Abstract
Reductions in measures of dendritic morphology in the agranular insular cortex have been identified as consequences of prenatal exposure to moderate levels of ethanol in the rat. Motivated by the strong connectivity between this region of frontal cortex and the striatum and a growing body of data linking specific components of the mesocortical/limbic system to effects of ethanol and ethanol self-administration, the current study investigated the effects of moderate fetal ethanol exposure on the dendritic morphology of medium spiny neurons (MSNs) in several regions of the striatum. Throughout gestation, pregnant rat dams either consumed a saccharin solution (control) or achieved average daily blood ethanol concentrations of 84 mg% via voluntary consumption of a 5% ethanol solution. The brains of adult male offspring were extracted and processed for Golgi-Cox staining. MSNs from the dorsomedial striatum, dorsolateral striatum and the nucleus accumbens core and shell were sampled for analysis. Relative to saccharin controls, robust reductions in dendritic length and branching, but not spine density, were observed in the shell of the nucleus accumbens in fetal-ethanol-exposed rats. No significant prenatal ethanol effects were found in the other regions of the striatum. These findings suggest that exposure to moderate levels of ethanol in utero can have profound effects on brain regions related to reward processing and provide possible clues relevant to understanding increased self-administration of drugs of abuse in animals exposed to ethanol during brain development.
Keywords: prenatal alcohol, Golgi-Cox, medium spiny neurons, mesolimbic dopamine system
Introduction
Exposure to ethanol during early development can have profound effects on behavior, cognition and related neurobiological processes throughout the lifespan (Kodituwakku, 2007; Streissguth et al., 1991). Fetal Alcohol Spectrum Disorders (FASDs) encompass a range of diagnoses including Fetal Alcohol Syndrome (FAS; Abel and Berman, 1994; Clarren and Smith, 1978; Jones and Smith, 1973, 1975; Jones et al., 1973) characterized by severe neurological and morphological alterations, and those assigned to lesser-affected children (see Hoyme et al., 2005), including Partial FAS, Alcohol Related Birth Defects (ARBD), and Alcohol Related Neurodevelopmental Disorder (ARND). The incidence of FAS is relatively low (0.1%; Abel, 1995), however, moderate ethanol consumption (1–2 drinks per day) occurs in 5–20% of pregnant women (Day et al., 1993) and is estimated to affect a much larger number of children (Centers for Disease Control and Prevention Report, 1994). Although more subtle, deficits associated with moderate exposure may, nonetheless, have significant negative consequences that persist throughout life (Conry, 1990; Streissguth et al., 1990, 1991).
Animal models of heavy exposure to ethanol during development typically involve binge-like exposure that results in high blood ethanol concentrations in pregnant dams (e.g. ~250mg/dL; Thomas et al., 2009) that produce profound neurochemical (Goodlett and Horn, 2001), functional (Cui et al., 2010), and behavioral (Kelly et al., 2000) deficits and gross morphological changes including robust reductions in brain and body weight (Thomas et al., 2009), dendritic structure (Berman et al., 1996; Hannigan and Pilati, 1991), and regional brain volume (Livy et al., 2003; Mooney and Napper, 2005). Animal models of moderate fetal ethanol exposure involve paradigms that produce far lower levels of blood ethanol concentrations (e.g. ~85mg/dL; Savage et al., 2010) and have revealed more subtle, but reliable, neurochemical, functional, and behavioral deficits in the absence of whole brain or regional structural alterations characteristic of heavy exposure (Savage et al., 1991, 2010; Sutherland et al., 1997, 2000; Varaschin et al., 2010). Recent work in our laboratory using a rodent model of moderate ethanol exposure has revealed reduced measures of dendritic branching, length and spine density in frontal cortical neurons of adult animals in the absence of reductions in body or brain weight (Hamilton et al., 2010a). More substantial fetal ethanol-related reductions in experience-dependent immediate early gene (IEG) expression were observed in the same regions of frontal cortex (Hamilton et al., 2010a,b), which is consistent with previous work from our group showing comparatively clear functional and neurochemical alterations following moderate ethanol exposure in contrast to more modest or absent morphological, structural and volumetric alterations (Allan et al., 1997; Galindo et al., 2004; Sutherland et al., 1997; Varaschin et al., 2010). The reductions in dendritic branching, length and spine density in frontal cortex pyramidal neurons noted above represent at least one level at which there are detectable structural alterations as a consequence of moderate fetal ethanol exposure. In light of these observations, the current study explored other brain regions for which alterations in dendritic morphology may be observed following moderate prenatal ethanol exposure. Based on the strong interconnectivity between the frontal cortex and the striatum (Voorn et al., 2004), the well established link between the frontal cortex and striatum in reward processing (Schoenbaum et al., 2006), the role of the striatum in self-administration of ethanol and other drugs of abuse (Rewal et al., 2009), as well as recent literature indicating that prenatal ethanol exposure has been found to influence voluntary ethanol consumption in adulthood (Barbier et al., 2008, 2009), the striatum represents an important region of interest for investigation of changes in dendritic morphology related to moderate fetal ethanol exposure in utero.
The striatum is comprised of distinct heterogeneous components that are primarily divided into dorsal and ventral subregions. The dorsal striatum is further divided into the Dorsolateral Striatum (DLS) and Dorsomedial Striatum (DMS) based on structural and functional dissociations (Yin and Knowlton, 2006). Exposure to ethanol has been shown to alter plasticity in DMS neurons in vitro (Yin et al., 2007). The ventral striatum is similarly differentiated into the shell and core region of the Nucleus Accumbens (Acb), which have been found to be differentially affected by exposure to drugs of abuse (Engleman et al., 2009; Hutchison et al., 2006), including alcohol. Functional dissociations between the core and shell have been established based on behavior in normal rats (Chaudhri et al., 2010), as well as changes in dendritic morphology as a result of chronic-intermittent ethanol exposure (Zhou et al., 2007), and intra-cranial self administration of ethanol (Engleman et al., 2009) in alcohol-preferring P rats.
Several studies have found that prenatal ethanol exposure led to increased ethanol consumption later in life. Barbier and collaborators (2008; 2009) found that moderate exposure during the third trimester equivalent period of development led to increased ethanol consumption in adulthood, and also altered IEG expression in the frontal cortex and striatum. Similar results have been found in rats exposed to ethanol during adolescence (Vetter et al., 2007). Thus, it appears that early exposure to ethanol could alter brain circuitry related to reward and motivation. Given the well-established role of the Acb in drug addiction, reward, and motivation (Reviewed by Everitt et al., 2008), the current experiments were undertaken to examine changes in the dendritic morphology of medium spiny neurons (MSNs) in the DLS, DMS, Acb shell, and Acb core in adult rats exposed to moderate levels of ethanol during prenatal brain development.
Materials and Methods
Subjects
The brains analyzed in the present study were collected as part of a previous study on dendritic morphology in the frontal cortex; They were obtained from rats that served in a control condition for which the only deviation from standard laboratory housing was routine video-taping of behavior in the home cage (see Hamilton et al., 2010a, Experiment 1). Subjects were 16 male Long-Evans rats (Harlan Industries, Indianapolis, IN), bred at the University of New Mexico Health Sciences Center Animal Resource Facility (HSC-ARF) exposed to saccharin or ethanol in utero (breeding protocol described below). Sixteen additional rats were acquired directly from Harlan and served as cage-mates for the 16 principal subjects1. Cage-mates were matched for sex, age, and body weight. Rats were pair-housed after weaning in standard plastic cages with food and water available ad libitum. Bedding, food, and water were changed at 48-hour intervals. For each rat the cage-mate remained the same for the duration of the study. All procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of New Mexico and were conducted in accordance with the NIH Guide for Care and Use of Laboratory Animals.
Procedures
Breeding and Voluntary Ethanol Consumption During Gestation
All breeding procedures were conducted in the University of New Mexico HSC-ARF. Three to four-month-old Long-Evans rat breeders (Harlan Industries, Indianapolis, IN) were single-housed in plastic cages at 22°C and kept on a reverse 12-hour light/dark schedule (lights on from 2100 to 0900 hours) with Purina Breeder Block rat chow and tap water ad libitum. After at least one week of acclimation to the animal facility, all female rats were provided 0.066% saccharin in tap water for four hours each day from 1000 to 1400 hours. On Days 1 and 2, the saccharin water contained 0% ethanol, on Days 3 and 4, saccharin water contained 2.5% ethanol (v/v). On Day 5 and thereafter, saccharin water contained 5% ethanol (v/v). Daily four-hour consumption of ethanol was monitored for at least two weeks and mean daily ethanol consumption was determined for each female breeder. Following two weeks of daily ethanol consumption females that drank less than one standard deviation below the mean of the entire group were removed from the study. The remainder of the females were assigned to either a saccharin control or 5% ethanol drinking group and matched such that the mean pre-pregnancy ethanol consumption by each group was comparable.
Subsequently, females were placed with proven male breeders until pregnant as evidenced by the presence of a vaginal plug. Female rats did not consume ethanol during the breeding procedure. Beginning on Gestational Day 1, rat dams were provided saccharin water containing either 0% or 5% ethanol for four hours a day, beginning precisely at 1000 hours (1 hour following the onset of the dark cycle). The volume of 0% ethanol saccharin water provided to the controls was matched to the mean volume of 5% ethanol in saccharin water consumed by the ethanol-drinking group, which remained relatively consistent at 16mL per four-hour drinking period over multiple breeding rounds. Rat chow was available ad libitum during both the drinking and non-drinking periods. Maternal weight gain during pregnancy and offspring birth-weight did not differ based on prenatal diet (Hamilton et al., 2010a; Savage et al., 2010). Daily four-hour ethanol consumption was recorded for each dam. Ethanol consumption ceased at birth, and all litters were weighed and culled to 10 pups. Offspring were weaned at 24 days of age and transferred from the HSC-ARF to the Psychology ARF where they were pair-housed for the remainder of the study as described in the subjects section. No more than one offspring from a single breeding pair was assigned to any particular experimental condition. In order to estimate maternal BACs produced with this voluntary drinking protocol, maternal serum ethanol levels were determined in 12 rat dams concurrent with the breeding round for offspring used in the present study. Due to potential for stress effects associated with blood collection all measurements were performed in a separate set of dams that were not used to generate experimental offspring. The detailed methods for quantification of blood ethanol concentrations for daily 4-hour consumption are published elsewhere (Hamilton et al., 2010a; Savage et al., 2010).
Golgi-Cox Staining and Analysis
At approximately postnatal day 130, rats that were exposed to ethanol or saccharin were deeply anesthetized with an overdose of sodium pentobarbital and perfused transcardially with 0.9% (wt/vol) saline, resulting in exsanguination. The brains were extracted, weighed, and immersed in Golgi-Cox solution (Glaser and Van der Loos, 1981) for 14 days and subsequently immersed in 30% (wt/vol) sucrose for at least 3 days. The brains were then cut into coronal sections (200µm thick) on a vibrating microtome, mounted on slides, and stained according to the procedures described by Gibb and Kolb (1998).
Medium spiny neurons from the nucleus accumbens (shell and core) and dorsal striatum (dorsolateral and dorsomedial regions) were selected for analysis (see Figure 1). The brain regions of interest were first identified at low power (100X magnification), and neurons were traced at 250X (final magnification) using the camera lucida technique on an Olympus light microscope (Model BX51) equipped with a drawing attachment. Sampling for all regions included sections ranging from 1.0–1.7 mm anterior to Bregma. Selection was limited to neurons that were not obscured by stain precipitate, blood vessels, astrocytes, or other artifacts, and had intact dendritic fields that were well impregnated and visible within a single section. All tracings were performed by experimenters who were blind to prenatal diet conditions. 10 neurons were drawn for each region of interest (5 per hemisphere) for each animal. Outcome measures were averaged for each rat, collapsing across hemispheres.
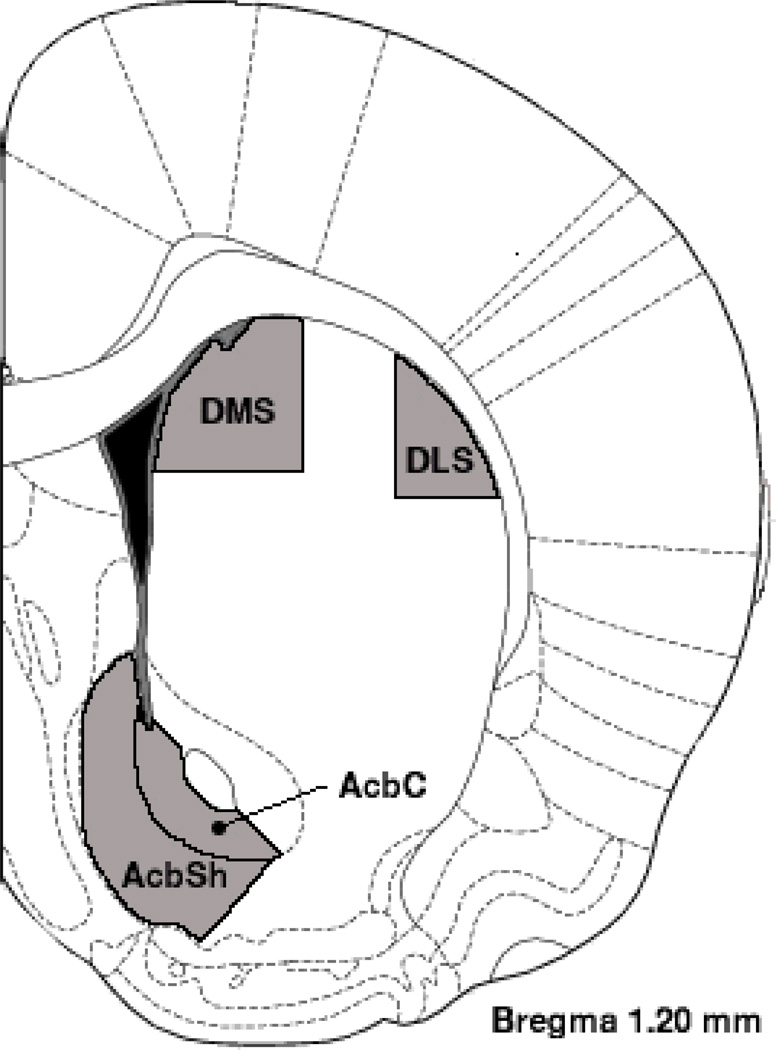
Figure 1.
Coronal diagram (1.20 mm anterior to Bregma) illustrating the four regions from which medium spiny neurons were analyzed: DMS (dorsomedial striatum), DLS (dorsolateral striatum), AcbC (Accumbens Core), and AcbSh (Accumbens Shell). Sampling from AcbSh was primarily limited to ventral and medial aspects and sampling from AcbC was limited to the ventral aspect (ventral to the anterior commissure). Sampling for all regions included sections ranging from 1.0–1.7 mm anterior to Bregma.
Dendritic branching was measured by counting bifurcations for each dendrite (Coleman and Riesen, 1968). Dendritic segments prior to the first bifurcation from the soma were designated as first-order branches and branch order was incremented by 1 for each subsequent bifurcation on a given dendritic branch. The number of first-through sixth-order (and higher) branches were quantified and an estimate of total branches was determined from these values. Dendritic length was measured using a Sholl analysis of ring intersections (Sholl, 1981). A series of concentric rings at 20µm increments (calibrated to the final magnification of 250X) printed on a transparency was centered over the soma. The total number of intersections between each ring and dendritic branches was counted and converted to estimates of dendritic length as a function of distance from the soma (i.e., for each 20µm segment) and overall dendritic length. Spine density was measured by tracing a dendritic terminal tip (>20µm in length) at high power (2000X final magnification), and quantifying the number of single-, double-, triple-, and quadruple-headed spines (per 10µm); Total spine density per 10µm was calculated from these values. Spine density was quantified on five terminal segments for each hemisphere on third-order (or greater) branches.
Results
All analyses were performed using SPSS (version 18 for Macintosh). Analyses of variance (ANOVAs) were conducted for each dependent measure with prenatal diet condition as a single factor unless otherwise noted. A type I error rate of .05 was adopted for all significance tests and estimates of effect size (partial η2) are reported for significant effects. Golgi-Cox staining in one of the brains from the ethanol group was not sufficient for quantification and one rat from the saccharin condition died during the course of the study, and therefore was not processed for Golgi-Cox staining. This resulted in a total of 14 brains, 7 per prenatal diet condition for which analyses were performed.
Voluntary Drinking Paradigm
Effects of daily four-hour ethanol consumption on female dams (weight gain, ethanol consumption, and serum ethanol concentration) and offspring (litter size and pup birth weight) for this group of animals have been published previously (see Hamilton et al., 2010a). Rat dams stably consumed an average of 2.72 ± 0.13 grams of ethanol/kg body weight over the four-hour interval each day (approximately 16 mLs of 5% ethanol in 0.066% saccharin water). This pattern and level of ethanol consumption produced a mean maternal serum ethanol concentration of 84 ± 5.5 mg/dL during the third week of gestation. Ethanol consumption did not affect maternal weight gain during pregnancy, litter size, or offspring birth weight (Hamilton et al., 2010a; Savage et al., 2010).
Brain Weight
Brain weights at the time of extraction were comparable for the two diet conditions [MSaccharin = 2.21g, MEthanol = 2.21g; F(1,12) < 1, p = 0.98].
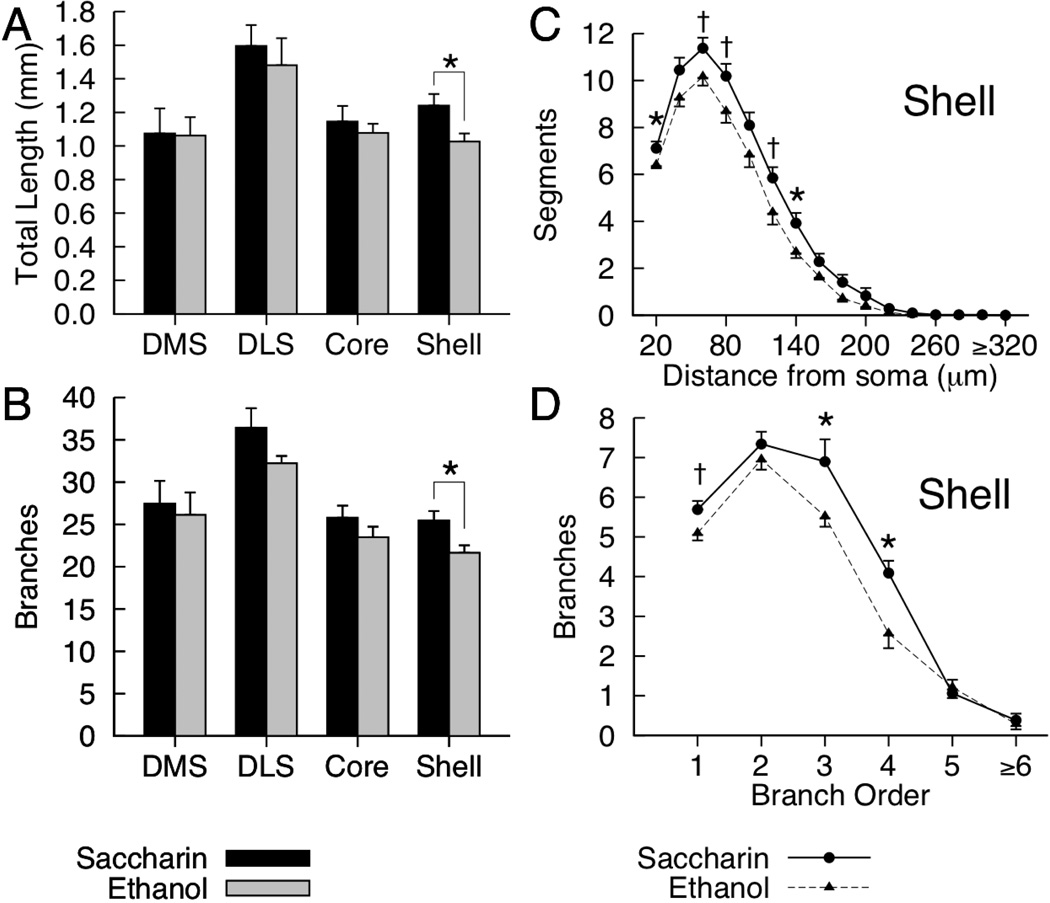
Dendritic Length and Branching
Mean dendritic length and branching for the four regions of interest are presented in Figures 2A and 2B. Significant ethanol-related reductions in overall dendritic length were observed in the nucleus accumbens shell [F(1,12) = 6.34, p = 0.027, partial η2 = .346], however, no differences related to prenatal diet were observed in the core or either region of the dorsal striatum [all ps > .45]. Repeated-measures ANOVAs on the number of dendritic segments as a function of distance from the soma revealed a significant Diet X Distance interaction for the nucleus accumbens shell [F(15, 180) = 2.58, p = 0.002, partial η2 = .177], but not in the other regions [all ps > 0.64]. Although there were greater numbers of dendritic segments in saccharin-exposed rats at all distances from the soma in the shell, the interaction was due to significant ethanol-related reductions in dendritic segments that were observed only for a small subset of distances from the soma (see Figure 2C). Ethanol-exposed rats had significantly fewer dendritic segments 20µm [F(1,12) = 5.10, p = 0.043, partial η2 = .298] and 140µm [F(1,12) = 6.09, p = .03, partial η2 = .337] from the soma. Ethanol-related reductions in dendritic segments 60µm, 80µm, and 120µm from the soma approached significance [ps .052–.06, see Figure 2C; all other ps > .07].
Figure 2.
Mean (+SEM) total dendritic length (A) and total branches (B) for medium spiny neurons in the DMS (dorsomedial striatum), DLS (dorsolateral striatum), nucleus accumbens core and shell of saccharin- and ethanol-exposed rats. For the nucleus accumbens shell, figures C and D represent mean (and SEM) dendritic length as a function of distance from soma (C), and total branches for first through sixth (and greater) branch orders (D) for saccharin- and ethanol-exposed rats. [* p < .05, †p = .05–.06.]
Significant ethanol-related reductions in total dendritic branches were observed in the nucleus accumbens shell [F(1,12) = 7.09, p = .021, partial η2 = .37]. No differences in total branches for saccharin- and ethanol-exposed rats were observed in the core or either region of the dorsal striatum [all ps > .11]. Repeated-measures ANOVAs on the number of branches for each branch order (first through sixth and greater) revealed a significant Diet X Branch Order interaction for the nucleus accumbens shell [F(5, 60) = 3.03, p = .017, partial η2 = .202], but not in the other regions [all ps > .77]. The interaction for the accumbens shell was due to significant ethanol-related reductions in third-[F(1, 12) = 4.93, p = .046, partial η2 = .291] and fourth-order [F(1, 12) = 9.70, p = .009, partial η2 = .447] branches (see Figure 2D) in the absence of significant differences between diet conditions for other orders. There was, however, an ethanol-related reduction in first-order branches that approached significance [p = .06, see Figure 2D; all other ps > .35].
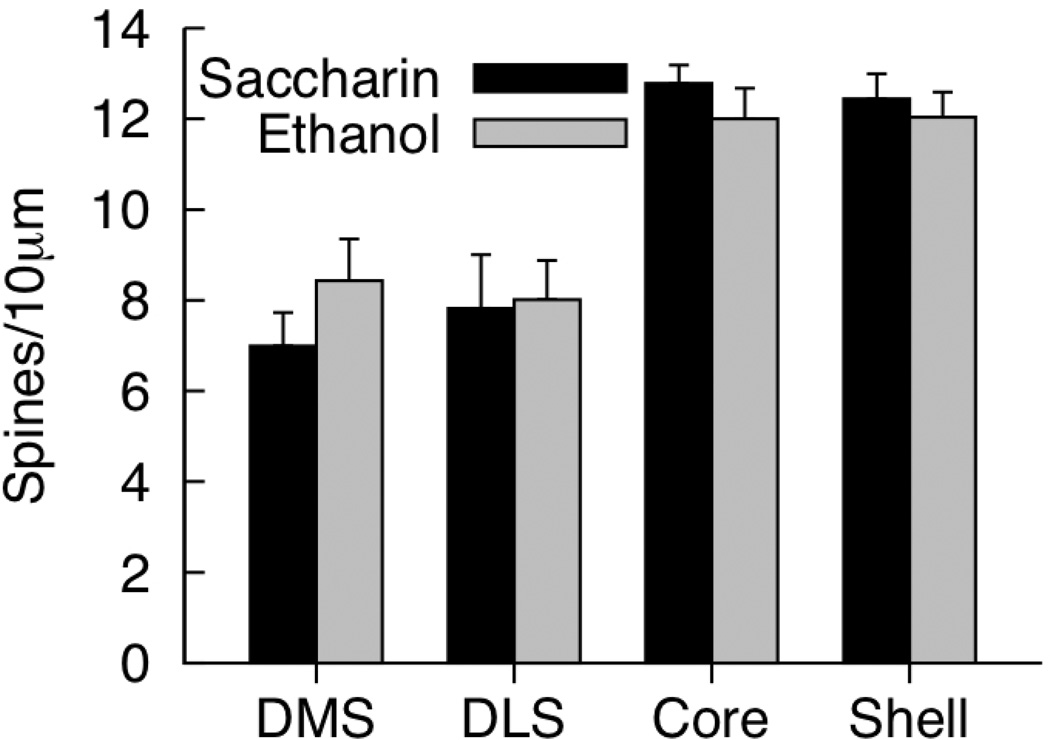
Spine Density
Mean total spine density for the four regions of interest are presented in Figure 3. There were no significant effects of prenatal diet on total spine density for any region of interest [all ps > .25]. Spines with three or greater heads were rarely observed (data not shown). Separate ANOVAs for density of single-, double-, triple-, and quadruple-headed spines in each region failed to detect effects of prenatal diet [data not shown; all ps > .19].
Figure 3.
Mean (+SEM) total density for medium spiny neurons in the DMS (dorsomedial striatum), DLS (dorsolateral striatum), nucleus accumbens core and shell of saccharin- and ethanol-exposed rats. [* p < .05, †p = .05–.06.]
Discussion
Prenatal exposure to moderate levels of ethanol was associated with a robust reduction in dendritic branching and length on medium spiny neurons (MSNs) in the nucleus accumbens (Acb) shell. These observations do not reflect a generalized reduction, as no differences between fetal-ethanol and saccharin rats were observed for measures of dendritic arborization in the other regions of the striatum selected for analysis including the Acb core, dorsolateral striatum (DLS), and dorsomedial striatum (DMS). Although reductions in fetal-ethanol exposed rats for both dendritic length and branching in the DLS approached significance (Figure 2A, 2B), the associated effect sizes were small compared to those noted in the Acb shell. Our analyses indicate that we would have to quadruple our group sizes in order for this effect to reach statistical significance. Dendritic length and branching in the Acb shell were reduced in ethanol-exposed rats by approximately 20% relative to saccharin controls (see Fig. 2A, 2B), which represents the largest morphological/structural alteration we have observed in adult rats exposed to moderate levels of ethanol. More modest fetal-ethanol-related reductions in dendritic arborization have been observed in pyramidal neurons of the agranular insular cortex (Hamilton et al., 2010a, approximately 4–5% reductions relative to saccharin controls), with no major effects of ethanol exposure on overall dendritic arborization of medial prefrontal cortex pyramidal neurons (Hamilton et al., 2010a; Whitcher and Klintsova, 2008). The present results indicate that alterations in mesolimbic dopamine circuitry are important consequences of moderate fetal ethanol exposure that are observable in adult animals long after the period of initial exposure. Fetal ethanol-related alterations within the mesocortical/limbic dopamine system may have important functional consequences related to reward processing, learning, behavioral effects of drug exposure, and drug self-administration and addiction, and may partially explain why prenatal ethanol exposure is associated with increased self-administration of drugs, including ethanol, that persists into adulthood (Barbier et al., 2008, 2009).
Prior work from our laboratory has revealed functional and neurochemical consequences of moderate prenatal ethanol exposure that are considerably more robust than morphological and structural alterations, with no observable effects of moderate fetal ethanol on body weight and whole brain weight (Hamilton et al., 2010a,b). The present observations indicate that changes in dendritic length and branching are important consequences of moderate fetal ethanol exposure, and represent the first clear morphological alteration associated with moderate fetal ethanol exposure we have observed. Identification of the specific functional and physiological consequences of these changes in dendritic morphology will require additional research, however, the specific patterns of alterations observed here suggest several potential targets for directed study. Acb shell MSNs of ethanol-exposed rats had fewer dendritic segments between 20–140µm from the soma and fewer 3rd and 4th order branches than MSNs of saccharin-exposed animals. The reduction in branching and length comparatively further from the soma suggests decreased representation of and responsiveness to distal afferents that may be expected to manifest in overall reductions in activity related to afferent signals. Previous studies have demonstrated reductions in dendritic spine density in the Acb shell in alcohol preferring P rats following adult exposure to chronic intermittent alcohol (Zhou et al., 2007), as well as reductions in spine density of cortical pyramidal neurons following prenatal or early postnatal ethanol exposure in rodents (Cui et al., 2010; Hamilton et al., 2010a; Whitcher and Klintsova, 2008). Ethanol-related decreases in dendritic spine density (single and multi-headed spines) measured at terminal tips were not observed in any of the regions analyzed here, however, the reduction in dendritic length indicates an overall reduction the total number of spines on Acb shell MSNs of ethanol-exposed animals. Dendritic spines on MSNs are the primary loci of glutamatergic (Kemp and Powell, 1971) and dopaminergic (Freund et al., 1984) synapses in the striatum, with cholinergic synapses from local interneurons primarily occupying sites on the soma and dendritic shafts (Izzo and Bolam, 1988). In other brain regions, each receptor system has been shown to be altered in ethanol-exposed animals (Allan et al., 1997, 1998; Kelly and Dillingham, 1994; Savage et al., 1998; Zhou et al., 1991), and in relation to self-administration of ethanol and other drugs (Besheer et al., 2010; Oleson et al., 2011; Tuesta et al., 2011). At present, it is not clear how alterations in these receptor systems might be related to changes in dendritic morphology in the Acb. Given the overall reduction in synaptic space on Acb shell MSNs, alterations in the overall number of each receptor type and associated physiological consequences may be expected, however, at present the effects of moderate prenatal ethanol exposure on the number, density, and function of these receptors in the Acb shell is not known.
Recent theories have posited that the Acb shell serves as the initial point of reward-guided learning in the striatum (Yin et al., 2008). That is, activity in the Acb shell could alter processing downstream in the Acb core, DMS, and DLS. Alterations in these spiraling networks within the striatum could have effects on reward processing as well as addictive behavior. Given that alterations in the Acb shell have been found to influence ethanol consumption (Engleman et al., 2009; Rewal et al., 2009; Zhou et al., 2007), altered reward-guided learning, especially related to drugs of abuse, might be a result of moderate fetal ethanol exposure. The Acb shell has also been shown to be critical for the processing of contextual information related to ethanol consumption in rats (Chaudhri et al., 2010). Dysfunctions in contextual processing could result in disruptions in learning about new information in the striatum, including impairments in extinction and increased context-induced craving. These deficits in the Acb shell could also increase the rate of learning in the DMS and DLS, which are suggested to be involved in response-outcome (R-O) and stimulus-response (S-R) learning, respectively (Yin et al., 2008). One consequence of such a scenario is that habit formation in the DLS could be accelerated (Yin & Knowlton, 2006), resulting in compulsive drug seeking behavior at an earlier stage than would be expected in normal animals.
The possible relationships between alterations in dendritic morphology and behavioral and cognitive deficits observed in fetal ethanol-exposed animals is an important topic for future investigation. Of particular interest for future work are behavioral consequences of fetal ethanol exposure that can be linked directly to the Acb shell. Among these are increases in self-administration of alcohol and other drugs, and reward-related behavior in general. The Acb shell plays a key role in gating feeding behavior (Krause et al., 2010) and motivation and addiction related to rewarding stimuli, particularly with respect to alcohol and other drugs (Chaudhri et al., 2010). Several studies have found that rats exposed to ethanol either in utero or in the early postnatal period more readily consume ethanol or stimulant drugs than controls (Barbier et al., 2008, 2009). The results reported here suggest one possible neural mechanism for increased proclivity for responding to rewarding stimuli, and investigation of drinking behavior in rats exposed to moderate levels of ethanol and the relationship to MSNs in the striatum is currently being pursued in our laboratory. Future studies should also consider other stimulant drugs and effects in other brain reward circuits including the Medial Forebrain Bundle of the thalamus (Hernandez et al., 2006) in order to determine the specificity of this potential alteration in reward processing. Another issue to be addressed in future studies is the developmental trajectory of this effect in the Acb shell. Although the results reported here support the idea that there may be critical periods for development in reward circuitry, other studies have shown that exposure to ethanol in adolescence can alter drug-related behavior in adulthood (Barbier et al., 2008), and can also alter ventral tegmental area (VTA) synaptic plasticity in mature rats (Bernier et al., 2011). The VTA is the point of origin of the mesolimbic dopamine reward system that terminates in the Acb (Hyman et al., 2006). Thus it would seem that brain reward systems can be modulated by exposure to drugs at any time, however, these effects might be amplified during development in utero. Another area of focus for future research involves recording the behavior of Acb neurons in vivo. Overall reductions in spontaneous and evoked activity in Acb neurons of fetal ethanol-exposed animals would be expected based on the results reported here, and specific changes in neuronal firing, particularly with respect to reward processing and self-administration behavior, would be of primary importance for better understanding the relationship between Acb function and behavioral processes that engage this circuitry.
Another important issue for future work concerns possible reduced experience-dependent structural plasticity in striatal neurons of ethanol-exposed animals. Both heavy and moderate ethanol exposure during brain development have been linked to reductions in normal experience-dependent structural changes in dendrites and spines related to various forms of enrichment (Hannigan, 1996; Hamilton et al., 2010a; Whitcher and Klintsova, 2008). These studies have focused on hippocampus and neocortical regions, however, experience-dependent changes in Acb MSNs have been extensively investigated in other domains. For example, enriched housing and exposure to stimulants have repeatedly been linked with increased dendritic arborization and spine density of Acb shell MSNs (Brown et al., 2001; Hamilton and Kolb, 2005; Kolb et al., 2003; Nimchinsky et al., 2002; Robinson and Kolb, 1999), which is in the opposite direction of the fetal-ethanol-related described here. It is tempting to speculate that the effects of fetal ethanol exposure on Acb MSNs may have persistent consequences on responses to drugs and other forms of experience, although this will require additional research. As noted, many forms of experience increase Acb MSN dednritic arborization, thus, it may be possible to enhance dendritic arborization in fetal-ethanol-exposed rats through enriched housing or exposure to stimulants, however, prior research has failed to observe normal experience-dependent increases in dendritic morphology in other regions following pre- or early postnatal ethanol exposure (Berman et al., 1996; Hamilton et al., 2010a). Presumably, a reduced capacity for experience-dependent structural plasticity in fetal-ethanol-exposed rats would result in a more dramatic difference in Acb MSN morphology between controls and fetal ethanol rats, with enriched rearing and chronic stimulant exposure representing what are,perhaps, the most obvious candidates for illustrating this possibility.
Several aspects of the design of the current study place some limitations on interpretation and generalization of the results and indicate several parametric and methodological factors that should be further investigated in future work. The present study only evaluated dendritic morphology in adult rats exposed to ethanol during prenatal brain development. It is tempting to conclude that the observed reductions in Acb shell branching and length represent persistent consequences of ethanol exposure, however, it is possible that these effects only emerged later in life and were not present throughout development. Investigation of MSN morphology throughout development would address this issue. The present study was also limited to analysis of males. This decision was based on the fact that our prior observations of fetal ethanol-related alterations in frontal cortical neurons were observed in males, but not females. Future work should address whether there are sex differences in fetal ethanol-related effects on MSN morphology, and whether any such differences can be further distinguished based on developmental trajectory, estrous, and relationship to behavior. The ethanol exposure paradigm employed here was limited to a chronic intermittent exposure protocol using a limited range of blood ethanol concentrations and one protocol for the timing of ethanol exposure.Zhou et al. (2007) demonstrated that ethanol-exposure in adult P rats that have been bred to voluntarily consume higher doses of ethanol differentially altered Acb shell or core MSNs based on whether the exposure was continuous or included intermittent periods of ethanol deprivation. Whether continuous or intermittent prenatal ethanol exposure is an important determinant of the pattern of fetal ethanol-related effects on MSN dendritic morphology in Acb is currently not known and should be examined in future investigations. Clearly, ethanol dose and the precise timing of ethanol exposure have been identified as important factors in determining the nature and magnitude of the consequences of ethanol exposure (Goodlett and Horn, 2001; Goodlett et al., 2005; Livy et al., 2003; Mooney and Napper, 2005; Mooney and Varlinskaya, 2011). The present study employed a limited range of ethanol doses with mean dam BACs tightly distributed around 84 mg% and with ethanol exposure occurring throughout gestation and terminated at birth. Future work should systematically investigate the role of dose and timing factors on MSN dendritic morphology and related effects on physiology, neurochemistry and behavior.
In summary, the present findings demonstrate a significant reduction in dendritic branching and length on the principal neurons of the Acb shell of adult rats exposed to moderate levels of ethanol in utero. These effects were not observed in other regions of the striatum, including the Acb core, DLS, or DMS. Given the involvement of the Acb shell in drug self-administration and processing or drug-related stimuli, these observations suggest that exposure to ethanol early in development can have a profound and long-lasting effects on brain areas related to reward and addiction, and identify the Acb shell as an important region of interest for future studies aimed at better understanding the neurobiological bases of fetal-ethanol-related deficits in behavior and cognition.
Supplementary Material
Acknowledgments
Research support provided by NIH grant AA015356 to D.A.H., AA017068 to D.D.S, and the Quad-L foundation. M.O.P. supported by BBSRC (UK) International Scientific Interchange Scheme.
Footnotes
This housing condition represents a control condition from a larger study that evaluated the influence of repeated exposure to novel cage-mates on the morphology of frontal cortex neurons Hamilton et al. (2010a).
References
- Abel EL. An update on incidence of FAS: FAS is not an equal opportunity birth defect. Neurotoxicol. Teratol. 1995;17:437–443. doi: 10.1016/0892-0362(95)00005-c. [DOI] [PubMed] [Google Scholar]
- Abel EL, Berman RF. Long-term behavioral effects of prenatal alcohol exposure in rats. Neurotoxicol. Teratol. 1994;16:467–470. doi: 10.1016/0892-0362(94)90124-4. [DOI] [PubMed] [Google Scholar]
- Allan A, Wu H, Paxton L, Savage D. Prenatal ethanol exposure alters the modulation of the gamma-aminobutyric acid(A) receptor-gated chloride ion channel in adult rat offspring. J. Pharmacol. Exp. Ther. 1998;284:250–257. [PubMed] [Google Scholar]
- Allan AM, Weeber EJ, Savage DD, Caldwell KK. Effects of prenatal ethanol exposure on phospholipase C-beta 1 and phospholipase A2 in hippocampus and medial frontal cortex of adult rat offspring. Alcohol. Clin. Exp. Res. 1997;21:1534–1541. [PubMed] [Google Scholar]
- Barbier E, Houchi H, Warnault V, Pierrefiche O, Daoust M, Naassila M. Effects of prenatal and postnatal maternal ethanol on offspring response to alcohol and psychostimulants in long evans rats. Neurosci. 2009;161:427–440. doi: 10.1016/j.neuroscience.2009.03.076. [DOI] [PubMed] [Google Scholar]
- Barbier E, Pierrefiche O, Vaudry D, Vaudry H, Daoust M, Naassila M. Long-term alterations in vulnerability to addiction to drugs of abuse and in brain gene expression after early life ethanol exposure. Neuropharmacol. 2008;55:1199–1211. doi: 10.1016/j.neuropharm.2008.07.030. [DOI] [PubMed] [Google Scholar]
- Berman RF, Hannigan JH, Sperry MA, Zajac CS. Prenatal alcohol exposure and the effects of environmental enrichment on hippocampal dendritic spine density. Alcohol. 1996;13:209–216. doi: 10.1016/0741-8329(95)02049-7. [DOI] [PubMed] [Google Scholar]
- Bernier BE, Whitaker LR, Morikawa H. Previous ethanol experience enhances synaptic plasticity of NMDA receptors in the ventral tegmental area. J. Neurosci. 2011;31:5205–5212. doi: 10.1523/JNEUROSCI.5282-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besheer J, Grondin JJ, Cannady R, Sharko AC, Faccidomo S, Hodge CW. Metabotropic glutamate receptor 5 activity in the nucleus accumbens is required for the maintenance of ethanol self-administration in a rat genetic model of high alcohol intake. Biol. Psychiatry. 2010;67:812–822. doi: 10.1016/j.biopsych.2009.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RW, Gonzalez CLR, Whishaw IQ, Kolb B. Nicotine improvement of Morris water task performance after fimbria-fornix lesion is blocked by mecamylamine. Behav. Brain Res. 2001;119:185–192. doi: 10.1016/s0166-4328(00)00355-7. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Report. Frequent alcohol consumption among women of childbearing age - behavioral risk factor surveillance system. JAMA. 1994;271:1820–1821. [PubMed] [Google Scholar]
- Chaudhri N, Sahuque LL, Schairer WW, Janak PH. Separable roles of the nucleus accumbens core and shell in context- and cue-induced alcohol-seeking. Neuropsychopharmacol. 2010;35:783–791. doi: 10.1038/npp.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarren SK, Smith DW. The fetal alcohol syndrome. N. Engl. J. Med. 1978;298:1063–1067. doi: 10.1056/NEJM197805112981906. [DOI] [PubMed] [Google Scholar]
- Coleman PD, Riesen AH. Evironmental effects on cortical dendritic fieldsIRearing in the dark. J Anat. 1968;102:363–374. [PMC free article] [PubMed] [Google Scholar]
- Conry J. Neuropsychological deficits in Fetal Alcohol Syndrome and fetal alcohol effects. Alcohol. Clin. Exp. Res. 1990;14:650–655. doi: 10.1111/j.1530-0277.1990.tb01222.x. [DOI] [PubMed] [Google Scholar]
- Cui Z-J, Zhao K-B, Zhao H-J, Yu D-M, Niu Y-L, Zhang J-S, Deng J-B. Prenatal Alcohol Exposure Induces Long-Term Changes in Dendritic Spines and Synapses in the Mouse Visual Cortex. Alcohol Alcohol. 2010;45:312–319. doi: 10.1093/alcalc/agq036. [DOI] [PubMed] [Google Scholar]
- Day NL, Cottreau CM, Richardson GA. The epidemiology of alcohol, marijuana, and cocaine use among women of childbearing age and pregnant women. Clin Obstet Gynecol. 1993;36:232–245. doi: 10.1097/00003081-199306000-00005. [DOI] [PubMed] [Google Scholar]
- Engleman EA, Ding ZM, Oster SM, Toalston JE, Bell RL, Murphy JM, McBride WJ, Rodd ZA. Ethanol is self-administered into the nucleus accumbens shell, but not the core: evidence of genetic sensitivity. Alcohol. Clin. Exp. Res. 2009;33:2162–2171. doi: 10.1111/j.1530-0277.2009.01055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, Robbins TW. Review. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos. Trans. R. Soc. Lond. B, Biol. Sci. 2008;363:3125–3135. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF, Powell JF, Smith AD. Tyrosine hydroxylase-immunoreactive boutons in synaptic contact with identified striatonigral neurons, with particular reference to dendritic spines. Neuroscience. 1984;13:1189–1215. doi: 10.1016/0306-4522(84)90294-x. [DOI] [PubMed] [Google Scholar]
- Galindo R, Frausto S, Wolff C, Caldwell KK, Perrone-Bizzozero NI, Savage DD. Prenatal ethanol exposure reduces mGluR5 receptor number and function in the dentate gyrus of adult offspring. Alcohol. Clin. Exp. Res. 2004;28:1587–1597. doi: 10.1097/01.alc.0000141815.21602.82. [DOI] [PubMed] [Google Scholar]
- Gibb R, Kolb B. A method for vibratome sectioning of Golgi-Cox stained whole rat brain. J Neurosci Methods. 1998;79:1–4. doi: 10.1016/s0165-0270(97)00163-5. [DOI] [PubMed] [Google Scholar]
- Glaser EM, Van der Loos H. Analysis of thick brain sections by obverse-reverse computer microscopy: application of a new, high clarity Golgi-Nissl stain. J Neurosci Methods. 1981;4:117–125. doi: 10.1016/0165-0270(81)90045-5. [DOI] [PubMed] [Google Scholar]
- Goodlett CR, Horn KH. Mechanisms of alcohol-induced damage to the developing nervous system. Alcohol Res Health. 2001;25:175–184. [PMC free article] [PubMed] [Google Scholar]
- Goodlett CR, Horn KH, Zhou FC. Alcohol teratogenesis: mechanisms of damage and strategies for intervention. Exp. Biol. Med. (Maywood) 2005;230:394–406. doi: 10.1177/15353702-0323006-07. [DOI] [PubMed] [Google Scholar]
- Hamilton DA, Akers KG, Rice JP, Johnson T, Candelaria-Cook FT, Maes LI, Rosenberg M, Valenzuela CF, Savage DD. Prenatal exposure to moderate levels of ethanol alters social behavior in adult rats: Relationship to structural plasticity and immediate early gene expression in frontal cortex. Behav. Brain Res. 2010a;207:290–304. doi: 10.1016/j.bbr.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton DA, Candelaria-Cook FT, Akers KG, Rice JP, Maes LI, Rosenberg M, Valenzuela CF, Savage DD. Patterns of social-experience-related c-fos and Arc expression in the frontal cortices of rats exposed to saccharin or moderate levels of ethanol during prenatal brain development. Behav. Brain Res. 2010b;214:66–74. doi: 10.1016/j.bbr.2010.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton DA, Kolb B. Differential effects of nicotine and complex housing on subsequent experience-dependent structural plasticity in the nucleus accumbens. Behav. Neurosci. 2005;119:355–365. doi: 10.1037/0735-7044.119.2.355. [DOI] [PubMed] [Google Scholar]
- Hannigan JH. What research with animals is telling us about alcohol-related neurodevelopmental disorder. Pharmacol. Biochem. Behav. 1996;55:489–499. doi: 10.1016/s0091-3057(96)00251-1. [DOI] [PubMed] [Google Scholar]
- Hannigan JH, Pilati ML. The effects of chronic postweaning amphetamine on rats exposed to alcohol in utero: Weight gain and behavior. Neurotoxicol. Teratol. 1991;13:649–656. doi: 10.1016/0892-0362(91)90049-3. [DOI] [PubMed] [Google Scholar]
- Hernandez G, Hamdani S, Rajabi H, Conover K, Stewart J, Arvanitogiannis A, Shizgal P. Prolonged rewarding stimulation of the rat medial forebrain bundle: neurochemical and behavioral consequences. Behav. Neurosci. 2006;120:888–904. doi: 10.1037/0735-7044.120.4.888. [DOI] [PubMed] [Google Scholar]
- Hoyme H, May P, Kalberg W, Kodituwakku P, Gossage J, Trujillo P, Buckley D, Miller J, Aragon A, Khaole N, Viljoen D, Jones K, Robinson L. A practical clinical approach to diagnosis of fetal alcohol spectrum disorders: Clarification of the 1996 Institute of Medicine Criteria. Pediatrics. 2005;115:39–47. doi: 10.1542/peds.2004-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison KE, Ray L, Sandman E, Rutter MC, Peters A, Davidson D, Swift R. The effect of olanzapine on craving and alcohol consumption. Neuropsychopharmacol. 2006;31:1310–1317. doi: 10.1038/sj.npp.1300917. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu. Rev. Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Izzo PN, Bolam JP. Cholinergic synaptic input to different parts of spiny striatonigral neurons in the rat. J. Comp. Neurol. 1988;269:219–234. doi: 10.1002/cne.902690207. [DOI] [PubMed] [Google Scholar]
- Jones KL, Smith DW. Recognition of the Fetal Alcohol Syndrome in early infancy. Lancet. 1973;302:999–1001. doi: 10.1016/s0140-6736(73)91092-1. [DOI] [PubMed] [Google Scholar]
- Jones KL, Smith DW. The Fetal Alcohol Syndrome. Teratol. 1975;12:1–10. doi: 10.1002/tera.1420120102. [DOI] [PubMed] [Google Scholar]
- Jones KL, Smith DW, Ulleland CN, Streissguth P. Pattern of malformation in offspring of chronic alcoholic mothers. Lancet. 1973;1:1267–1271. doi: 10.1016/s0140-6736(73)91291-9. [DOI] [PubMed] [Google Scholar]
- Kelly SJ, Day N, Streissguth AP. Effects of prenatal alcohol exposure on social behavior in humans and other species. Neurotoxicol Teratol. 2000;22:143–149. doi: 10.1016/s0892-0362(99)00073-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly SJ, Dillingham RR. Sexually dimorphic effects of perinatal alcohol exposure on social interactions and amygdala DNA and DOPAC concentrations. Neurotoxicol Teratol. 1994;16:377–384. doi: 10.1016/0892-0362(94)90026-4. [DOI] [PubMed] [Google Scholar]
- Kemp JM, Powell TP. The site of termination of afferent fibres in the caudate nucleus. Philos. Trans R. Soc. Lond. B, Biol. Sci. 1971;262:413–427. doi: 10.1098/rstb.1971.0104. [DOI] [PubMed] [Google Scholar]
- Kodituwakku PW. Defining the behavioral phenotype in children with fetal alcohol spectrum disorders: A review. Neurosci. Biobehav. Rev. 2007;31:192–201. doi: 10.1016/j.neubiorev.2006.06.020. [DOI] [PubMed] [Google Scholar]
- Kolb B, Gorny G, Li Y, Samaha AN, Robinson TE. Amphetamine or cocaine limits the ability of later experience to promote structural plasticity in the neocortex and nucleus accumbens. Proc. Natl. Acad. SciU.SA. 2003;100:10523–10528. doi: 10.1073/pnas.1834271100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause M, German PW, Taha SA, Fields HL. A pause in nucleus accumbens neuron firing is required to initiate and maintain feeding. J. Neurosci. 2010;30:4746–4756. doi: 10.1523/JNEUROSCI.0197-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livy DJ, Miller EK, Maier SE, West JR. Fetal alcohol exposure and temporal vulnerability: effects of binge-like alcohol exposure on the developing rat hippocampus. Neurotoxicol Teratol. 2003;25:447–458. doi: 10.1016/s0892-0362(03)00030-8. [DOI] [PubMed] [Google Scholar]
- Mooney SM, Napper RM. Early postnatal exposure to alcohol reduces the number of neurons in the occipital but not the parietal cortex of the rat. Alcohol. Clin. Exp. Res. 2005;29:683–691. doi: 10.1097/01.alc.0000158936.40150.5a. [DOI] [PubMed] [Google Scholar]
- Mooney SM, Varlinskaya EI. Acute prenatal exposure to ethanol and social behavior: effects of age, sex, and timing of exposure. Behav. Brain Res. 2011;216:358–364. doi: 10.1016/j.bbr.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimchinsky EA, Sabatini BL, Svoboda K. Structure and function of dendritic spines. Ann. Rev. Physiol. 2002;64:313–353. doi: 10.1146/annurev.physiol.64.081501.160008. [DOI] [PubMed] [Google Scholar]
- Oleson EB, Richardson JM, Roberts DC. A novel IV cocaine self-administration procedure in rats: differential effects of dopamine, serotonin, and GABA drug pre-treatments on cocaine consumption and maximal price paid. Psychopharmacology (Berl.) 2011;214:567–577. doi: 10.1007/s00213-010-2058-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rewal M, Jurd R, Gill TM, He DY, Ron D, Janak PH. Alpha4-containing GABAA receptors in the nucleus accumbens mediate moderate intake of alcohol. J. Neurosci. 2009;29:543–549. doi: 10.1523/JNEUROSCI.3199-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Alterations in the morphology of dendrites and dendritic spines in the nucleus accumbens and prefrontal cortex following repeated treatment with amphetamine or cocaine. Eur. J. Neurosci. 1999;11:1598–1604. doi: 10.1046/j.1460-9568.1999.00576.x. [DOI] [PubMed] [Google Scholar]
- Savage D, Cruz L, Duran L, Paxton L. Prenatal ethanol exposure diminishes activity-dependent potentiation of amino acid neurotransmitter release in adult rat offspring. Alcohol. Clin. Exp. Res. 1998;22:1771–1777. [PubMed] [Google Scholar]
- Savage DD, Montano CY, Otero MA, Paxton LL. Prenatal ethanol exposure decreases hippocampal NMDA-sensitive [3H]-glutamate binding site density in 45-day-old rats. Alcohol. 1991;8:193–201. doi: 10.1016/0741-8329(91)90806-8. [DOI] [PubMed] [Google Scholar]
- Savage DD, Rosenberg MJ, Wolff CR, Akers KG, El-Emawy A, Staples MC, Varaschin RK, Wright CA, Seidel JL, Caldwell KK, Hamilton DA. Effects of a novel cognition-enhancing agent on fetal ethanol-induced learning deficits. Alcohol. Clin. Exp. Res. 2010;34:1793–1802. doi: 10.1111/j.1530-0277.2010.01266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Roesch MR, Stalnaker TA. Orbitofrontal cortex, decision-making and drug addiction. Trends Neurosci. 2006;29:116–124. doi: 10.1016/j.tins.2005.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sholl DA. The organization of the cerebral cortex. Methuen, London: 1981. [Google Scholar]
- Streissguth AP, Aase JM, Clarren SK, Randels SP, LaDue RA, Smith DF. Fetal alcohol syndrome in adolescents and adults. JAMA. 1991;265:1961–1967. [PubMed] [Google Scholar]
- Streissguth AP, Barr HM, Sampson PD. Moderate prenatal alcohol exposure: effects on child IQ and learning problems at age 7 1/2 years. Alcohol. Clin. Exp. Res. 1990;14:662–669. doi: 10.1111/j.1530-0277.1990.tb01224.x. [DOI] [PubMed] [Google Scholar]
- Sutherland RJ, McDonald RJ, Savage DD. Prenatal exposure to moderate levels of ethanol can have long-lasting effects on hippocampal synaptic plasticity in adult offspring. Hippocampus. 1997;7:232–238. doi: 10.1002/(SICI)1098-1063(1997)7:2<232::AID-HIPO9>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Sutherland RJ, McDonald RJ, Savage DD. Prenatal exposure to moderate levels of ethanol can have long-lasting effects on learning and memory in adult offspring. Psychobiol. 2000;28:532–539. doi: 10.1002/(SICI)1098-1063(1997)7:2<232::AID-HIPO9>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Thomas JD, Abou EJ, Dominguez HD. Prenatal choline supplementation mitigates the adverse effects of prenatal alcohol exposure on development in rats. Neurotoxicol Teratol. 2009;31:303–311. doi: 10.1016/j.ntt.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuesta L, Fowler CD, Kenny PJ. Recent advances in understanding nicotinic receptor signaling mechanisms that regulate drug self-administration behavior. Biochem Pharmacol. 2011:1. doi: 10.1016/j.bcp.2011.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varaschin RK, Rosenberg MJ, Akers KG, Hamilton DA, Savage DD. Effects of the Cognition-Enhancing Agent ABT-239 on Fetal Ethanol-induced Deficits in Dentate Gyrus Synaptic Plasticity. J. Pharmacol. Exp. Ther. 2010;334:191–198. doi: 10.1124/jpet.109.165027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter CS, Doremus-Fitzwater TL, Spear LP. Time course of elevated ethanol intake in adolescent relative to adult rats under continuous, voluntary-access conditions. Alcohol. Clin. Exp. Res. 2007;31:1159–1168. doi: 10.1111/j.1530-0277.2007.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voorn P, Vanderschuren LJ, Groenewegen HJ, Robbins TW, Pennartz CM. Putting a spin on the dorsal-ventral divide of the striatum. Trends Neurosci. 2004;27:468–474. doi: 10.1016/j.tins.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Whitcher LT, Klintsova AY. Postnatal binge-like alcohol exposure reduces spine density without affecting dendritic morphology in rat mPFC. Synapse. 2008;62:566–573. doi: 10.1002/syn.20532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nat. Rev. Neurosci. 2006;7:464–476. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]
- Yin HH, Park BS, Adermark L, Lovinger DM. Ethanol reverses the direction of long-term synaptic plasticity in the dorsomedial striatum. Eur. J. Neurosci. 2007;25:3226–3232. doi: 10.1111/j.1460-9568.2007.05606.x. [DOI] [PubMed] [Google Scholar]
- Zhou FC, Anthony B, Dunn KW, Lindquist WB, Xu ZC, Deng P. Chronic alcohol drinking alters neuronal dendritic spines in the brain reward center nucleus accumbens. Brain Res. 2007;1134:148–161. doi: 10.1016/j.brainres.2006.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou FC, Bledsoe S, Lumeng L, Li TK. Immunostained serotonergic fibers are decreased in selected brain regions of alcohol-preferring rats. Alcohol. 1991;8:425–431. doi: 10.1016/s0741-8329(91)90034-t. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.