Abstract
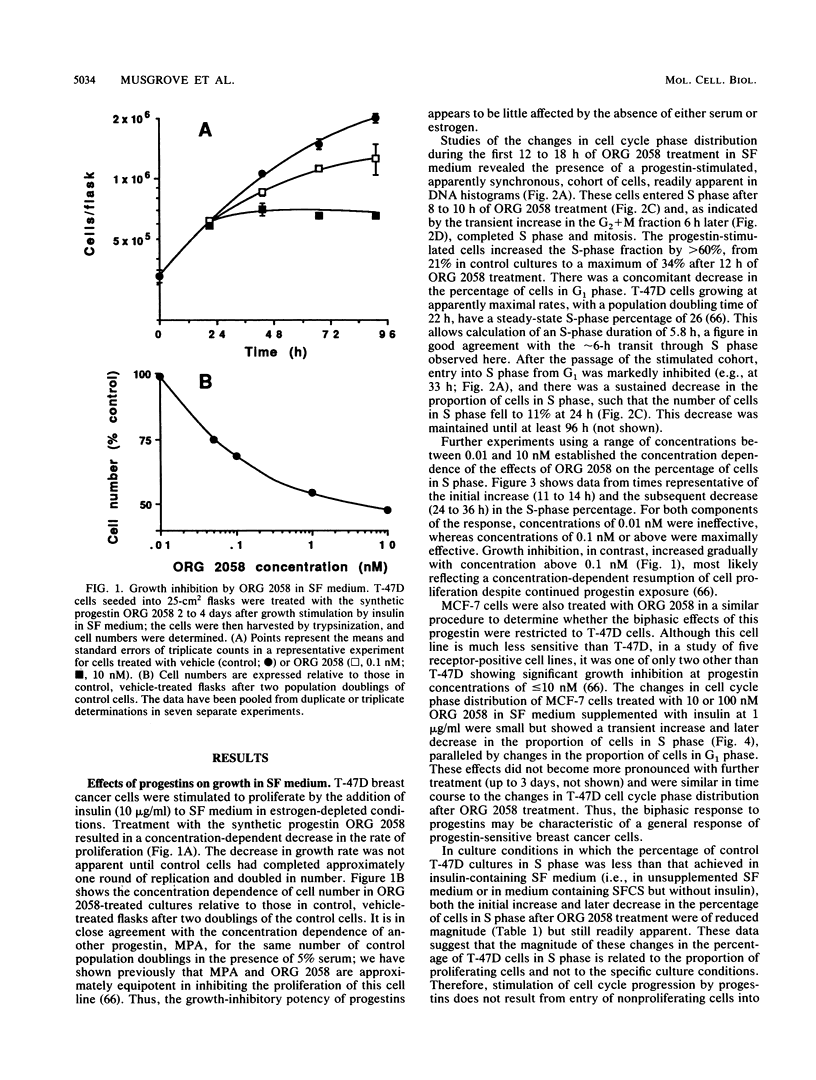
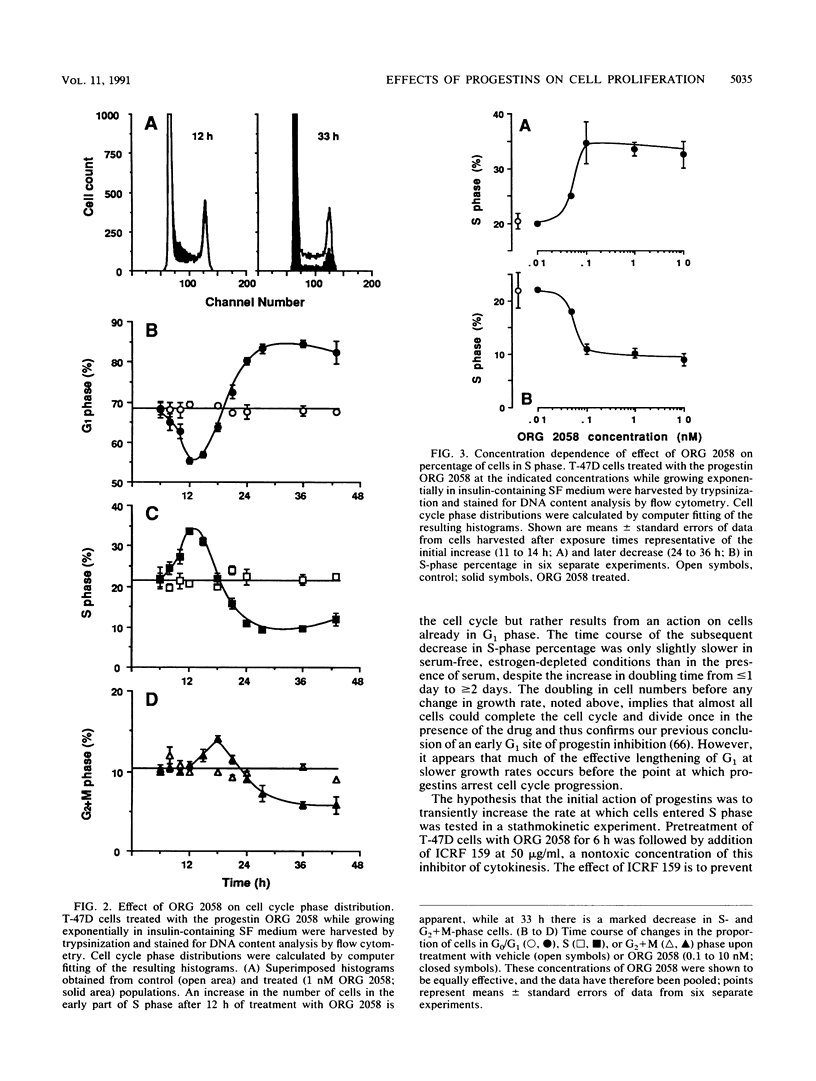
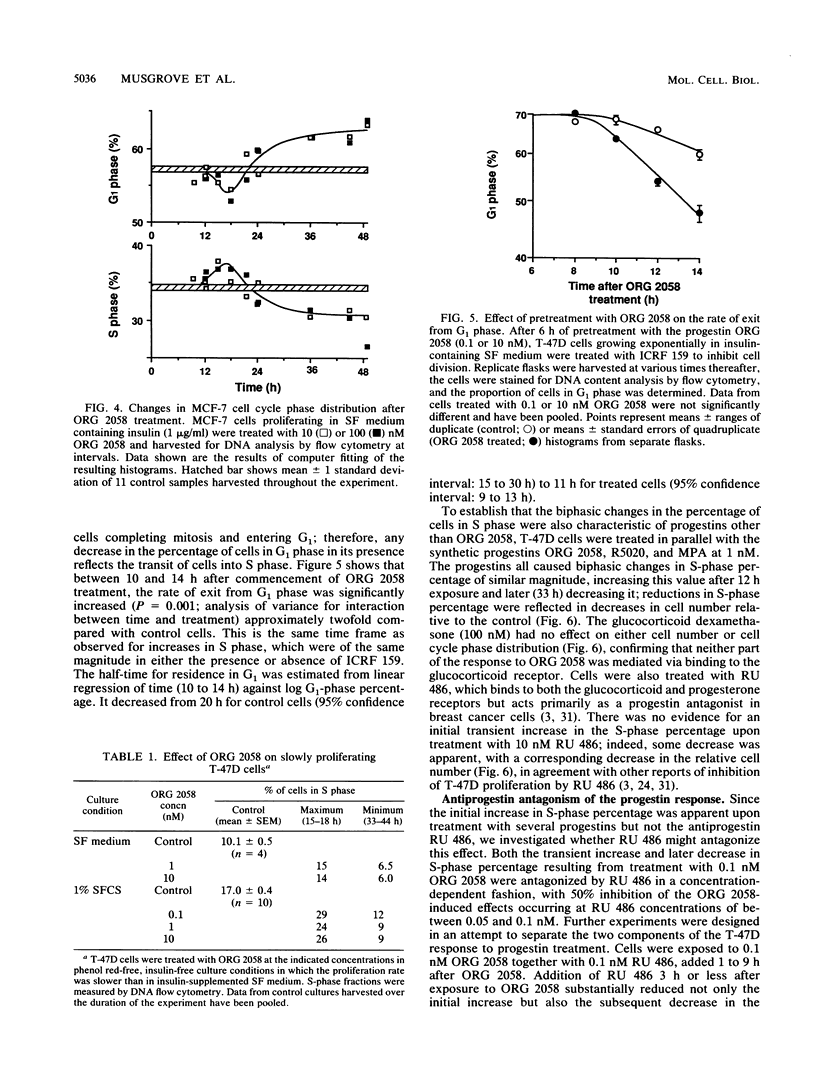
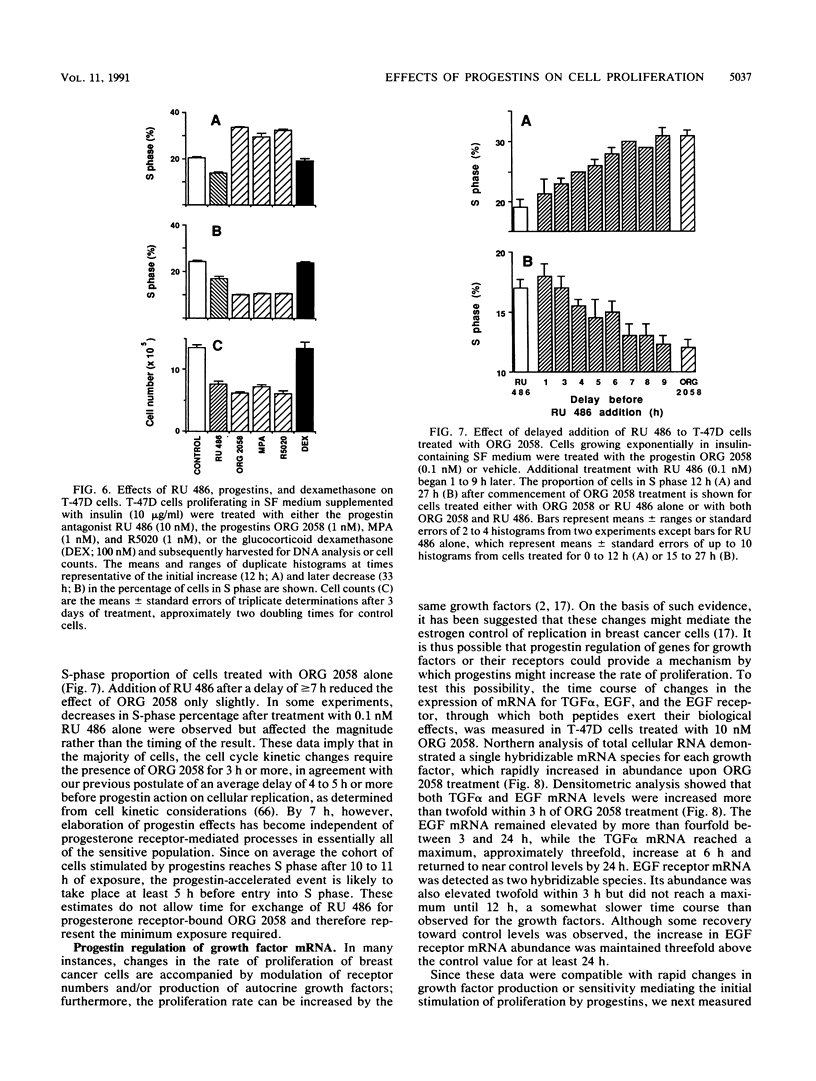
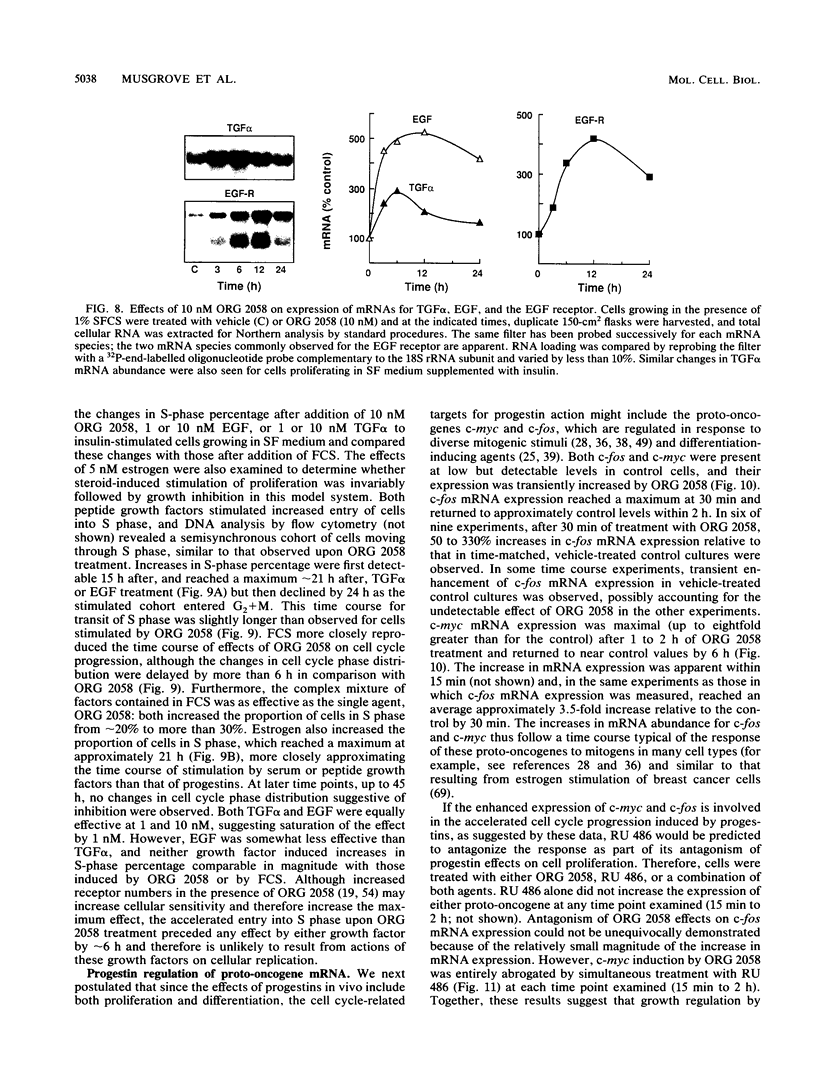
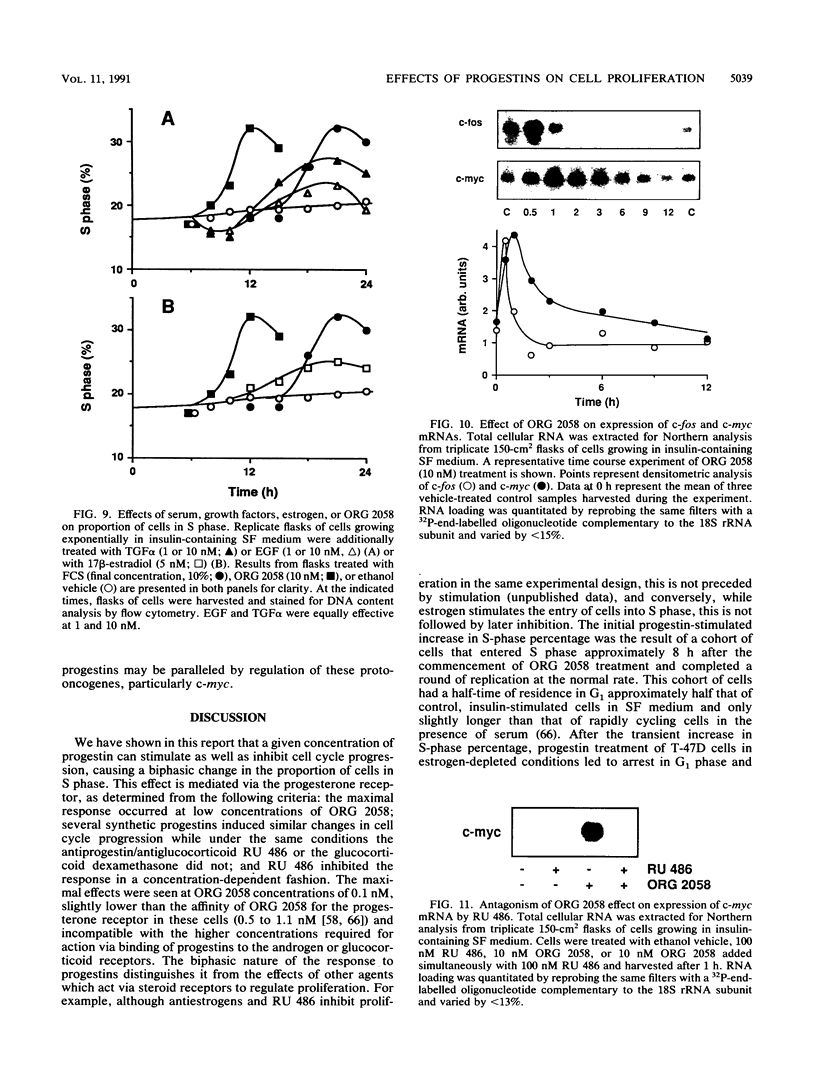
This study documents a biphasic change in the rate of cell cycle progression and proliferation of T-47D human breast cancer cells treated with synthetic progestins, consisting of an initial transient acceleration in transit through G1, followed by cell cycle arrest and growth inhibition. Both components of the response were mediated via the progesterone receptor. The data are consistent with a model in which the action of progestins is to accelerate cells already progressing through G1, which are then arrested early in G1 after completing a round of replication, as are cells initially in other phases of the cell cycle. Such acceleration implies that progestins act on genes or gene products which are rate limiting for cell cycle progression. Increased production of epidermal growth factor and transforming growth factor alpha, putative autocrine growth factors in breast cancer cells, does not appear to account for the initial response to progestins, since although the mRNA abundance for these growth factors is rapidly induced by progestins, cells treated with epidermal growth factor or transforming growth factor alpha did not enter S phase until 5 to 6 h later than those stimulated by progestin. The proto-oncogenes c-fos and c-myc were rapidly but transiently induced by progestin treatment, paralleling the well-known response of these genes to mitogenic signals in other cell types. The progestin antagonist RU 486 inhibited progestin regulation of both cell cycle progression and c-myc expression, suggesting that this proto-oncogene may participate in growth modulation by progestins.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander I. E., Clarke C. L., Shine J., Sutherland R. L. Progestin inhibition of progesterone receptor gene expression in human breast cancer cells. Mol Endocrinol. 1989 Sep;3(9):1377–1386. doi: 10.1210/mend-3-9-1377. [DOI] [PubMed] [Google Scholar]
- Arteaga C. L., Osborne C. K. Growth factors as mediators of estrogen/antiestrogen action in human breast cancer cells. Cancer Treat Res. 1991;53:289–304. doi: 10.1007/978-1-4615-3940-7_14. [DOI] [PubMed] [Google Scholar]
- Bardon S., Vignon F., Chalbos D., Rochefort H. RU486, a progestin and glucocorticoid antagonist, inhibits the growth of breast cancer cells via the progesterone receptor. J Clin Endocrinol Metab. 1985 Apr;60(4):692–697. doi: 10.1210/jcem-60-4-692. [DOI] [PubMed] [Google Scholar]
- Beato M. Gene regulation by steroid hormones. Cell. 1989 Feb 10;56(3):335–344. doi: 10.1016/0092-8674(89)90237-7. [DOI] [PubMed] [Google Scholar]
- Bell G. I., Fong N. M., Stempien M. M., Wormsted M. A., Caput D., Ku L. L., Urdea M. S., Rall L. B., Sanchez-Pescador R. Human epidermal growth factor precursor: cDNA sequence, expression in vitro and gene organization. Nucleic Acids Res. 1986 Nov 11;14(21):8427–8446. doi: 10.1093/nar/14.21.8427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthois Y., Katzenellenbogen J. A., Katzenellenbogen B. S. Phenol red in tissue culture media is a weak estrogen: implications concerning the study of estrogen-responsive cells in culture. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2496–2500. doi: 10.1073/pnas.83.8.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden R. T., Hissom J. R., Moore M. R. Growth stimulation of T47D human breast cancer cells by the anti-progestin RU486. Endocrinology. 1989 May;124(5):2642–2644. doi: 10.1210/endo-124-5-2642. [DOI] [PubMed] [Google Scholar]
- Braunsberg H., Coldham N. G., Leake R. E., Cowan S. K., Wong W. Actions of a progestogen on human breast cancer cells: mechanisms of growth stimulation and inhibition. Eur J Cancer Clin Oncol. 1987 May;23(5):563–571. doi: 10.1016/0277-5379(87)90321-x. [DOI] [PubMed] [Google Scholar]
- Campisi J., Gray H. E., Pardee A. B., Dean M., Sonenshein G. E. Cell-cycle control of c-myc but not c-ras expression is lost following chemical transformation. Cell. 1984 Feb;36(2):241–247. doi: 10.1016/0092-8674(84)90217-4. [DOI] [PubMed] [Google Scholar]
- Chalbos D., Chambon M., Ailhaud G., Rochefort H. Fatty acid synthetase and its mRNA are induced by progestins in breast cancer cells. J Biol Chem. 1987 Jul 25;262(21):9923–9926. [PubMed] [Google Scholar]
- Chan Y. L., Gutell R., Noller H. F., Wool I. G. The nucleotide sequence of a rat 18 S ribosomal ribonucleic acid gene and a proposal for the secondary structure of 18 S ribosomal ribonucleic acid. J Biol Chem. 1984 Jan 10;259(1):224–230. [PubMed] [Google Scholar]
- Clarke C. L., Sutherland R. L. Progestin regulation of cellular proliferation. Endocr Rev. 1990 May;11(2):266–301. doi: 10.1210/edrv-11-2-266. [DOI] [PubMed] [Google Scholar]
- Coppola J. A., Cole M. D. Constitutive c-myc oncogene expression blocks mouse erythroleukaemia cell differentiation but not commitment. Nature. 1986 Apr 24;320(6064):760–763. doi: 10.1038/320760a0. [DOI] [PubMed] [Google Scholar]
- Derynck R., Roberts A. B., Winkler M. E., Chen E. Y., Goeddel D. V. Human transforming growth factor-alpha: precursor structure and expression in E. coli. Cell. 1984 Aug;38(1):287–297. doi: 10.1016/0092-8674(84)90550-6. [DOI] [PubMed] [Google Scholar]
- Diamond M. I., Miner J. N., Yoshinaga S. K., Yamamoto K. R. Transcription factor interactions: selectors of positive or negative regulation from a single DNA element. Science. 1990 Sep 14;249(4974):1266–1272. doi: 10.1126/science.2119054. [DOI] [PubMed] [Google Scholar]
- Dickson R. B., Lippman M. E. Estrogenic regulation of growth and polypeptide growth factor secretion in human breast carcinoma. Endocr Rev. 1987 Feb;8(1):29–43. doi: 10.1210/edrv-8-1-29. [DOI] [PubMed] [Google Scholar]
- Dubik D., Dembinski T. C., Shiu R. P. Stimulation of c-myc oncogene expression associated with estrogen-induced proliferation of human breast cancer cells. Cancer Res. 1987 Dec 15;47(24 Pt 1):6517–6521. [PubMed] [Google Scholar]
- Ewing T. M., Murphy L. J., Ng M. L., Pang G. Y., Lee C. S., Watts C. K., Sutherland R. L. Regulation of epidermal growth factor receptor by progestins and glucocorticoids in human breast cancer cell lines. Int J Cancer. 1989 Oct 15;44(4):744–752. doi: 10.1002/ijc.2910440432. [DOI] [PubMed] [Google Scholar]
- Fink K. L., Wieben E. D., Woloschak G. E., Spelsberg T. C. Rapid regulation of c-myc protooncogene expression by progesterone in the avian oviduct. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1796–1800. doi: 10.1073/pnas.85.6.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freter C. E., Lippman M. E., Cheville A., Zinn S., Gelmann E. P. Alterations in phosphoinositide metabolism associated with 17 beta-estradiol and growth factor treatment of MCF-7 breast cancer cells. Mol Endocrinol. 1988 Feb;2(2):159–166. doi: 10.1210/mend-2-2-159. [DOI] [PubMed] [Google Scholar]
- Freytag S. O. Enforced expression of the c-myc oncogene inhibits cell differentiation by precluding entry into a distinct predifferentiation state in G0/G1. Mol Cell Biol. 1988 Apr;8(4):1614–1624. doi: 10.1128/mcb.8.4.1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambrell R. D., Jr Proposal to decrease the risk and improve the prognosis of breast cancer. Am J Obstet Gynecol. 1984 Sep 15;150(2):119–132. doi: 10.1016/s0002-9378(84)80002-2. [DOI] [PubMed] [Google Scholar]
- Gill P. G., Vignon F., Bardon S., Derocq D., Rochefort H. Difference between R5020 and the antiprogestin RU486 in antiproliferative effects on human breast cancer cells. Breast Cancer Res Treat. 1987 Oct;10(1):37–45. doi: 10.1007/BF01806133. [DOI] [PubMed] [Google Scholar]
- Gonda T. J., Metcalf D. Expression of myb, myc and fos proto-oncogenes during the differentiation of a murine myeloid leukaemia. Nature. 1984 Jul 19;310(5974):249–251. doi: 10.1038/310249a0. [DOI] [PubMed] [Google Scholar]
- Graham M. L., 2nd, Krett N. L., Miller L. A., Leslie K. K., Gordon D. F., Wood W. M., Wei L. L., Horwitz K. B. T47DCO cells, genetically unstable and containing estrogen receptor mutations, are a model for the progression of breast cancers to hormone resistance. Cancer Res. 1990 Oct 1;50(19):6208–6217. [PubMed] [Google Scholar]
- Greenberg M. E., Greene L. A., Ziff E. B. Nerve growth factor and epidermal growth factor induce rapid transient changes in proto-oncogene transcription in PC12 cells. J Biol Chem. 1985 Nov 15;260(26):14101–14110. [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984 Oct 4;311(5985):433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- Greene L. A., Tischler A. S. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hissom J. R., Bowden R. T., Moore M. R. Effects of progestins, estrogens, and antihormones on growth and lactate dehydrogenase in the human breast cancer cell line T47D. Endocrinology. 1989 Jul;125(1):418–423. doi: 10.1210/endo-125-1-418. [DOI] [PubMed] [Google Scholar]
- Horwitz K. B., Freidenberg G. R. Growth inhibition and increase of insulin receptors in antiestrogen-resistant T47DCO human breast cancer cells by progestins: implications for endocrine therapies. Cancer Res. 1985 Jan;45(1):167–173. [PubMed] [Google Scholar]
- Horwitz K. B. The antiprogestin RU38 486: receptor-mediated progestin versus antiprogestin actions screened in estrogen-insensitive T47Dco human breast cancer cells. Endocrinology. 1985 Jun;116(6):2236–2245. doi: 10.1210/endo-116-6-2236. [DOI] [PubMed] [Google Scholar]
- Jonat C., Rahmsdorf H. J., Park K. K., Cato A. C., Gebel S., Ponta H., Herrlich P. Antitumor promotion and antiinflammation: down-modulation of AP-1 (Fos/Jun) activity by glucocorticoid hormone. Cell. 1990 Sep 21;62(6):1189–1204. doi: 10.1016/0092-8674(90)90395-u. [DOI] [PubMed] [Google Scholar]
- Judge S. M., Chatterton R. T., Jr Progesterone-specific stimulation of triglyceride biosynthesis in a breast cancer cell line (T-47D). Cancer Res. 1983 Sep;43(9):4407–4412. [PubMed] [Google Scholar]
- Kelly K., Cochran B. H., Stiles C. D., Leder P. Cell-specific regulation of the c-myc gene by lymphocyte mitogens and platelet-derived growth factor. Cell. 1983 Dec;35(3 Pt 2):603–610. doi: 10.1016/0092-8674(83)90092-2. [DOI] [PubMed] [Google Scholar]
- Koga M., Musgrove E. A., Sutherland R. L. Modulation of the growth-inhibitory effects of progestins and the antiestrogen hydroxyclomiphene on human breast cancer cells by epidermal growth factor and insulin. Cancer Res. 1989 Jan 1;49(1):112–116. [PubMed] [Google Scholar]
- Kruijer W., Cooper J. A., Hunter T., Verma I. M. Platelet-derived growth factor induces rapid but transient expression of the c-fos gene and protein. Nature. 1984 Dec 20;312(5996):711–716. doi: 10.1038/312711a0. [DOI] [PubMed] [Google Scholar]
- Kruijer W., Schubert D., Verma I. M. Induction of the proto-oncogene fos by nerve growth factor. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7330–7334. doi: 10.1073/pnas.82.21.7330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachman H. M., Skoultchi A. I. Expression of c-myc changes during differentiation of mouse erythroleukaemia cells. Nature. 1984 Aug 16;310(5978):592–594. doi: 10.1038/310592a0. [DOI] [PubMed] [Google Scholar]
- Larsson L. G., Gray H. E., Tötterman T., Pettersson U., Nilsson K. Drastically increased expression of MYC and FOS protooncogenes during in vitro differentiation of chronic lymphocytic leukemia cells. Proc Natl Acad Sci U S A. 1987 Jan;84(1):223–227. doi: 10.1073/pnas.84.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. S., Koga M., Sutherland R. L. Modulation of estrogen receptor and epidermal growth factor receptor mRNAs by phorbol ester in MCF 7 breast cancer cells. Biochem Biophys Res Commun. 1989 Jul 14;162(1):415–421. doi: 10.1016/0006-291x(89)92013-5. [DOI] [PubMed] [Google Scholar]
- Lee W. M., Schwab M., Westaway D., Varmus H. E. Augmented expression of normal c-myc is sufficient for cotransformation of rat embryo cells with a mutant ras gene. Mol Cell Biol. 1985 Dec;5(12):3345–3356. doi: 10.1128/mcb.5.12.3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longacre T. A., Bartow S. A. A correlative morphologic study of human breast and endometrium in the menstrual cycle. Am J Surg Pathol. 1986 Jun;10(6):382–393. doi: 10.1097/00000478-198606000-00003. [DOI] [PubMed] [Google Scholar]
- Lucibello F. C., Slater E. P., Jooss K. U., Beato M., Müller R. Mutual transrepression of Fos and the glucocorticoid receptor: involvement of a functional domain in Fos which is absent in FosB. EMBO J. 1990 Sep;9(9):2827–2834. doi: 10.1002/j.1460-2075.1990.tb07471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malden L. T., Novak U., Kaye A. H., Burgess A. W. Selective amplification of the cytoplasmic domain of the epidermal growth factor receptor gene in glioblastoma multiforme. Cancer Res. 1988 May 15;48(10):2711–2714. [PubMed] [Google Scholar]
- Mitchell R. L., Zokas L., Schreiber R. D., Verma I. M. Rapid induction of the expression of proto-oncogene fos during human monocytic differentiation. Cell. 1985 Jan;40(1):209–217. doi: 10.1016/0092-8674(85)90324-1. [DOI] [PubMed] [Google Scholar]
- Moore M. R., Hathaway L. D., Bircher J. A. Progestin stimulation of thymidine kinase in the human breast cancer cell line T47D. Biochim Biophys Acta. 1991 Feb 22;1096(2):170–174. doi: 10.1016/0925-4439(91)90056-f. [DOI] [PubMed] [Google Scholar]
- Murphy L. C., Dotzlaw H. Endogenous growth factor expression in T-47D, human breast cancer cells, associated with reduced sensitivity to antiproliferative effects of progestins and antiestrogens. Cancer Res. 1989 Feb 1;49(3):599–604. [PubMed] [Google Scholar]
- Murphy L. C., Dotzlaw H. Regulation of transforming growth factor alpha and transforming growth factor beta messenger ribonucleic acid abundance in T-47D, human breast cancer cells. Mol Endocrinol. 1989 Apr;3(4):611–617. doi: 10.1210/mend-3-4-611. [DOI] [PubMed] [Google Scholar]
- Murphy L. C., Murphy L. J., Dubik D., Bell G. I., Shiu R. P. Epidermal growth factor gene expression in human breast cancer cells: regulation of expression by progestins. Cancer Res. 1988 Aug 15;48(16):4555–4560. [PubMed] [Google Scholar]
- Murphy L. C., Murphy L. J., Shiu R. P. Progestin regulation of EGF-receptor mRNA accumulation in T-47D human breast cancer cells. Biochem Biophys Res Commun. 1988 Jan 15;150(1):192–196. doi: 10.1016/0006-291x(88)90504-9. [DOI] [PubMed] [Google Scholar]
- Murphy L. J., Sutherland R. L., Stead B., Murphy L. C., Lazarus L. Progestin regulation of epidermal growth factor receptor in human mammary carcinoma cells. Cancer Res. 1986 Feb;46(2):728–734. [PubMed] [Google Scholar]
- Musgrove E. A., Wakeling A. E., Sutherland R. L. Points of action of estrogen antagonists and a calmodulin antagonist within the MCF-7 human breast cancer cell cycle. Cancer Res. 1989 May 1;49(9):2398–2404. [PubMed] [Google Scholar]
- Müller R., Bravo R., Burckhardt J., Curran T. Induction of c-fos gene and protein by growth factors precedes activation of c-myc. Nature. 1984 Dec 20;312(5996):716–720. doi: 10.1038/312716a0. [DOI] [PubMed] [Google Scholar]
- Paek I., Axel R. Glucocorticoids enhance stability of human growth hormone mRNA. Mol Cell Biol. 1987 Apr;7(4):1496–1507. doi: 10.1128/mcb.7.4.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochownik E. V., Kukowska J., Rodgers C. c-myc antisense transcripts accelerate differentiation and inhibit G1 progression in murine erythroleukemia cells. Mol Cell Biol. 1988 Sep;8(9):3683–3695. doi: 10.1128/mcb.8.9.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddel R. R., Alexander I. E., Koga M., Shine J., Sutherland R. L. Genetic instability and the development of steroid hormone insensitivity in cultured T 47D human breast cancer cells. Cancer Res. 1988 Aug 1;48(15):4340–4347. [PubMed] [Google Scholar]
- Reddel R. R., Murphy L. C., Sutherland R. L. Factors affecting the sensitivity of T-47D human breast cancer cells to tamoxifen. Cancer Res. 1984 Jun;44(6):2398–2405. [PubMed] [Google Scholar]
- Rosenfeld M. G., O'Malley B. W. Steroid hormones: effects on adenyl cyclase activity and adenosine 3',5'-momophosphate in target tissues. Science. 1970 Apr 10;168(3928):253–255. doi: 10.1126/science.168.3928.253. [DOI] [PubMed] [Google Scholar]
- Rudkin B. B., Lazarovici P., Levi B. Z., Abe Y., Fujita K., Guroff G. Cell cycle-specific action of nerve growth factor in PC12 cells: differentiation without proliferation. EMBO J. 1989 Nov;8(11):3319–3325. doi: 10.1002/j.1460-2075.1989.tb08493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santen R. J., Manni A., Harvey H., Redmond C. Endocrine treatment of breast cancer in women. Endocr Rev. 1990 May;11(2):221–265. doi: 10.1210/edrv-11-2-221. [DOI] [PubMed] [Google Scholar]
- Sarup J. C., Rao K. V., Fox C. F. Decreased progesterone binding and attenuated progesterone action in cultured human breast carcinoma cells treated with epidermal growth factor. Cancer Res. 1988 Sep 15;48(18):5071–5078. [PubMed] [Google Scholar]
- Schüle R., Rangarajan P., Kliewer S., Ransone L. J., Bolado J., Yang N., Verma I. M., Evans R. M. Functional antagonism between oncoprotein c-Jun and the glucocorticoid receptor. Cell. 1990 Sep 21;62(6):1217–1226. doi: 10.1016/0092-8674(90)90397-w. [DOI] [PubMed] [Google Scholar]
- Scott R. E., Florine D. L., Wille J. J., Jr, Yun K. Coupling of growth arrest and differentiation at a distinct state in the G1 phase of the cell cycle: GD. Proc Natl Acad Sci U S A. 1982 Feb;79(3):845–849. doi: 10.1073/pnas.79.3.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland R. L., Hall R. E., Pang G. Y., Musgrove E. A., Clarke C. L. Effect of medroxyprogesterone acetate on proliferation and cell cycle kinetics of human mammary carcinoma cells. Cancer Res. 1988 Sep 15;48(18):5084–5091. [PubMed] [Google Scholar]
- Topper Y. J., Freeman C. S. Multiple hormone interactions in the developmental biology of the mammary gland. Physiol Rev. 1980 Oct;60(4):1049–1106. doi: 10.1152/physrev.1980.60.4.1049. [DOI] [PubMed] [Google Scholar]
- Travers M. T., Knowler J. T. Oestrogen-induced expression of oncogenes in the immature rat uterus. FEBS Lett. 1987 Jan 19;211(1):27–30. doi: 10.1016/0014-5793(87)81267-x. [DOI] [PubMed] [Google Scholar]
- Vignon F., Bardon S., Chalbos D., Rochefort H. Antiestrogenic effect of R5020, a synthetic progestin in human breast cancer cells in culture. J Clin Endocrinol Metab. 1983 Jun;56(6):1124–1130. doi: 10.1210/jcem-56-6-1124. [DOI] [PubMed] [Google Scholar]
- Weisz A., Bresciani F. Estrogen induces expression of c-fos and c-myc protooncogenes in rat uterus. Mol Endocrinol. 1988 Sep;2(9):816–824. doi: 10.1210/mend-2-9-816. [DOI] [PubMed] [Google Scholar]
- Westin E. H., Wong-Staal F., Gelmann E. P., Dalla-Favera R., Papas T. S., Lautenberger J. A., Eva A., Reddy E. P., Tronick S. R., Aaronson S. A. Expression of cellular homologues of retroviral onc genes in human hematopoietic cells. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2490–2494. doi: 10.1073/pnas.79.8.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilding G., Lippman M. E., Gelmann E. P. Effects of steroid hormones and peptide growth factors on protooncogene c-fos expression in human breast cancer cells. Cancer Res. 1988 Feb 15;48(4):802–805. [PubMed] [Google Scholar]
- Yang-Yen H. F., Chambard J. C., Sun Y. L., Smeal T., Schmidt T. J., Drouin J., Karin M. Transcriptional interference between c-Jun and the glucocorticoid receptor: mutual inhibition of DNA binding due to direct protein-protein interaction. Cell. 1990 Sep 21;62(6):1205–1215. doi: 10.1016/0092-8674(90)90396-v. [DOI] [PubMed] [Google Scholar]
- van der Burg B., van Selm-Miltenburg A. J., de Laat S. W., van Zoelen E. J. Direct effects of estrogen on c-fos and c-myc protooncogene expression and cellular proliferation in human breast cancer cells. Mol Cell Endocrinol. 1989 Jul;64(2):223–228. doi: 10.1016/0303-7207(89)90149-4. [DOI] [PubMed] [Google Scholar]