Abstract
For nearly a decade, our research group has had the privilege of developing and mining a multi-center, microarray-based, genome-wide expression database of critically ill children (≤ 10 years of age) with septic shock. Using bioinformatic and systems biology approaches, the expression data generated through this discovery-oriented, exploratory approach have been leveraged for a variety of objectives, which will be reviewed. Fundamental observations include wide spread repression of gene programs corresponding to the adaptive immune system, and biologically significant differential patterns of gene expression across developmental age groups. The data have also identified gene expression-based subclasses of pediatric septic shock having clinically relevant phenotypic differences. The data have also been leveraged for the discovery of novel therapeutic targets, and for the discovery and development of novel stratification and diagnostic biomarkers. Almost a decade of genome-wide expression profiling in pediatric septic shock is now demonstrating tangible results. The studies have progressed from an initial discovery-oriented and exploratory phase, to a new phase where the data are being translated and applied to address several areas of clinical need.
INTRODUCTION
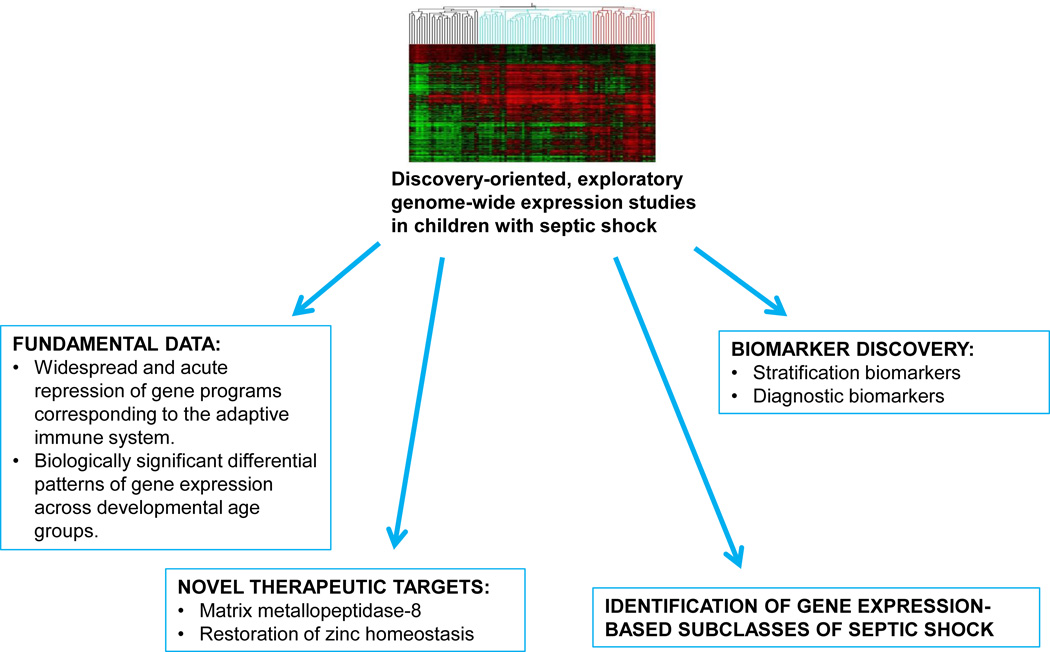
For nearly a decade now, our research group has had the privilege of developing and mining a multi-center, microarray-based, genome-wide expression database of critically ill children (≤ 10 years of age) with septic shock. The expression data are based on whole blood-derived RNA and are focused on the initial, acute presentation to the pediatric intensive care unit (PICU) with a clinical diagnosis of septic shock. Comparator groups include age matched normal controls, critically ill children meeting criteria for sepsis, and critically ill children meeting criteria for the systemic inflammatory response syndrome (SIRS), based on pediatric specific definitions (1). Using bioinformatic and systems biology approaches, the expression data generated through this discovery-oriented, exploratory approach have been leveraged for a variety of objectives, which are summarized in Figure 1 and will be reviewed below. Analogous studies involving adult populations were recently reviewed (2).
Figure 1.
Schematic depicting how genome-wide expression data from children with septic have been leveraged for a variety of objectives.
GENOME-LEVEL RESPONSE OF PEDIATRIC SEPTIC SHOCK AND REPRESSION OF ADAPTIVE IMMUNITY-RELATED GENES
The long-standing paradigm for understanding the pathophysiology of septic shock is centered on a dysfunctional innate immune system, wherein excessive and uncontrolled pro-inflammatory responses lead to direct tissue and organ injury (3, 4). While this paradigm remains valid, the vast majority of clinical trials focused on controlling or modulating this excessive inflammatory response have failed to demonstrate efficacy, thus leading to the derivation of alternative paradigms for understanding the pathophysiology of human sepsis (5). One such alternative paradigm focuses on dysfunction of the adaptive immune system, wherein a form of immune suppression or “immune paralysis” accounts for the negative consequences of sepsis (6–10).
Genome-level expression patterns in children with septic shock strongly support this concept of immune suppression (2, 11–17). Specifically, pediatric septic shock is characterized by wide spread repression of gene programs corresponding to various major components of the adaptive immune system, including the T cell receptor signaling pathway, T cell function, B cell function, and the MHC antigen presentation pathway. This pattern of gene repression is evident within the first 24 hours of presentation to the PICU with septic shock, and persists at least into the third day of PICU admission. Analogous studies in adults with septic shock, and in adults suffering from major traumatic injuries, have also demonstrated similar patterns of gene repression corresponding to the adaptive immune system (18–22). Accordingly, novel therapeutic strategies for septic shock are now being contemplated, which focus on restoration of the adaptive immune system, rather than inhibition of the innate immune system and the inflammatory response (23, 24).
INFLUENCE OF DEVELOPMENT ON THE GENOME-LEVEL RESPONSE OF PEDIATRIC SEPTIC SHOCK
A variety of clinical and experimental data indicate that developmental age influences the immune system, and hence the host response to sepsis (4, 25–28). A recent study examined the acute, genome-level expression patterns across four developmental age groups with septic shock: neonate (≤ 28 days of age), infant (1 month through 1 year of age), toddler (2 – 5 years of age), and school-age (≥ 6 years of age) (17).
In comparisons to age-matched controls, the neonate and school age groups had the largest number of uniquely regulated genes corresponding to several key inflammation- and immunity-related pathways. In direct comparisons across the developmental age groups, there were minimal differences between the infant, toddler, and school-age groups. In contrast, age-specific differential gene expression was most profound in the neonate group, which predominately demonstrated reduced expression of several key pathways of both the innate and adaptive immune response. This observation does not appear to be an artifact of differential white blood cell counts. The expression data were analyzed for the presence of signature probe sets for neutrophils, lymphocytes, and monocytes. The signature probe sets were present to a similar degree across the four developmental age groups, thus suggesting that the relative contributions of the three major leukocyte subsets to the gene expression profiles were not substantially different across the four developmental age groups.
One striking example involved expression of genes related to the triggering receptor expressed on myeloid cells-1 (TREM-1) signaling pathway, for which there has been recent interest as a therapeutic target in sepsis (29). Genes corresponding to the TREM-1 signaling pathway were not expressed in the neonate group, whereas they were highly expressed in the other three, older developmental age groups. These data illustrate how development must be taken into consideration when developing novel, immunity-based therapies for sepsis.
DISCOVERY OF GENE EXPRESSION-BASED SUBCLASSES
Septic shock is a heterogeneous syndrome, rather than a discrete, uniform disease process (5). This high level of heterogeneity implies the potential existence of disease “subclasses” predicated on distinct patterns of gene expression. A series of three recent studies indicate that gene expression-based subclasses of pediatric septic shock may indeed exist and that the subclasses have clinically important phenotypic differences (30–32).
The first study in this series attempted to identify subclasses of pediatric septic shock based exclusively on differential patterns of gene expression (30). A list of over 6,000 differentially regulated genes was generated using statistical and expression filters targeted at subclass discovery. The differentially regulated genes were then subjected to unsupervised hierarchical clustering and three putative gene expression-based subclasses (subclasses “A”, “B”, and “C”) were identified based on the first- and second-order cluster branches. Post-hoc analysis of the gene expression-based subclasses revealed that patients in subclass A had a significantly higher degree of illness severity and a significantly higher mortality rate, compared to patients in subclasses B and C.
The subclass-defining gene signature was subsequently distilled to the top 100 class predictor genes, and these genes corresponded to T cell receptor signaling, B cell receptor signaling, glucocorticoid receptor signaling, and peroxisome proliferator-activated receptor-α (PPARα) activation (30). Importantly, the genes corresponding to these signaling pathways were generally repressed in the subclass A patients, which also had the highest degree of illness severity and the highest mortality rate among the three identified subclasses. Recently, these observations were experimentally corroborated by the demonstration that PPARα deficient mice are more susceptible to the untoward effects of experimental sepsis (33).
In the second study of this series, the 100 class defining-genes were depicted using gene expression mosaics that allow for intuitive pattern recognition of otherwise complex gene expression patterns (32, 34, 35). Clinicians with no formal bioinformatic training and minimal instruction were able to allocate patients to the correct gene expression-based subclasses with a high degree of accuracy, thus demonstrating the potential feasibility of bringing gene expression-based subclassification of pediatric septic shock to the bedside. In the final study of this series, the gene expression mosaics were used to allocate a new, test cohort of patients into each of the three putative subclasses (31). This validation study corroborated that patients in subclass A have a higher degree of illness severity, compared to patients in subclasses B and C. Collectively, this series of studies indicates that gene expression-based subclasses of pediatric septic shock exist, that the subclasses can be readily identified by clinicians using gene expression mosaics, that the class-defining genes are biologically relevant, and that the subclasses have clinically relevant phenotypic differences.
DISCOVERY OF NOVEL THERAPEUTIC TARGETS
A common goal for discovery-oriented clinical studies centered on genome-wide expression patterns involves the discovery of novel therapeutic targets. The pediatric genome-wide expression studies have thus far identified two promising areas for therapeutic intervention, which have been corroborated experimentally: inhibition of matrix metallopeptidase-8 (MMP-8) activity and restoration of zinc homeostasis.
MMP-8 belongs to a family of 25 individual endopeptidases having different cellular sources, expression conditions, and target substrates (36). MMP-8 is also known as neutrophil collagenase given its original identification as a neutrophil product that remodels the extracellular matrix (ECM) via cleavage of collagen type I. However, it is now apparent that multiple cell types have the ability to express MMP-8, particularly in the setting of inflammatory conditions. In addition, MMP-8 can also cleave a wide range of non-collagenous substrates, including serine protease inhibitors and several chemokines.
MMP-8 is consistently the highest expressed gene in children with septic shock, and the degree of expression and proteolytic activity positively correlate with the degree of illness severity and mortality, but do not correlate with differential white blood cell counts (37). Similar observations have been reported in adults with sepsis (38). These observations have led to a series of experiments designed to formally test the role of MMP-8 in a murine model of sepsis (37). MMP-8 null mice have a significant survival advantage in experimental sepsis, compared to wild-type animals. This survival advantage correlates with an attenuated inflammatory response, without compromising clearance of bacteria. In addition, this phenotype can be replicated by treating wild-type mice with a pharmacologic inhibitor of MMP-8 activity, after induction of sepsis. Collectively, these data indicate that MMP-8 inhibition is a potential, novel therapeutic strategy for sepsis and this assertion is supported by the availability of clinically approved pharmaceutical compounds that effectively inhibit MMP-8 activity (36, 39).
Zinc is an essential trace element and critically important for normal functioning of both the innate and adaptive immune systems (40). A consistent observation across multiple gene expression studies is that pediatric septic shock is characterized by wide spread repression of gene families that either directly participate in zinc homeostasis, or are directly dependent on zinc homeostasis for normal function (11, 12, 14–16, 30). These observations suggest that altered zinc homeostasis may be a prominent feature of pediatric septic shock. Indeed, low serum zinc concentrations have been documented in pediatric septic shock, and non-survivors have lower serum zinc concentrations compared to survivors (16). Low serum zinc concentrations have also been reported in other forms of critical illness in both adults and children (41–43).
These observations have collectively fostered the concept that zinc supplementation may be a low cost and effective therapeutic strategy for septic shock (41, 44). In animal models of sepsis, zinc supplementation confers a survival benefit, and the survival benefit correlates with enhanced bacterial clearance as well as modulation of excessive inflammation (45–47). Two recent studies involving children with either pneumonia or bacteremia, in developing countries, demonstrated that zinc supplementation significantly reduced infection-related complications, including mortality (48, 49). A study involving critically ill children in the United States tested the efficacy of oral zinc supplementation, in combination with oral selenium, glutamine, and metoclopramide, as a means of preventing nosocomial infection or sepsis (24). There was no efficacy in the overall study population for the primary study end point (time to development of nosocomial infection/sepsis), but a secondary analysis restricted to patients with baseline immune dysfunction suggested a beneficial effect in reducing nosocomial infections. Thus, zinc supplementation as an adjunctive therapeutic strategy for septic shock remains an intriguing concept that needs to be further tested. There are currently two ongoing Phase 1 trials of intravenous zinc supplementation designed to further address this question (ClinicalTrials.gov: NCT01062009 and NCT01162109).
DISCOVERY OF STRATIFICATION BIOMARKERS
The ability to stratify outcome risk is fundamental to effective clinical practice and clinical research, and there is great deal of interest in using biomarkers to stratify outcome risk in septic shock (5, 50–52). Effective stratification biomarkers could benefit the field of septic shock in three broad areas: risk stratification for clinical trials, informing decision making for individual patients, and as a metric for quality improvement efforts. The genome-wide expression studies in pediatric septic shock have served as effective tools for the discovery of candidate stratification biomarkers.
The first genome-wide expression study in pediatric septic shock generated a list of genes differentially expressed between survivors and non-survivors, thus providing an initial working list of candidate stratification biomarkers (16). Interleukin-8 (IL-8), the principal human chemo-attractant for neutrophils, was among these differentially expressed genes and validation assays confirmed that serum IL-8 protein levels were indeed increased in non-survivors, compared to survivors (16). This observation led to a subsequent study directly testing the ability of serum IL-8 levels to predict 28-day mortality vs. survival (53). This study demonstrated that an IL-8 level ≤ 220 pg/ml, within 24 hours of presentation to the PICU with septic shock, had a negative predictive value for mortality of 95%. This observation was subsequently validated in an independent test cohort. Consequently, it has been proposed that serum IL-8 levels can be used to exclude low risk patients from future pediatric septic shock interventional trials as a means to improve the risk to benefit ratio of a therapeutic intervention. Interestingly, IL-8 does not seem to be an effective stratification biomarker in adult septic shock, thus suggesting important biological and physiological differences between adult and pediatric septic shock (54).
While the negative predictive value of serum IL-8 alone is high, the other test characteristics of IL-8 (i.e. sensitivity, specificity, and positive predictive value) are not sufficiently robust to develop a comprehensive pediatric septic shock stratification tool meeting a wide variety of clinical and research needs. Given the complexity of septic shock, it is possible that a multi-biomarker based stratification system could better address all of these needs. Accordingly, the genome-wide expression data in pediatric septic shock have been leveraged to select, objectively, a panel of 12 candidate stratification biomarkers (51). Using classification and regression tree (CART) methodology, the candidate stratification biomarkers have been used to derive and test a decision tree-based model that robustly predicts outcome in children with septic shock (55). The model has been tested in 355 subjects and has the following test characteristics for predicting 28-day all-cause mortality: sensitivity 93%, specificity 74%, positive predictive value 32% (in the context of an overall mortality rate of ~10%), and negative predictive value 99%. While the model requires further testing, the test characteristics indicate that it has substantial potential to serve a wide variety of stratification needs in pediatric septic shock.
DISCOVERY OF DIAGNOSTIC BIOMARKERS
The development of diagnostic biomarkers is another area of interest in field of pediatric critical care medicine, and again, genome-wide expression data have been leveraged for the discovery of two classes of candidate diagnostic biomarkers: biomarkers to diagnose the development of septic shock associated kidney injury (SSAKI) and biomarkers to diagnose the presence of bacterial infection in critically ill children.
Acute kidney injury (AKI) is a common complication of critical illness and septic shock, and consequently there is substantial interest in the development of biomarkers that diagnose AKI before a rise in serum creatinine, as a means to institute potentially renal protective therapies in a more timely manner (56). Commonly used biomarkers for AKI may not be as effective in the context of septic shock, compared to other etiologies of AKI (56–58). Accordingly, there remains a need for the development of AKI diagnostic biomarkers specific to septic shock.
Genome-wide expression data have been leveraged for the discovery of 21 unique genes that can predict the development of SSAKI with a sensitivity of 98% and a specificity of 80% (59). The products of several of these genes (i.e. proteins) can be readily measured in the serum compartment, and preliminary data indicate that the serum protein concentrations can also predict the development of SSAKI (59). Studies are currently in progress to determine if a panel of these candidate, serum-based, diagnostic markers can further improve the ability to diagnose SSAKI.
A daily clinical conundrum in the PICU is the differentiation of SIRS secondary to infection (i.e. sepsis) from SIRS secondary to non-infectious etiologies (i.e. “sterile inflammation”). Procalcitonin (PCT) has emerged as a potential diagnostic biomarker to meet this need, but its performance in critically ill populations has been challenged (60).
The most recent analysis of the pediatric genome-wide expression data has been targeted toward the identification of diagnostic biomarkers to identify the presence of bacterial infection in critically ill children (61). The analysis compared critically ill children with SIRS having documented negative bacterial cultures (i.e. the non-infected group), to critically ill children with SIRS having documented positive bacterial cultures (i.e. the infected group). There were 221 gene probes differentially regulated between the non-infected group and the infected group. A leave-one-out cross validation procedure based on a Support Vector Machines learning algorithm and the 221 differentially regulated gene probes was able to differentiate the two groups with 86% accuracy. Epstein-Barr virus induced gene 3 (EBI3) had the highest predictive strength among these 221 gene probes.
EBI3 is a subunit of the heterodimeric cytokine, IL-27, and IL-27 has been recently linked to the pathophysiology of sepsis (62, 63). Since IL-27 can be readily measured in the serum compartment, the ability of IL-27 to diagnose bacterial infection in critically ill children was tested and compared to the performance of PCT (61). Serum IL-27 levels had a >90% specificity and positive predictive value for diagnosing bacterial infection in this cohort of critically ill children and the overall performance of IL-27 was generally better than that of PCT. In addition, a decision tree incorporating IL-27 and PCT performed better than either biomarker alone. Thus, IL-27 has potential as an effective “rule-in” test for bacterial infection in critically ill children. Additional studies are being planned to test further the ability of IL-27 to serve as a biomarker for bacterial infection.
POTENTIAL LIMITATIONS
The majority of the studies reviewed above are based on whole blood-derived RNA. Thus, the source of RNA is a mixed population of white blood cells. While this approach has facilitated the accumulation of a large number of biological samples from multiple institutions, it has the potential to confound data in as much as differential patterns of gene expression could reflect differential white blood cell counts, rather than intrinsic differences in mRNA expression. This potential confounder has been addressed by multiple complementary approaches, including corrections for differential white blood cell counts (11, 12), interrogation of gene expression data for leukocyte subset-specific gene probes (15, 17, 64), the generation of expression data using leukocyte subset-specific mRNA (15), and the ability to validate gene expression signatures in formal validation cohorts (11, 31). In addition, the gene expression data generated through these studies have been successfully leveraged for the development of serum protein biomarkers (51–53, 55, 59, 65) and have been validated in animal models of sepsis (33, 37, 47). Thus, while the potential confounder of using whole blood-derived RNA remains an important issue, it would appear that the data generated thus far primarily reflect intrinsic differences in gene expression rather than an artifact of mRNA derived from a mixed population of white blood cells.
CONCLUSION
Almost a decade of genome-wide expression profiling in pediatric septic shock is now demonstrating tangible results. The studies have progressed from an initial discovery-oriented and exploratory phase, to a new phase where the data are being translated and applied to address several areas of clinical need. These include the identification of potential novel therapeutic targets and pathways, discovery of candidate diagnostic and stratification biomarkers, and the possibility of clinically relevant and clinically feasible gene expression-based subclassification. The challenges moving ahead include robust validation studies, which will require large-scale collaborations.
ACKNOWLEDGMENTS
The author thanks the following co-investigators and institutions for submitting biological samples and clinical data for the gene expression database, which could not have been developed without their generous collaboration: Natalie Z. Cvijanovich, Children’s Hospital and Research Center Oakland, Oakland, CA; Mark Hall, Nationwide Children’s Hospital, Columbus, OH; Geoffrey L. Allen, Children’s Mercy Hospital, Kansas City, MO; Neal J. Thomas, Penn State Hershey Children’s Hospital, Hershey, PA; Robert J. Freishtat, Children’s National Medical Center, Washington, DC; Nick Anas, Children’s Hospital of Orange County, Orange, CA; Keith Meyer, Miami Children’s Hospital, Miami, FL; Paul A. Checchia, Texas Children’s Hospital, Houston, TX; Richard Lin, The Children’s Hospital of Philadelphia, Philadelphia, PA; Thomas P. Shanley, CS Mott Children’s Hospital at the University of Michigan, Ann Arbor, MI; Michael T. Bigham, Akron Children’s Hospital, Akron, OH; Anita Sen, Morgan Stanley Children’s Hospital, Columbia University Medical Center, New York, NY; Jeffrey Nowak, Children’s Hospital and Clinics of Minnesota, Minneapolis, MN; Michael Quasney, Children’s Hospital of Wisconsin, Milwaukee, WI; Jared W. Henricksen, Primary Children’s Medical Center, Salt Lake City, UT; Arun Chopra, St Christopher’s Hospital for Children, Philadelphia, PA.
Statement of financial support: National Institutes of Health grants R01GM099773, R01GM096994, and RC1HL100474.
Footnotes
Disclosures: The Cincinnati Children’s Hospital Research Foundation and the author have submitted provisional patent applications for themes discussed in this manuscript. They include interleukin-8 as a stratification biomarker, a multi-biomarker–based stratification model, and interleukin-27 as a diagnostic biomarker.
REFERENCES
- 1.Goldstein B, Giroir B, Randolph A. International pediatric sepsis consensus conference: Definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005;6:2–8. doi: 10.1097/01.PCC.0000149131.72248.E6. [DOI] [PubMed] [Google Scholar]
- 2.Wong HR. Clinical review: Sepsis and septic shock - the potential of gene arrays. Crit Care. 2012;16:204. doi: 10.1186/cc10537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cornell TT, Wynn J, Shanley TP, Wheeler DS, Wong HR. Mechanisms and regulation of the gene-expression response to sepsis. Pediatrics. 2010;125:1248–1258. doi: 10.1542/peds.2009-3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wynn J, Cornell TT, Wong HR, Shanley TP, Wheeler DS. The host response to sepsis and developmental impact. Pediatrics. 2010;125:1031–1041. doi: 10.1542/peds.2009-3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marshall JC. Sepsis: rethinking the approach to clinical research. J Leukoc Biol. 2008;83:471–482. doi: 10.1189/jlb.0607380. [DOI] [PubMed] [Google Scholar]
- 6.Boomer JS, To K, Chang KC, et al. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA. 2011;306:2594–2605. doi: 10.1001/jama.2011.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hotchkiss RS, Opal S. Immunotherapy for sepsis--a new approach against an ancient foe. N Engl J Med. 2010;363:87–89. doi: 10.1056/NEJMcibr1004371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Felmet KA, Hall MW, Clark RS, Jaffe R, Carcillo JA. Prolonged lymphopenia, lymphoid depletion, and hypoprolactinemia in children with nosocomial sepsis and multiple organ failure. J Immunol. 2005;174:3765–3772. doi: 10.4049/jimmunol.174.6.3765. [DOI] [PubMed] [Google Scholar]
- 9.Hall MW, Knatz NL, Vetterly C, et al. Immunoparalysis and nosocomial infection in children with multiple organ dysfunction syndrome. Intensive Care Med. 2011;37:525–532. doi: 10.1007/s00134-010-2088-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hotchkiss RS, Coopersmith CM, McDunn JE, Ferguson TA. The sepsis seesaw: tilting toward immunosuppression. Nat Med. 2009;15:496–497. doi: 10.1038/nm0509-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cvijanovich N, Shanley TP, Lin R, et al. Validating the genomic signature of pediatric septic shock. Physiol Genomics. 2008;34:127–134. doi: 10.1152/physiolgenomics.00025.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shanley TP, Cvijanovich N, Lin R, et al. Genome-level longitudinal expression of signaling pathways and gene networks in pediatric septic shock. Mol Med. 2007;13:495–508. doi: 10.2119/2007-00065.Shanley. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wong HR. Genetics and genomics in pediatric septic shock. Crit Care Med. 2012;40:1618–1626. doi: 10.1097/CCM.0b013e318246b546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong HR, Cvijanovich N, Allen GL, et al. Genomic expression profiling across the pediatric systemic inflammatory response syndrome, sepsis, and septic shock spectrum. Crit Care Med. 2009;37:1558–1566. doi: 10.1097/CCM.0b013e31819fcc08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong HR, Freishtat RJ, Monaco M, Odoms K, Shanley TP. Leukocyte subset-derived genomewide expression profiles in pediatric septic shock. Pediatr Crit Care Med. 2010;11:349–355. doi: 10.1097/PCC.0b013e3181c519b4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong HR, Shanley TP, Sakthivel B, et al. Genome-level expression profiles in pediatric septic shock indicate a role for altered zinc homeostasis in poor outcome. Physiol Genonics. 2007;30:146–155. doi: 10.1152/physiolgenomics.00024.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wynn JL, Cvijanovich NZ, Allen GL, et al. The influence of developmental age on the early transcriptomic response of children with septic shock. Mol Med. 2011;17:1146–1156. doi: 10.2119/molmed.2011.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang BM, Huang SJ, McLean AS. Genome-wide transcription profiling of human sepsis: a systematic review. Crit Care. 2010;14:R237. doi: 10.1186/cc9392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang BM, McLean AS, Dawes IW, Huang SJ, Cowley MJ, Lin RC. Gene-expression profiling of gram-positive and gram-negative sepsis in critically ill patients. Crit Care Med. 2008;36:1125–1128. doi: 10.1097/CCM.0b013e3181692c0b. [DOI] [PubMed] [Google Scholar]
- 20.Tang BM, McLean AS, Dawes IW, Huang SJ, Lin RC. The use of gene-expression profiling to identify candidate genes in human sepsis. Am J Respir Crit Care Med. 2007;176:676–684. doi: 10.1164/rccm.200612-1819OC. [DOI] [PubMed] [Google Scholar]
- 21.Tang BM, McLean AS, Dawes IW, Huang SJ, Lin RC. Gene-expression profiling of peripheral blood mononuclear cells in sepsis. Crit Care Med. 2009;37:882–888. doi: 10.1097/CCM.0b013e31819b52fd. [DOI] [PubMed] [Google Scholar]
- 22.Xiao W, Mindrinos MN, Seok J, et al. J Exp Med. 2011;208:2581–2590. doi: 10.1084/jem.20111354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Unsinger J, McGlynn M, Kasten KR, et al. Il-7 promotes t cell viability, trafficking, and functionality and improves survival in sepsis. J Immunol. 184:3768–3779. doi: 10.4049/jimmunol.0903151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carcillo JA, Dean JM, Holubkov R, et al. The randomized comparative pediatric critical illness stress-induced immune suppression (CRISIS) prevention trial. Pediatr Crit Care Med. 2012;13:165–173. doi: 10.1097/PCC.0b013e31823896ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wynn JL, Levy O. Role of innate host defenses in susceptibility to early-onset neonatal sepsis. Clin Perinatol. 2010;37:307–337. doi: 10.1016/j.clp.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wynn JL, Scumpia PO, Winfield RD, et al. Defective innate immunity predisposes murine neonates to poor sepsis outcome but is reversed by TLR agonists. Blood. 2008;112:1750–1758. doi: 10.1182/blood-2008-01-130500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cuenca AG, Wynn JL, Kelly-Scumpia KM, et al. Critical role for cxc ligand 10/cxc receptor 3 signaling in the murine neonatal response to sepsis. Infect Immun. 2011;79:2746–2754. doi: 10.1128/IAI.01291-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wynn JL, Scumpia PO, Delano MJ, et al. Increased mortality and altered immunity in neonatal sepsis produced by generalized peritonitis. Shock. 2007;28:675–683. doi: 10.1097/SHK.0b013e3180556d09. [DOI] [PubMed] [Google Scholar]
- 29.Bouchon A, Facchetti F, Weigand MA, Colonna M. TREM-1 amplifies inflammation and is a crucial mediator of septic shock. Nature. 2001;410:1103–1107. doi: 10.1038/35074114. [DOI] [PubMed] [Google Scholar]
- 30.Wong HR, Cvijanovich N, Lin R, et al. Identification of pediatric septic shock subclasses based on genome-wide expression profiling. BMC Med. 2009;7:34. doi: 10.1186/1741-7015-7-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wong HR, Cvijanovich NZ, Allen GL, et al. Validation of a gene expression-based subclassification strategy for pediatric septic shock. Crit Care Med. 2011;39:2511–2517. doi: 10.1097/CCM.0b013e3182257675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wong HR, Wheeler DS, Tegtmeyer K, et al. Toward a clinically feasible gene expression-based subclassification strategy for septic shock: Proof of concept. Crit Care Med. 2010;38:1955–1961. doi: 10.1097/CCM.0b013e3181eb924f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Standage SW, Caldwell CC, Zingarelli B, Wong HR. Reduced peroxisome proliferator-activated receptor alpha expression is associated with decreased survival and increased tissue bacterial load in sepsis. Shock. 2012;37:164–169. doi: 10.1097/SHK.0b013e31823f1a00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eichler GS, Huang S, Ingber DE. Gene expression dynamics inspector (gedi): For integrative analysis of expression profiles. Bioinformatics. 2003;19:2321–2322. doi: 10.1093/bioinformatics/btg307. [DOI] [PubMed] [Google Scholar]
- 35.Guo Y, Eichler GS, Feng Y, Ingber DE, Huang S. Towards a holistic, yet gene-centered analysis of gene expression profiles: A case study of human lung cancers. J Biomed Biotechnol. 2006;2006:1–11. doi: 10.1155/JBB/2006/69141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vanlaere I, Libert C. Matrix metalloproteinases as drug targets in infections caused by gram-negative bacteria and in septic shock. Clin Microbiol Rev. 2009;22:224–239. doi: 10.1128/CMR.00047-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Solan PD, Dunsmore KE, Denenberg AG, Odoms K, Zingarelli B, Wong HR. A novel role for matrix metalloproteinase-8 in sepsis. Crit Care Med. 2012;40:379–387. doi: 10.1097/CCM.0b013e318232e404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lauhio A, Hastbacka J, Pettila V, et al. Serum mmp-8, -9 and timp-1 in sepsis: High serum levels of mmp-8 and timp-1 are associated with fatal outcome in a multicentre, prospective cohort study. Hypothetical impact of tetracyclines. Pharmacol Res. 2011;64:590–594. doi: 10.1016/j.phrs.2011.06.019. [DOI] [PubMed] [Google Scholar]
- 39.Fink MP. Matrix metalloproteinase-8 as a potential drug target for the therapy of sepsis. Crit Care Med. 2012;40:655–656. doi: 10.1097/CCM.0b013e31823b97d7. [DOI] [PubMed] [Google Scholar]
- 40.Prasad AS. Zinc: Mechanisms of host defense. J Nutr. 2007;137:1345–1349. doi: 10.1093/jn/137.5.1345. [DOI] [PubMed] [Google Scholar]
- 41.Heyland DK, Jones N, Cvijanovich NZ, Wong H. Zinc supplementation in critically ill patients: A key pharmaconutrient? J Parenter Enteral Nutr. 2008;32:509–519. doi: 10.1177/0148607108322402. [DOI] [PubMed] [Google Scholar]
- 42.Besecker BY, Exline MC, Hollyfield J, et al. A comparison of zinc metabolism, inflammation, and disease severity in critically ill infected and noninfected adults early after intensive care unit admission. Am J Clin Nutr. 2011;93:1356–1364. doi: 10.3945/ajcn.110.008417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cvijanovich NZ, King JC, Flori HR, Gildengorin G, Wong HR. Zinc homeostasis in pediatric critical illness. Pediatr Crit Care Med. 2009;10:29–34. doi: 10.1097/PCC.0b013e31819371ce. [DOI] [PubMed] [Google Scholar]
- 44.Knoell DL, Liu MJ. Impact of zinc metabolism on innate immune function in the setting of sepsis. Int J Vitam Nutr Res. 2010;80:271–277. doi: 10.1024/0300-9831/a000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bao S, Liu MJ, Lee B, et al. Zinc modulates the innate immune response in vivo to polymicrobial sepsis through regulation of nf-kappab. Am J Physiol Lung Cell Mol Physiol. 2010;298:L744–L754. doi: 10.1152/ajplung.00368.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Knoell DL, Julian MW, Bao S, et al. Zinc deficiency increases organ damage and mortality in a murine model of polymicrobial sepsis. Crit Care Med. 2009;37:1380–1388. doi: 10.1097/CCM.0b013e31819cefe4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nowak JE, Harmon K, Caldwell CC, Wong HR. Prophylactic zinc supplementation reduces bacterial load and improves survival in a murine model of sepsis. Pediatr Crit Care Med. 2012;13:e323–e329. doi: 10.1097/PCC.0b013e31824fbd90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Srinivasan MG, Ndeezi G, Mboijana CK, et al. Zinc adjunct therapy reduces case fatality in severe childhood pneumonia: A randomized double blind placebo-controlled trial. BMC Med. 2012;10:14. doi: 10.1186/1741-7015-10-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bhatnagar S, Wadhwa N, Aneja S, et al. Zinc as adjunct treatment in infants aged between 7 and 120 days with probable serious bacterial infection: A randomised, double-blind, placebo-controlled trial. Lancet. 2012;379:2072–2078. doi: 10.1016/S0140-6736(12)60477-2. [DOI] [PubMed] [Google Scholar]
- 50.Marshall JC, Reinhart K. Biomarkers of sepsis. Crit Care Med. 2009;37:2290–2298. doi: 10.1097/CCM.0b013e3181a02afc. [DOI] [PubMed] [Google Scholar]
- 51.Kaplan JM, Wong HR. Biomarker discovery and development in pediatric critical care medicine. Pediatr Crit Care Med. 2011;12:165–173. doi: 10.1097/PCC.0b013e3181e28876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Standage SW, Wong HR. Biomarkers for pediatric sepsis and septic shock. Expert Rev Anti Infect Ther. 2011;9:71–79. doi: 10.1586/eri.10.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wong HR, Cvijanovich N, Wheeler DS, et al. Interleukin-8 as a stratification tool for interventional trials involving pediatric septic shock. Am J Resp Crit Care Med. 2008;178:276–282. doi: 10.1164/rccm.200801-131OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Calfee CS, Thompson BT, Parsons PE, Ware LB, Matthay MA, Wong HR. Plasma interleukin-8 is not an effective risk stratification tool for adults with vasopressor-dependent septic shock. Crit Care Med. 2010;38:1436–1441. doi: 10.1097/CCM.0b013e3181de42ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wong HR, Salibury S, Xiao Q, et al. The pediatric sepsis biomarker risk model. Crit Care. 2012;16:R174. doi: 10.1186/cc11652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Basu RK, Devarajan P, Wong H, Wheeler DS. An update and review of acute kidney injury in pediatrics. Pediatr Crit Care Med. 2011;12:339–347. doi: 10.1097/PCC.0b013e3181fe2e0b. [DOI] [PubMed] [Google Scholar]
- 57.Wheeler DS, Devarajan P, Ma Q, et al. Serum neutrophil gelatinase-associated lipocalin (NGAL) as a marker of acute kidney injury in critically ill children with septic shock. Crit Care Med. 2008;36:1297–1303. doi: 10.1097/CCM.0b013e318169245a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mishra J, Dent C, Tarabishi R, et al. Neutrophil gelatinase-associated lipocalin (ngal) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365:1231–1238. doi: 10.1016/S0140-6736(05)74811-X. [DOI] [PubMed] [Google Scholar]
- 59.Basu RK, Standage SW, Cvijanovich NZ, et al. Identification of candidate serum biomarkers for severe septic shock-associated kidney injury via microarray. Crit Care. 2011;15:R273. doi: 10.1186/cc10554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tang BM, Eslick GD, Craig JC, McLean AS. Accuracy of procalcitonin for sepsis diagnosis in critically ill patients: Systematic review and meta-analysis. Lancet Infect Dis. 2007;7:210–217. doi: 10.1016/S1473-3099(07)70052-X. [DOI] [PubMed] [Google Scholar]
- 61.Wong HR, Cvijanovich NZ, Hall M, et al. Interleukin-27 is a novel candidate diagnostic biomarker for bacterial infection in critically ill children. Crit Care. 2012;16:R213. doi: 10.1186/cc11847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wojno ED, Hunter CA. New directions in the basic and translational biology of interleukin-27. Trends Immunol. 2012;33:91–97. doi: 10.1016/j.it.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wirtz S, Tubbe I, Galle PR, et al. Protection from lethal septic peritonitis by neutralizing the biological function of interleukin 27. J Exp Med. 2006;203:1875–1881. doi: 10.1084/jem.20060471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sun L, Gorospe JR, Hoffman EP, Rao AK. Decreased platelet expression of myosin regulatory light chain polypeptide (myl9) and other genes with platelet dysfunction and cbfa2/runx1 mutation: Insights from platelet expression profiling. J Thromb Haemost. 2007;5:146–154. doi: 10.1111/j.1538-7836.2006.02271.x. [DOI] [PubMed] [Google Scholar]
- 65.Nowak JE, Wheeler DS, Harmon KK, Wong HR. Admission chemokine (c-c motif) ligand 4 levels predict survival in pediatric septic shock. Pediatr Crit Care Med. 2010;11:213–216. doi: 10.1097/PCC.0b013e3181b8076c. [DOI] [PMC free article] [PubMed] [Google Scholar]