Abstract
While much is known about the factors that control each component of the terrestrial nitrogen (N) cycle, it is less clear how these factors affect total N availability, the sum of organic and inorganic forms potentially available to microorganisms and plants. This is particularly true for N-poor ecosystems such as drylands, which are highly sensitive to climate change and desertification processes that can lead to the loss of soil nutrients such as N. We evaluated how different climatic, abiotic, plant and nutrient related factors correlate with N availability in semiarid Stipa tenacissima grasslands along a broad aridity gradient from Spain to Tunisia. Aridity had the strongest relationship with N availability, suggesting the importance of abiotic controls on the N cycle in drylands. Aridity appeared to modulate the effects of pH, plant cover and organic C (OC) on N availability. Our results suggest that N transformation rates, which are largely driven by variations in soil moisture, are not the direct drivers of N availability in the studied grasslands. Rather, the strong relationship between aridity and N availability could be driven by indirect effects that operate over long time scales (decades to millennia), including both biotic (e.g. plant cover) and abiotic (e.g. soil OC and pH). If these factors are in fact more important than short-term effects of precipitation on N transformation rates, then we might expect to observe a lagged decrease in N availability in response to increasing aridity. Nevertheless, our results suggest that the increase in aridity predicted with ongoing climate change will reduce N availability in the Mediterranean basin, impacting plant nutrient uptake and net primary production in semiarid grasslands throughout this region.
Introduction
Nitrogen (N) is, after water, the most important factor limiting plant growth, net primary production and microbial decomposition in drylands [1]–[2]. Thus, it is important to develop a predictive understanding of the factors controlling the amount of available N within an ecosystem, which includes both the inorganic (nitrate and ammonium) and organic forms that can be assimilated by plants and microorganisms [3]–[4]. The amount of available N in an ecosystem is controlled by the cumulative effects of microbially-driven N inputs through N-fixation, by the mineralization of N from organic matter, and by N-losses through leaching and gas emissions [5]–[6].
Although temperature and moisture are strong drivers of N transformation rates in soils [7]–[8], the net effect of climate on N availability remains unclear [5]–[6]. For example, N mineralization and decomposition increase with temperature and soil moisture, but high rainfall levels may also enhance denitrification by promoting anaerobic conditions and nitrate leaching [5], [8], [9]–[10]. Some studies have shown that increases in mean annual precipitation enhance the concentration of available N in the field [11]–[12], but others have found the opposite [9]–[10], or have reported inconsistent responses [13]–[14]. Studies focusing on the effects of temperature on N dynamics have also shown inconsistent results [2]. Some experiments show that increases in temperature enhance N availability [8], [15], but others found no significant effects of temperature on the concentration of N in soils [16]. The contradictory results observed to date may be due to differences in plant species, soil types and ecosystems studied, which may determine the effects of temperature and precipitation on soil N availability.
Environmental variables such as plant cover, soil pH, texture and organic matter are also major drivers of N cycling [6], [12], [17]. The uptake of N by plants, as well as the litter and root exudates they produce, affects N concentration in soils [2], [18]. Sandy soils typically exhibit lower denitrification rates, but higher N losses after rainfall events, than clay soils [5], [19]. Plants typically increase the amount of litter mass and soil water moisture under their canopies compared to adjacent bare soil microsites, affecting N mineralization and depolymerization processes [20]–[21]. Acidic soil pH and high organic C concentrations may favor fungal communities, which are involved in depolymerization and decomposition processes, increasing the amount of available N [6], [19], [22]. Finally, the C:N ratio modulates the immobilization and mineralization of N [3], [22].
Despite the emergence of new paradigms for soil N cycling that emphasize the importance of both organic and inorganic N forms [3]–[4], [18], and the growing literature on the topic, we lack an integrated understanding of the most important determinants of N availability. This is particularly true for N-poor ecosystems such as drylands (arid, semiarid and dry-subhumid ecosystems; [22]–[24]). In addition, these areas are more open in terms of their N cycling relative to more humid systems, where most studies have been performed, because they support high N losses relative to N turnover [25]. Drylands are of paramount importance for the Earth system, as they cover about 41% of Earth’s land surface and support over 38% of the total global human population [26]. Improving our knowledge of the factors driving N availability in drylands can greatly enhance our ability to understand how ongoing climate change may affect their functioning [17], and can help to establish “early warning” signals of the onset of desertification processes [26], which often start with the loss of soil nutrients such as N [27].
This study had two main objectives. First, we aimed to evaluate the relationships between N availability (measured as the sum of extractable inorganic and organic N forms) and climatic (aridity), abiotic (soil pH and sand content), plant (average interdistance between plant patches) and nutrient related (organic carbon, N mineralization rate, N transformation rate and the C:N ratio) factors in arid and semiarid grasslands dominated by the tussock grass Stipa tenacissima L. along a broad aridity gradient from Spain to Tunisia. They are one of the most widespread and representative ecosystems of the semiarid regions of the Mediterranean basin [28], which is considered highly vulnerable to climate change and desertification [29]. Our second objective was to evaluate whether S. tenacissima tussocks modify the relative importance of climate, abiotic, plant and nutrient related factors as potential modulators of N availability. This species is able to create “resource islands” [20], [30]–[31] and modify microclimate, infiltration and soil water availability dynamics [32]–[34] as compared to adjacent bare ground areas. As such, they can potentially alter how climate and other factors affect N availability.
Methods
Study Area
This study was carried out in 22 sites located in Spain, Morocco and Tunisia (See map S1), which cover most of the geographic distribution range of S. tenacissima grasslands in the Mediterranean basin [28]. All of the sites were located in areas of arid or semiarid climate; total annual precipitation and mean temperature ranged from 141 mm to 465 mm, and from 12.5°C to 20°C, respectively (Table S1). Slope and elevation ranged between 1° and 22°, and between 172 m a.s.l. and 1427 m a.s.l., respectively (Table S1). All sites were located on calcareous soils, and on south-facing slopes. Perennial vegetation cover ranged between 8% and 64%, and was in all cases an open steppe dominated by S. tenacissima, with shrub species such as Quercus coccifera L., Rosmarinus officinalis L. and Thymus vulgaris L. in Spain, Cistus clusii and Helianthemum apenninum L. (Mill.) in Morocco, and Artemisia herba-alba Asso and Hammada scoparia (Pomel) Iljin in Tunisia.
Sampling Design and Measurements
At each site, we established a 30 m×30 m plot representative of the dominant vegetation. In the upper left corner of each plot, we located a 30 m transect oriented downslope for the vegetation survey. Three parallel transects of the same length, spaced 8 m apart across the slope, were added. Along each transect, we collected a continuous record of vegetated patches and bare ground areas (i.e. devoid of vascular vegetation). From these transects, we obtained total plant cover, the average interdistance between plant patches and the number of plant patches per 10 m of transect. Vegetation surveys were carried out during 2005 and 2006 (Spain) and in 2010 (Morocco and Tunisia).
We sampled soils in Spain and North Africa (Morocco and Tunisia) during 2006 and 2010, respectively, using a stratified random procedure. Five 50 cm×50 cm quadrats were randomly placed at each of two microsites: bare ground areas and S. tenacissima tussocks (Bare and Stipa microsites hereafter). A composite sample consisting of five 145 cm3 soil cores (0–7.5 cm depth) was collected from each quadrat, bulked and homogenized in the field. After collection, soils were transported to the laboratory and air-dried at room temperature for four weeks. Previous studies have found that soil biochemical properties are minimally affected by air-drying in semiarid Mediterranean soils [35]. No specific permissions were required for our field activities, except for the site located in Djebel Bou-Hedma Biosphere Reserve and National Park (Tunisia). The required authorization to work at this site was obtained from the Ministère de l’Agriculture, Direction générale des forêts, Tunisia’s authority that manages the Djebel Bou-Hedma Biosphere Reserve and National Park. The study did not involve handling or collection of endangered species.
Organic C (OC) was determined following Anderson and Ingramm [36]. Total N was measured with a CN analyzer (Leco CHN628 Series, Leco Corporation, St Joseph, MI, USA). Total available N was colorimetrically determined from K2SO4 extracts as the sum of ammonium, nitrate and dissolved organic nitrogen following Delgado-Baquerizo et al. [37]. Potential net N transformation (production of available N) and mineralization (production of inorganic-N) rates were measured by determination of the total available and mineral N before and after incubation in the laboratory at 80% of water holding capacity and 30°C for 14 days [23]. Soil pH was measured in all the soil samples with a pH meter, in a 1∶2.5 mass: volume soil and water suspension. One composite sample each per microsite (Bare and Stipa) and plot were analyzed for sand, clay and silt content according to Kettler et al. [38].
The UNEP (1992) aridity index (AI = P/PET, where P is annual precipitation and PET is annual potential evapotranspiration) of each site was gathered from the Worldclim global database [39]–[41], as described in Maestre et al. [17]. The AI decreases as aridity increases, and to avoid confusing readers we used the inverse of AI in this work. Thus, aridity = 1-AI. This index is strongly related to both annual average rainfall (R2 = −0.98) and temperature (R2 = 0.80) in our study sites.
Statistical Analyses
Due to the low number of study sites as compared to number of climatic, abiotic, plant and nutrient variables studied, and the significant correlations between some of the variables from each group (Table S2), we reduced the dimensionality of our dataset by selecting one representative variable for each group (climatic, abiotic, plant and nutrient). We first conducted correlation analyses between the total available N and the different climatic (aridity), abiotic (pH and sand content), plant (total plant and bare cover, average interdistance between plant patches, plant patch area and number of plant patches per 10 m of transect) and nutrient (OC, C:N ratio, potential net mineralization and potential net N transformation rate) variables studied in both Stipa and Bare microsites (Table S3). We then retained for further analyses those variables significantly related to available N in both Stipa and Bare microsites. Aridity, pH and OC were retained as the climatic, abiotic and nutrient variables for subsequent analyses. All of the plant variables were significant related to N availability (Table S3). Thus, we carried out a principal component analysis (PCA) to unify these variables into a single plant component. The first component of this PCA (plant-ax1), which explained the 83.3% of the variance and was the only axis with an eigenvalue higher than 1 (4.167), was retained for further analyses. This component was negatively related to coverage of bare ground and the average plant patch interdistance (r = −0.983 and −0.889, respectively P<0.01 in both cases), but positively related to the Stipa tenacissima coverage, plant patch area and number of plant patches per 10 m of transect, respectively (r = 0.932, 0.894 and 0.861, respectively, P<0.01 in all cases).
With this reduced set of independent variables (aridity, pH, OC and plant-ax1), we then used a multi-model inference approach based on information theory and ordinary least squares regression [42] to evaluate their relative importance as drivers of N availability (dependent variable). We ranked all the models that could be generated with our independent variables according to the second-order Akaike information criterion (AICc), calculated as described in Fotheringham et al. [43]. The AICc of each model was then transformed to ΔAICc, which is the difference between AICc of each model and the minimum AICc found for the set of models compared. The ΔAICc values were also used to obtain the Akaike weights of each model (wi), according to Burnham & Anderson [42]. Akaike’s weights were also used to define the relative importance of each predictor across the full set of models evaluated by summing wi values of all models that include the predictor of interest, taking into account the number of models in which each predictor appears [42]. As we were interested in evaluating whether S. tenacissima tussocks modified the importance of the different drivers of N availability evaluated, multi-model analyses were carried out for Bare and Stipa microsites separately. These analyses were conducted with the software SAM 4.0 [44].
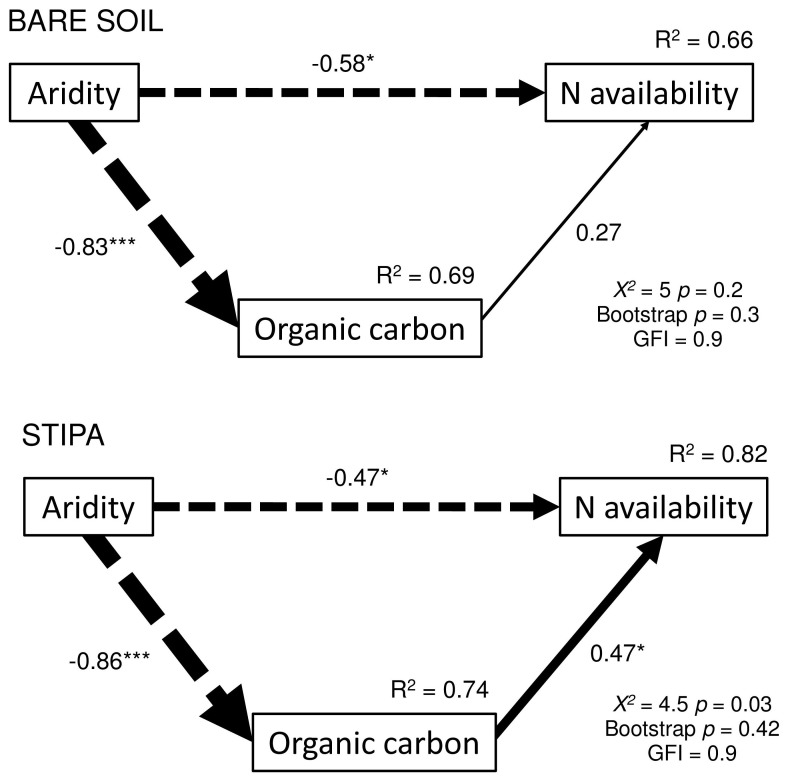
Aridity and OC, which were highly correlated, had a high explanatory power (Fig. 1) and importance (Fig. 2) among the different predictors of available N that we evaluated (Table S2). Thus, to tease out their relative importance as drivers of N availability, we used structural equation modeling (SEM, [45]). This tool has emerged as a synthesis of path analysis, factor analysis, and maximum likelihood techniques, and has been thoroughly used in the ecological sciences a causal inference tool [46]–[47]. It can test the plausibility of a directed, causal model like that proposed here. Another important capability of SEM is its ability to partition direct and indirect effects that one variable may have on another, and estimate the strengths of these multiple effects. Unlike regression or ANOVA, SEM offers the ability to separate multiple pathways of influence and view them as a system [45]. Thus, the use of SEM is useful for investigating the complex networks of relationships found in natural ecosystems [47]. Path coefficients were obtained using the maximum likelihood estimation technique. These coefficients are interpreted as the size of an effect that one variable exerts upon another. Because of our SEM was saturated (the number of degrees of freedom was zero), no probability level could be assigned to the chi-square statistic, making the model untestable. Thus, the free covariance weight between Aridity and the best solution was chosen through maximization of the maximum likelihood function. The goodness of fit of SEM models was checked following Schermelleh-Engel et al. [48]. SEM models were separately conducted for Bare and Stipa microsites with the software AMOS 20 (IBM SPSS Inc, Chicago, IL, USA).
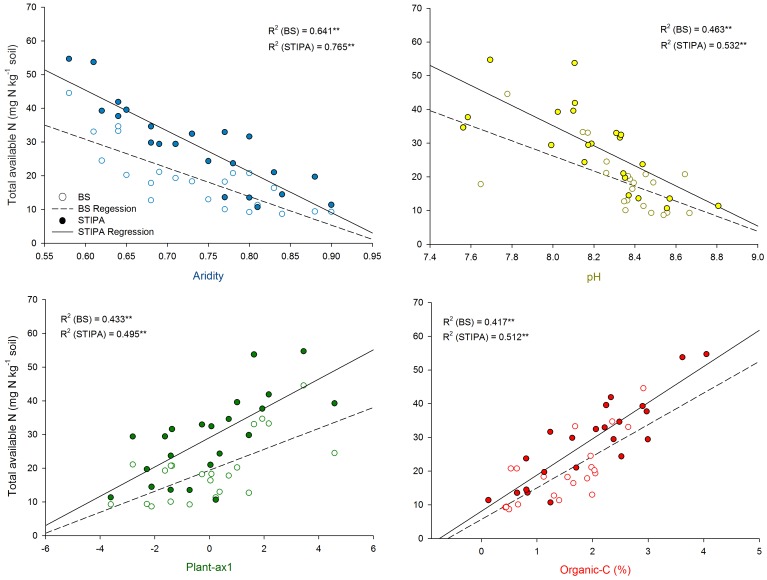
Figure 1. Relationships between total nitrogen (N) availability and aridity, pH, plant-ax1 (first component of a PCA including the cover of bare and plant microsites, average plant patch interdistance, area of plant patches and number of plant patches per 10 m of transect) and organic carbon for both Stipa tenassicima (STIPA) and Bare ground (BS) microsites.
Every data point is the average of five replicated soil samples. Significance levels are as follows: *p<0.05, **p<0.01 and ***p<0.001.
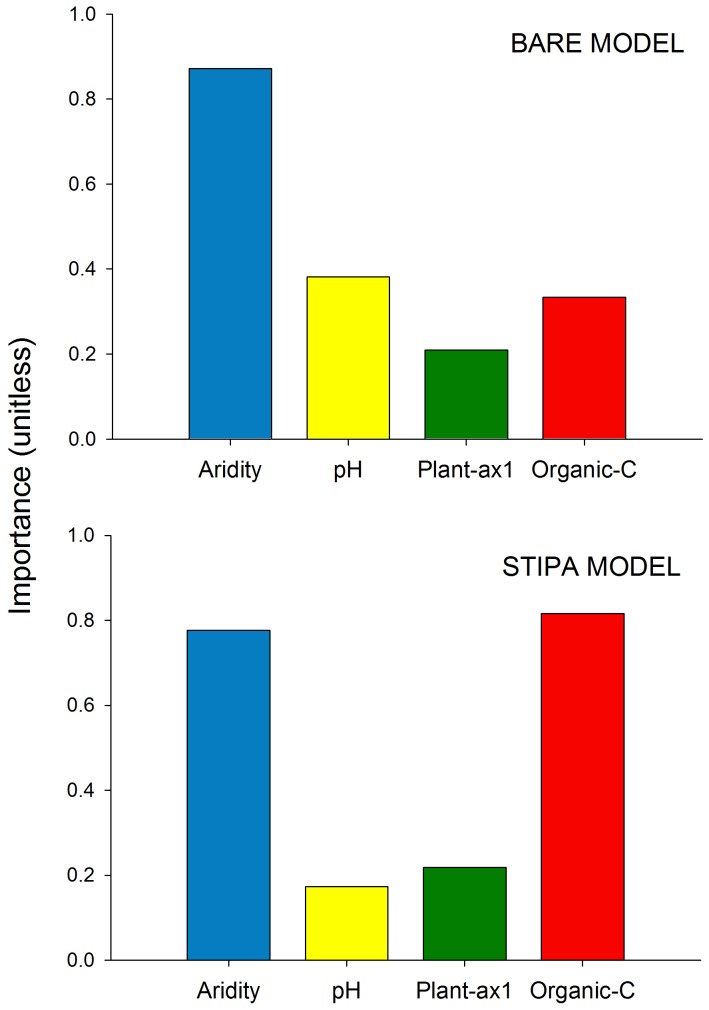
Figure 2. Relative importance of aridity, pH, organic C, and plant-ax1 (first component of a PCA including the cover of bare and plant microsites, average plant patch interdistance, area of plant patches and number of plant patches per 10 m of transect) variables as drivers of variations in N availability.
Results are shown for: i) bare ground microsites, and ii) Stipa tenacissima microsites. The height of each bar is the sum of the Akaike weights of all models that included the predictor of interest, taking into account the number of models in which each predictor appears.
Results
Soil characteristics changed consistently across the aridity gradient studied. Soil pH increased with increasing aridity for both Stipa and Bare microsites (p<0.01; Table S2). OC decreased with increases in aridity for both microsites (p<0.01; Table S2), but the soil C:N ratio was not related to aridity (p>0.05; Table S2). The cover of Stipa, and both the number and area of plant patches were negatively related to aridity (p<0.01; Table S2), while the average interdistance between consecutive plant patches and the cover of bare ground areas increased concomitantly with aridity (p<0.01; Table S2).
Total N availability decreased with aridity for both Stipa (p<0.01) and Bare (p<0.01) microsites (p<0.01; Table S3; Fig. 1). N availability was correlated with both soil and plant characteristics. For example, N availability was positively correlated with pH and OC in both Stipa and Bare microsites (p<0.01;Table S3; Fig. 1). The relationship between N availability and sand content was only significant in the Stipa microsites (p = 0.01; Table S3). We did not find a significant relationship between N availability and the C:N ratio (Table S3). The plant-ax1 was positively related to N availability in both microsites (p<0.01; Table S3; Fig. 1). Total N availability did not appear to be driven by N supply rates, as the relationships between N availability and either potential net mineralization or N transformation rates were non-significant (p>0.05; Table S3).
Multi-model analyses showed that aridity and OC were the most important variables affecting N availability. Aridity explained more of the variability in N availability in Bare microsites (Fig. 2; Table 1), but OC was the most important predictor of N availability in Stipa microsites (Fig. 2; Table 1). The SEMs were satisfactorily fitted to our data, as indicated by goodness-of-fit statistics (Fig. 3; [48]). These analyses revealed that the effects of OC on N availability were largely modulated by aridity, which had larger total effects on N availability than OC in both Bare and Stipa microsites (Fig. 3).
Table 1. Top eight best-fitting regression models, ranked according to their AICc value, are presented.
| BARE | |||||||
| Aridity | pH | Plant-ax1 | Organic-C | R2 | AICc | ΔAIC | Wi |
| X | 0.635 | 146.24 | 0 | 0.31 | |||
| X | X | 0.673 | 146.89 | 0.64 | 0.22 | ||
| X | X | 0.658 | 147.82 | 1.58 | 0.14 | ||
| X | X | 0.638 | 149.09 | 2.85 | 0.07 | ||
| X | X | X | 0.68 | 149.8 | 3.55 | 0.05 | |
| X | X | X | 0.674 | 150.17 | 3.92 | 0.04 | |
| X | 0.561 | 150.31 | 4.07 | 0.03 | |||
| X | X | 0.605 | 151.01 | 4.77 | 0.04 | ||
| STIPA | |||||||
| Aridity | pH | Plant-ax1 | Organic-C | R2 | AICc | ΔAIC | Wi |
| X | X | 0.82 | 145.83 | 0 | 0.41 | ||
| X | 0.77 | 148.32 | 2.49 | 0.12 | |||
| X | 0.76 | 148.51 | 2.68 | 0.11 | |||
| X | X | X | 0.82 | 149.03 | 3.2 | 0.08 | |
| X | X | X | 0.82 | 149.09 | 3.27 | 0.08 | |
| X | X | 0.79 | 149.2 | 3.37 | 0.08 | ||
| X | X | 0.77 | 151 | 5.17 | 0.03 | ||
| X | X | 0.77 | 151.28 | 5.45 | 0.01 |
AICc measures the relative goodness of fit of a given model; the lower its value, the more likely the model to be correct. Aridity, pH, plant-ax1 and organic C were included in these models. Bare = data from bare ground soils only, and Stipa = data from Stipa tenacissima soils only.
Figure 3. Structural equation models showing the direct and indirect effects of aridity and organic carbon on the total nitrogen availability for the Stipa tenacissima (STIPA) and bare ground (BARE SOIL) microsites.
Continuous and dashed arrows indicate positive and negative relationships, respectively. Width of arrows is proportional to the strength of path coefficients. The proportion of variance explained (R2) appears above every response variable in the model. Goodness-of-fit statistics for each model are shown in the lower right corner. Significance levels are as follows: *p<0.05, **p<0.01 and ***p<0.001.
Discussion
Evaluating which abiotic and biotic factors drive the N cycle has been an important research focus over the last two decades. It has been motivated by the importance of N for the functioning of the Earth system, and by anthropogenic activities affecting N cycling in a range of ecosystems [42]. Yet, most of the studies carried out on this topic have focused on the effects of single variables, and relatively few of them have been carried out in drylands [11], [13]–[14].
Our results indicate that aridity is the most important driver of N availability in the studied grasslands, highlighting the importance of abiotic controls of the N cycle in drylands. This availability was strongly correlated with multiple soil and plant variables (Table S2), which are also highly related to different aspects of the N cycle [5]–[6]. Aridity also appears to be a strong driver of OC, which was strongly correlated with N availability in this study. Increases in OC may enhance the activity of heterotrophic fungi and bacterial communities that carry out depolymerization and mineralization processes, increasing N availability [22].
Plant and soil properties were also correlated with N availability. Reductions in aridity along the environmental gradient were associated with increases in the plant-ax1, i.e. in the cover, area and number of S. tenacissima patches (Table S2). As plant cover increases across the aridity gradient, N and C inputs to the soil from litter are also likely to increase, as indicated by higher decomposition rates under S. tenacissima canopies than on surrounding bare ground areas [49]–[50]. The link between plant cover and N availability is likely to be most evident in ecosystems with low organic matter content, such as those studied, where existing substrate pools in the soil are small relative to the inputs of nutrients entering the soil from plant detritus [51]. Increases in rainfall (lower aridity conditions) may favor N leaching and soil formation processes, decreasing the pH and sand content of the soil, which may increase the retention of OC and favor fungal communities involved in N mineralization and depolymerization processes [5]–[6]. We observed an increase in N availability with decreases in soil pH and increases in OC contents, consistent with previous studies [6], [22]. However, the relationship between N availability and OC in drylands is not straightforward because N availability should be more related to microbial biomass turnover and atmospheric inputs than to soil organic matter conent [52]–[54].
Other factors, such as potential net N mineralization and transformation rates, and the C:N ratio, were poorly related to N availability. These results were unexpected, as N transformation rates (which include depolymerization and N mineralization) should be strongly linked to N availability [18]. Our results suggest that potential net N transformation rates, conducted under laboratory conditions (30°C and 80% of water holding capacity), may not be a good indicator of soil N availability in arid and semiarid ecosystems because they do not take into account the effects of drying-rewetting cycles on microbial and plant processes, including rapid microbial turnover and plant nutrient uptake and losses through leaching or gaseous emissions [23], [55]. A decrease in the C:N ratio has been suggested to increase N mineralization [22]. However, this ratio did not show a significant relationship with the amount of available N in this study. This finding suggests that the C:N ratio may not be a good indicator of N availability in this system, possibly because this ratio includes both labile and recalcitrant C, the latter being less available for microorganisms [2], [22].
Important differences were found among microsites when modeling N availability. While aridity was the most important driver of changes in N availability in the Bare model, OC was as important as aridity in Stipa microsites. Soils at this microsite had more OC than soils in Bare microsites in most of the sites surveyed (14 out of 22 sites, Table S4). Similar results have been found in other S. tenacissima steppes (e.g. [20]). Increasing OC content may drive increased biomass of heterotrophic microbial communities (fungi and bacteria), which drive N mineralization and depolymerization processes, increasing N availability and favoring a fertility island effect under the canopy of S. tenacissima [2], [6], [20], [30]–[34]. The effect of S. tenacissima on microclimate and soils beneath their canopies, including lower temperature and higher moisture compared to bare ground areas [20], [33], may have also promoted N mineralization and depolymerization processes.
Studies aiming to evaluate how N availability changes along regional climatic gradients have been carried out in drylands from the USA [14], Argentina [12] and South Africa [11]. However, none of them considered both organic and inorganic N availability. Our results indicate that aridity is the most important factor modulating total N availability in Mediterranean S. tenacissima grasslands. In addition, four different organic (DON and amino acids) and inorganic (ammonium and nitrate) N forms were positively related to N availability but negatively related to aridity, showing that decreasing N availability with increasing aridity may affect both organic and inorganic N forms available to plants and microorganisms (Figure S1 and S2). This suggests that changes in precipitation regimes are likely to affect N availability, albeit the temporal scale of this response is uncertain. Climate change models predict reductions in rainfall and increases in temperature throughout the semiarid and arid areas of the Mediterranean basin [29]. While soil moisture is a proximate driver of N transformation rates, our results suggest that N transformation rates are not the most important drivers of N availability. Rather, the strong relationship between aridity and N availability could be driven by indirect effects that operate over longer time scales (decades to millennia) including both biotic (e.g. plant cover) and abiotic (e.g. soil OC and pH). If these factors are in fact more important than short-term effects of precipitation on N transformation rates, then we might expect to observe a lagged decrease in N availability in response to increasing aridity. If N availability does decrease in response to climate change, it will have important harmful effects on plant nutrition and net primary production within S. tenacissima grasslands, and will also negatively impact other key supporting (e.g. soil conservation, [50]), provisioning (grazing, [56]) and cultural (e.g. game hunting, [57]) ecosystem services provided by this ecosystem throughout the Mediterranean basin.
Supporting Information
Pearsońs relationships between organic (DON and amino acids) and inorganic (ammonium and nitrate) N forms with aridity for both Stipa tenassicima (STIPA) and Bare soil (BS) microsites. Every data point is the average of five soil samples. Significance levels are as follows: *p<0.05, **p<0.01 and ***p<0.001. Ammonium, nitrate and DON were measured as described by Delgado-Baquerizo et al. (1). Amino acids were determined by following Chantigny et al. (2).
(DOC)
Relationships between organic (DON and amino acids) and inorganic (ammonium and nitrate) N forms with total available N for both Stipa tenassicima (STIPA) and Bare soil (BS) microsites. Every data point is the average of five soil samples. Significance levels are as follows: *p<0.05, **p<0.01 and ***p<0.001. Ammonium, nitrate and DON were measured as described by Delgado-Baquerizo et al. (1). Amino acids were determined by following Chantigny et al. (2).
(DOC)
Location, climatic, physical and main soil chemical characteristics in the studied sites. MAT = Mean annual temperature; MAP = Mean annual precipitation; Stipa = coverage of Stipa.
(DOC)
Pearson correlations coefficients. between the different climatic (aridity), abiotic (pH; SAC: % of sand content), plant (CBA: % of coverage of bare ground; CHE: % of coverage of Stipa tenacissima; PA: plant patch area [m2]; API: Average plant patch interdistance [m]; NP: number of plant patches per 10 m of transect) and nutrient (Organic-C [%]; MIN; potential net mineralization rate [mg N kg−1 soil day−1]; NRT: potential net N transformation rate [mg N kg−1 soil day−1]; ratio C:N) variables. Significance levels are as follows: *p<0.05 and **and p<0.01.
(DOC)
Pearson correlations coefficients. between available N and climatic (aridity), abiotic (pH; SAC: sand content), plant (CBA: coverage of bare ground; CHE: coverage of Stipa tenacissima; PA: plant patch area; API: Average plant patch interdistance; NP: number of plant patches per 10 m of transect) and nutrient (Organic-C; MIN; potential net mineralization rate; NRT: potential net N transformation rate; ratio C:N) variables. Significance levels are as follows: *p<0.05 and **and p<0.01.
(DOC)
Summary results of the semi-parametric PERMANOVA analyses carried out with organic carbon. PERMANOVA uses permutation tests to obtain p values, does not rely on the assumptions of traditional parametric ANOVA, and can handle experimental designs such as employed here (1). The model used evaluated the effects of plot (PL as random factor) and microsite (MI as fixed factor) on organic carbon.When significant interactions between factors were found, separate PERMANOVA analysis were conducted for each site. All analyses were carried out using the PERMANOVA+ module of the PRIMER software (PRIMER-E Ltd., Plymounth Marine Laboratory, UK). RES = residuals.
(DOC)
Acknowledgments
We thank Wahida Ghiloufi and Mohamed Chaieb for their help during the surveys carried out in Tunisia.
Funding Statement
This research is supported by the European Research Council (ERC) under the European Community’s Seventh Framework Programme (FP7/2007–2013)/ERC Grant agreement n° 242658 (BIOCOM), and by the Ministry of Science and Innovation of the Spanish Government, Grant n°CGL2010-21381. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Craine JM, Morrow C, Fierer N (2007) Microbial nitrogen limitation increases decomposition. Ecology 88: 2105–2113. [DOI] [PubMed] [Google Scholar]
- 2.Robertson GP, Groffman P (2007) Nitrogen transformations. Microbiology Biochemistry Ecology Springer New York New York USA.
- 3. Schimel JP, Bennett J (2004) Nitrogen mineralization: challenges of a changing paradigm. Ecology 85: 591–602. [Google Scholar]
- 4. Medina-Roldan E, Bardgett RD (2012) Inter-Specific Competition but ot Different Soil Microbial Communities, Affects N Chemical Forms Uptake by Competing Graminoids of Upland Grasslands. PLoS ONE 7 (12): e51193 doi:10.1371/journal.pone.0051193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nieder R, Benbi DK (2008) Carbon and Nitrogen in the Terrestrial Environment. Springer New York New York USA.
- 6.Butterbach-Bahl K, Gundersen P (2011) Nitrogen processes in terrestrial ecosystems. The European Nitrogen Assessment Sources Effects Policy Perspectives. Cambridge University Press New York USA.
- 7. Beier C, Emmett BA, Peñuelas J, Schmidt IK, Tietema A, et al. (2008) Carbon and nitrogen cycles in European ecosystems respond differently to global warming. Science of the Total Environment 407: 692–697. [DOI] [PubMed] [Google Scholar]
- 8. Szukics U, Abell GCJ, Hodl V, Mitter B, Asessitsch AH, et al. (2010) Nitrifiers and denitrifiers respond rapidly to changed moisture and increasing temperature in a pristine forest soil. FEMS Microbiology Ecology 72: 395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Austin AT, Vitousek PM (1998) Nutrient dynamics on a precipitation gradient in Hawai’i. Oecologia 113: 519–529. [DOI] [PubMed] [Google Scholar]
- 10. Schuur EAG, Matson PA (2001) Net primary productivity and nutrient cycling across a mesic to wet precipitation gradient in Hawaiian montane forest. Oecologia 128: 431–442. [DOI] [PubMed] [Google Scholar]
- 11. Austin AT, Sala OE (2002) Carbon nitrogen dynamics across a natural precipitation gradient in Patagonia Argentina. Journal of Vegetation Science 13: 351–360. [Google Scholar]
- 12. Luo T, Pan Y, Ouyang Y, Shi H, Luo P, et al. (2004) Leaf area index and net primary productivity along subtropical to alpine gradients in the Tibetan Plateau. Global Ecology and Biogeography 13: 345–358. [Google Scholar]
- 13. Aranibar JN, Otter L, Macko SA, Feral CJW, Epstein HE, et al. (2004) Nitrogen cycling in the soil-plant system along a precipitation gradient in the Kalahari sands. Global Change Biology 10: 359–373. [Google Scholar]
- 14. McCulley RL, Burke IC, Lauenroth WK (2009) Conservation of nitrogen increases with precipitation across a major grassland gradient in the central Great Plains of North America Oecologia. 159: 571–581. [DOI] [PubMed] [Google Scholar]
- 15. Thiagalingam K, Kanehiro Y (1973) Effect of temperature on nitrogen transformation in Hawaiian soils. Plant and soil 38: 177–189. [Google Scholar]
- 16. Barrett JE, Burke IC (2002) Nitrogen retention in semiarid ecosystems across a soil organic-matter gradient. Ecological Applications 12: 878–890. [Google Scholar]
- 17. Maestre FT, Quero JL, Gotelli NJ, Escudero A, Ochoa V, et al. (2012) Plant species richness ecosystem multifunctionality in global drylands. Science 335: 214–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schimel JP, Bennett J, Fierer N (2005) Microbial community composition and soil N cycling: is there really a connection?. Biological diversity function in soils Cambridge University Press New York New York USA.
- 19. Kooijman AM, van Mourik JM, Schilder MLM (2009) The relationship between Nmineralization or microbial biomass N with micromorphological properties in beech forest soils with different texture pH. Biology and Fertility of Soils 45: 449–459. [Google Scholar]
- 20. Maestre FT, Bautista S, Cortina J, Bellot J (2001) Potential of using facilitation by grasses to establish shrubs on a semiarid degraded steppe. Ecological Applications 11: 1641–1655. [Google Scholar]
- 21. Delgado-Baquerizo M, Castillo-Monroy AP, Maestre FT, Gallardo A (2010) Change in the dominance of N forms within a semi-arid ecosystem. Soil Biology Biochemistry 42: 376–378. [Google Scholar]
- 22. Cookson WR, Müller C, O’Brien PA, Murphy DV, Grienson PF (2006) Nitrogen dynamics in an australian semiarid grassland soil. Ecology 87: 2047–2057. [DOI] [PubMed] [Google Scholar]
- 23. Delgado-Baquerizo M, Gallardo A (2011) Depolymerization and mineralization rates at 12 Mediterranean sites with varying soil N availability A test for the Schimel Bennett model. Soil Biology and Biochemistry 43: 693–696. [Google Scholar]
- 24. Delgado-Baquerizo M, Covelo F, Gallardo A (2011) Dissolved Organic Nitrogen in Mediterranean Ecosystems. Pedosphere 21: 302–308. [Google Scholar]
- 25. Swap RJ, Aranibar JN, Dowty PR, Gilhooly WP, Macko SP (2004) Natural abundance of 13C 15N in C3 and C4 vegetation of southern Africa patterns implications. Global Change Biology 10: 350–358. [Google Scholar]
- 26. Reynolds JF, Smith DM, Lambin EF, Turner BL, Mortimore M, et al. (2007) Global desertification: Building a science for dryland development. Science 316: 847–851. [DOI] [PubMed] [Google Scholar]
- 27.Li FR, Zhao WZ, Liu JL (2009) Degraded vegetation and wind erosion influence soil carbon, nitrogen and phosphorus accumulation in sandy grasslands. Plant and Soil: 317 79–92.
- 28. Le Houérou HN (2001) Biogeography of the arid steppeland north of the Sahara. Journal of Arid Environments 48: 103–128. [Google Scholar]
- 29. Gao XJ, Giorgi F (2008) Increased aridity in the Mediterranean region under greenhouse gas forcing estimated from high resolution simulations with a regional climate model. Global Planet Change 62: 195–209. [Google Scholar]
- 30. Reynolds JF, Virginia RA, Kemp PR, Soyza AG, Tremmel DC (1999) Impact of drought on desert shrubs: effects of seasonality and degree of resource island development. Ecological Monographs 69: 69–106. [Google Scholar]
- 31. Maestre FT, Cortina J (2004) Insights into ecosystem composition and function in a sequence of degraded semiarid steppes. Restoration Ecology 12: 494–502. [Google Scholar]
- 32. Cerda A (1997) The effect of patchy distribution of Stipa tenacissima L. on runoff and erosion. Journal of Arid Environments 36: 37–51. [Google Scholar]
- 33. Maestre FT, Bautista S, Cortina J (2003) Positive, negative and net effects in grass-shrub interactions in Mediterranean semiarid grasslands. Ecology 84: 3186–3197. [Google Scholar]
- 34. Eldridge D, Bowker MA, Maestre FT, Alonso P, Mau RL, et al. (2010) Interactive effects of three ecosystem engineers on infiltration in a semi–arid Mediterranean grassland. Ecosystems 13: 499–510. [Google Scholar]
- 35. Zornoza R, Mataix-Solera J, Guerrero C, Arcenegui V, Mataix-Beneyto J (2009) Storage effects on biochemical properties of air-dried soil samples from southeastern Spain. Arid Land Research Management 23: 213–222. [Google Scholar]
- 36.Anderson JM, Ingramm JSI (1993) Tropical soil biology fertility a handbook of methods second edition CABI Wallingford UK.
- 37.Delgado-Baquerizo, M Maestre FT, Gallardo A (2012) Biological soilcrust increases the resistance of soil nitrogen dynamics to changes in temperature in a semi-arid ecosystem. Plant and soil DOI: 101007/s11104-012-1404-3.
- 38. Kettler TA, Doran JW, Gilbert TL (2001) Simplified method for soil particle-size determination to accompany soil-quality analyses. Soil Science Society of America Journal 65: 849. [Google Scholar]
- 39. Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A (2005) Very high resolution ninterpolated climate surfaces for global areas. International Journal of Climatology 25: 1965. [Google Scholar]
- 40.UNEP (1992) World Atlas of Desertification Edward Arnold London UK.
- 41.Zomer RJ, Trabucco A, Straaten OV, Bossio DA (2006) Carbon, Land and Water: A Global Analysis of the Hydrologic Dimensions of Climate Change Mitigation Through Afforestation/Reforestation. International Water Management Institute (IWMI) Research Report 101, IWMI, Colombo, Sri Lanka.
- 42.Burnham KP, Anderson DR (2002) Model Selection Multimodel Inference A Practical Information-Theoretic Approach second edition Springer New York USA.
- 43.Fotheringham AS, Brunsdon C, Charlton M (2002) Geographically Weighted Regression: The Analysis of Spatially Varying Relationships. Wiley, Hoboken, NJ.
- 44. Rangel TF, Diniz-Filho JAF, Bini LM (2010) SAM a comprehensive application for Spatial Analysis in Macroecology Ecography. 33: 46–50. [Google Scholar]
- 45.Grace JB (2006) Structural Equation Modeling and Natural Systems Cambridge University Press New York USA.
- 46.Shipley B (2001) Cause and correlation in biology: A User’s guide to path analysis structural equations and casual inference. Cambridge University Press Cambridge UK.
- 47. Grace JB, Anderson TM, Olff H, Scheiner SM (2010) On the specification of structural equation models for ecological systems. Ecological Monographs 80: 67–87. [Google Scholar]
- 48. Schermelleh-Engel, Moosbrugger KH, Müller H (2003) Evaluating the fit of structural equation models tests of significance descriptive goodness-of-fit measures. Methods of Psychological Research Online 8: 23–74. [Google Scholar]
- 49. Vitousek PM, Aber JD, Howarth RW, Likens GE, Matson PA, et al. (1997) Human Alteration of the global nitrogen cycle: sources and consequences. Ecological Applications 7: 737–750. [Google Scholar]
- 50. Puigdefábregas J, Solé-Benet A, Gutiérrez L, Del Barrio G, y Boer M (1999) Scales and processes of water and sediment redistribution in drylands: results from the Rambla Honda field site in Southeast Spain. Earth-Science Reviews 48: 39–70. [Google Scholar]
- 51. Zak DR, Holmes WE, White DC, Peacock AD, Tilman D (2003) Plant diversity soil microbial communities and ecosystem function: are there any links?. Ecology 84: 2042–2050. [Google Scholar]
- 52. Singh JS, Raghubanshi AS, Singh RS, Srisvatava SC (1989) Microbial biomass acts as a source of plant nutrients in dry tropical forest and savanna. Nature 338: 499–500. [Google Scholar]
- 53. Schimel JP, Balser TC, Wallenstein M (2007) Microbial stress-response physiology and its implications for ecosystem function. Ecology. 88: 1386–1394. [DOI] [PubMed] [Google Scholar]
- 54. Austin AT, Yahdjian L, Stark JM, Belnap J, Porporato A, et al. (2004) Water pulses and biogeochemical cycles in arid and semiarid ecosystems. Oecologia 141: 221–235. [DOI] [PubMed] [Google Scholar]
- 55.Allen SE, Grimshaw HM, Rowland AP (1986) Chemical analysis. Methods in Plant Ecology. Blackwell Scientific Oxford 285–344.
- 56. Genin D, Khorchani T, Hammadi M (2007) Improving nutritive value of a North African range grass Stipa tenacissima effect of dung ash urea treatment on digestion by goats. Animal Feed Science and Technology 136: 1–10. [Google Scholar]
- 57. Maestre FT, Bowker MA, Puche MD, Hinojosa MB, Martínez I, et al. (2009) Shrub encroachment can reverse desertification in Mediterranean semiarid grasslands. Ecology Letters 12: 930–941. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Pearsońs relationships between organic (DON and amino acids) and inorganic (ammonium and nitrate) N forms with aridity for both Stipa tenassicima (STIPA) and Bare soil (BS) microsites. Every data point is the average of five soil samples. Significance levels are as follows: *p<0.05, **p<0.01 and ***p<0.001. Ammonium, nitrate and DON were measured as described by Delgado-Baquerizo et al. (1). Amino acids were determined by following Chantigny et al. (2).
(DOC)
Relationships between organic (DON and amino acids) and inorganic (ammonium and nitrate) N forms with total available N for both Stipa tenassicima (STIPA) and Bare soil (BS) microsites. Every data point is the average of five soil samples. Significance levels are as follows: *p<0.05, **p<0.01 and ***p<0.001. Ammonium, nitrate and DON were measured as described by Delgado-Baquerizo et al. (1). Amino acids were determined by following Chantigny et al. (2).
(DOC)
Location, climatic, physical and main soil chemical characteristics in the studied sites. MAT = Mean annual temperature; MAP = Mean annual precipitation; Stipa = coverage of Stipa.
(DOC)
Pearson correlations coefficients. between the different climatic (aridity), abiotic (pH; SAC: % of sand content), plant (CBA: % of coverage of bare ground; CHE: % of coverage of Stipa tenacissima; PA: plant patch area [m2]; API: Average plant patch interdistance [m]; NP: number of plant patches per 10 m of transect) and nutrient (Organic-C [%]; MIN; potential net mineralization rate [mg N kg−1 soil day−1]; NRT: potential net N transformation rate [mg N kg−1 soil day−1]; ratio C:N) variables. Significance levels are as follows: *p<0.05 and **and p<0.01.
(DOC)
Pearson correlations coefficients. between available N and climatic (aridity), abiotic (pH; SAC: sand content), plant (CBA: coverage of bare ground; CHE: coverage of Stipa tenacissima; PA: plant patch area; API: Average plant patch interdistance; NP: number of plant patches per 10 m of transect) and nutrient (Organic-C; MIN; potential net mineralization rate; NRT: potential net N transformation rate; ratio C:N) variables. Significance levels are as follows: *p<0.05 and **and p<0.01.
(DOC)
Summary results of the semi-parametric PERMANOVA analyses carried out with organic carbon. PERMANOVA uses permutation tests to obtain p values, does not rely on the assumptions of traditional parametric ANOVA, and can handle experimental designs such as employed here (1). The model used evaluated the effects of plot (PL as random factor) and microsite (MI as fixed factor) on organic carbon.When significant interactions between factors were found, separate PERMANOVA analysis were conducted for each site. All analyses were carried out using the PERMANOVA+ module of the PRIMER software (PRIMER-E Ltd., Plymounth Marine Laboratory, UK). RES = residuals.
(DOC)