Abstract
BACKGROUND
Everyday life demands continuous flexibility in thought and behavior. Here we examined whether individual differences in dopamine function are related to variability in the effects of amphetamine on one aspect of flexibility – task switching.
METHODS
Forty healthy human participants performed a task-switching paradigm following placebo and oral amphetamine administration. [18F]fallypride was used to measure D2/D3 baseline receptor availability and amphetamine-stimulated dopamine release.
RESULTS
The majority of the participants showed amphetamine-induced benefits through reductions in switch costs. However, such benefits were variable. Individuals with higher baseline thalamic and cortical receptor availability and striatal dopamine release showed greater reductions in switch costs following amphetamine than individuals with lower levels. The relationship between dopamine receptors and stimulant-enhanced flexibility was partially mediated by striatal dopamine release.
CONCLUSIONS
These data indicate that the impact of the psychostimulant on cognitive flexibility is influenced by the status of dopamine within a thalamocorticostriatal network. Beyond demonstrating a link between this dopaminergic network and the enhancement in task switching, these neural measures accounted for unique variance in predicting the psychostimulant-induced cognitive enhancement. The present results suggest that there may be measurable aspects of variability in the dopamine system that predispose certain individuals to benefit from and hence use psychostimulants for cognitive enhancement.
Keywords: dopamine, flexibility, cognitive control, amphetamine, striatum, thalamus, prefrontal cortex, parietal cortex, PET
Introduction
Cognitive flexibility refers to the broad set of skills used in everyday life to adjust behavior according to the changing demands of the environment. These skills have been associated with corticostriatal brain regions (1–8) modulated by the biogenic amines dopamine (DA), serotonin, and norepinephrine (9; 10). The ability to switch tasks represents an important component of cognitive flexibility, as it is essential for adaptively adjusting behavior in response to changing internal or external needs. This ability can be quantified as the additional amount of time it takes to switch to a new task relative to repeating the same task (“switch cost”) (11). Deficits in this ability arise in a number of neuropsychiatric disorders including attention deficit hyperactivity disorder (12) and Parkinson’s Disease (13), as well as in normal aging (14).
Task switching has both theoretically (15) and empirically been associated with the neuromodulator DA. Although manipulations of the DA system affect switch costs (12; 13; 16–18), responses to such manipulations are variable across participants. Understanding such variability is critical for evaluating the utility of agents aimed at modulating the DA system in a given individual. This issue takes on particular importance for psychostimulants given their widespread licit and illicit usage for promoting attention and alertness (19).
Genetic studies suggest that heritable individual variability in the function or expression of DA signaling pathway components, including catechol-O-methyltransferase, the DA transporter, and D2 receptors may contribute to individual differences in switch costs (20–23). However, to date these genetic studies have not directly measured DA functioning; thus, the relationship between variability in DA and individual differences in behavior are inferred rather than empirically observed. Indeed, knowledge of the cognitive correlates of individual differences in DA functioning of specific brain regions is largely absent from the human literature, as the cognitive genetic association studies are typically unable to provide information about regional specificity, and most DA PET imaging studies use low-affinity radioligands (e.g., [11C]raclopride) that are only suitable for assessing binding potential in the striatum.
In the present study, we examined individual differences in psychostimulant enhancement of cognitive flexibility, specifically focusing on task switching (11), by combining pharmacological manipulation of the DA system with PET imaging of DA D2/D3 receptors in healthy young adults. Given prior studies suggesting that the ability of DA agonists to enhance task-switching behavior may depend on interindividual variation in DA networks (20) we examined whether measures of receptor availability and psychostimulant-induced DA release predict individual differences in the cognitive benefits of amphetamine (d-AMPH) administration.
Methods and Materials
Participants
Forty neurologically and psychiatrically healthy, right-handed adult human participants (mean age = 22.4, range 18–33; 21 men, 19 women) with estimated IQ greater than 80 and no history of substance abuse were studied as part of an ongoing investigation of individual differences in striatal and extrastriatal DA release. All participants provided written informed consent approved by the Vanderbilt University Institutional Review Board. Female participants were studied during the early follicular phase of their menstrual cycle. Full screening and study eligibility details are provided in Supplement 1.
Task-Switching
All participants completed a classic task switching paradigm (24) (Figure S1). The task included predictable switches on every other trial (see Methods in Supplement 1 for more details). There were a total of 352 trials per session. Switch costs were calculated as the difference between the average reaction time on switch trials (magnitude to odd-even; odd-even to magnitude) and the average reaction time on repetition trials (odd-even to odd-even; magnitude to magnitude) collapsing across two different response-to-stimulus intervals (see Results in Supplement 1 for details).
Participants completed one round of the switch task (352 trials) on placebo and a second round (352 trials) on a 0.43 mg/Kg oral dose of d-AMPH. Participants performed the task approximately 90 minutes after ingesting the drug and before entering the PET scanner. This time delay was selected as the likely peak time for observing behavioral effects of the drug. We selected d-AMPH as the pharmacological agent because of its widespread use (both licit and illicit) as a cognitive enhancer, and due to its ability to stimulate DA release in a manner that reliably displaces our radiotracer [18F]fallypride. It is this latter property that allows us to measure individual differences in DA responses to d-AMPH in both striatal and extrastriatal regions (25). The sessions were separated by an average of 18.5 days (SD = 19.8, range = 1–85). Number of days between sessions was uncorrelated with changes from placebo to d-AMPH for both mean reaction time, r = .08, p = .61, and switch cost, r = .05, p = .77. Basic speed of processing and motor measures were also collected during both the placebo and d-AMPH sessions and included digit symbol coding, symbol search, finger tapping, and toe tapping to ensure that observations were specific to task switching and did not reflect simple psychomotor processing speed. A subset of 10 of these participants completed two rounds of the task (separated by the same number of days as their original two sessions) several years later to estimate the effect of task repetition in the absence of a drug (see Results in Supplement 1). This follow-up was conducted 4–5 years after the tasks were completed the first time, so it is unlikely that any initial exposure to the task carried over across this interval. An additional, separate sample of 10 healthy young adults (ages 21–30) performed the task twice but did not receive d-AMPH or undergo PET imaging (see Results in Supplement 1).
Positron emission tomography acquisition and preprocessing
All PET images were acquired using [18F]fallypride. Unlike other D2/D3 ligands, [18F]fallypride allows stable estimates of D2/D3 binding in both striatal and extrastriatal regions (26; 27) with high test-retest reliability (see Methods in Supplement 1 for more details). Protocols for PET image acquisition and analysis were derived from a larger ongoing study and have been previously published (26; 28; 29).
Participants received two PET scans using [18F]fallypride. The first scan was a baseline placebo scan; the second scan was performed while the participant received a d-AMPH challenge. PET imaging was performed on a GE Discovery LS scanner located at Vanderbilt University Medical Center that was upgraded to a Discovery STE system during the course of the study (10 participants on LS, 30 participants on STE). All participants received their baseline and d-AMPH scans on the same scanner. Prior analyses with the same dataset reveal no significant differences between scanners (28). Nevertheless, scanner was included here as a covariate in all analyses. Following reconstruction both scanners had similar in-plane and through-plane resolution. [18F]fallypride was produced in the radiochemistry laboratory attached to the PET unit, following synthesis and quality control procedures described in US Food and Drug Administration IND 47,245. Scans were timed to start 3 hours after 0.43 mg/Kg oral d-AMPH administration, which was timed to coincide with the period of peak plasma d-AMPH. 3-D emission acquisitions scans were performed following a 5.0 mCi slow bolus injection of [18F]fallypride (specific activity greater than 3000 Ci/mmol). Serial scans were started simultaneously with the bolus injection of [18F]fallypride and were obtained for approximately 3.5 hours, with two 15-minute breaks for participant comfort. CT transmission scans were collected for attenuation correction prior to each of the three emission scans.
Each participant’s serial PET scans were first corrected for motion across scanning periods and then co-registered to the participant’s structural T1-weighted MRI image (see Supplement 1 for MRI details). Regional D2/D3 binding potential (non-displaceable; BPND) was calculated on a voxel-wise basis using the full reference region method (30), with cerebellum chosen as the reference region because of its relative lack of D2/D3 receptors (31). Using the full reference region method, we have shown near perfect (r=0.99) correlation with modeled estimates using a metabolite corrected plasma input function (32). Although this approach is slower computationally than the simplified (three-parameter) tissue reference method, it robustly estimates the key variable of interest (binding potential), and we have observed excellent convergence of modeled fits in regions with both high and low DA D2/D3 receptor levels. Voxel-wise kinetic modeling was executed using Interactive Data Language. Individual voxelwise images of percent-change in [18F]fallypride binding from placebo to d-AMPH (representing percent-change in DA release) were created by subtracting each participant’s d-AMPH scan from their placebo scan and dividing the resulting imaging by the placebo scan.
Individual difference analyses
Prior to group analyses, a composite PET binding potential/T1-weighted MR image was created for each participant and warped to MNI space. The transformation matrix from this warping was then applied to the binding potential maps in order to bring all participants data into a common space. Individual difference analyses of the PET data were performed in SPM8 by separately regressing participants’ switch cost scores against their D2/D3 binding (placebo) and d-AMPH-induced DA release (percent-change) images. In all cases, age, sex, and scanner were included as covariates. SPMs were thresholded at a height of t > 3; cluster threshold was set to 30 contiguous voxels. Only voxels within clusters surviving a cluster extent correction for multiple comparisons (pFDR < 0.05) at this threshold are reported. The topological FDR default in SPM8 was used here, but the results are unchanged if the correction is changed to FWE.
Structural equation modeling
A structural equation model was used to illustrate the relationships between DA binding potential and release across a thalamic, cortical, and striatal network and their influence on d-AMPH-induced cognitive flexibility. For latent variables, one of three paths to the manifest variables was fixed to an unstandardized coefficient of 1 during model fitting.
Results
Behavioral Results
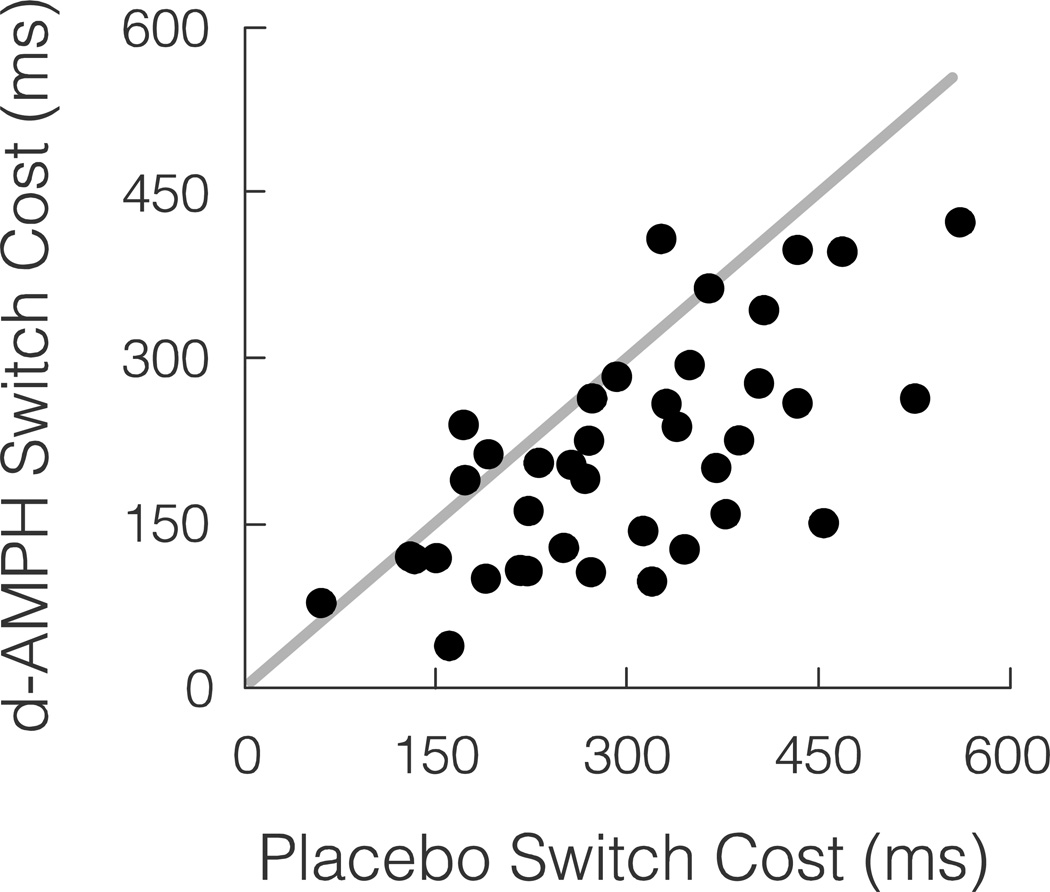
The majority of participants showed a behavioral benefit of d-AMPH. In addition to an overall decrease in mean reaction time under d-AMPH (744 ms, SD = 157) compared to placebo (837 ms, SD = 196), mean switch costs were also reduced under d-AMPH (210 ms, SD = 101) compared to placebo (299 ms, SD = 115). The average reduction in switch cost from placebo to d-AMPH (placebo – d-AMPH = 89 ms, SD = 89) was significant across the sample, t39 = 6.32, p < .0001. For comparison of switch costs under placebo and d-AMPH, see Figure 1. Additionally, the magnitude of the d-AMPH increase in cognitive flexibility was predicted by baseline switch costs, r = .53, p < .001, such that individuals with larger switch costs under placebo showed greater behavior change.
Figure 1.
The behavioral measure of inflexibility (switch cost = SC) was reduced with an oral dose of amphetamine for most participants. ms = milliseconds. Grey line is unity line (placebo switch cost = d-AMPH switch cost). N = 40
Neuroimaging Results
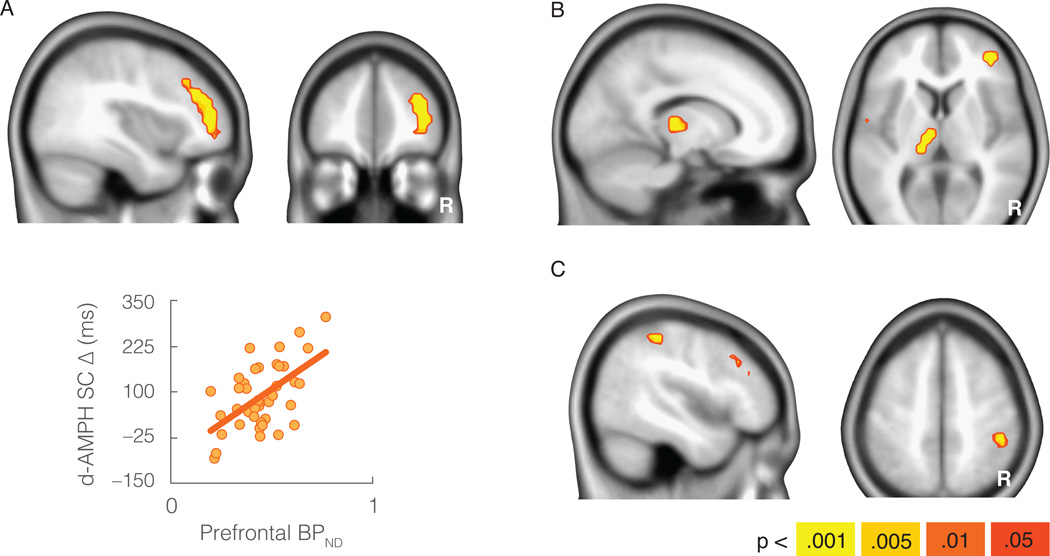
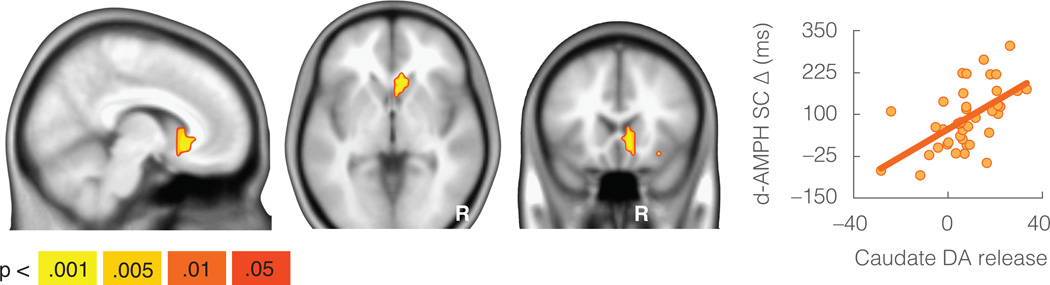
The behavioral increase in flexibility (i.e., average reduction in switch cost from placebo to d-AMPH) was also associated with baseline DA D2/D3 receptor availability (as indexed by [18F]fallypride binding potential, non-displaceable: BPND) in the lateral prefrontal cortex, left thalamus, and right inferior parietal lobule (p < .05 whole-brain corrected). In each region higher levels of BPND were associated with larger d-AMPH-associated cognitive benefits (see Figure 2 and Table S1). Increases in flexibility were also associated with DA release in the striatum (p < .05 whole-brain corrected) as indexed by the change in [18F]fallypride BPND following d-AMPH. Higher levels of DA release in anteromedial aspects of the caudate head were associated with larger cognitive benefits (see Figure 3 and Table S1). Although this cluster appears to extend into the white matter immediately anterior to the caudate, note that the spatial resolution of the PET data is much lower than the MRI template used as the underlay in the figures. No regions showed effects in the opposite direction (i.e., negative correlation between BPND and cognitive benefit) at the whole-brain threshold. There were also no regions that were significantly associated with switch cost at baseline (placebo) at the cluster-corrected whole-brain threshold.
Figure 2.
Increases in cognitive flexibility (reductions in switch cost) were associated with dopamine D2/D3 binding potential in the lateral frontal cortex (a), thalamus (b), and parietal cortex (c). Scatterplot y-axis is size of switch cost reduction from placebo to d-AMPH in milliseconds (d-AMPH SC Δ). Scatterplot is displayed only as a sample depiction of effects to illustrate the absence of outliers. N = 40
Figure 3.
Increases in cognitive flexibility (reductions in switch cost) were associated with dopamine release in the caudate. Scatterplot y-axis is size of switch cost reduction from placebo to d-AMPH in milliseconds (d-AMPH SC Δ). Scatterplot is displayed only as a sample depiction of effects to illustrate the absence of outliers. N = 40
The associations between DA receptors and release in these regions and increased flexibility were specific and not simply a general cognitive or motor benefit. The relationships with baseline BPND and d-AMPH-induced release in these regions remained significantly associated with the d-AMPH behavioral benefit after controlling for performance on digit symbol coding, symbol search, finger tapping, or toe tapping tasks. The d-AMPH improvement in performance was significant for digit symbol coding, t38 = 3.06, p < .005, and symbol search, t39 = 8.20, p < .001, but non-significant for finger tapping, t39 = 1.68, p = .10, and toe tapping, t39 = 1.53, p = .13. Importantly, these basic measures of motor change post d-AMPH were not associated with changes in switch costs (all |r|<.19, p>.26). Follow-up analyses also revealed that motor change measures were not significantly associated with prefrontal cortical BPND (all |r|<.29, p>.08), parietal cortical BPND (all |r|<.26, p>.12), thalamic BPND (all |r|<.26, p>.13), or caudate DA release (all |r|<.15, p>.36). Follow-up quadratic (inverted-U) effects were tested with data extracted from regions of interest in the lateral prefrontal cortex, parietal cortex, thalamus, and caudate. These measures of DA receptors (cortex, thalamus) and release (caudate) for each regions of interest were average BPND values across each cluster identified in the whole brain analyses examining individual differences in the drug effect. All relationships were linear, with no evidence of an additional inverted-U relationship between the neural measures and behavior change in any of these regions.
Stepwise regression analyses examined whether the neural measures explained additional variance in predicting behavioral benefits of d-AMPH over baseline performance levels alone (see Table 1). As reported above, in the first step placebo switch costs (i.e. baseline performance) explained a significant amount of variance in the d-AMPH behavioral benefit. For step two, a composite of thalamic-cortical BPND was created by averaging the z-scored measures of BPND across the thalamus, lateral frontal cortex, and parietal cortex (BPND measures in these three regions were highly correlated consistent with past analyses (26); all r > .58, p < .0001). In step two, significantly more variance in behavior change was explained by the addition of thalamic-cortical BPND (R2 change = .29), F1, 34 = 23.59, p < .0001. In step three, significantly more variance was explained by adding caudate DA release to the model from step two (R2 change = .06), F1, 33 = 5.31, p < .05. Importantly, the behavioral measure of the drug benefit used in this regression model is based on a subtraction from the baseline behavioral measure. Thus, the evidence that the size of the d-AMPH-induced reduction in switch cost is partially explained by the baseline switch cost is not surprising. However, this analysis demonstrates that the subcortical and cortical PET measures explain variance above and beyond that provided by baseline behavioral performance alone. Note that we use the term thalamic-cortical to refer to this correlated network of thalamic and cortical regions where receptor availability is associated with the effect of d-AMPH. We are not using the term to refer to the glutamatergic thalamocortical projections from thalamus to cortex.
Table 1.
Behavioral and neural predictors of amphetamine-induced cognitive flexibility.
| d-AMPH Switch Cost Reduction | |||
|---|---|---|---|
| Step 1 | Step 2 | Step 3 | |
| Placebo Switch Cost | 0.52 (0.14) | 0.30 (0.12) | 0.26 (0.12) |
| 3.64*** | 2.51* | 2.29* | |
| Thalamic-Cortical DA BPND | — | 0.76 (0.16) | 0.54 (0.18) |
| 4.86*** | 3.10** | ||
| Caudate DA Release | — | — | 0.30 (0.13) |
| 2.30* | |||
| Age | −0.07 (0.15) | 0.17 (0.13) | 0.09 (0.12) |
| −0.49 | 1.31 | 0.74 | |
| Sex | −0.03 (0.14) | −0.13 (0.11) | −0.11 (0.11) |
| −0.21 | −1.12 | −1.02 | |
| Scanner | 0.05 (0.15) | 0.25 (0.12) | 0.21 (0.12) |
| 0.34 | 1.97 | 1.80 | |
| R2 | .29* | .58*** | .64*** |
| Adjusted R2 | .21* | .52*** | .57*** |
| Observations | 40 | 40 | 40 |
BPND = binding potential, non-displaceable. top: coefficient (s.e.m.); bottom: t-statistic,
p<.05,
p<.01,
p<.001
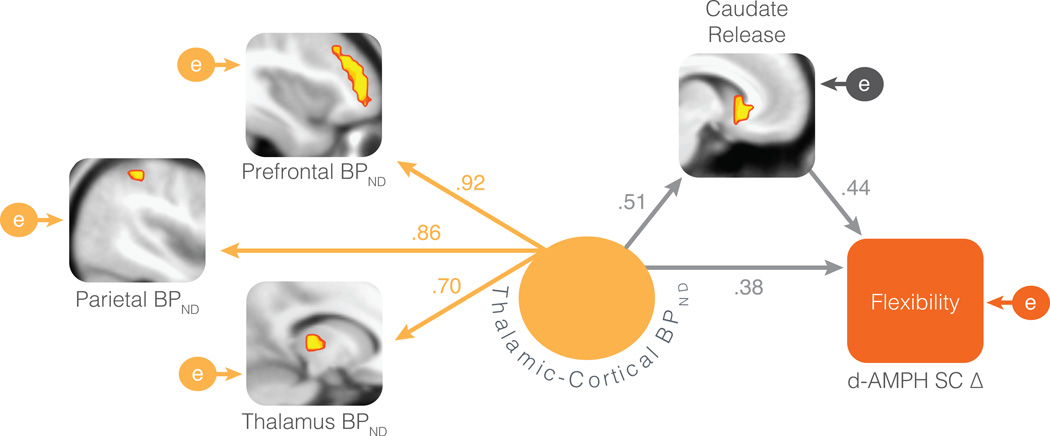
In the full model (step three) thalamic-cortical BPND and caudate DA release were significant predictors of increased flexibility (Table 1), suggesting that they each contribute at least some unique variance. A structural equation model was used to formalize both the relationships between these regions and their unique effects on behavior change. A model whereby thalamic-cortical BPND (represented as a single latent variable indexed by thalamic, frontal, and parietal BPND) and caudate DA release are significantly correlated but also uniquely predict psychostimulant-enhanced cognitive flexibility provided a good fit to the data (χ24 = 4.69, p = .32; CFI = .99, RMSEA = .066). The model is displayed in Figure 4 with path coefficients. All paths in the model are significant at p < .05. Although both thalamic-cortical BPND and striatal DA release are significantly associated with behavior change, the model also demonstrates a partial mediation effect such that the influence of thalamic-cortical BPND on stimulant-enhanced flexibility is partially mediated by the impact of thalamic-cortical BPND on DA release in the striatum (Sobel test = 2.20, p < .05).
Figure 4.
Structural equation model of the thalamocorticostriatal network effects on psychostimulant-enhanced cognitive flexibility. Path coefficients are standardized regression betas. The path between the thalamic-cortical latent variable and the thalamus manifest variable was fixed to an unstandardized coefficient of 1 during estimation. All paths are significant at p < .05. Encircled e’s are residual error variance terms. d-AMPH SC Δ = switch cost reduction from placebo to d-AMPH (expressed as an increase in flexibility)
Discussion
The present study indicates that the psychostimulant d-AMPH generally enhances task switching in healthy young adults, with improvements exceeding those that can be explained by simple motor effects alone. The overall enhancement in task switching is consistent with both theoretical accounts of a role for DA in allowing switching of behavioral outputs (15) and prior evidence that L-DOPA and methylphenidate reduce switch costs (12; 13) in patient populations. However, to our knowledge this is the first study to demonstrate the beneficial effect of d-AMPH on switch costs in healthy human participants. As expected there were significant individual differences in the degree to which d-AMPH reduced switch costs. Critically, the extent to which participants showed improvement in cognitive flexibility following d-AMPH was associated with individual differences in a dopaminergic network of cortical and subcortical brain regions.
Improvements in performance from placebo to d-AMPH were predicted by D2/D3 receptor availability in the lateral frontal and parietal cortices and thalamus. The localization of these relationships in lateral frontal and parietal regions is consistent with lesion studies and functional and structural imaging literature on task switching (3–5; 7; 8; 22). The evidence that individuals with a greater number of available (unoccupied) D2/D3 receptors show larger benefits of d-AMPH for task switching suggests that having more sites for DA to act in the thalamus and cortex aids in its enhancement of cognitive function.
The effects of cortical D2 engagement may be understood within existing models of prefrontal DA action (33). According to this model, D1 and D2 receptors alter the response properties of prefrontal neurons, such that D1 receptors enhance the maintenance of information in working memory buffers by suppressing the ability of distracters to engage local circuitry while D2 receptors lower the barriers for new or different information to gain access to these buffers, promoting flexibility. Updating has been shown to be linked more strongly to D2 receptor stimulation than D1 receptor stimulation (34; 35). In the context of task switching, enhancing D2 signaling may improve task switching by facilitating the accessibility of new information to working memory, thus preventing unnecessarily long maintenance of the prior task rule.
It is likely that any enhanced signaling here is occurring within an optimal range, as excessive D2 signaling will degrade attentional performance by promoting distractibility (35; 36). It should also be noted that d-AMPH will impact D1 receptors. The D2-sensitive ligand used in the present study does not provide evidence about the relative D1 and D2 dominance in the cortex. Although there is strong pharmacological and genetic evidence for a selective relationship between D2 receptor function and flexibility (20), we did not measure D1 receptors here, and, at present, data linking PET measures to these specific state conditions is lacking.
Critically, a positive relationship was also found between DA release in the caudate and the psychostimulant enhancement of task switching. It has been suggested that striatal DA may play a more central role than cortical DA in flexibility (37). Striatal DA has been shown to influence the neural efficiency of other dorsolateral striatal and prefrontal cortical regions, which directly influence switching behavior. Striatal DA may also serve a gating function on cortical connections (38; 39) which may aid in the continuous alternation of responses required by the task.
Although we found specific associations between DA receptors and release and the cognitive benefit of d-AMPH, it is important to acknowledge that the effects of these stimulants are not limited to the DA system. D-AMPH and methylphenidate also act on the norepinephrine transporter. Thus, this behavioral benefit is also likely to be partially dependent on other neurochemical systems (10; 40). Nevertheless, a significant portion of the variance in behavior change was accounted for by individual differences in DA binding potential and release (adjusted R2 range was .20–.38 across individual regions). We did not assess other neurochemical systems here, so it is not possible to examine potential relationships between behavior change and variability in the norepinephrine system or directly compare the size of the effects between neuromodulators.
Although prior studies have examined relationships between DA drug effects on other flexibility measures (e.g., reversal learning) and striatal DA function (41; 42), the goal of the whole-brain approach in the present study was to better characterize the role of a broader DA network. A structural equation model was used to test the hypothesized associations between these cortical and subcortical effects and their relationship with psychostimulant-enhanced flexibility. The model supports the hypothesis that these regions compose a unified network but also make independent contributions to behavior change. The set of regions in this network is remarkably consistent with documented thalamocorticostriatal circuits (43; 44). Although thalamic activation has been reported in studies of task switching (1; 2; 8), it is rarely discussed. The results of the present study support an important role for the thalamus in this dopaminergic network (43; 45–47). In prior discussions of the role of corticostriatal circuits in switching there is an implicit assumption that the loops proceed through thalamic relays. However, the observation that D2/D3 binding potential in the thalamus influences the results suggests that there could be a more explicit influence of the thalamus that extends beyond an automatic relay. As such, we adopt the term thalamocorticostriatal in describing this network. Of course, a complete circuit model of these thalamic, cortical, and striatal regions necessarily includes GABAergic and glutamatergic pathways that we did not measure here. Our use of the “network” terminology refers to the correlated DA receptor and release effects in thalamic, cortical, and striatal regions.
The results also demonstrate a partial mediation effect such that the thalamic and cortical receptor influence on stimulant-enhanced flexibility is partially mediated by striatal DA release. The relationship between thalamic and cortical binding potential and striatal DA release is specified directionally for two reasons. First, the measure of DA release that we use in this study is d-AMPH-induced and depends on baseline levels of DA receptors. Second, directionality is anatomically predicted by well-known dorsolateral prefrontal loops that include direct pathways from the prefrontal cortex to the striatum. Although we model directionality, more generally, it is possible that this relationship could be bidirectional. Although previous studies and theory have emphasized the opponent roles of the striatum and prefrontal cortex for supporting flexibility and stability, respectively (35), the present results suggest that both striatal and cortical regions may be serving complementary functions.
Some limitations in the study should be noted. The order of administration of placebo and drug was not counter-balanced. Although two sets of additional behavioral data suggest that the drug effects are larger than the task repetition effects (see Results in Supplement 1), we cannot completely rule out the possibility that a portion of the variance in performance across participants may also be related to individual differences in learning ability (41; 42). Future studies will need to collect both measures of flexibility and learning to formally examine these potential relationships. Also, the present sample was composed entirely of healthy young adults. It is not clear if the same relationships would be observed in a clinical sample (48; 49). Another important limitation is that the behavioral change was only examined over a short time scale from a single dose of d-AMPH. We did not measure repeated exposure to the stimulant. It is possible that the flexibility benefits may plateau over time, or even reverse with sensitization (50). A related limitation of using a single dose of d-AMPH in this study is that we cannot establish dose-response curves with the available data.
An increasing number of otherwise healthy adults use psychostimulants for cognitive enhancement (19). Despite the potential for addiction (51), individuals will continue to use d-AMPH and other psychostimulants to manipulate their attention and arousal (52; 53). The present results suggest that there may be measurable aspects of variability in the DA system that predispose certain individuals to benefit from and hence use psychostimulants for cognitive enhancement. These factors may also influence the risk for abusing such medications. In a recent study we found that increased DA release in the striatum was associated with increased self-reported desire for more d-AMPH (28). Although we did not specifically measure the perceived cognitive benefits here, it is possible that such perceived cognitive effects of initial stimulant exposure may be an important part of the subjective experience that contributes to the later development of addiction (54).
Supplementary Material
Acknowledgments
Thanks to Evan Shelby and Ashley Schwartzman for assistance with data collection, and Chrystyna Kouros and Lauren Atlas for advice on structural equation modeling. G.R.S.L. was supported by National Institute of Mental Health training grant T32-MH018921, National Institute on Aging post-doctoral NRSA F32-AG039131, and National Institute on Aging Pathway to Independence Award K99-AG042596 during data analysis and manuscript preparation. This research was funded by National Institute on Drug Abuse grant R01-DA019670 to D.H.Z. and was supported by CTSA AWARD No. UL1TR000445 from the National Center for Advancing Translational Science. Its contents are solely the responsibility of the authors and do not necessarily represent official views of the NCATS or NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
All authors report no biomedical financial interest or potential conflicts of interest.
References
- 1.Kimberg DY, Aguirre GK, D'Esposito M. Modulation of task-related neural activity in task-switching: an fMRI study. Brain Res Cogn Brain Res. 2000;10:189–196. doi: 10.1016/s0926-6410(00)00016-1. [DOI] [PubMed] [Google Scholar]
- 2.Sohn MH, Ursu S, Anderson JR, Stenger VA, Carter CS. The role of prefrontal cortex and posterior parietal cortex in task switching. PNAS. 2000;97:13448–13453. doi: 10.1073/pnas.240460497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aron AR, Monsell S, Sahakian BJ, Robbins TW. A componential analysis of task-switching deficits associated with lesions of left and right frontal cortex. Brain. 2004;127:1561–1573. doi: 10.1093/brain/awh169. [DOI] [PubMed] [Google Scholar]
- 4.Cools R, Ivry RB, D'Esposito M. The human striatum is necessary for responding to changes in stimulus relevance. J Cogn Neurosci. 2006;18:1973–1983. doi: 10.1162/jocn.2006.18.12.1973. [DOI] [PubMed] [Google Scholar]
- 5.Badre D, Wagner AD. Computational and neurobiological mechanisms underlying cognitive flexibility. PNAS. 2006;103:7186–7191. doi: 10.1073/pnas.0509550103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Esterman M, Chiu Y-C, Tamber-Rosenau BJ, Yantis S. Decoding cognitive control in human parietal cortex. PNAS. 2009;106:17974–17979. doi: 10.1073/pnas.0903593106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gold BT, Powell DK, Xuan L, Jicha GA, Smith CD. Age-related slowing of task switching is associated with decreased integrity of frontoparietal white matter. Neurobiol Aging. 2010;31:512–522. doi: 10.1016/j.neurobiolaging.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim C, Johnson NF, Cilles SE, Gold BT. Common and distinct mechanisms of cognitive flexibility in prefrontal cortex. J Neurosci. 2011;31:4771–4779. doi: 10.1523/JNEUROSCI.5923-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robbins TW. Shifting and stopping: fronto-striatal substrates, neurochemical modulation and clinical implications. Philos Trans R Soc Lond, B, Biol Sci. 2007;362:917–932. doi: 10.1098/rstb.2007.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kehagia AA, Murray GK, Robbins TW. Learning and cognitive flexibility: frontostriatal function and monoaminergic modulation. Curr Opin Neurobiol. 2010;20:199–204. doi: 10.1016/j.conb.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 11.Monsell S. Task switching. Trends Cogn Sci. 2003;7:134–140. doi: 10.1016/s1364-6613(03)00028-7. [DOI] [PubMed] [Google Scholar]
- 12.Kramer AF, Cepeda NJ, Cepeda ML. Methylphenidate effects on task-switching performance in attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2001;40:1277–1284. doi: 10.1097/00004583-200111000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Cools R, Barker RA, Sahakian BJ, Robbins TW. L-Dopa medication remediates cognitive inflexibility, but increases impulsivity in patients with Parkinson's disease. Neuropsychologia. 2003;41:1431–1441. doi: 10.1016/s0028-3932(03)00117-9. [DOI] [PubMed] [Google Scholar]
- 14.Wasylyshyn C, Verhaeghen P, Sliwinski MJ. Aging and task switching: a meta-analysis. Psychol Aging. 2011;26:15–20. doi: 10.1037/a0020912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oades RD. The role of noradrenaline in tuning and dopamine in switching between signals in the CNS. Neuroscience and Biobehavioral Reviews. 1985;9:261–282. doi: 10.1016/0149-7634(85)90050-8. [DOI] [PubMed] [Google Scholar]
- 16.Evenden JL, Robbins TW. The effects of d-amphetamine, chlordiazepoxide and alpha-flupenthixol on food-reinforced tracking of a visual stimulus by rats. Psychopharmacology. 1985;85:361–366. doi: 10.1007/BF00428202. [DOI] [PubMed] [Google Scholar]
- 17.Crofts HS, Dalley JW, Collins P, Van Denderen JC, Everitt BJ, Robbins TW, et al. Differential effects of 6-OHDA lesions of the frontal cortex and caudate nucleus on the ability to acquire an attentional set. Cereb Cortex. 2001;11:1015–1026. doi: 10.1093/cercor/11.11.1015. [DOI] [PubMed] [Google Scholar]
- 18.Mehta MA, Manes FF, Magnolfi G, Sahakian BJ, Robbins TW. Impaired set-shifting and dissociable effects on tests of spatial working memory following the dopamine D2 receptor antagonist sulpiride in human volunteers. Psychopharmacology. 2004;176:331–342. doi: 10.1007/s00213-004-1899-2. [DOI] [PubMed] [Google Scholar]
- 19.McCabe SE, Knight JR, Teter CJ, Wechsler H. Non-medical use of prescription stimulants among US college students: prevalence and correlates from a national survey. Addiction. 2005;100:96–106. doi: 10.1111/j.1360-0443.2005.00944.x. [DOI] [PubMed] [Google Scholar]
- 20.van Holstein M, Aarts E, van der Schaaf ME, Geurts DEM, Verkes RJ, Franke B, et al. Human cognitive flexibility depends on dopamine D2 receptor signaling. Psychopharmacology. 2011;218:567–578. doi: 10.1007/s00213-011-2340-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Colzato LS, Waszak F, Nieuwenhuis S, Posthuma D, Hommel B. The flexible mind is associated with the catechol-O-methyltransferase (COMT) Val158Met polymorphism: evidence for a role of dopamine in the control of task-switching. Neuropsychologia. 2010;48:2764–2768. doi: 10.1016/j.neuropsychologia.2010.04.023. [DOI] [PubMed] [Google Scholar]
- 22.Stelzel C, Basten U, Montag C, Reuter M, Fiebach CJ. Frontostriatal involvement in task switching depends on genetic differences in d2 receptor density. J Neurosci. 2010;30:14205–14212. doi: 10.1523/JNEUROSCI.1062-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aarts E, Roelofs A, Franke B, Rijpkema M, Fernández G, Helmich RC, et al. Striatal dopamine mediates the interface between motivational and cognitive control in humans: evidence from genetic imaging. Neuropsychopharmacology. 2010;35:1943–1951. doi: 10.1038/npp.2010.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rogers R, Monsell S. Costs of a predictible switch between simple cognitive tasks. Journal of experimental psychology: General. 1995;124:207–231. [Google Scholar]
- 25.Riccardi P, Li R, Ansari MS, Zald D, Park S, Dawant B, et al. Amphetamine-induced displacement of [18F] fallypride in striatum and extrastriatal regions in humans. Neuropsychopharmacology. 2006;31:1016–1026. doi: 10.1038/sj.npp.1300916. [DOI] [PubMed] [Google Scholar]
- 26.Zald DH, Woodward ND, Cowan RL, Riccardi P, Ansari MS, Baldwin RM, et al. The interrelationship of dopamine D2-like receptor availability in striatal and extrastriatal brain regions in healthy humans: a principal component analysis of [18F]fallypride binding. NeuroImage. 2010;51:53–62. doi: 10.1016/j.neuroimage.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Christian BT, Narayanan T, Shi B, Morris ED, Mantil J, Mukherjee J. Measuring the in vivo binding parameters of [18F]-fallypride in monkeys using a PET multiple-injection protocol. J Cereb Blood Flow Metab. 2004;24:309–322. doi: 10.1097/01.WCB.0000105020.93708.DD. [DOI] [PubMed] [Google Scholar]
- 28.Buckholtz JW, Treadway MT, Cowan RL, Woodward ND, Li R, Ansari MS, et al. Dopaminergic network differences in human impulsivity. Science. 2010;329:532. doi: 10.1126/science.1185778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buckholtz JW, Treadway MT, Cowan RL, Woodward ND, Benning SD, Li R, et al. Mesolimbic dopamine reward system hypersensitivity in individuals with psychopathic traits. Nat Neurosci. 2010;13:419–421. doi: 10.1038/nn.2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lammertsma AA, Bench CJ, Hume SP, Osman S, Gunn K, Brooks DJ, et al. Comparison of methods for analysis of clinical [11C]raclopride studies. J Cereb Blood Flow Metab. 1996;16:42–52. doi: 10.1097/00004647-199601000-00005. [DOI] [PubMed] [Google Scholar]
- 31.Hall H, Sedvall G, Magnusson O, Kopp J, Halldin C, Farde L. Distribution of D1- and D2-dopamine receptors, and dopamine and its metabolites in the human brain. Neuropsychopharmacology. 1994;11:245–256. doi: 10.1038/sj.npp.1380111. [DOI] [PubMed] [Google Scholar]
- 32.Kessler RM, Mason NS, Jones C, Ansari MS, Manning RF, Price RR. [18F]N-allyl-5-fluoropropylepidepride (fallypride): radiation dosimetry, quantification of striatal and extrastriatal dopamine receptors in man. NeuroImage. 2000;11:S32. [Google Scholar]
- 33.Seamans JK, Yang CR. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol. 2004;74:1–58. doi: 10.1016/j.pneurobio.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 34.Wang M, Vijayraghavan S, Goldman-Rakic PS. Selective D2 receptor actions on the functional circuitry of working memory. Science. 2004;303:853–856. doi: 10.1126/science.1091162. [DOI] [PubMed] [Google Scholar]
- 35.Cools R, D'Esposito M. Inverted-U-shaped dopamine actions on human working memory and cognitive control. Biol Psychiatry. 2011;69:e113–e125. doi: 10.1016/j.biopsych.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mattay VS, Goldberg TE, Fera F, Hariri AR, Tessitore A, Egan MF, et al. Catechol O-methyltransferase val158-met genotype and individual variation in the brain response to amphetamine. PNAS. 2003;100:6186–6191. doi: 10.1073/pnas.0931309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cools R. Dopaminergic control of the striatum for high-level cognition. Curr Opin Neurobiol. 2011;21:402–407. doi: 10.1016/j.conb.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 38.van Schouwenburg MR, Ouden den HEM, Cools R. The human basal ganglia modulate frontal-posterior connectivity during attention shifting. J Neurosci. 2010;30:9910–9918. doi: 10.1523/JNEUROSCI.1111-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McNab F, Klingberg T. Prefrontal cortex and basal ganglia control access to working memory. Nat Neurosci. 2008;11:103–107. doi: 10.1038/nn2024. [DOI] [PubMed] [Google Scholar]
- 40.Berridge CW, Stalnaker TA. Relationship between low-dose amphetamine-induced arousal and extracellular norepinephrine and dopamine levels within prefrontal cortex. Synapse. 2002;46:140–149. doi: 10.1002/syn.10131. [DOI] [PubMed] [Google Scholar]
- 41.Clatworthy PL, Lewis SJG, Brichard L, Hong YT, Izquierdo D, Clark L, et al. Dopamine release in dissociable striatal subregions predicts the different effects of oral methylphenidate on reversal learning and spatial working memory. J Neurosci. 2009;29:4690–4696. doi: 10.1523/JNEUROSCI.3266-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cools R, Frank MJ, Gibbs SE, Miyakawa A, Jagust WJ, D'Esposito M. Striatal dopamine predicts outcome-specific reversal learning and its sensitivity to dopaminergic drug administration. J Neurosci. 2009;29:1538–1543. doi: 10.1523/JNEUROSCI.4467-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haber SN. The primate basal ganglia: parallel and integrative networks. Journal of Chemical Neuroanatomy. 2003;26:317–330. doi: 10.1016/j.jchemneu.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 44.Alexander GE, Crutcher MD, DeLong MR. Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor, "prefrontal" and “limbic” functions. Prog. Brain Res. 1990;85:119–146. [PubMed] [Google Scholar]
- 45.Mink JW. The basal ganglia: focused selection and inhibition of competing motor programs. Prog Neurobiol. 1996;50:381–425. doi: 10.1016/s0301-0082(96)00042-1. [DOI] [PubMed] [Google Scholar]
- 46.Sánchez-González MA, García-Cabezas MA, Rico B, Cavada C. The primate thalamus is a key target for brain dopamine. J Neurosci. 2005;25:6076–6083. doi: 10.1523/JNEUROSCI.0968-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.García-Cabezas MA, Martínez-Sánchez P, Sánchez-González MA, Garzón M, Cavada C. Dopamine innervation in the thalamus: monkey versus rat. Cereb Cortex. 2009;19:424–434. doi: 10.1093/cercor/bhn093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Advokat C. What are the cognitive effects of stimulant medications? Emphasis on adults with attention-deficit/hyperactivity disorder (ADHD) Neuroscience and Biobehavioral Reviews. 2010;34:1256–1266. doi: 10.1016/j.neubiorev.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 49.Hermens DF, Cooper NJ, Kohn M, Clarke S, Gordon E. Predicting stimulant medication response in ADHD: evidence from an integrated profile of neuropsychological, psychophysiological and clinical factors. J Integr Neurosci. 2005;4:107–121. doi: 10.1142/s0219635205000653. [DOI] [PubMed] [Google Scholar]
- 50.Featherstone RE, Rizos Z, Kapur S, Fletcher PJ. A sensitizing regimen of amphetamine that disrupts attentional set-shifting does not disrupt working or long-term memory. Behav. Brain Res. 2008;189:170–179. doi: 10.1016/j.bbr.2007.12.032. [DOI] [PubMed] [Google Scholar]
- 51.Swanson JM, Volkow ND. Increasing use of stimulants warns of potential abuse. Nature. 2008;453:586. doi: 10.1038/453586a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Greely H, Sahakian B, Harris J, Kessler RC, Gazzaniga M, Campbell P, et al. Towards responsible use of cognitive-enhancing drugs by the healthy. Nature. 2008;456:702–705. doi: 10.1038/456702a. [DOI] [PubMed] [Google Scholar]
- 53.Maher B. Poll results: look who's doping. Nature. 2008;452:647–675. doi: 10.1038/452674a. [DOI] [PubMed] [Google Scholar]
- 54.Lambert NM, McLeod M, Schenk S. Subjective responses to initial experience with cocaine: an exploration of the incentive-sensitization theory of drug abuse. Addiction. 2006;101:713–725. doi: 10.1111/j.1360-0443.2006.01408.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.