Abstract
Bone marrow transplantation has resulted in life-saving sustained T cell reconstitution in many SCID infants, but correction of B cell function has been more problematic. At the annual meeting of the Primary Immunodeficiency Treatment Consortium held in Boston, MA on April 27, 2012, a debate was held regarding the use of pre-transplant conditioning versus no pre-transplant conditioning in an effort to address this problem. Reviews of the literature were made by both debaters and there was agreement that there was a higher rate of B cell chimerism and a lower number of patients who required ongoing IG replacement therapy in centers that used pre-transplant conditioning. However, there were still patients who required IG replacement in those centers, so pre-transplant conditioning did not guarantee development of B cell function. RB presented data on B cell function according to the molecular defect of the patient, and showed that patients with IL7Rα, ADA and CD3 chain gene mutations can have normal B cell function post-transplantation with only host B cells. EH presented a statistical analysis of B cell function in published reports and showed that only a conditioning regimen that contained busulfan was significantly associated with better B cell function post-transplantation. The question is whether the risk of immediate and longterm toxicity in using busulfan is justified, particularly in SCID patients with DNA repair defects and in very young SCID newborns who will be detected by newborn screening.
Keywords: Severe combined immunodeficiency, conditioning regimen, haematopoietic stem cell transplantation, B cell function, immunoglobulin therapy
While bone marrow transplantation has resulted in life-saving sustained T cell reconstitution in most SCID infants,1 correction of B cell function has been more problematic.2 It has been suggested that the need for post-transplantation immunoglobulin (IG) replacement is due to a lack of donor B cell engraftment, leading some centers to use pre-transplant chemoablative conditioning in an effort to achieve this. However, data to support the efficacy of achieving this with conditioning have been far from clear. Few studies have been published regarding longterm B cell function in patients with SCID who have received bone marrow transplants.3-15 A review by the RB of 19 reports from Europe and the United States published over the past two decades found that the percentage of survivors with B cell chimerism and/or function was higher and the percentage requiring IG replacement was lower at those Centers that used pre-transplant conditioning.2 However there were substantial numbers of patients requiring IG replacement at all centers, so pre-transplant conditioning does not guarantee development of B cell function.3;16 More importantly, survival rates were higher when neither pre-transplant conditioning nor post-transplantation immunosuppressive drugs were used for graft-versus-host disease (GVHD) prophylaxis.2;17;18 In most of the reviewed reports, there was incomplete information about the underlying molecular defects that caused SCID in those subjects.
This article contains the substance of a debate held at the annual meeting of the Primary Immunodeficiency Treatment Consortium (PIDTC) in April of 2012, where RB was the proponent of no conditioning and EH the proponent of conditioning, and the arguments posed within are given in the order they were presented.
No Conditioning
RB and her colleagues have recently published the results of a longitudinal study on B cell function in 125 surviving SCIDs according to their molecular type who received bone marrow transplants without pre-transplant chemotherapy or post-transplantation GVHD immunosuppressive drugs at her center over a 28 year period.19 Only 17 of the survivors received HLA-identical marrow, while the other 104 received rigorously T cell-depleted haploidentical parental marrow. Table 1 shows the number and percentages with donor B cell chimerism and the number and percentages of patients of each molecular type who currently require IVIG treatment. The molecular defects with the highest percentages of donor B cell chimerism were X-linked SCIDs, of which twenty-one (36%) had donor B cells, and ADA-Def SCIDs of which 6 (33%) had donor B cell chimerism, with smaller percentages of donor B cell chimerism found among the other molecular types. Eighty-nine (71%) of the patients do not have donor B cell chimerism. Nevertheless, only 61 (48.8%) of the 125 survivors require immunoglobulin (IG) replacement therapy. Thus, 28 of the survivors without B cell chimerism do not require IG replacement. Sixty-two percent of those requiring IG replacement are X-linked SCIDs; 38 of the 58 X-SCID patients are currently receiving it and 37 of them do not have B cell chimerism. Other molecular types with a high percentage receiving IG replacement are RAG-Def SCIDs (83%) and autosomal recessive SCIDs of unknown molecular type (73%). By contrast, only 1 (6%) of the 17 patients with IL7Rα-Def SCID, 4 (22%) of the 18 ADA-Def SCIDs and none of the CD3-Def SCIDs require IG replacement. Only 1 of the 17 IL7Rα-Def SCIDs, 6 of the 18 ADA-Def SCIDs and none of the CD3 chain deficient SCIDs have donor B cell chimerism. Thus, host B cells appear to be able to cooperate with donor T cells and function normally in these particular SCID defects.
Table 1.
B Cell Chimerism and 416 Need for IG Treatment*
| # (%) with | # (%) | |
|---|---|---|
| Donor B Cell | On | |
| Group** | Chimerism | IG Treatment |
| X-linked N=58 |
21(36) | 38(66) |
| Jak3 Def N=8 |
2(25) | 3(38) |
| IL-7R Def N=17 |
1(6) | 1(6) |
| ADA Def N=18 |
6(33) | 4(22) |
| CD3 Chain N=3 |
0 | 0 |
| RAG ½ N=6 |
1(17) | 5(83) |
| AutoRec N=11 |
3(27) | 8(73) |
| CD45Def N=1 |
0 | 1(100) |
| Artemis N=1 |
1(100) | 0 |
| CHH N=1 |
0 | 1(100) |
| Unknown N=1 |
1(100) | 0 |
| Totals N=125 |
36(29) | 61(49) |
Reproduced with permission from reference # 19.
The distribution of the 17 HLA identical transplants is as follows: 5 among the X-linked, 5 among the ADA deficient, 3 among the autosomal recessive, 2 among the Jak3 deficient, 1 among the IL-7Ra deficient and 1 among 460 the RAG2 deficient.
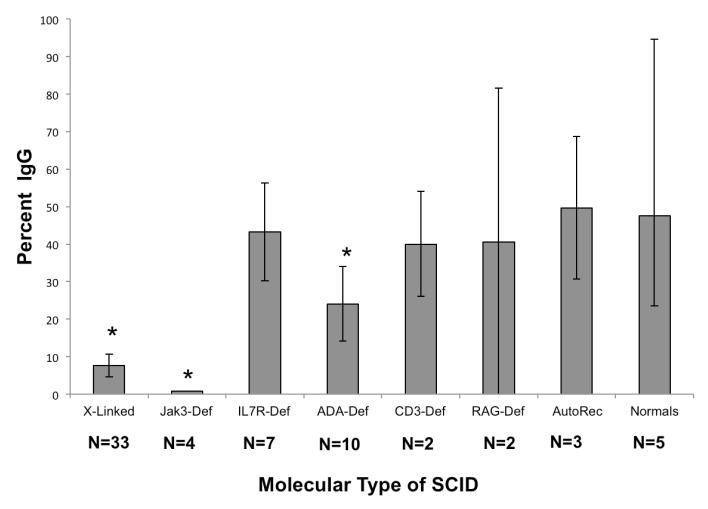
To examine the reason that B cell function did not occur in some types of SCID despite the presence of T cell chimerism, a number of studies were performed.19 These included blood immunoglobulin measurements; antibody titers to standard vaccines, blood group antigens and bacteriophage φ X 174; flow cytometry to examine for markers of immaturity, memory, switched memory B cells and BAFF receptor expression; B cell chimerism; B cell spectratyping; and B cell proliferation. One of the most significant findings was the inability of the B cells in X-linked and Jak3-deficient SCIDs to isotype switch in response to immunization with bacteriophage φ X 174, whereas this happened normally in the other types of SCID who were immunized with this neoantigen (Figure 1).20
Fig. 1.
Antibody responses to bacteriophage φ X174 in all 61 SCID patients immunized with this vaccine post-transplantation. The mean percentages of IgG antibody made after the second immunization were significantly lower than normal in Jak3Def (p<0.0001), X-Linked (p<0.0001) SCIDs and ADA-Def (p=0.0475) SCIDs. Those with Jak3Def SCID had an even lower mean percentage of IgG antibody those with X-linked SCID (p=0.0156). All other comparisons were not significantly different from normal controls.20 All patients have donor T cells and good T cell function.
We were also able to assess the percentage of switched memory B cells in 55 of the patients (Table 2). As can be seen, the molecular types of SCID with the lowest percentages of switched memory B cells are the X-SCIDs who are still receiving IG therapy (p=<0001), the autosomal recessive SCIDs of unknown molecular type (p=<0001), the ADA-Def SCIDs (p=0.0017) and the CD45-Def SCID. The percentages of switched memory B cells are normal for all of the other molecular types except the RAG-Def, Artemis-Def and CHH patients (not listed) who do not have any B cells.
Table 2.
Percentages of Switched Memory B Cells
| Molecular Type SCID | N | Mean | St. Dev. | S.E.M. |
|---|---|---|---|---|
| ADA-Deficient | 6 | 27.67H | 10.60 | 4.33 |
| CD3 Chain Deficient | 2 | 56.75 | 1.85 | 1.31 |
| IL7Ralpha-Deficient | 8 | 48.79 | 12.41 | 4.39 |
| ARSCID | 4 | 11.05HH | 3.69 | 1.85 |
| CD45 Deficient | 1 | 8.40 | NA** | NA** |
| Jak3-Deficient | 3 | 30.87 | 13.99 | 8.08 |
| X-SCID on IG | 21 | 11.41HH | 11.16 | 2.44 |
| X- SCID not on IG | 10 | 34.68 | 22.26 | 7.04 |
Normal adult mean % and range: 464 51.4 (32.3-70.5)
NA= not applicable for statistics as there is only 1 patient of this type.
p=0.0017
p<0.0001
Reproduced with permission from reference # 19.
In summary, these studies found that the most important factor determining whether B cell function develops in SCID T cell chimeras is the underlying molecular defect. In some types, host B cells function normally, so there is no need to use ablative measures to ensure donor B cell chimerism. In those molecular types where host B cell function did not develop, donor B cell chimerism was necessary to achieve B cell function. However, donor B cell chimerism did develop in 36 (29%) of the 125 patients despite no pre-transplant chemotherapy and 21 of the 36 were X-linked SCIDs, the most common molecular type of SCID and also the most problematic in terms of B cell reconstitution.
Now that newborn screening has been recommended by the Secretary of HHS, it will be important that SCID infants diagnosed at birth be treated as soon as possible after birth before they become infected with viruses that are difficult to treat.21 The off-quoted 94% survival rate if SCIDs are transplanted prior to 3.5 months of life was based on results at RB’s institution, where none of the SCIDs received pre-transplant conditioning.14;17;18 The fact that many centers do use chemotherapy prior to transplantation in SCID infants, presents a concerning situation when it comes to treating SCIDs detected by newborn screening. It will be important that they avoid pre-transplant chemotherapy, particularly myeloablative conditioning, if at all possible, as chemotherapy is most toxic in the neonatal period. Omitting toxic chemoablative agents prior to bone marrow transplantation in SCID allows the patients to avoid later infertility, veno-occlusive disease and damage to the lungs, endocrine organs, or brain.22-24
For Conditioning
The optimal outcomes of haematopoietic stem cell transplantation (HSCT) for the treatment of SCID are not only survival but also for patients to lead normal lives without the need for further treatment. Retrospective studies conducted with large cohorts have taught us that this outcome is indeed possible.8;13;25-28 However, the same retrospective studies have shown that many patients don’t have normal B cell function and still require intravenous immunoglobulin (IVIg) substitution therapy. Neven et al8 have shown in a retrospective study of 90 long-term SCID survivors of HSCT, using multivariate Poisson analysis, that abnormal B cell function was associated with poor outcomes. Therefore, even if the development of normal B cell function is not required in terms of survival, it remains a logical goal to strive for in HSCT-treated SCID patients.
The main goal of HSCT in SCID is to establish T cell engraftment and normal T cell function; and the question of the use of a CR versus no CR with regard to B cell reconstitution is interesting because it has not been proven that the CR influences T cell reconstitution and survival despite data showing that the use of a CR is associated with better survival in B-SCID.25;26
A review of the literature that addressed B cell immune reconstitution in long-term survivors was performed. Considering only studies in which at least 5 patients in a group of interest were analyzed, 14 publications were reviewed and data on normal B cell function as a function of the CR and B cell chimerism were extracted and presented in Tables 3-5.2;3;6-13;25;27-29 Of these, two references were excluded, Antoine et al25 and Neven et al,8 since the data required for the assessment were not included in these reports. In some studies, the data on normal B cell function as a function of the CR and B cell chimerism were simply extracted and used as is, while for other studies, the data contained in the Tables and body of the manuscripts required re-analysis. Patients were considered to have normal B cell function if they were off IVIg replacement therapy. CRs used among the studies were very heterogeneous. Since a part of the question debated here concerns the necessity or not of using a myeloablative CR to obtain B cell engraftment and B cell function, it was decided that patients who received a CR that did not contain busulfan were grouped with patients who did not receive any CR. Pooled Odds ratio (standard and Peto Odds Ratio - OR) as association estimates were calculated using a random effects model with inverse-variance weighting using the DerSimonian and Laird method.30 Statistical analyses used Stata/SE 11 software (StataCorp, College Station, TX).
Table 3.
B Cell Chimerism and B Cell Function after HLA-Identical 472 Related HSCT In SCID
| Study Reference | n | B cell chimerism§ |
Normal B cell function |
% (n) Normal B cell function |
|---|---|---|---|---|
| Haddad et al3 | 5 | 1 D 4 R |
1/1 2/4 |
60% (3) |
| *Grunebaum et al27 | 12a | 3 D 9 R |
3/3 8/9 |
90% (11) |
| Mazzolari et al7 | 8b | 3 D 5 R |
3/3 5/5 |
100% (8) |
| Friedrich et al29 | 6 | 6 D | 5/6 | 80 % (5) |
| Patel et al9 | 5 | 1 Dd | 3 | 60% (3) |
| Patel et al10 | 5c | 3 D 2 R |
3/3 1/2 |
80% (4) |
| Buckley et al13 | 12 | 5 D 7 R |
nd | nd |
| O’Marcaigh et al6 | 6 | 1 D 5 R |
1/1 0/5 |
17% (1) |
| Total | 59 (CR = 9) |
23/55 (42%) D
32/55 (58%) R |
17/18 (94%)
e
16/25 (64%) e |
74 % (35/47) |
D, Donor. R, Recipient. CR, Conditioning regimen. nd, no data.
3 of 12 patients received a CR
1 of 8 patients received a CR
5 of 5 patients received a CR.
Only 1 of 5 patients evaluated for B cell chimerism.
For the 43 patients in which both B cell function and B cell chimerism were available, 94% of these with B cell engraftment had normal B cell function as compared to 64% of these with host B cells (pooled Peto OR=13.2; 95% CI: 1.5-117.5; p=0.02). However, this association could be ascribed to data from only one study in Athabascan SCIDs in which B cell function was absent in all 5 (probably Artemis SCID) patients without B cell engraftment.6
For B cell chimerism, in most studies it was reported that B cells were of recipient or 486 donor type while in others it was reported in terms of “mixed” in which case we 487 considered that there was an engraftment, or of “traces” in which case we considered 488 that there was no engraftment. When a percentage was given, all patients with donor 489 percentages greater than 10% were included in the category “Donor” in Tables 3 and 5.
Table 5.
B Cell Function And B Cell Chimerism In SCID Patients With Donors Other Than HLA-Identical Related Donors
| Study reference | No Busulfan | Busulfan | ||||
|---|---|---|---|---|---|---|
| n | B cell chimerism§ | Off IVIg | n | B cell chimerism§ | Off IVIg** | |
| Haddad et al3 | 8 | 2 D 6 R |
2/2 D 2/6 R |
9 | 1 D 8 R |
1/1 D 3/8 R |
| Grunebaum et al27 | 1 | 0 D 1 R |
0/1 R | 43 | 35 D 8 R |
23/23 D 2/2 R |
| Mazzolari et al7 | 5 | 0 D 5 R |
1/5 R | 27 | 24 D 3 R |
23/24 D 3/3 R |
| Slatter et al11 | 2 | 0 D 2 R |
0/2 R | 32 | 17 D or M 15 R (5 T) |
17/17 D 8/15 R |
| Friedrich et al29 | 12 | 2 D 10 R |
2/2 D 2/10 R |
13 | 11 D 2 R |
11/11 D 1/2 R |
| Patel et al9 | 7 | 3 D (90-12-11*) 4 R |
2/3 D 2/4 R |
nd | nd | nd |
| Patel et al10 | nd | nd | nd | 12 | 9 D (100-45-29-19*) 3 R |
7/9 D 1/3 R |
| Dvorak et al12 | 10 | 1 D 9 R (or T) |
1/1 D 3/9 R |
nd | nd | nd |
| O’Marcaigh et al6 | 9 | 0 D 9 R |
1/9 R | 3 | 2 D 1 R |
2/2 D 0/1 R |
| Total | 54 |
8 D (15%)
46 R (85%) |
7/8 (88%)
11/46 (24%) |
139 |
99 D (71%)
40 R (29%) |
84/87 (97%)
18/34 (53%) |
For B cell chimerism, see the legend of Table 3
Corresponds to the chimerism of B cells given in percentage in the manuscript each time for a patient.
For many patients the information was not available in the manuscripts explaining the discrepancy sometimes observed between the number of patients with a given chimerism and the number of patients for which B cell function (off IVIg replacement) was given.
D, Donor. R, Recipient. M, Mixed. T, Traces. nd, no data.
Analysis of the literature
The question of the use of a CR versus no CR should only be debated in the absence of an HLA-identical related donor, since survival in that situation without a CR can be as high as 100% with most patients having normal B cell function and an engraftment of donor B cells in 40 to 50% of them (Table 3).
The pertinent issue is the need for using a CR versus no CR for B cell reconstitution in SCID patients with donors other than HLA-identical related donors. On this matter there are several key questions.
Is normal B cell function more frequent if a busulfan-containing CR has been used?
This question is indeed crucial and there are no published data that specifically address it. Nevertheless, compilation of data in the literature seems to show that use of a CR is associated with a higher frequency of normal B cell function in long-term survivors (Table 4). Indeed, in 7 published studies one can find that, in the presence of a busulfan-containing CR, B cell function was found to be normal in 85% of 139 patients overall (Table 4), ranging from a high of 96% (26/27),7 to a low of 44% (4/9).3 While in the absence of a CR (or with a non-busulfan-containing CR), B cell function was found to be normal in a much lower percentage of patients: 33% of 57 patients overall, ranging from a high of only 50% (4/8 and 5/10, respectively) for 2 of the 8 studies3;9 to a low of 0% (0/1 and 0/2, respectively) in 2 studies.11;27 Normal B cell function was thus significantly associated with the use of a busulfan-containing CR (pooled OR=7.9; 95% CI: 3.0-21.0; p<0.0001). In a study by Buckley et al,13 which included the largest cohort of patients who did not receive any CR, but that was not considered in the primary analysis because HLA-identical related HSCTs were mixed with other HSCTs, 37% (27/72) of patients had normal B cell function. Also, in the multicenter study by Haddad et al,28 48% (13/27) of patients who did not receive any CR had normal B cell function while it was normal in 64% (57/89) of patients who did receive a CR, although data from this study, retrieved from personal notes from the author, was not included either. Even when these 2 studies with large cohorts were taken into account in the analysis (Table 4), there was a doubling in the percent of patients with normal B cell function (77%; 175/228) who had received a CR compared to those patients who had not received a CR (38%; 59/156) and the data remained significant (pooled OR=3.7; 95% CI: 2.0-6.0; p<0.0001).
Table 4.
Influence of Conditioning Regimen on B Cell 490 Function in SCID Patients with Donors Other Than HLA-Identical Related Donors
| Study reference | No CR (or no myeloablative CR) | CR (containing Bu) | ||
|---|---|---|---|---|
| n | Normal B cell function % (n) |
n | Normal B cell function % (n) |
|
| Haddad et al3 | 8 | 50% (4) | 9 | 44% (4) |
| *Grunebaum et al27 | 1 | 0%(0) | 43 | 95% (41) |
| Mazzolari et al7 | 5 | 20% (1) | 27 | 96% (26) |
| Slatter et al11 | 2 | 0% (0) | 32 | 78% (25) |
| Friedrich et al29 | 12 | 33% (4) | 13 | 92% (12) |
| Patel et al9 | 10 | 50% (5) | nd | nd |
| Patel et al10 | nd | nd | 12 | 67% (8) |
| Dvorak et al12 | 10 (nma)a |
40% (4) | nd | nd |
| O’Marcaigh et al6 | 9 (nma)a |
11% (1) | 3 | 67% (2) |
| Buckley et al13 | 72 | 37%b (27) | nd | nd |
| †Haddad et al28 | 27 | 45%b (13) | 89* | 64%* (57) |
| Total 1 b | 57 | 33% (19) | 139 | 85% (118) |
| Total 2 b | 156 | 37% (59) | 228 | 77% (175) |
nma, non myeloablative CR. nd, no data.
Total 1 does not include the data from Buckley et al13 and Haddad et al28 while Total 2 includes the data from all references.
The information regarding the dose of busulfan used in the CR was obtained by directly contacting the first author of this manuscript.
The information on B cell function as a function of the CR was unpublished data from EH.
Is it possible to have normal B cell function in the absence of B cell engraftment?
To answer this question, data from 9 studies presented in Table 5 were evaluated. In the absence of a CR, out of a total of 54 evaluable patients, 46 (85%) did not achieve B cell engraftment, and of these only 24% (11/46) had normal B cell function; while among the 15% of patients (8/54) with B cell engraftment, 88% (7/8) had normal B cell function. B cell engraftment was thus significantly associated with a normal B cell function in the absence of a CR (pooled OR=6.2; 95% CI: 1.3-30.9; p=0.02). In the presence of a CR, the percentage of patients that did not have any B cell engraftment was greatly reduced to 29% (40/139) yet of these, 53% had normal B cell function while among the 71% of patients (99/139) with B cell engraftment, 96% (84 out of 87 evaluable patients) had normal B cell function. Thus, B cell engraftment was also significantly associated with normal B cell function in the presence of a CR (pooled OR=8.8; 95% CI: 3.1-24.5; p<0.0001). Therefore, yes it is possible to have normal B cell function in the absence of B cell engraftment that can occur both in the absence or presence of a CR, but it is much more likely to occur in the presence of B cell engraftment (pooled OR=13.3; 95% CI: 5.6-31.6; p<0.0001).
Does a CR induce B cell engraftment more frequently?
The data in Table 5 shows that B cell engraftment increased from 15% (8/54) of patients without a CR to 71% of patients (99/139) who received a CR. If we compare the individual studies this ranged from a high of 89%7 to a low of 53%;11 with the exception of one study3 (11%; 1/9 patients) in which, interestingly, only patients with B+ SCID were analyzed.
This analysis of the literature clearly shows that the use of a CR is associated with a greater chance that the patient will have B cell engraftment and function. It is true that these data also show that a CR does not guarantee B cell engraftment. In addition, patients transplanted without a CR do not receive any GvHD prophylaxis.2 However, there are no data demonstrating or suggesting that T cell reconstitution occurs more rapidly in the absence of a CR.
Issues to consider for the use of a CR
Despite the fact that some of the combined published study data are statistically significant, one should consider these numbers with caution since individual study data taken from the analyzed manuscripts were shown not to be significant for the questions raised in this manuscript, for several reasons: 1) the cohorts studied were too small to reach any significance; 2) only one CR strategy was used (CR or no CR) at one center making impossible any comparison; 3) this was not the objective of the study and B cell function as a function of the CR was not analyzed; or 4) HLA identical related HSCTs were mixed together with other HSCTs. Another difficulty is the fact that CRs differed between and also within studies. Should we consider that patients receiving an 8 mg/Kg total dose of busulfan be analyzed together with those receiving a total dose of 16 mg/Kg? Interestingly, the studies in which the percentage of patients with normal B cell function was higher7;27 were those in which patients received busulfan 16mg/kg and cyclophosphamide 200 mg/kg (Bu16Cy200).
Furthermore, all SCID patients should not be analyzed the same way. Indeed, it is possible that B cell engraftment and B cell function could be different in different molecular types of SCID. Unfortunately, the information given in the literature did not allow us to separate the data for the different SCID groups at the clinical or molecular level.
The higher number of SCID infants who are diagnosed early will force us to address whether or not we should consider the use of a CR in this patient population. Myers et al14 have shown that for 21 neonatal infants with SCID who received an HLA-identical related BMT (n=2) or a T cell-depleted HLA-haploidentical BMT (n=19) without any CR, survival was 95%. Only 7 out of the 20 long-term survivors did not require IVIg. Therefore, one could consider that a CR would be unnecessary for these patients. On the other hand, it is also possible that this high survival rate was due to the good clinical condition in which these neonates were transplanted and, therefore, a CR could potentially be used without causing GvHD or mortality but rather induce better long term B cell function. The use of a CR in neonates, however, is further complicated by the fact that little is known regarding the pharmacokinetics of busulfan in neonates.
Altogether, from a clinical standpoint, we can consider that the literature provides information that tends to show that a CR is beneficial in terms of B cell reconstitution for SCID patients, and that perhaps the use of a fully myeloablative CR (i.e. with Bu16) is the best strategy to adopt to obtain the greatest chance for B cell reconstitution. One may question whether a CR or the intensity of a CR should be discussed according to the molecular type of SCID. Certainly, in SCID patients who have abnormal DNA repair such as Artemis, one could question the long-term toxicity of a myeloablative CR. Also, one should await upcoming retrospective studies that will specifically address the long-term severe neurological and developmental side effects in patients with DNA repair abnormalities and whether these long-term complications are associated with the use of a CR or more specifically a myeloablative CR.
These questions and hypotheses should be addressed in well-designed retrospective studies using registries with enough patients to ask precise questions such as which type of CR is better for a given type of SCID instead of the global question posed in this debate, i.e. CR versus no CR in SCID that, even with statistically significant data, does not allow one to choose the best strategy for a given patient.
Acknowledgements
This work was supported by a U54 grant U54AI082973 from the National Institute of Allergy and Infectious Diseases for the Primary Immunodeficiency Treatment Consortium (PIDTC), an R13 grant 5R13AI094943 in support of the 2012 annual meeting of the PIDTC, and the NIH Office of Rare Diseases Research of the National Center for Advancing Translational Sciences.
ABBREVIATIONS
- BMT
Bone marrow transplantation
- Bu
Busulfan
- CR
Conditioning regimen
- Cy
Cyclophosphamide
- GvHD
Graft-versus-host disease
- HLA
Human leukocyte antigens
- HSCT
Haematopoietic stem cell transplantation
- IVIg
Intravenous immunoglobulin
- OR
Odds ratio
- PID
Primary immunodeficiency
- PIDTC
Primary immunodeficiency treatment consortium
- RAG
Recombination activating gene
- SCID
Severe combined immunodeficiency
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sarzotti-Kelsoe M, Win CM, Parrott RE, Cooney M, Moser BK, Roberts JL, et al. Thymic output, T-cell diversity, and T-cell function in long-term human SCID chimeras. Blood. 2009;114(7):1445–53. doi: 10.1182/blood-2009-01-199323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buckley RH. B-cell function in severe combined immunodeficiency after stem cell or gene therapy: a review. J Allergy Clin Immunol. 2010;125(4):790–7. doi: 10.1016/j.jaci.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haddad E, Deist FL, Aucouturier P, Cavazzana-Calvo M, Blanche S, Basile GD, et al. Long-term chimerism and B-cell function after bone marrow transplantation in patients with severe combined immunodeficiency with B cells: A single-center study of 22 patients. Blood. 1999;94(8):2923–30. [PubMed] [Google Scholar]
- 4.Dror Y, Gallagher R, Wara DW, Colombe BW, Merino A, Benkerrou M, et al. Immune reconstitution in severe combined immunodeficiency disease after lectin-treated, T cell depleted haplocompatible bone marrow transplantation. Blood. 1993;81:2021–30. [PubMed] [Google Scholar]
- 5.van Leeuwen JE, van Tol MJ, Joosten AM, Schellekens PT, van den Bergh RL, Waaijer JL, et al. Relationship between patterns of engraftment in peripheral blood and immune reconstitution after allogeneic bone marrow transplantation for (severe) combined immunodeficiency. Blood. 1994;84:3936–47. [PubMed] [Google Scholar]
- 6.O’Marcaigh AS, DeSantes K, Hu D, Pabst H, Horn B, Li L, et al. Bone marrow transplantation for T-B-severe combined immunodeficiency disease in Athabascan-speaking native Americans. Bone Marrow Transplant. 2001;27(7):703–9. doi: 10.1038/sj.bmt.1702831. [DOI] [PubMed] [Google Scholar]
- 7.Mazzolari E, Forino C, Guerci S, Imberti L, Lanfranchi A, Porta F, et al. Long-term immune reconstitution and clinical outcome after stem cell transplantation for severe T-cell immunodeficiency. J Allergy Clin Immunol. 2007;120(4):892–9. doi: 10.1016/j.jaci.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 8.Neven B, Leroy S, Decaluwe H, Le Deist F, Picard C, Moshous D, et al. Long-term outcome after hematopoietic stem cell transplantation of a single-center cohort of 90 patients with severe combined immunodeficiency. Blood. 2009;113(17):4114–24. doi: 10.1182/blood-2008-09-177923. [DOI] [PubMed] [Google Scholar]
- 9.Patel NC, Chinen J, Rosenblatt HM, Hanson IC, Brown BS, Paul ME, et al. Long-term outcomes of nonconditioned patients with severe combined immunodeficiency transplanted with HLA-identical or haploidentical bone marrow depleted of T cells with anti-CD6 mAb. J Allergy Clin Immunol. 2008;122(6):1185–93. doi: 10.1016/j.jaci.2008.10.030. [DOI] [PubMed] [Google Scholar]
- 10.Patel NC, Chinen J, Rosenblatt HM, Hanson IC, Krance RA, Paul ME, et al. Outcomes of patients with severe combined immunodeficiency treated with hematopoietic stem cell transplantation with and without preconditioning. J Allergy Clin Immunol. 2009;124(5):1062–9. doi: 10.1016/j.jaci.2009.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Slatter MA, Brigham K, Dickinson AM, Harvey HL, Barge D, Jackson A, et al. Long-term immune reconstitution after anti-CD52-treated or anti-CD34-treated hematopoietic stem cell transplantation for severe T-lymphocyte immunodeficiency. J Allergy Clin Immunol. 2007;121:361–7. doi: 10.1016/j.jaci.2007.10.035. [DOI] [PubMed] [Google Scholar]
- 12.Dvorak CC, Hung GY, Horn B, Dunn E, Oon CY, Cowan MJ. Megadose CD34(+) cell grafts improve recovery of T cell engraftment but not B cell immunity in patients with severe combined immunodeficiency disease undergoing haplocompatible nonmyeloablative transplantation. Biol Blood Marrow Transplant. 2008;14(10):1125–33. doi: 10.1016/j.bbmt.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 13.Buckley RH, Schiff SE, Schiff RI, Markert L, Williams LW, Roberts JL, et al. Hematopoietic stem cell transplantation for the treatment of severe combined immunodeficiency. N Engl J Med. 1999;340:508–16. doi: 10.1056/NEJM199902183400703. [DOI] [PubMed] [Google Scholar]
- 14.Myers LA, Patel DD, Puck JM, Buckley RH. Hematopoietic stem cell transplantation for severe combined immunodeficiency in the neonatal period leads to superior thymic output and improved survival. Blood. 2002;99(3):872–8. doi: 10.1182/blood.v99.3.872. [DOI] [PubMed] [Google Scholar]
- 15.Recher M, Berglund LJ, Avery DT, Cowan MJ, Gennery AR, Smart J, et al. IL-21 is the primary common gamma chain-binding cytokine required for human B-cell differentiation in vivo. Blood. 2011;118(26):6824–35. doi: 10.1182/blood-2011-06-362533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Himelstein BP, Puck J, August C, Pierson G, Bunin N. T cell depleted maternal bone marrow transplantaton for siblngs with X-linked severe combined immunodeficiency. J Pediatr. 1993;122:289–91. doi: 10.1016/s0022-3476(06)80135-6. [DOI] [PubMed] [Google Scholar]
- 17.Buckley RH. Transplantation of Hematopoietic Stem Cells in Human Severe Combined Immunodeficiency: Longterm Outcomes. Immunologic Research. 2011;49:25–43. doi: 10.1007/s12026-010-8191-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Railey MD, LoKhnygina Y, Buckley RH. Long Term Clinical Outcome of Patients with Severe Combined Immunodeficiency who Received Related Donor Bone Marrow Transplants without Pre-transplant Chemotherapy or Post-transplant GVHD Prophylaxis. J Pediatr. 2009;155:834–40. doi: 10.1016/j.jpeds.2009.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buckley RH, Win CM, Moser BK, Parrott RE, Sajaroff E, Sarzotti-Kelsoe M. Post-Transplantation B Cell Function in Different Molecular Types of SCID. J Clin Immunol. 2012 doi: 10.1007/s10875-012-9797-6. Published Online, September 24, 2012. (DOI) 10.1007/s10875-012-9797-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ochs HD, Davis SD, Wedgwood RJ. Immunologic responses to bacteriophage phi-X 174 in immunodeficiency diseases. J Clin Invest. 1971;50(12):2559–68. doi: 10.1172/JCI106756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buckley R. The long quest for neonatal screening for SCID. J Allerg Clin Immunol. 2012;129:597–604. doi: 10.1016/j.jaci.2011.12.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koppelmans V, Breteler MM, Boogerd W, Seynaeve C, Gundy C, Schagen SB. Neuropsychological performance in survivors of breast cancer more than 20 years after adjuvant chemotherapy. J Clin Oncol. 2012;30(10):1080–6. doi: 10.1200/JCO.2011.37.0189. [DOI] [PubMed] [Google Scholar]
- 23.Titman P, Pink E, Skucek E, O’Hanlon K, Cole TJ, Gaspar J, et al. Cognitive and behavioural abnormalities in children following haematopoietic stem cell transplantation for severe congenital immunodeficiencies. Blood. 2008;112:3907–13. doi: 10.1182/blood-2008-04-151332. [DOI] [PubMed] [Google Scholar]
- 24.Sanders JE, the Seattle Marrow Transplant Group . Late Effects of Treatment for Childhood Cancer. Wiley-Liss Publishing Ltd; New York, NY: 1992. Effects of bone marrow transplantation on reproductive function; pp. 95–101. [Google Scholar]
- 25.Antoine C, Muller S, Cant A, Cavazzana-Calvo M, Veys P, Vossen J, et al. Long-term survival and transplantation of haemopoietic stem cells for immunodeficiencies: report of the European experience 1968-99. Lancet. 2003;361:553–60. doi: 10.1016/s0140-6736(03)12513-5. [DOI] [PubMed] [Google Scholar]
- 26.Bertrand Y, Landais P, Friedrich W, Gerritsen B, Morgan G, Fasth A, et al. Influence of severe combined immunodeficiency phenotype on the outcome of HLA non-identical T cell-depleted bone marrow transplantation. J Pediatr. 1999;134:740–8. doi: 10.1016/s0022-3476(99)70291-x. [DOI] [PubMed] [Google Scholar]
- 27.Grunebaum E, Mazzolari E, Porta F, Dallera D, Atkinson A, Reid B, et al. Bone marrow transplantation for severe combined immune deficiency. JAMA. 2006;295(5):508–18. doi: 10.1001/jama.295.5.508. [DOI] [PubMed] [Google Scholar]
- 28.Haddad E, Landais P, Friedrich W, Gerritsen B, Cavazzana-Calvo M, Morgan G, et al. Long-term immune reconstitution and outcome after HLA-nonidentical T-cell-depleted bone marrow transplantation for severe combined immunodeficiency: a European retrospective study of 116 patients. Blood. 1998;91(10):3646–53. [PubMed] [Google Scholar]
- 29.Friedrich W, Honig M, Muller SM. Long-term follow-up in patients with severe combined immunodeficiency treated by bone marrow transplantation. Immunol Res. 2007;38(1-3):165–73. doi: 10.1007/s12026-007-0030-2. [DOI] [PubMed] [Google Scholar]
- 30.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]