Abstract
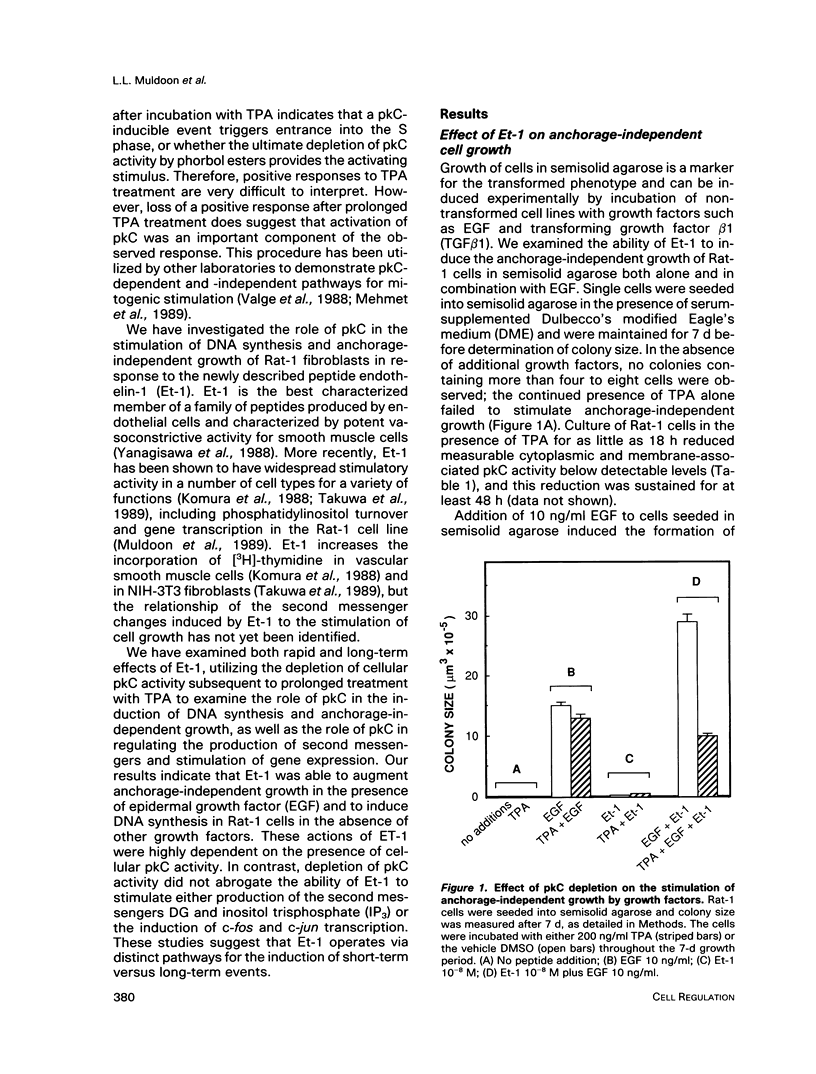
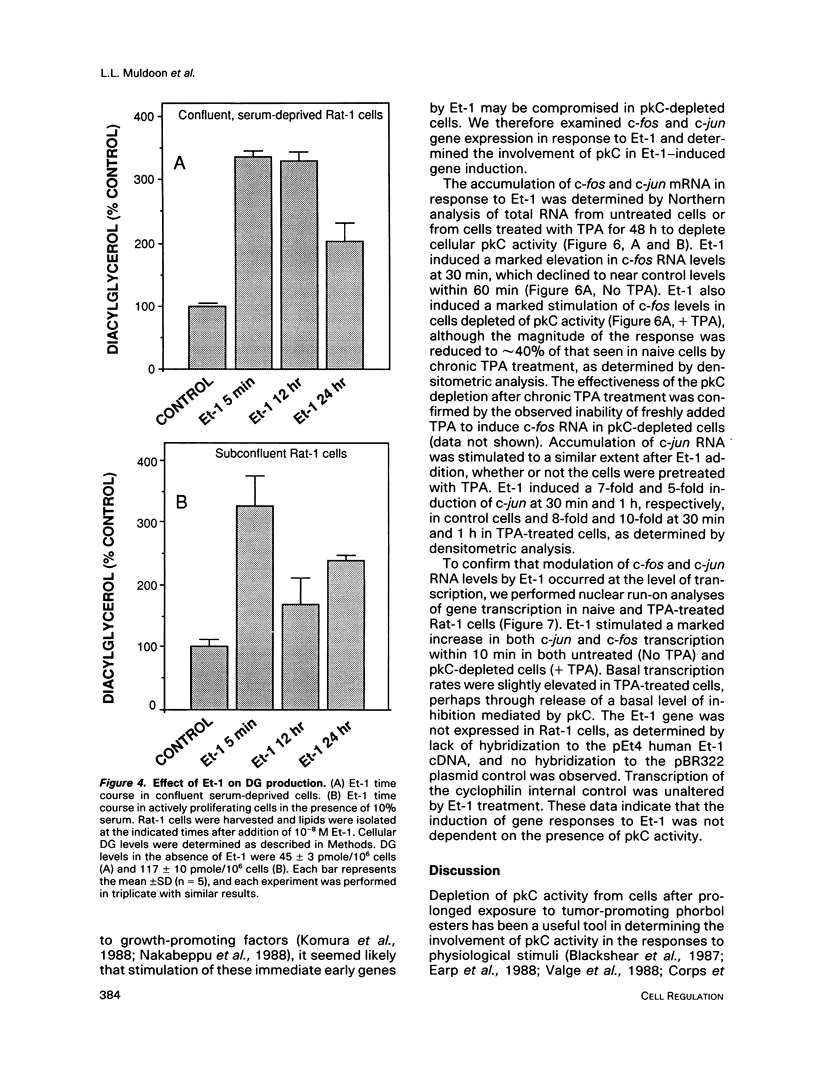
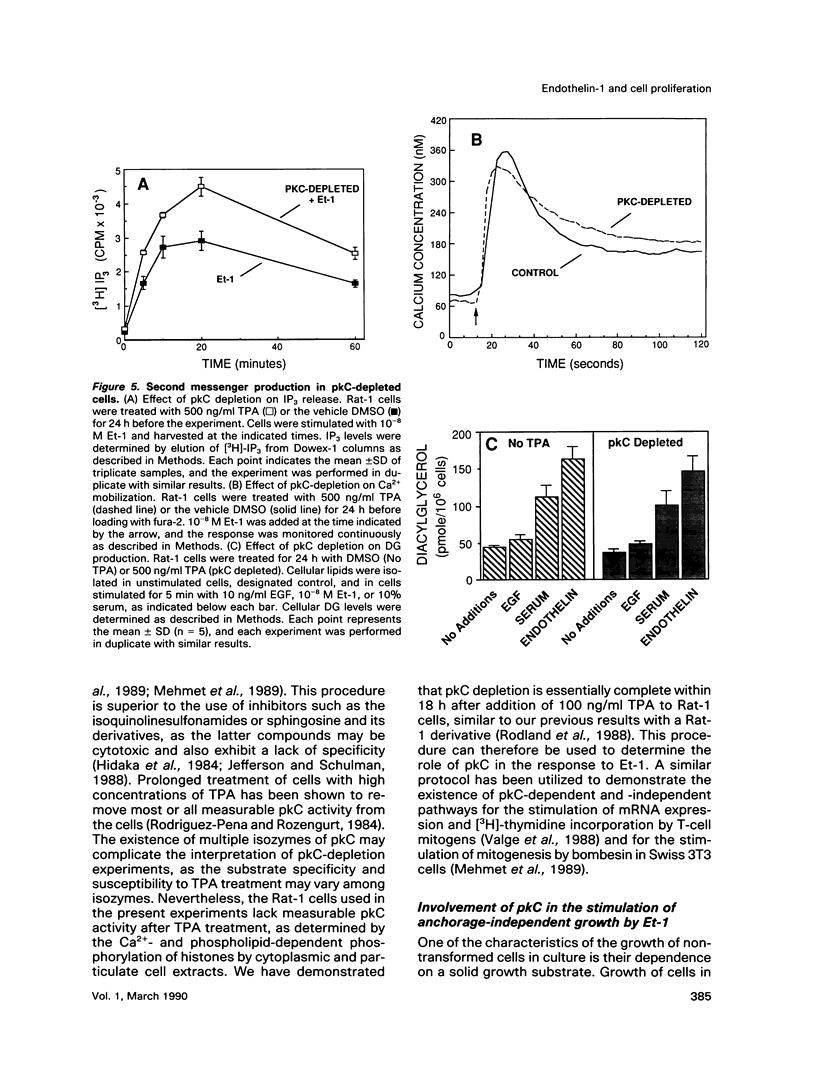
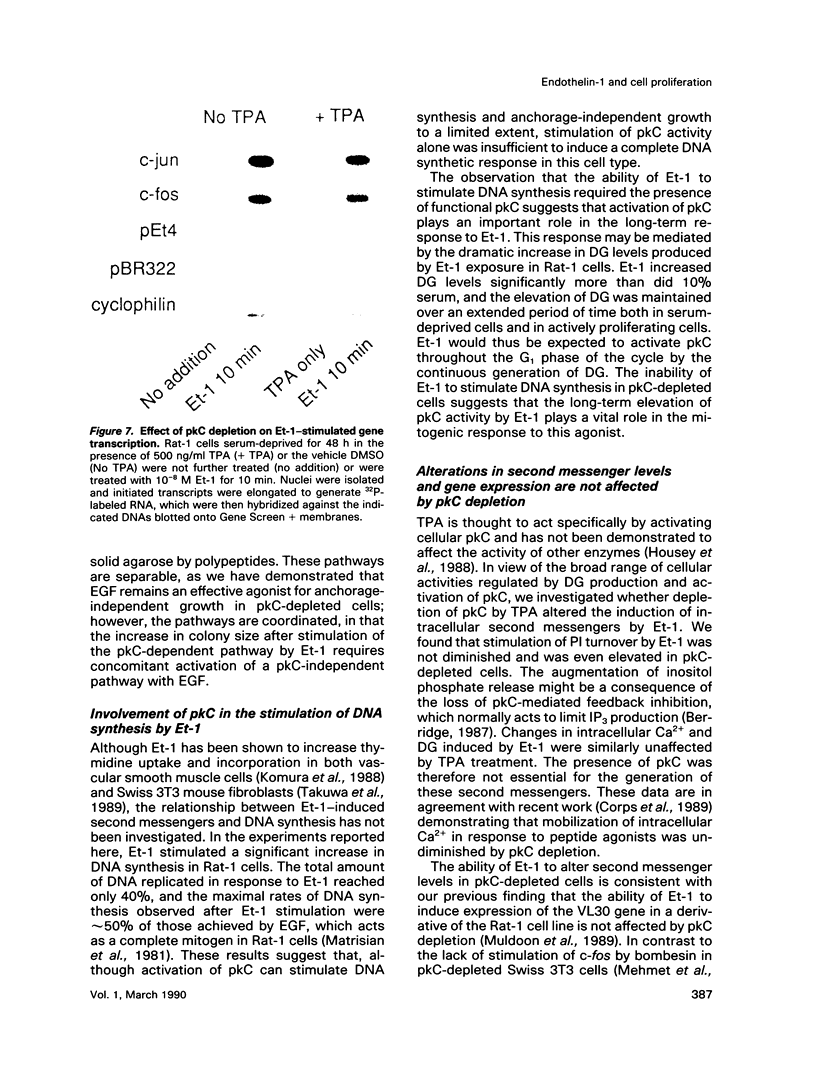
Stimulation of quiescent cultured fibroblasts with a variety of growth-promoting factors induces release of diacylglycerol (DG) and subsequent activation of protein kinase C (pkC), but the role of pkC in the induction of DNA synthesis and cell proliferation remains unclear. We have investigated the involvement of pkC in the response of Rat-1 fibroblasts to the newly described peptide endothelin-1 (Et-1), an agonist that is secreted by the vascular endothelium and that may play a role in the proliferative response of cells in the vessel wall. Addition of Et-1 to serum-deprived Rat-1 cells promoted DNA synthesis in the absence of additional factors and stimulated anchorage-independent growth in the presence of epidermal growth factor (EGF), indicating that Et-1 has many of the characteristics of a mitogen. The ability of Et-1 to stimulate both DNA synthesis and anchorage-independent growth was markedly reduced by the depletion of cellular pkC activity induced by prolonged exposure to 12-O-tetradecanoylphorbol-13-acetate (TPA). In contrast, the ability of Et-1 to induce both second messenger production and transcription of c-fos and c-jun was largely independent of cellular pkC activity. Production of DG in response to Et-1 persisted for greater than 12 h and may account for the ability of Et-1 to augment the G1-S phase transition. Although these observations indicate that functional pkC is not an essential component of the proximal pathway leading to rapid changes in gene transcription and second messenger production in response to Et-1 treatment, the data suggest that activation of pkC is an essential component of the downstream events responsible for the stimulation of cell proliferation and anchorage-independent growth in Rat-1 cells exposed to Et-1.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballester R., Rosen O. M. Fate of immunoprecipitable protein kinase C in GH3 cells treated with phorbol 12-myristate 13-acetate. J Biol Chem. 1985 Dec 5;260(28):15194–15199. [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- Bishop R., Martinez R., Weber M. J., Blackshear P. J., Beatty S., Lim R., Herschman H. R. Protein phosphorylation in a tetradecanoyl phorbol acetate-nonproliferative variant of 3T3 cells. Mol Cell Biol. 1985 Sep;5(9):2231–2237. doi: 10.1128/mcb.5.9.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackshear P. J., Stumpo D. J., Huang J. K., Nemenoff R. A., Spach D. H. Protein kinase C-dependent and -independent pathways of proto-oncogene induction in human astrocytoma cells. J Biol Chem. 1987 Jun 5;262(16):7774–7781. [PubMed] [Google Scholar]
- Claycomb W. C., Moses R. L. Growth factors and TPA stimulate DNA synthesis and alter the morphology of cultured terminally differentiated adult rat cardiac muscle cells. Dev Biol. 1988 Jun;127(2):257–265. doi: 10.1016/0012-1606(88)90313-2. [DOI] [PubMed] [Google Scholar]
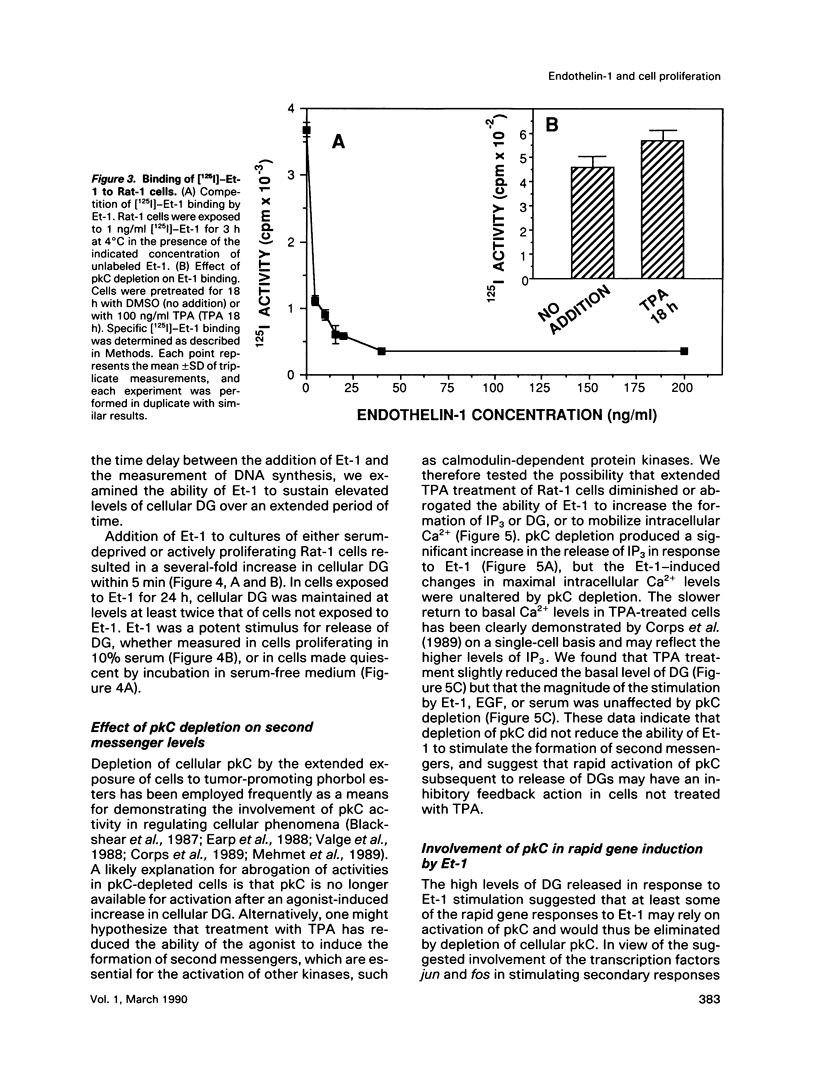
- Corps A. N., Cheek T. R., Moreton R. B., Berridge M. J., Brown K. D. Single-cell analysis of the mitogen-induced calcium responses of normal and protein kinase C-depleted Swiss 3T3 cells. Cell Regul. 1989 Nov;1(1):75–86. doi: 10.1091/mbc.1.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dion L. D., Gindhart T. D., Colburn N. H. Four-day duration of tumor promoter exposure required to transform JB6 promotion-sensitive cells to anchorage independence. Cancer Res. 1988 Dec 15;48(24 Pt 1):7126–7131. [PubMed] [Google Scholar]
- Earp H. S., Hepler J. R., Petch L. A., Miller A., Berry A. R., Harris J., Raymond V. W., McCune B. K., Lee L. W., Grisham J. W. Epidermal growth factor (EGF) and hormones stimulate phosphoinositide hydrolysis and increase EGF receptor protein synthesis and mRNA levels in rat liver epithelial cells. Evidence for protein kinase C-dependent and -independent pathways. J Biol Chem. 1988 Sep 25;263(27):13868–13874. [PubMed] [Google Scholar]
- Hidaka H., Inagaki M., Kawamoto S., Sasaki Y. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry. 1984 Oct 9;23(21):5036–5041. doi: 10.1021/bi00316a032. [DOI] [PubMed] [Google Scholar]
- Housey G. M., Johnson M. D., Hsiao W. L., O'Brian C. A., Murphy J. P., Kirschmeier P., Weinstein I. B. Overproduction of protein kinase C causes disordered growth control in rat fibroblasts. Cell. 1988 Feb 12;52(3):343–354. doi: 10.1016/s0092-8674(88)80027-8. [DOI] [PubMed] [Google Scholar]
- Issandou M., Rozengurt E. Diacylglycerols, unlike phorbol esters, do not induce homologous desensitization or down-regulation of protein kinase C in Swiss 3T3 cells. Biochem Biophys Res Commun. 1989 Aug 30;163(1):201–208. doi: 10.1016/0006-291x(89)92121-9. [DOI] [PubMed] [Google Scholar]
- Jefferson A. B., Schulman H. Sphingosine inhibits calmodulin-dependent enzymes. J Biol Chem. 1988 Oct 25;263(30):15241–15244. [PubMed] [Google Scholar]
- Kariya K., Fukumoto Y., Tsuda T., Yamamoto T., Kawahara Y., Fukuzaki H., Takai Y. Antiproliferative action of protein kinase C in cultured rabbit aortic smooth muscle cells. Exp Cell Res. 1987 Dec;173(2):504–514. doi: 10.1016/0014-4827(87)90290-4. [DOI] [PubMed] [Google Scholar]
- Komuro I., Kurihara H., Sugiyama T., Yoshizumi M., Takaku F., Yazaki Y. Endothelin stimulates c-fos and c-myc expression and proliferation of vascular smooth muscle cells. FEBS Lett. 1988 Oct 10;238(2):249–252. doi: 10.1016/0014-5793(88)80489-7. [DOI] [PubMed] [Google Scholar]
- Machida C. M., Muldoon L. L., Rodland K. D., Magun B. E. Transcriptional modulation of transin gene expression by epidermal growth factor and transforming growth factor beta. Mol Cell Biol. 1988 Jun;8(6):2479–2483. doi: 10.1128/mcb.8.6.2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magun B. E., Bowden G. T. Effects of the tumor promoter TPA on the induction of DNA synthesis in normal and RSV-transformed rat fibroblasts. J Supramol Struct. 1979;12(1):63–72. doi: 10.1002/jss.400120107. [DOI] [PubMed] [Google Scholar]
- Matrisian L. M., Bowden G. T., Magun B. E. Mechanism of synergistic induction of DNA synthesis by epidermal growth factor and tumor promoters. J Cell Physiol. 1981 Sep;108(3):417–425. doi: 10.1002/jcp.1041080316. [DOI] [PubMed] [Google Scholar]
- Matrisian L. M., Larsen B. R., Finch J. S., Magun B. E. Further purification of epidermal growth factor by high-performance liquid chromatography. Anal Biochem. 1982 Sep 15;125(2):339–351. doi: 10.1016/0003-2697(82)90015-x. [DOI] [PubMed] [Google Scholar]
- Mehmet H., Moore J. P., Sinnett-Smith J. W., Evan G. I., Rozengurt E. Dissociation of c-fos induction from protein kinase C-independent mitogenesis in Swiss 3T3 cells. Oncogene Res. 1989;4(3):215–222. [PubMed] [Google Scholar]
- Meyn R. E., Hewitt R. R., Humphrey R. M. Evaluation of S phase synchronization by analysis of DNA replication in 5-bromodeoxyuridine. Exp Cell Res. 1973 Nov;82(1):137–142. doi: 10.1016/0014-4827(73)90255-3. [DOI] [PubMed] [Google Scholar]
- Muldoon L. L., Jamieson G. A., Jr, Kao A. C., Palfrey H. C., Villereal M. L. Mitogen stimulation of Na+-H+ exchange: differential involvement of protein kinase C. Am J Physiol. 1987 Aug;253(2 Pt 1):C219–C229. doi: 10.1152/ajpcell.1987.253.2.C219. [DOI] [PubMed] [Google Scholar]
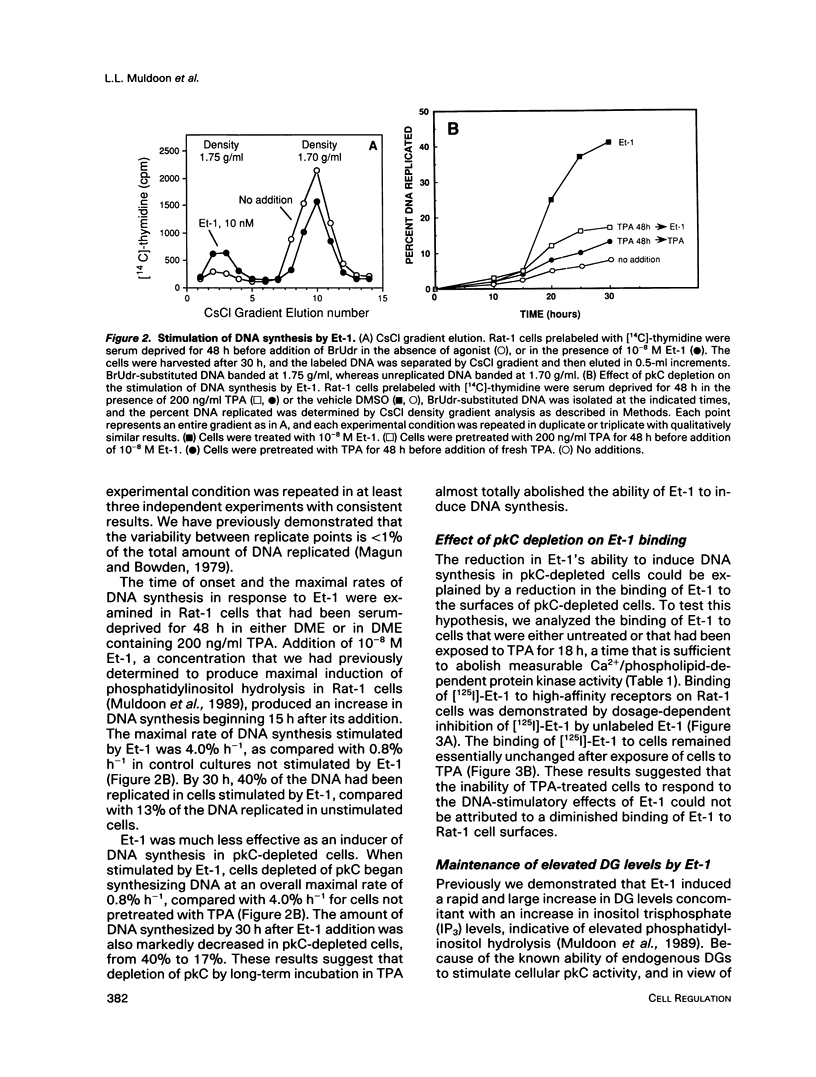
- Muldoon L. L., Rodland K. D., Forsythe M. L., Magun B. E. Stimulation of phosphatidylinositol hydrolysis, diacylglycerol release, and gene expression in response to endothelin, a potent new agonist for fibroblasts and smooth muscle cells. J Biol Chem. 1989 May 25;264(15):8529–8536. [PubMed] [Google Scholar]
- Nakabeppu Y., Ryder K., Nathans D. DNA binding activities of three murine Jun proteins: stimulation by Fos. Cell. 1988 Dec 2;55(5):907–915. doi: 10.1016/0092-8674(88)90146-8. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- O'Keefe E. J., Pledger W. J. A model of cell cycle control: sequential events regulated by growth factors. Mol Cell Endocrinol. 1983 Aug;31(2-3):167–186. doi: 10.1016/0303-7207(83)90147-8. [DOI] [PubMed] [Google Scholar]
- Pardee A. B. G1 events and regulation of cell proliferation. Science. 1989 Nov 3;246(4930):603–608. doi: 10.1126/science.2683075. [DOI] [PubMed] [Google Scholar]
- Preiss J., Loomis C. R., Bishop W. R., Stein R., Niedel J. E., Bell R. M. Quantitative measurement of sn-1,2-diacylglycerols present in platelets, hepatocytes, and ras- and sis-transformed normal rat kidney cells. J Biol Chem. 1986 Jul 5;261(19):8597–8600. [PubMed] [Google Scholar]
- Roberts A. B., Frolik C. A., Anzano M. A., Sporn M. B. Transforming growth factors from neoplastic and nonneoplastic tissues. Fed Proc. 1983 Jun;42(9):2621–2626. [PubMed] [Google Scholar]
- Rodland K. D., Muldoon L. L., Dinh T. H., Magun B. E. Independent transcriptional regulation of a single VL30 element by epidermal growth factor and activators of protein kinase C. Mol Cell Biol. 1988 May;8(5):2247–2250. doi: 10.1128/mcb.8.5.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Pena A., Rozengurt E. Disappearance of Ca2+-sensitive, phospholipid-dependent protein kinase activity in phorbol ester-treated 3T3 cells. Biochem Biophys Res Commun. 1984 May 16;120(3):1053–1059. doi: 10.1016/s0006-291x(84)80213-2. [DOI] [PubMed] [Google Scholar]
- Takuwa N., Takuwa Y., Yanagisawa M., Yamashita K., Masaki T. A novel vasoactive peptide endothelin stimulates mitogenesis through inositol lipid turnover in Swiss 3T3 fibroblasts. J Biol Chem. 1989 May 15;264(14):7856–7861. [PubMed] [Google Scholar]
- Valge V. E., Wong J. G., Datlof B. M., Sinskey A. J., Rao A. Protein kinase C is required for responses to T cell receptor ligands but not to interleukin-2 in T cells. Cell. 1988 Oct 7;55(1):101–112. doi: 10.1016/0092-8674(88)90013-x. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M., Kurihara H., Kimura S., Tomobe Y., Kobayashi M., Mitsui Y., Yazaki Y., Goto K., Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988 Mar 31;332(6163):411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]