Abstract
Oxidative stress may play a role in the pathogenesis of depression. We tested the hypothesis that urinary F2 isoprostane, a robust marker of oxidative stress, was increased in patients with depression and associated with symptoms and response to treatment. Urinary F2 isoprostane was compared in 18 patients with depression and 36 age and sex matched control subjects. In patients, we tested the association between oxidative stress, depression questionnaires and antidepressant treatment. Urinary F2 isoprostane excretion was significantly higher in patients with depression than in control subjects. This association remained significant after adjustment for age, sex and BMI. Depression symptom severity scores were not correlated with F2 isoprostane excretion. Nine patients were treated with sertraline or bupropion for eight weeks. Depression severity rating scale scores decreased significantly and F2 isoprostane excretion increased. The increase in F2 isoprostane excretion was inversely correlated with the improvement in Hamilton Depression Rating 17 items. In conclusion, oxidative stress is increased in patients with depression. However, although treatment with either bupropion or sertraline reduces the symptoms of depression, it may increase F2 isoprostane excretion. These results suggest that alternative mechanisms, beyond oxidative stress, may be involved in the development of depression and subsequent responses to treatment.
Keywords: depression, oxidative stress, F2 isoprostanes, antidepressants, sertraline, bupropion
1. Introduction
Depression is a heterogeneous clinical disorder and its cause is not clear. Several mechanisms have been implicated in its pathogenesis. These include genetic predisposition, deficiency of monoamines, abnormalities in circadian rhythm, hypercortisolemia, and increased inflammatory cytokines.(Belmaker and Agam, 2008) In addition, oxidative stress has been postulated to have an important role in the pathogenesis of depression.(Ng et al. 2008)
Oxidative stress is the imbalance between oxidant and antioxidants in favor of the oxidants, leading to a disruption of the redox signaling and control and/or molecular damage.(Sies and Jones D.P. 2007) There are several clinical conditions associated with increased oxidative stress, and different methods to assess it. The discovery of F2 isoprostanes, products derived from lipid peroxidation, and the development of methods to quantify them accurately, have provided a robust marker of oxidative stress in vivo. (Morrow et al. 1999;Lawson et al. 1999;Cracowski et al. 2002; Nourooz-Zadeh et al. 2008)
Previous studies have linked increased measures of oxidative stress with affective disorders. Ozcan et al used superoxide dismutase (SOD) glutathione peroxidase (GSH-Px) and catalase (CAT) activities and malondialdehyde (MDA) and nitric oxide (NO) levels as measures of oxidative stress. Because enzymatic function was decreased and MDA levels increased in patients with affective disorders, the authors concluded that the antioxidant system was impaired during mood episodes. (Ozcan et al. 2004) Others have used different techniques, such lower total oxidant capacity and higher total oxidant status in patients with major depression.(Cumurcu et al. 2009) Furthermore, using an oxidative stress index, defined as the percent ratio of total peroxide plasma concentration to the total antioxidant potential, investigators have found an association between oxidative stress with higher Hamilton Depression Rating Scales. (Yanik et al. 2004) However, many of the techniques lack sensitivity and specificity to measure oxidative stress in vivo. Thus, we measured F2-isoprostanes, a technique recognized by experts as the most accurate method to evaluate oxidative stress. (Milne et al. 2007)
Despite the therapeutic effects of antidepressants, there are conflicting data regarding antidepressants and oxidative stress. Recent reports suggest that antidepressants may have antioxidant properties. For example, venlafaxine treatment produced anti-anxiety effects and attenuated oxidative damage (Kumar et al. 2010) and fluoxetine acted as an antioxidant in melanoma-induced oxidative changes. (Kirkova et al. 2010) However, because fluoxetine is a fluorinated compound, its hepatotoxicity is thought to be mediated by increased levels of oxidative stress (Inkielewicz-Stepniak ,2011) and pro- oxidant properties, through coenzyme Q deficiency, have been attributed to amitriptyline. (Bautista-Ferrufino et al. 2011)
Thus, we tested the hypothesis that oxidative stress, as determined by F2 isoprostane excretion, was increased in patients with depression and was correlated with severity of symptoms and response to treatment.
2. METHODS
2.1. Study subjects
We studied 18 patients with major depressive episodes (MDE), diagnosed by the Structured Clinical Interview for DSM-IV (First et al. 1996) and confirmed by diagnostic interview with a senior psychiatrist (RMS). Patients were free of psychotropic use for at least 1 month prior to study, and at least 2 months for fluoxetine. Patients were older than 18 years of age and were recruited from newspaper and Vanderbilt community email announcements. Medical status was evaluated by physical examination and a routine laboratory screen, including pregnancy testing and urine drug screen. Any primary non-MDE comorbid diagnosis or substance abuse in the prior six months was cause for exclusion. Those who were currently using tobacco, had any sign of acute or chronic medical or neurological illness, or were pregnant were excluded from the study. Thirty-six non- smoking control subjects were selected by frequency-matching for age and sex from a cohort of control subjects without inflammatory disease or depression. The study was approved by the Institutional Review Board of Vanderbilt University Hospital, and all subjects provided written informed consent.
2.2. F2 isoprostanes
2,3-dinor-5,6-dihydro-15-F2-Isoprostane, a urine metabolite and robust method of oxidative stress, was measured using gas chromatography/mass spectrometry as previously described.(Morrow and Roberts ,1994;Roberts and Morrow ,2000)
2.3. Questionnaires
All patients were interviewed for the Hamilton Depression Rating Scale, which has 17 items (Hamilton ,1967) and is widely used for the assessment of depression severity. Items are rated on a five point scale (0–4) except for items on weight loss, appetite, sleep disturbance, general somatic symptoms, loss of libido and insight that were rated on a three point scale (0–2).(Morriss et al. 2008) Patients were also evaluated with a modified version of the Hamilton Depression Rating Scale that includes 25 items.(Mazure et al. 1986) Higher scores in both instruments indicate higher levels of depression. Patients also completed the Profile of Mood States (POMS) instrument that includes 65 items. Each item is rated on a five-point scale. Scores are reported as global as well as six individual subscales (tension/anxiety, depression/dejection, anger/hostility, vigor, fatigue, and confusion).(Garland et al. 2007)
2.4. Treatment
A subgroup of nine patients with depression completed an open-label, random-assignment, 8 week treatment with either sertraline (n=6, 100 mg/day) or bupropion (n= 3, 300 mg mg/day). This subgroup of patients had a follow-up visit at week that included both Hamilton scores and POMS. Urine samples were collected before and after 8 weeks of therapy, frozen at –40 °C until isoprostane concentrations were measured.
2.5. Statistical analysis
Based on previous data showing that the mean F2 isoprostane excretion in control subjects is 2.2 ng/mg creatinine with a standard deviation (SD) of 1.4,(Avalos et al. 2007) a post hoc calculation determined that a study with 18 patients with depression and 36 control subjects would have 92% power to detect a difference of 1.4 ng/mg creatinine in F2 isoprostane excretion with a two-sided significance level of 5%. Given the variability between individuals in F2 isoprostane excretion, a difference of 1 SD between control subjects and those with depression was considered likely to indicate a biologically important difference.
Baseline characteristics and outcome measures were compared between patients and controls using Wilcoxon rank-sum tests. The relationship between questionnaire scores and F2 isoprostane excretion in patients with depression was evaluated using Spearman's rank correlation test. Pre and post-treatment results were analyzed with the use of Wilcoxon-sign rank test. All analyses used a 5% two sided level of significance and were performed using STATA 10.0 (STATA corp, Texas).
3. RESULTS
3.1. Patients
Patients and control subjects were of similar age (mean age 32.2±10.0 years and 32.3±7.6 years, respectively, p=0.78) and sex (67% female in both groups). Control subjects had higher BMI (27.0±4.8 kg/m2) than depressed patients (25.2±7.3 kg/m2, p=0.05). No patients or control subjects smoked.
3.2. F2 isoprostanes and depression
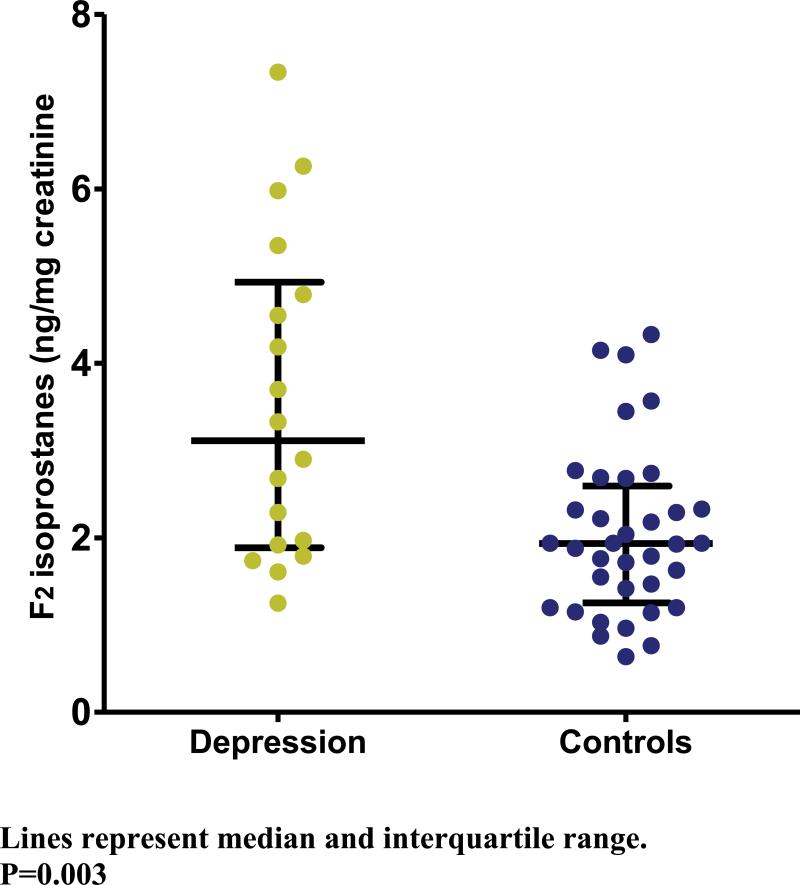
Urinary F2 isoprostane excretion was significantly higher in patients with depression [3.1 (1.9-4.8) ng/mg creatinine] than in control subjects [2.0 (1.3-2.5) ng/mg creatinine] (p=0.003). (Figure 1) A linear regression model with logarithmically transformed F2 isoprostane concentrations as the dependent variable, and depression as independent variable, confirmed this association [Beta coefficient 0.52 (0.23-0.81), p=0.001], and it remained significant after adjustment for age and sex [Beta coefficient 0.52 (0.24-0.80), p<0.001), and after further adjustment for BMI [Beta coefficient 0.47 (0.19-0.75), p=0.001].
Figure 1.
Urinary F2-isoprostane excretion in patients with depression and nes represent median and interquartile range P=0.003.
3.3. F2 isoprostanes and symptoms of depression
At baseline, when depression scores were high, urinary F2 isoprostanes were not significantly correlated with mood or depressive symptoms as reported by the POMS or the Hamilton scores respectively. (Table 1) In the 9 patients treated with antidepressants symptoms of depression and mood improved significantly. The median score for tension/anxiety decreased from 7 to 0, for depression/dejection from 35 to 4, for anger/hostility from 9 to 3, fatigue from 19 to 4, confusion from 10 to 2, and vitality increased from 4 to 8 (all p<0.05). The scores from both versions of the Hamilton also decreased significantly (p=0.01). (Table 2)
Table 1.
Median [IQR] depression scale scores and their correlation with F2 isoprostane excretion in patients with depression at baseline
| Scale | Rho | P-value* |
|---|---|---|
| POMS-T | -0.09 | 0.70 |
| POMS-D | 0.07 | 0.78 |
| POMS-A | -0.02 | 0.95 |
| POMS-V | -0.20 | 0.43 |
| POMS-F | -0.01 | 0.98 |
| POMS-C | 0.09 | 0.73 |
| Hamilton 17 (n=9) | 0.64 | 0.06 |
| Hamilton 25 (n=9) | 0.29 | 0.44 |
Table 2.
Mood and depression scores in nine patients with depression at baseline and after treatment with antidepressants
| Scale | Baseline (n=9) | After Treatment (n=9) | P-value* |
|---|---|---|---|
| POMS-T | 7 (4 - 9) | 0 (-1 – 2) | 0.01 |
| POMS-D | 35 (33 – 17) | 4 (1 - 18) | 0.01 |
| POMS-A | 9 (2 – 16) | 3 (0 – 3) | 0.01 |
| POMS-V | 4 (3 - 5) | 8 (6 – 12) | 0.05 |
| POMS-F | 19 (7 - 22) | 4 (2 - 6) | 0.02 |
| POMS-C | 10 (8 - 12) | 2 (1 – 7) | 0.01 |
| Hamilton 17 | 19 (18 – 23) | 5 (2 – 8) | 0.01 |
| Hamilton 25 | 35 ( 26 – 38) | 7 (5 – 15) | 0.01 |
Wilcoxon signed-rank test
3.4. Use of antidepressants and F2 isoprostanes
F2 isoprostane excretion increased from 2.9 (1.8-4.2) to 4.9 (2.0-6.3) ng/mg creatinine (p=0.04), after treatment with either sertraline or bupropion. This trend was present for both sertraline [F2 isoprostanes increased from 2.8 (1.7-3.3) to 4.3 (2.0-5.5) ng/mg creatinine] and bupropion [F2 isoprostanes increased from 4.2 (1.8-7.3) to 6.3 (1.7-6.7) ng/mg creatinine].
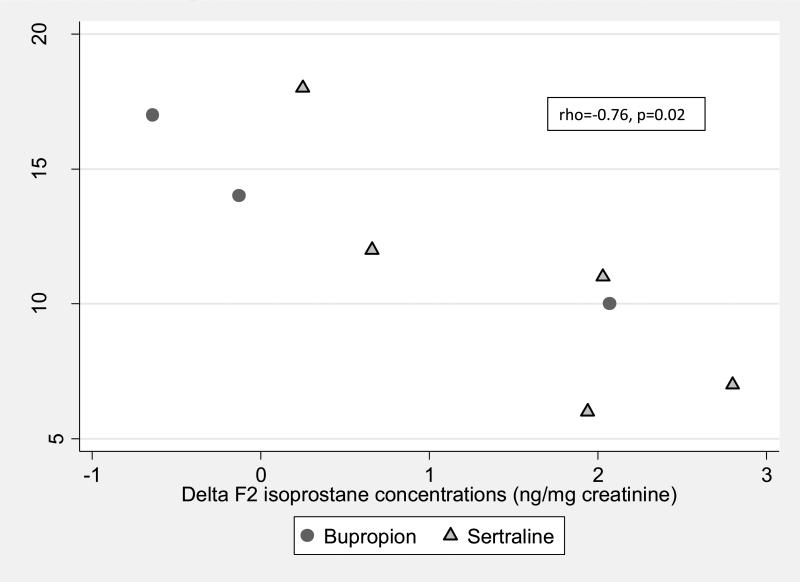
Depression symptom scores and F2 isoprostane excretion after treatment did not correlate (p all >0.15) except for a significant negative association between the POMS vitality score and F2 isoprostane excretion (rho -0.83, p= 0.005), suggesting that patients who were more vital had low levels of oxidative stress. However, the increase in F2 isoprostane excretion and the improvement in the 17 item Hamilton depression score between the baseline and after treatment visit were significantly correlated (rho=-0.76, p=0.02). (Figure 2)
Figure 2.
Correlations between changes in the 17 item Hamilton score and changes in F2isoprostanes after treatment with antidepressants
4. DISCUSSION
The major findings of this study are that (1) oxidative stress as measured by F2 isoprostane excretion is increased in patients with depression compared to age and sex matched control subjects, and (2) F2 isoprostane excretion increases after effective treatment of depression.
Free radicals generated by oxidative stress result in lipid peroxidation and consequently tissue damage. This effect may be even more important in highly vulnerable organs such as the brain. The brain is vulnerable because of its high rate of oxygen use, its modest antioxidant defenses and because it is rich in lipids that provide substrate for lipid oxidation.(Ng et al. 2008)
Concordant with our finding of increased oxidative stress in patients with depression, a few studies have reported that patients with major depression have increased concentrations of malondialdehyde (Bilici et al. 2001;Sarandol et al. 2007) and 4-hydroxy-2-nonenal concentrations.(Selley,2004) However, these methods do not optimally measure the degree of oxidative stress present in vivo.
The discovery of F2 isoprostanes, derived from free radical mediated lipid peroxidation, and the development of sensitive and specific mass spectrometry methods to measure them have provided a robust and sensitive marker of oxidative stress in vivo.(Morrow et al. 1999;Lawson et al. 1999;Cracowski et al. 2002) A recent study demonstrated that patients with depression had higher serum concentrations of F2 isoprostanes.(Yager et al. 2010) However, serum concentrations of F2 isoprostanes may be affected by serum lipid concentrations with uncertainty regarding the need to further adjust for HDL, LDL, arachidonate or total lipid levels.(Halliwell and Lee ,2010) Thus, our study has used state-of-the-art methodology to measure oxidative stress in patients with depression.
In addition to demonstrating increased oxidative stress in patients with depression, our findings suggest that effective treatment with antidepressants is associated with increase rather than decrease oxidative stress. Consistent with our results, a preclinical study suggested an increase in oxidative stress after antidepressant exposure. Using rat glioma and human astrocytoma cell lines, Slamon found decreased concentrations of the antioxidant glutathione following toxic doses of antidepressants (Slamon and Pentreath ,2000) and suggested that oxidative stress may play a role in the cytotoxicity observed. Subsequently, other studies have shown decreases in markers of oxidative stress following antidepressant treatment.(Bilici et al. 2001;Kotan et al. 2011) Further study of dosage and time course effects may be indicated.
Although the number of patients we studied was small, the finding of increased isoprostanes excretion after treatment is interesting, and if confirmed, may suggest that oxidative stress is not causally related to the symptoms of depression, or alternatively, that there is a substantial time lag between symptom improvement and a decrease in oxidative stress.
Another potential explanation for the improvement in depression scores despite an increase in oxidative stress was that effective treatment for depression could result in another change associated with increased oxidative stress. For example, obesity is associated with increased F2 isoprostane excretion, and it is possible that improvement of depression may have improved appetite and resulted in weight gain, although based on clinical observations this would be unlikely to occur over 8 weeks and does not appear to be a plausible explanation.
This study has some limitations. First, the sample size was small; though enough to test our hypothesis that levels of isoprostanes were increased in patients with depression. Second, markers of inflammation and metabolic variables beyond body mass index were not collected; therefore, we could not examine if the association of oxidative stress and depression was independent of inflammation.
Third, while some studies have shown increased markers of oxidative stress in peripheral blood of patients with depression; the association of oxidative stress measured directly from the central nervous system is less clear. With the goal to better understand this, Teyssier et al obtained postmortem prefrontal cortex from patients with depression and control subjects and measured gene expression of stress response, but did not find any associations.(Teyssier et al. 2011) Our study did not aim to look at markers of oxidative stress in tissue.
In conclusion, oxidative stress is increased in patients with depression but may not be causal since treatment with either bupropion or sertraline significantly reduced the symptoms of depression but increased F2 isoprostane excretion.
Acknowledgements
We thank Emily A. Bryant, B.S., M.Sc., for her assistance in collecting and organizing the data.
This work was supported by grants HL05082; K23 MH01828 to RMS; CTSA 1 UL1 RR024975, P60 AR056116 and GM007569 from the National Institutes of Health.
CPC was supported by the Vanderbilt Physician Scientist Development Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- Avalos I, Chung CP, Oeser A, Milne GL, Morrow JD, Gebretsadik T, Shintani A, Yu C, Stein CM. Oxidative stress in systemic lupus erythematosus: relationship to disease activity and symptoms. Lupus. 2007;16:195–200. doi: 10.1177/0961203306075802. [DOI] [PubMed] [Google Scholar]
- Bautista-Ferrufino MR, Cordero MD, Sanchez-Alcazar JA, Illanes M, Fernandez-Rodriguez A, Navas P, de MM. Amitriptyline induces coenzyme Q deficiency and oxidative damage in mouse lung and liver. Toxicology Letters. 2011;204:32–37. doi: 10.1016/j.toxlet.2011.03.033. [DOI] [PubMed] [Google Scholar]
- Belmaker RH, Agam G. Major depressive disorder. NewEngland Journal of Medicine. 2008;358:55–68. doi: 10.1056/NEJMra073096. [DOI] [PubMed] [Google Scholar]
- Bilici M, Efe H, Koroglu MA, Uydu HA, Bekaroglu M, Deger O. Antioxidative enzyme activities and lipid peroxidation in major depression: alterations by antidepressant treatments. Journal of Affective Disorders. 2001;64:43–51. doi: 10.1016/s0165-0327(00)00199-3. [DOI] [PubMed] [Google Scholar]
- Cracowski JL, Durand T, Bessard G. Isoprostanes as a biomarker of lipid peroxidation in humans: physiology, pharmacology and clinical implications. Trends in Pharmacological Science. 2002;23:360–366. doi: 10.1016/s0165-6147(02)02053-9. [DOI] [PubMed] [Google Scholar]
- Cumurcu BE, Ozyurt H, Etikan I, Demir S, Karlidag R. Total antioxidant capacity and total oxidant status in patients with major depression: impact of antidepressant treatment. Psychiatry and Clinical Neurosciences. 2009;63:639–645. doi: 10.1111/j.1440-1819.2009.02004.x. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Clinician Version (SCID-CV) American Psychiatric Press, Inc; Washington, D.C.: 1996. [Google Scholar]
- Garland SN, Carlson LE, Cook S, Lansdell L, Speca M. A non-randomized comparison of mindfulness-based stress reduction and healing arts programs for facilitating post-traumatic growth and spirituality in cancer outpatients. Supportive Care Cancer. 2007;15:949–961. doi: 10.1007/s00520-007-0280-5. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Lee CY. Using isoprostanes as biomarkers of oxidative stress: some rarely considered issues. Antioxidants & Redox Signaling. 2010;13:145–156. doi: 10.1089/ars.2009.2934. [DOI] [PubMed] [Google Scholar]
- Hamilton M. Development of a rating scale for primary depressive illness. British Journal of Social and Clinical Psychology. 1967;6:278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- Inkielewicz-Stepniak I. Impact of fluoxetine on liver damage in rats. Pharmacological Reports. 2011;63:441–447. doi: 10.1016/s1734-1140(11)70510-2. [DOI] [PubMed] [Google Scholar]
- Kirkova M, Tzvetanova E, Vircheva S, Zamfirova R, Grygier B, Kubera M. Antioxidant activity of fluoxetine: studies in mice melanoma model. Cell Biochemistry and Function. 2010;28:497–502. doi: 10.1002/cbf.1682. [DOI] [PubMed] [Google Scholar]
- Kotan VO, Sarandol E, Kirhan E, Ozkaya G, Kirli S. Effects of long-term antidepressant treatment on oxidative status in major depressive disorder: a 24-week follow-up study. Progress in Neuropsychopharmacology and Biological Psychiatry. 2011;35:1284–1290. doi: 10.1016/j.pnpbp.2011.03.021. [DOI] [PubMed] [Google Scholar]
- Kumar A, Garg R, Gaur V, Kumar P. Venlafaxine involves nitric oxide modulatory mechanism in experimental model of chronic behavior despair in mice. Brain Research. 2010;1311:73–80. doi: 10.1016/j.brainres.2009.11.050. [DOI] [PubMed] [Google Scholar]
- Lawson JA, Rokach J, Fitzgerald GA. Isoprostanes: formation, analysis and use as indices of lipid peroxidation in vivo. The Journal of Biological Chemistry. 1999;274:24441–24444. doi: 10.1074/jbc.274.35.24441. [DOI] [PubMed] [Google Scholar]
- Mazure C, Nelson JC, Price LH. Reliability and validity of the symptoms of major depressive illness. Archives of General Psychiatry. 1986;43:451–456. doi: 10.1001/archpsyc.1986.01800050053006. [DOI] [PubMed] [Google Scholar]
- Milne GL, Sanchez SC, Musiek ES, Morrow JD. Quantification of F2-isoprostanes as a biomarker of oxidative stress. Nature Protocols. 2007;2:221–226. doi: 10.1038/nprot.2006.375. [DOI] [PubMed] [Google Scholar]
- Morriss R, Leese M, Chatwin J, Baldwin D. Inter-rater reliability of the Hamilton Depression Rating Scale as a diagnostic and outcome measure of depression in primary care. Journal of Affective Disorders. 2008;111:204–213. doi: 10.1016/j.jad.2008.02.013. [DOI] [PubMed] [Google Scholar]
- Morrow JD, Chen Y, Brame CJ, Yang J, Sanchez SC, Xu J, Zackert WE, Awad JA, Roberts LJ. The isoprostanes: unique prostaglandin-like products of free-radical-initiated lipid peroxidation. Drug Metabolism Reviews. 1999;31:117–139. doi: 10.1081/dmr-100101910. [DOI] [PubMed] [Google Scholar]
- Morrow JD, Roberts LJ. Mass spectrometry of prostanoids: F2-isoprostanes produced by non-cyclooxygenase free radical-catalyzed mechanism. Methods in Enzymology. 1994;233:163–174. doi: 10.1016/s0076-6879(94)33019-0. [DOI] [PubMed] [Google Scholar]
- Ng F, Berk M, Dean O, Bush AI. Oxidative stress in psychiatric disorders: evidence base and therapeutic implications. International Journal of Neuropsychopharmacology. 2008;11:851–876. doi: 10.1017/S1461145707008401. [DOI] [PubMed] [Google Scholar]
- Nourooz-Zadeh J. Key issues in F2-isoprostane analysis. Biochemical Society Transactions. 2008;36:1060–1065. doi: 10.1042/BST0361060. [DOI] [PubMed] [Google Scholar]
- Ozcan ME, Gulec M, Ozerol E, Polat R, Akyol O. Antioxidant enzyme activities and oxidative stress in affective disorders. International Clinical Psychopharmacology. 2004;19:89–95. doi: 10.1097/00004850-200403000-00006. [DOI] [PubMed] [Google Scholar]
- Roberts LJ, Morrow JD. Measurement of F(2)-isoprostanes as an index of oxidative stress in vivo. Free Radical Biology & Medicine. 2000;28:505–513. doi: 10.1016/s0891-5849(99)00264-6. [DOI] [PubMed] [Google Scholar]
- Sarandol A, Sarandol E, Eker SS, Erdinc S, Vatansever E, Kirli S. Major depressive disorder is accompanied with oxidative stress: short-term antidepressant treatment does not alter oxidative-antioxidative systems. Human Psychopharmacology. 2007;22:67–73. doi: 10.1002/hup.829. [DOI] [PubMed] [Google Scholar]
- Selley ML. Increased (E)-4-hydroxy-2-nonenal and asymmetric dimethylarginine concentrations and decreased nitric oxide concentrations in the plasma of patients with major depression. Journal of Affective Disorders. 2004;80:249–256. doi: 10.1016/S0165-0327(03)00135-6. [DOI] [PubMed] [Google Scholar]
- Sies H, Jones DP. Encyclopedia of Stress. Elsevier; 2007. Oxidative Stress. pp. 45–48. [Google Scholar]
- Slamon ND, Pentreath VW. Antioxidant defense against antidepressants in C6 and 1321N1 cells. Chemico Biological Interactions. 2000;127:181–199. doi: 10.1016/s0009-2797(00)00172-1. [DOI] [PubMed] [Google Scholar]
- Teyssier JR, Ragot S, Chauvet-Gelinier JC, Trojak B, Bonin B. Expression of oxidative stress-response genes is not activated in the prefrontal cortex of patients with depressive disorder. Psychiatry Research. 2011;186:244–247. doi: 10.1016/j.psychres.2010.07.030. [DOI] [PubMed] [Google Scholar]
- Yager S, Forlenza MJ, Miller GE. Depression and oxidative damage to lipids. Psychoneuroendocrinology. 2010;35:1356–1362. doi: 10.1016/j.psyneuen.2010.03.010. [DOI] [PubMed] [Google Scholar]
- Yanik M, Erel O, Kati M. The relationship between potency of oxidative stress and severity of depression. Acta Neuropsychiatrica. 2004;16:200–203. doi: 10.1111/j.0924-2708.2004.00090.x. [DOI] [PubMed] [Google Scholar]