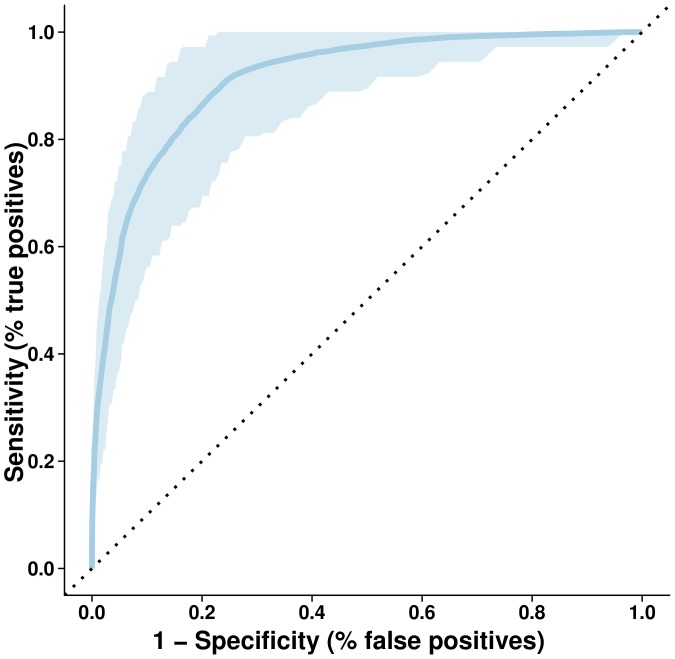
The ROC curve for the predictive model using broadband network characteristics is shown in Figure 4 . The model had a mean sensitivity of 0.96 [95% confidence interval (CI): 0.78 – 1.00], mean specificity of 0.95 [CI: 0.76 – 1.00], mean positive predictive value of 0.96 [CI: 0.82 – 1.00] and a mean negative predictive value of 0.96 [CI: 0.81 – 1.00]. The model performance was excellent, with an AUC of 0.89 [CI: 0.80 – 0.95]. We found similar results in terms of model performance if the analysis was repeated for specific frequency bands including delta band (0.5–4 Hz), theta band (4–8 Hz), alpha band (8–12 Hz), beta band (12–30 Hz) and gamma band (30–45 Hz) (data not shown).
Figure 4. (ROC curve).
ROC curve (dark blue) and 95% confidence interval for the network characteristics based on the broadband frequency.
In an additional sub analysis, we bootstrap validated the model specifically using only the subset of 8 epilepsy patients of whom routinely performed EEG recordings were judged normal, resulting in a sensitivity of 0.86 [CI: 0.64 – 1.00]. Similarly, we tested the subset of 3 controls in whom routinely performed EEG recordings contained aspecific abnormalities and in these patients the model was found to have a good performance as well with a specificity of 0.81 [CI: 0.33 – 1.00].
Conclusions
In this study we were able to build a highly accurate diagnostic prediction model to distinguish children with partial epilepsy from children who were judged to have had non-epileptic events, with network analysis on resting-state epochs of routinely performed interictal EEG recordings. The diagnostic power was high: sensitivity and specificity of 0.96 and 0.95 respectively. The values obtained by the diagnostic model clearly exceed the sensitivity and specificity based on epileptiform EEG activity only, namely 0.77 and 0.91 respectively.
There is a rising interest in understanding brain functioning from a network perspective. However, only few studies have aimed to explore the clinical value of these functional networks in epilepsy. For example, two studies have used a functional network approach to predict post-surgical outcome in epilepsy surgery. Wilke and colleagues studied invasive corticography recordings during epilepsy surgery to correlate betweenness centrality, to the resected cortical regions. They found that the betweenness centrality was correlated with the resected areas of patients who became seizure-free after surgery [38]. Yet another study, found that functional connectivity in patients with seizure-recurrence after epilepsy surgery was less lateralized compared to those who were seizure free [39], [40]. Although these results are promising, the study domain has been limited to epilepsy surgery and data acquisition has been applied to specific patients only.
This study was undertaken to bridge the gap between the field of more fundamental computational neuroscience and daily clinical practice. The use of network characteristics in diagnosing epilepsy extends the method used in a recent study [13]. Douw and colleagues used EEG functional connectivity to diagnose epilepsy after a first suspected seizure and found a specificity of 0.76 and sensitivity of 0.62. The higher sensitivity and specificity values obtained in our study argue for a multivariable prediction design and machine learning models, such as the random forest classifier, although one may argue that the high sensitivity and specificity may be partly due to the homogeneity of our patients. Douw and colleagues found the theta frequency band to be most sensitive in terms of prediction. In our study we found excellent results using the broadband frequency, suggesting that the discriminatory power is not restricted to one specific frequency band.
A potential limitation of our study is that the inclusion was restricted to children diagnosed with partial epilepsy. Hence, this prediction model can be applied in children with partial epilepsy only. In addition, selecting resting-state EEG epochs in very young children can be challenging since EEG recordings may have motion-induced artifacts. Clear instructions and longer registrations could potentially overcome this limitation. Clearly, its clinical value will increase with extended inclusion of adults and other epilepsy syndromes.
Early accurate diagnosis is particularly valuable in young children to inform and guide parents, to prompt treatment decisions and to limit the period of uncertainty and unnecessary risks [1]. To explore the true diagnostic value of our proposed predictive model, larger studies are required, especially using external validation, although we corrected for too optimistic model results using stringent internal validation. The use of freely available software packages for model development validation should facilitate the process of external validation [29], [30], [31], [32], [37]. Our prediction model, based on routinely performed EEG, is appealing due to the immediate clinical availability. Further, standard EEG epoch selection is straightforward, network calculations are relatively fast and software is freely available. Although current selection of epochs was performed manually, software is available to reduce the time spent to select ‘resting state’ epochs semi-automatically [41].
Indications for future research are multiple. Use of other modalities such as resting-state functional magnetic resonance imaging, high density EEG and magnetoencephalogram may lead to development of diagnostic models with even higher accuracy due to their superior spatial resolution of functional networks.
In conclusion, although more and larger studies are needed, this study clearly shows that functional network characteristics are promising and clinically useful in the early diagnosis of partial epilepsy in children after their first seizure(s).
Supporting Information
Clinical characteristics of 35 children with new onset partial epilepsy.
(DOCX)
Clinical characteristics of 35 controls.
(DOCX)
Acknowledgments
The authors would like to thank Anja Ezendam, EEG technician, UMC Utrecht, for her help in retrieving and selecting interictal EEG recordings for our study.
Funding Statement
EvD was supported by the Epilepsy Fund of the Netherlands (NEF 09-93), this project is performed under supervision of CJS, KPJB and FEJ. WMO was supported by the Epilepsy Fund of the Netherlands (NEF 08-10). Furthermore, CJS serves on the scientific board of the Epilepsy Fund of the Netherlands and as an associate editor of Clinical Neurophysiology. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Hamiwka LD, Singh N, Niosi J, Wirrell EC (2007) Diagnostic inaccuracy in children referred with "first seizure": role for a first seizure clinic. Epilepsia 48: 1062–1066. [DOI] [PubMed] [Google Scholar]
- 2. Niedermeyer E, Lopes da Silva F (1987) Electroencephalography: basic principles, clinical applications and related fields. Baltimore: Urban & Schwarzenberg. Inc [Google Scholar]
- 3. Goodin DS, Aminoff MJ, Laxer KD (1990) Detection of epileptiform activity by different noninvasive EEG methods in complex partial epilepsy. Annals of neurology 27: 330–334. [DOI] [PubMed] [Google Scholar]
- 4. Smith SJ (2005) EEG in the diagnosis, classification, and management of patients with epilepsy. J Neurol Neurosurg Psychiatry 76 Suppl 2ii2–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bassett DS, Bullmore ET (2009) Human brain networks in health and disease. Current opinion in neurology 22: 340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rubinov M, Sporns O (2010) Complex network measures of brain connectivity: uses and interpretations. Neuroimage 52: 1059–1069. [DOI] [PubMed] [Google Scholar]
- 7. Chavez M, Valencia M, Navarro V, Latora V, Martinerie J (2010) Functional modularity of background activities in normal and epileptic brain networks. Phys Rev Lett 104: 118701. [DOI] [PubMed] [Google Scholar]
- 8. Horstmann MT, Bialonski S, Noennig N, Mai H, Prusseit J, et al. (2010) State dependent properties of epileptic brain networks: comparative graph-theoretical analyses of simultaneously recorded EEG and MEG. Clin Neurophysiol 121: 172–185. [DOI] [PubMed] [Google Scholar]
- 9. Bettus G, Guedj E, Joyeux F, Confort-Gouny S, Soulier E, et al. (2009) Decreased basal fMRI functional connectivity in epileptogenic networks and contralateral compensatory mechanisms. Hum Brain Mapp 30: 1580–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liao W, Zhang Z, Pan Z, Mantini D, Ding J, et al. (2010) Altered functional connectivity and small-world in mesial temporal lobe epilepsy. PLoS One 5: e8525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vlooswijk MC, Vaessen MJ, Jansen JF, de Krom MC, Majoie HJ, et al. (2011) Loss of network efficiency associated with cognitive decline in chronic epilepsy. Neurology 77: 938–944. [DOI] [PubMed] [Google Scholar]
- 12. Boersma M, Smit DJ, de Bie HM, Van Baal GC, Boomsma DI, et al. (2011) Network analysis of resting state EEG in the developing young brain: structure comes with maturation. Hum Brain Mapp 32: 413–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Douw L, de Groot M, van Dellen E, Heimans JJ, Ronner HE, et al. (2010) 'Functional connectivity' is a sensitive predictor of epilepsy diagnosis after the first seizure. PLoS One 5: e10839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamilton JD (1994) Time series analysis: Princeton University Press. [Google Scholar]
- 15. Pereda E, Quiroga RQ, Bhattacharya J (2005) Nonlinear multivariate analysis of neurophysiological signals. Prog Neurobiol 77: 1–37. [DOI] [PubMed] [Google Scholar]
- 16. Ponten SC, Bartolomei F, Stam CJ (2007) Small-world networks and epilepsy: graph theoretical analysis of intracerebrally recorded mesial temporal lobe seizures. Clinical neurophysiology 118: 918–927. [DOI] [PubMed] [Google Scholar]
- 17. Altenburg J, Vermeulen RJ, Strijers RLM, Fetter WPF, Stam CJ (2003) Seizure detection in the neonatal EEG with synchronization likelihood. Clinical neurophysiology 114: 50–55. [DOI] [PubMed] [Google Scholar]
- 18. Ferri R, Stam CJ, Lanuzza B, Cosentino FII, Elia M, et al. (2004) Different EEG frequency band synchronization during nocturnal frontal lobe seizures. Clinical neurophysiology 115: 1202–1211. [DOI] [PubMed] [Google Scholar]
- 19. Stam CJ, Van Dijk BW (2002) Synchronization likelihood: an unbiased measure of generalized synchronization in multivariate data sets. Physica D: Nonlinear Phenomena 163: 236–251. [Google Scholar]
- 20. Stam CJ, Reijneveld JC (2007) Graph theoretical analysis of complex networks in the brain. Nonlinear Biomed Phys 1: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Newman MEJ (2005) Power laws, Pareto distributions and Zipf's law. Contemporary Physics 46: 29. [Google Scholar]
- 22. Clauset A, Shalizi CR, Newman MEJ (2007) Power-law distributions in empirical data. Arxiv preprint arXiv 07061062. [Google Scholar]
- 23. Opsahl T, Agneessens F, Skvoretz J (2010) Node centrality in weighted networks: Generalizing degree and shortest paths. Social Networks 32: 245–251. [Google Scholar]
- 24. Newman MEJ (2007) The mathematics of networks. The new palgrave encyclopedia of economics [Google Scholar]
- 25. Bonacich P (1987) Power and centrality: A family of measures. American journal of sociology 1170–1182. [Google Scholar]
- 26. Onnela JP, Saramäki J, Kertész J, Kaski K (2005) Intensity and coherence of motifs in weighted complex networks. Physical Review E 71: 065103. [DOI] [PubMed] [Google Scholar]
- 27. Saramäki J, Kivelä M, Onnela JP, Kaski K, Kertesz J (2007) Generalizations of the clustering coefficient to weighted complex networks. Physical Review E 75: 027105. [DOI] [PubMed] [Google Scholar]
- 28. Sporns O, Chialvo DR, Kaiser M, Hilgetag CC (2004) Organization, development and function of complex brain networks. Trends Cogn Sci 8: 418–425. [DOI] [PubMed] [Google Scholar]
- 29. Ripley BD (2001) The R project in statistical computing. MSOR Connections. The newsletter of the LTSN Maths, Stats & OR Network pp. 3. [Google Scholar]
- 30. Kung M, Wing J, Waton S, Williams A, Keefer C, Engelhart A (2007) Caret: classificaton and regression training. R package [Google Scholar]
- 31. Breiman L (2001) Random forests. Machine learning 45: 5–32. [Google Scholar]
- 32. Liaw A, Wiener M (2002) Classification and Regression by randomForest. R News 2: 5. [Google Scholar]
- 33. Zou KH, Warfield SK, Fielding JR, Tempany C, Wells WM (2003) Statistical validation based on parametric receiver operating characteristic analysis of continuous classification data1* 1. Academic radiology 10: 1359–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hanley JA, McNeil BJ (1982) The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 143: 29–36. [DOI] [PubMed] [Google Scholar]
- 35. Harrell Jr FE, Lee KL, Califf RM, Pryor DB, Rosati RA (1984) Regression modelling strategies for improved prognostic prediction. Statistics in Medicine 3: 143–152. [DOI] [PubMed] [Google Scholar]
- 36. Steyerberg EW, Bleeker SE, Moll HA, Grobbee DE, Moons KGM (2003) Internal and external validation of predictive models: a simulation study of bias and precision in small samples. Journal of clinical epidemiology 56: 441–447. [DOI] [PubMed] [Google Scholar]
- 37. Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, et al. (2011) pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics 12: 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wilke C, Worrell G, He B (2011) Graph analysis of epileptogenic networks in human partial epilepsy. Epilepsia 52: 84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Negishi M, Martuzzi R, Novotny EJ, Spencer DD, Constable RT (2011) Functional MRI connectivity as a predictor of the surgical outcome of epilepsy. Epilepsia 52: 1733–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ortega GJ, Menendez de la Prida L, Sola RG, Pastor J (2008) Synchronization clusters of interictal activity in the lateral temporal cortex of epileptic patients: intraoperative electrocorticographic analysis. Epilepsia 49: 269–280. [DOI] [PubMed] [Google Scholar]
- 41. Dalponte M, Bovolo F, Bruzzone L (2007) Automatic selection of frequency and time intervals for classification of EEG signals. Electronics Letters 43: 1406–1408. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Clinical characteristics of 35 children with new onset partial epilepsy.
(DOCX)
Clinical characteristics of 35 controls.
(DOCX)