Abstract
Thalamic nuclei associated with language including the ventral lateral, ventral anterior, intralaminar and mediodorsal form a hub that uniquely receives the output of the basal ganglia and cerebellum, and is connected with frontal (premotor and prefrontal) cortices through two parallel circuits: a thalamic pathway targets the middle frontal cortical layers focally, and the other innervates widely cortical layer 1, poised to recruit other cortices and thalamic nuclei for complex cognitive operations. Return frontal pathways to the thalamus originate from cortical layers 6 and 5. Information through this integrated thalamo-cortical system is gated by the inhibitory thalamic reticular nucleus and modulated by dopamine, representing a specialization in primates. The intricate dialogue of distinct thalamic nuclei with the basal ganglia, cerebellum, and specific dorsolateral prefrontal and premotor cortices associated with language, suggests synergistic roles in the complex but seemingly effortless sequential transformation of cognitive operations for speech production in humans.
Keywords: prefrontal cortex, premotor cortex, thalamic motor nuclei, ventral anterior thalamic nucleus, ventral lateral thalamic nucleus, mediodorsal nucleus, thalamic reticular nucleus, thalamic aphasia, parallel thalamo-cortical circuits, drivers, modulators, thalamic dopaminergic innervation, basal ganglia and thalamus, cerebellum and thalamus
Overview: Distributed neural circuits associated with language
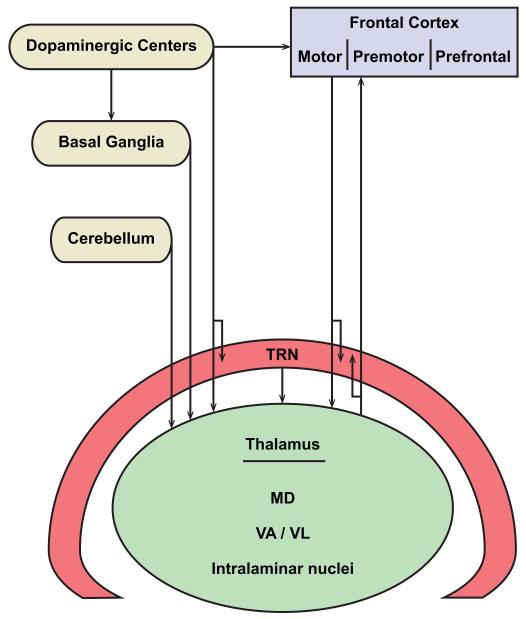
The complex neural processing for language in volves a large number of cortical areas and subcortical structures. Among the latter, damage to ventral lateral (VL), ventral anterior (VA), intralaminar, and mediodorsal (MD) thalamic nuclei consistently leads to language disturbances. This review centers on these thalamic nuclei which serve as a hub to link three structures associated with language: the frontal cortex, the basal ganglia and the cerebellum. Thalamic nuclei associated with language processes receive the output of the basal ganglia and the cerebellum and have bidirectional connections with the frontal cortex. As summarized in Figure 1, the focus here is on the anatomic organization of this integrated circuit, its monitoring by a fast and early processing inhibitory system mediated by the thalamic reticular nucleus (TRN), and modulation by dopamine, whose precise regulation is essential for cognitive operations [reviewed in (Arnsten & Li, 2005)].
Figure 1.
Thalamic nuclei that serve as a hub for distributed neural circuits associated with language. The nodes of networks that interact with the thalamus include: the frontal cortex, which consists of motor, premotor, and prefrontal areas; basal ganglia; cerebellum; dopaminergic groups from the mesencephalic substantia nigra pars compacta and the ventral tegmental area; and the inhibitory thalamic reticular nucleus (TRN).
The term frontal cortex refers to the large cortical expanse that includes the motor cortex at its posterior extent, and progressively more anteriorly the premotor and prefrontal cortices (Figs. 1-2). The lateral frontal cortex includes Broca’s region, which is associated with language. On the other hand, damage to prefrontal areas does not lead to aphasia, but based on the role of prefrontal areas in sequencing information in working memory, their integrity is essential for fluent speech.
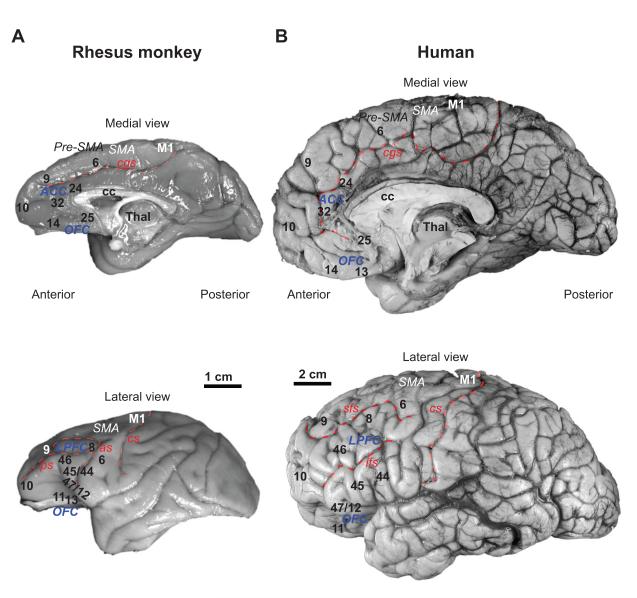
Figure 2.
The frontal cortex in the primate brain. A, B, Medial (top) and lateral (bottom) views of the rhesus monkey (A) and human (B) brains show cingulate, orbital, and lateral prefrontal, premotor cortices and the primary motor cortex. Abbreviations: ACC, anterior cingulate cortices; as, arcuate sulcus; cc, corpus callosum; cgs, cingulate sulcus; cs, central sulcus; ifs, inferior frontal sulcus; LPFC, lateral prefrontal cortices; M1, primary motor cortex; OFC, orbitofrontal cortices; Pre-SMA, anterior supplementary motor area; ps, principal sulcus; SMA, supplementary motor area; sfs, superior frontal sulcus; Thal, thalamus.
Classical neuropathological and modern imaging studies have also associated several post-Rolandic areas with language. Among these, temporal auditory areas that process sound stimuli project to frontal areas for speech articulation in areas 44/45 (Hickok & Poeppel, 2004) which make up Broca’s region on the left hemisphere, as well as to prefrontal areas 46 and 9, which are associated with working memory [reviewed in (Saur et al., 2008; Petrides & Pandya, 2009)]. Post-Rolandic parietal and temporal cortices associated with language on the left side include Wernicke’s areas, which are thought to process meaningful speech [reviewed in (Galaburda, LeMay, Kemper, & Geschwind, 1978; Toga & Thompson, 2003; Hickok & Poeppel, 2004; Stowe, Haverkort, & Zwarts, 2005; Bennett & Hacker, 2006; Hickok & Poeppel, 2007; Price, 2010)]. These parieto-temporal cortices are connected with the lateral posterior and pulvinar thalamic nuclei, which project widely to other cortices as well, including some occipital and prefrontal cortices [reviewed in (Jones, 2007)]. There is some evidence that damage to the pulvinar or lateral posterior nuclei also results in language disturbances (Crosson, 1999). This review is based only on thalamic nuclei associated with premotor and prefrontal cortices whose damage results in severe language disturbances. The focus is on the frontal cortical connections of these thalamic nuclei, and their relationship with the basal ganglia and distinct cerebellar sites associated with language. This review does not consider the various theories about language processing in the brain, or describe the large number of post-Rolandic cortices that have been associated with specific linguistic tasks in functional imaging studies. Temporal, parietal and occipital cortices have topographic connections with prefrontal and premotor cortices, as described elsewhere [e.g., (Barbas, 1992; Barbas, 2000a; Barbas, Ghashghaei, Rempel-Clower, & Xiao, 2002)].
Frontal cortices and language
Lateral areas 44 and 45 in the human brain are situated within the frontal language region of Broca and have a direct role in speech production. Area 44 is engaged also during silent speech (Grafton, Fadiga, Arbib, & Rizzolatti, 1997; Friedman et al., 1998). In the rhesus monkey brain area 44 lies within the premotor region and area 45 is located rostrally in the adjacent prefrontal cortex (Fig. 2A, B). Other premotor cortices include area 6, which is found behind and largely dorsal to areas 44 and 45. At its most dorsal extent area 6 gives way to the supplementary motor area (SMA), which extends to the medial surface. The SMA is situated within the dorsal and medial part of area 6 in Brodmann’s map (Brodmann, 1905; reviewed in Dum & Strick, 2002; Chouinard & Paus, 2006). On the medial surface, the cingulate motor areas are located on the cingulate gyrus below the SMA (or area 6 in Fig. 2, top). The pre-SMA is found anterior to the SMA on the medial and dorsal surface. Areas found below and anterior to the medial premotor cortices include the anterior cingulate cortex (ACC). The ACC has an important role in vocalization within an emotional context (Barbas, 2000b), and a key role in allocating attentional resources, both of which are important processes for language.
Situated in front and above lateral areas 44 and 45, dorsolateral prefrontal areas 46 and 9 have synergistic roles in cognitive processes and are engaged in language functions that require holding information within working memory. Areas 46, 9, and the ACC have strong connections with neighboring premotor cortices, and especially its dorsal and medial sectors via corticocortical connections, as well as through common nuclei in the thalamus [reviewed in (Ward, Jr. & McCulloch, 1947; Goldman-Rakic, Bates, & Chafee, 1992; Barbas, 2000a; Luebke, Barbas, & Peters, 2010)]. As discussed below, the key thalamic nuclei of these prefrontal and premotor cortices are robustly linked with the ba sal ganglia, and several sites within the same nuclei receive the output of the cerebellum.
Thalamic nuclei associated with language
The role of the thalamus in language began to be appreciated with the introduction of stereotactic surgery, conducted in an attempt to ameliorate tremor and other motor symptoms in disorders such as Parkinson’s disease. Patients with surgical lesions in the motor-related ventral lateral nucleus (VL; see Table 1 for nomenclature), showed deficits in naming objects and short-term verbal memory [reviewed in (Petrovici, 1980)]. The role of VL in language function was subsequently corroborated using the independent approach of electrical stimulation [reviewed in (Johnson & Ojemann, 2000)]. More recently, neuroimaging studies of aphasic patients with subcortical lesions have described deficits in language after damage to thalamic nuclei, including anomia, verbal paraphasias, reduced verbal output and fluency, with relative sparing of language comprehension (Nadeau & Crosson, 1997; Radanovic & Scaff, 2003). Similar deficits have been described after vascular lesions of the thalamus affecting the tuberothalamic and paramedian arteries, which supply the thalamic reticular nucleus (TRN) and several anterior, medial and ventral thalamic nuclei [reviewed in (Schmahmann, 2003)].
Table 1.
Motor thalamic nuclei in macaque monkey and human
|
Olszewski, 1952 macaque |
Hirai & Jones, 1989 human |
|---|---|
| VAmc | VAmc |
| VApc | VA |
| VLm | VMp |
| VLo | VLa |
| VLc | VLp dorsal part |
| Area X | VLp anteromedial part |
| VPLo | VLp ventral part |
The most commonly used nomenclature for the macaque monkey and human thalamus subdivides the ventral nuclei into anterior, lateral and posterior. The oral portion of the ventral posterior lateral nucleus (VPLo) of the macaque is considered here to be part of VL because of its pattern of subcortical and cortical connections. The VPLo nucleus of Olszewski (Olszewski, 1952) is equivalent to the ventral part of VL (VLp) in the human (Hirai & Jones, 1989), the most preferred target for thalamotomy to alleviate tremor. In this review we use the Hirai and Jones terminology to refer to studies in humans and the map of Olszewski of the macaque thalamus to describe connections.
The thalamic infarcts that most frequently produce aphasia involve the left side, consistent with the lateralization of language to the left hemisphere in the majority of individuals, and the predominant ipsilateral connections between the thalamus and cortex. Because vascular or other lesions in humans usually affect more than one nucleus, it is not possible to attribute specific deficits to distinct thalamic nuclei. The difficulties in pinpointing the specific damage after vascular lesions are compounded in cases where blood flow is significantly reduced in several brain areas without apparent damage to the system that is visible in images of the living brain [reviewed in (Nadeau & Crosson, 1997)]. In spite of these difficulties, the collective evidence from stereotactic surgery, electrical stimulation and imaging studies in aphasic patients with thalamic stroke implicate the VL, MD, intralaminar (especially the centromedian-parafascicular, CnMd-Pf) and the VA nuclei as the most likely candidates with a significant role in language [(Nadeau & Crosson, 1997; Carrera, Michel, & Bogousslavsky, 2004; Carrera & Bogousslavsky, 2006); reviewed in (Schmahmann, 2003)].
The above thalamic nuclei have several features in common: they receive the output of the basal ganglia and/or the cerebellum, two structures that have key roles in language processes. These nuclei are also connected with the frontal cortex, which includes the frontal language areas, other lateral and medial premotor areas as well as dorsolateral prefrontal areas implicated in cognitive tasks and sequential processing, which have an integral role in language processing, as elaborated below.
Thalamic nuclei connected with frontal cortices are innervated by the basal ganglia
Thalamic nuclei with a demonstrated role in language (VL, VA, MD and intralaminar) receive the output of the basal ganglia (Fig. 3, 4A) and have bidirectional connections with frontal language areas and the prefrontal cortex (Rosvold, 1972; Alexander, Delong, & Strick, 1986; Pribram, 1986; Groenewegen, Berendse, Wolters, & Lohman, 1990; Joel & Weiner, 1994; Mitchell, Cooper, & Griffiths, 1999; Middleton & Strick, 2000; Graybiel, 2000; Haber & McFarland, 2001; Parent, Levesque, & Parent, 2001; Anderson, 2001; Haber, 2003; Xiao & Barbas, 2004). The projections from the basal ganglia to the above thalamic nuclei emanate from the internal segment of the globus pallidus (GPi) and the substantia nigra pars reticulata (SNr) [reviewed in (Haber & McFarland, 2001; Groenewegen, 2003)]. The topographic association of frontal areas with the basal ganglia was demonstrated with the use of retrograde transneuronal tracers injected in premotor and prefrontal cortices. The viral tracer injected in a given frontal area was transported back to neurons in the thalamus and from the thalamus back to GPi/SNr. These studies showed that the amount of territory associated with prefrontal areas in GPi is comparable to the amount of volume occupied by projection neurons directed to the premotor and motor cortices through the thalamus [reviewed in (Middleton & Strick, 1994; Middleton & Strick, 2000; Middleton & Strick, 2002)].
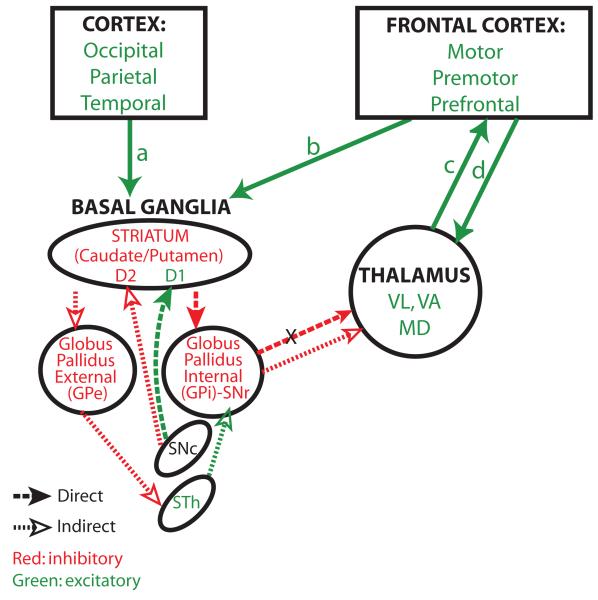
Figure 3.
Direct and indirect basal ganglia loops. The entire cortex projects to the basal ganglia. The diagram shows the direct and indirect pathways through the basal ganglia. Excitatory pathways are shown in green and inhibitory in red. Pathways that are common to the direct and indirect pathways are shown by solid green arrows. The cortex projects to the striatum (arrows a, b). Cortical axons terminate on inhibitory neurons of either the caudate or putamen, depending on their origin in the cortex. The direct pathway (heavy dotted line, solid arrowheads) projects from the striatum to GPi/SNr, which projects to the thalamus. Neurons in the GPi/SNr are inhibited by the direct pathway and therefore they can’t inhibit the thalamus (X, inhibited pathway). The thalamus remains free to project to the frontal cortex and back for movement. The indirect pathway (fine dotted line, silhouette arrowheads) takes a different route: from the striatum it projects and inhibits GPe. The GPe projects to the excitatory subthalamic nucleus (STN). The inhibited GPe leaves the STN free to activate GPi/SNr, which inhibits the thalamus, preventing activation and movement. Dopaminergic pathways from the substantia nigra pars compacta (SNc) have opposite effects on the two parallel pathways because they interact with distinct types of dopamine receptors found on neurons in the striatum. In the direct pathway, dopaminergic axons interact with D1 receptors, which are excitatory. In the indirect pathway, dopaminergic axons interact with D2 receptors, which are inhibitory. Abbreviations: GPe, external segment of the globus pallidus; GPi, internal segment of the globus pallidus. SNc, substantia nigra pars compacta; STN, subthalamic nucleus; MD, mediodorsal thalamic nucleus; VA, ventral anterior thalamic nucleus; VL, ventral lateral thalamic nucleus.
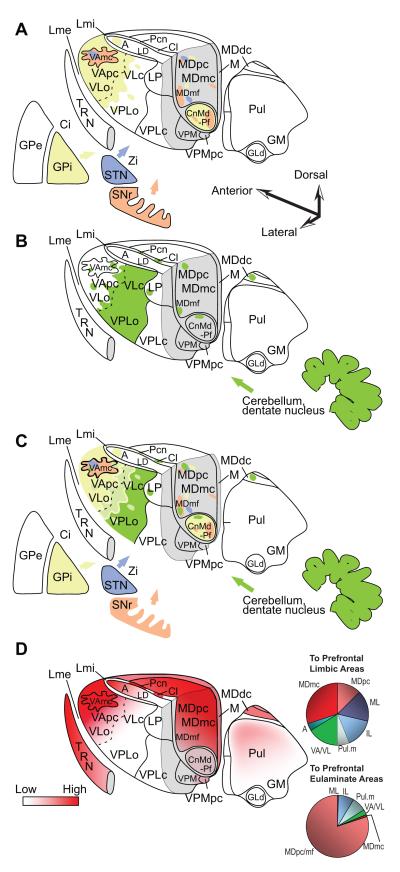
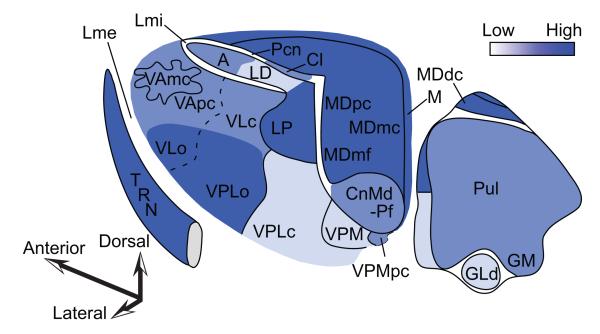
Figure 4.
Innervation of the primate thalamus from the basal ganglia, cerebellum, and connections with prefrontal cortices, based on pathway studies in non-human primates [e.g. (Francois, Tande, Yelnik, & Hirsch, 2002; Sidibe, Pare, & Smith, 2002; Rouiller, Liang, Babalian, Moret, & Wiesendanger, 1994; Sakai, Inase, & Tanji, 1996; Sakai, Stepniewska, Qi, & Kaas, 2000; Erickson, Melchitzky, & Lewis, 2004)]. A, Matched coloring shows projections from the output nuclei of the basal ganglia. B, Shows projections from the output of the cerebellum through the dentate nucleus. C, Overlap of A and B. D (left), Prefrontal connections with the thalamus. D (right), Relative distribution of thalamic projection neurons directed to cingulate and orbital limbic areas (top, limbic) and to the eulaminate areas 46, 8 and 9 (bottom). Quantitative data are from: (Barbas, Henion, & Dermon, 1991; Dermon & Barbas, 1994). Abbreviations: A, anterior nuclei; Ci, internal capsule; Cl, centrolateral nucleus; CnMd, centromedian nucleus; GLd, lateral dorsal nucleus geniculate; GM, medial nucleus geniculate; GPe, globus pallidus external; GPi, globus pallidus internal; LD, lateral dorsal nucleus; Lme, external lamina; Lmi, internal lamina; LP, lateral posterior nucleus; M, midline nuclei; MDdc/mc/mf/pc, the densocellular (dc), magnocellular (mc), multiform (mf), and parvicellular (pc) subdivisions of the mediodorsal (MD) nucleus; Pcn, paracentral nucleus; Pul, pulvinar nucleus; TRN, thalamic reticular nucleus; SNr, substantia nigra reticulata; STN, subthalamic nucleus; VAmc/pc, magnocellular (mc) and parvocellular (pc) subdivision of the ventral anterior (VA) nucleus; VL, ventrolateral nucleus; VLc, caudal ventrolateral nucleus; VLo, oral ventrolateral nucleus; VPLc, caudal subdivision of the ventral posterior-lateral nucleus; VPLo, oral subdivision of the ventral posterior-lateral nucleus;VPM, medial ventroposterior nucleus; VPMpc, parvocellular subdivision of the medial ventroposterior nucleus; Zi, zona incerta.
Detailed classical and recent neuroanatomical studies have established that the entire frontal cortex has a special relationship with the basal ganglia not enjoyed by other cortices. Thus, while all cortices project topographically to the input nuclei of the basal ganglia (the caudate or putamen), only the frontal cortex receives indirect feedback from the basal ganglia, mostly through the thalamic VL, VA and MD nuclei. This circuitry suggests that information from the entire cortex can be accessed selectively by the frontal cortex.
The relationship of the cortex with the basal ganglia is organized into two major loops that have opposite functions with respect to activation of motor (VL, VA) thalamic nuclei and MD, as summarized in Figure 3. A ‘direct’ pathway originates from excitatory cortical neurons in layer 5 and terminates on inhibitory neurons in the striatum (caudate or putamen). Striatal neurons innervate (and inhibit) the GPi/SNr, which are also inhibitory, resulting in disinhibition of thalamic nuclei. This circuit is thought to allow initiation of motor responses through bidirectional connections between the thalamus and frontal cortex. The indirect pathway originates from excitatory cortical neurons in layer 5 and innervates the striatum as well. But these striatal neurons project to the external segment of the globus pallidus (GPe), which innervates the excitatory subthalamic nucleus, which projects and activates inhibitory neurons of the GPi/SNr, resulting in inhibition of thalamic neurons (Fig. 3). The indirect pathway is thought to inhibit inappropriate movements.
The output of the basal ganglia to thalamic nuclei that project to frontal cortices likely has a key role in motor functions, including speech production, which depends on coordination of multiple cortical areas to translate sound into the complex process of speech. Parkinsonian patients have difficulty in initiating speech, which may be rooted to disruption of a loop through the basal ganglia, its projection to the thalamic nuclei VA/VL and MD and projection to premotor, and dorsal prefrontal cortices and the ACC (Xiao & Barbas, 2004). In this context, another thalamic nucleus, the anterior medial, which is connected robustly with limbic areas in the ACC, is innervated by the anterior tip of GPi as well (Xiao & Barbas, 2002b), which thus connects the basal ganglia with limbic cortices. This circuit may help explain the inability of Parkinsonian patients to change facial expression in emotional situations.
The basal ganglia have a key role in behavioral switching, timing and sequential processing [reviewed in (Lieberman, 2002; Graybiel, 2008; Coull, Cheng, & Meck, 2011)], which are essential components for language. Moreover, the basal ganglia show a high degree of plasticity as learned responses become habitual (Graybiel, 2008), as exemplified when children acquire language. However, debates abound as to whether the role of the basal ganglia in language is specific or assistive [e.g., (Chan, Ryan, & Bever, 2011)]. The problems of Parkinsonian patients in initiating speech, for example, may be part of the general problem of initiating any movement. In this context, the basal ganglia provide an excellent example of the use of a canonical circuitry in evolution for diverse functions (Lieberman, 2002). Parallel or partly overlapping circuits through the cortex and the basal ganglia underlie not only motor pr ocesses but also emotional and cognitive processes [reviewed in (Alexander, Delong, & Strick, 1986; Middleton & Strick, 2000; Haber, 2003; Graybiel, 2008)], which are essential for language. Problems with sentence processing in Parkinsonian patients may thus reflect disruption of circuits that link the striatum with frontal language areas 44/45, and the rostrally situated prefrontal cortex (Grossman, 1999).
Cerebellar role in language and its connections with language-related thalamic nuclei
Another major structure with a role in language is the cerebellum. Functional imaging studies have provided evidence that the lateral cerebellum, which has greatly expanded in evolution, is involved in high-order cognitive processes, including working memory, executive functions and language [reviewed in (Stoodley & Schmahmann, 2010)]. The cerebellar areas implicated in these functions include lobules IV, V and VI, which are linked with motor/premotor areas, and Crus I and II, which are connected with prefrontal and posterior parietal cortices [reviewed in (Stoodley & Schmahmann, 2010)].
The role of the cerebellum in language is intricately linked to a circuit that includes several thalamic nuclei associated with language, as described above, as well as with the projection zones of these thalamic nuclei to frontal language-related areas 44/45, adjacent dorsolateral prefrontal areas and medial areas in the ACC and the pre-SMA cortex. The cerebellar connections with frontal premotor and high-order association cortices are bidirectional and indirect, akin to the pattern described above for the basal ganglia. This indirect circuitry involves projections from the cortex to pontine nuclei, which project via the mossy fiber system to the cerebellar cortex. Return projections from the same cerebellar sites that receive frontal cortical input terminate in the dentate nucleus of the deep cerebellar nuclei [reviewed in (Strick, Dum, & Fiez, 2009)]. The deep cerebellar nuclei project to motor-related thalamic nuclei, including subdivisions of VL and ventral posterior lateral oralis (VPLo), area X, MD, and the intralaminar paracentral, central lateral and CnMd-Pf nuclei (Fig. 4B). These nuclei project widely to the frontal cortex, including the frontal language areas and the prefrontal cortex.
Using viral transneuronal tracers, it was found that prefrontal connections with the cerebellum are selective, involving mostly medial and lateral subdivisions of area 9, and dorsal area 46 [reviewed in (Strick, Dum, & Fiez, 2009)], the same areas that have the strongest connections with thalamic nuclei associated with the basal ganglia (Xiao & Barbas, 2004). Importantly, the ventral part of the deep cerebellar dentate nucleus, which is the source of transthalamic projections to contralateral areas 9 and dorsal area 46, also projects to the pre-SMA [reviewed in (Strick, Dum, & Fiez, 2009)]. Projections from the cerebellar cortex to the prefrontal cortex are contralateral, so that functional imaging studies involving linguistic processing or verbal working memory involve the right cerebellum and left frontal language areas and dorsolateral prefrontal cortices (Strick, Dum, & Fiez, 2009; Marvel & Desmond, 2010; Stoodley & Schmahmann, 2010).
Segregation and overlap of thalamic zones from the basal ganglia and cerebellum and connections with frontal cortex
Classical studies had emphasized that the basal ganglia and cerebellum target different parts of the thalamus, or at least different sectors of the same nuclei [for review of earlier literature and discussion see (Sakai, Inase, & Tanji, 1996)]. Figure 4A shows the main thalamic sites that receive the output of the basal ganglia and Figure 4B shows those that receive projections from the output of the cerebellum. Reevaluation of the relationship of projections from the two structures in macaque monkeys showed zones of preferential projections from the GPi to the ventral lateral oralis (VLo) and VA (especially its parvicellular sector, VApc), and from the cerebellar output to VPLo and area X. However, there were interdigitated and overlapping projections in the thalamus from these structures as well. The overall pattern suggests a mirror image in the principal thalamic targets of projections from each structure, with pallidal input being highest in VApc, followed by the ventral lateral oralis (VLo), ventral lateral caudalis (VLc), area X and VPLo, while projections from the cerebellar output show the opposite trend (Sakai, Inase, & Tanji, 1996). These relationships are depicted in the superimposed projections from the basal ganglia and the cerebellum in Figure 4C.
The above nuclei have robust connections with premotor and motor cortices. Ventral premotor areas situated within or behind the lower limb of the arcuate sulcus in macaque monkeys (areas 44, and subdivisions of ventral area 6), are connected with the VA as well as with the motor thalamic nuclei VLo, ventral lateral medialis (VLm) and area X (Djiok and Barbas, personal observations). The primary motor cortex (MI or area 4) has comparatively weaker connections with the VA and strong connections with the VL and VPLo (Barbas, Henion, & Dermon, 1991; Dermon & Barbas, 1994; McFarland & Haber, 2002).
The VA has bidirectional connections with premotor and motor cortices as well, as described in many studies [e.g., (Kievit & Kuypers, 1975; Kievit & Kuypers, 1977; Miyata & Sasaki, 1984; Jurgens, 1984; Wiesendanger & Wiesendanger, 1985; McFarland & Haber, 2002; Kultas-Ilinsky, Sivan-Loukianova, & Ilinsky, 2003)]. These connections involve VAmc as well as the VApc, with some degree of topographic specificity. For example, VApc has connections with the anterior part of dorsal premotor areas, including the SMA and the rostral part of the dorsal premotor cortex, as well as cingulate areas 24a-c. Caudal premotor areas have connections with the posterior part of VAmc.
Overlapping projections from the output of the basal ganglia and the cerebellum in the thalamus are also found in the lateral part of MD and in the intralaminar nuclei (Sakai, Inase, & Tanji, 1996), both of which have robust connections with dorsolateral prefrontal areas 9, 46 and 8. These cortices have a key role in cognitive operations, including working memory.
The thalamic nuclei associated with the prefrontal cortex are shown in Figure 4D. While the prefrontal cortex has classically been associated with the thalamic MD nucleus, it is also connected with several other nuclei in macaque monkeys, including the VA, VL, the anterior medial, intralaminar and midline nuclei and the medial pulvinar [e.g. (Kievit & Kuypers, 1977; Goldman-Rakic & Porrino, 1985; Preuss & Goldman-Rakic, 1987; Barbas, Henion, & Dermon, 1991; Morecraft, Geula, & Mesulam, 1992; Dermon & Barbas, 1994; Bachevalier, Meunier, Lu, & Ungerleider, 1997; Cavada, Company, Tejedor, Cruz-Rizzolo, & Reinoso-Suarez, 2000; Xiao & Barbas, 2002a; Xiao & Barbas, 2002b; Xiao & Barbas, 2004; Zikopoulos & Barbas, 2007b)]. Among these, the thalamic nuclei affected at least in some cases of thalamic aphasia include the VL, MD, VA, intralaminar CnMd-Pf and midline nuclei (Nadeau & Crosson, 1997; Carrera, Michel, & Bogousslavsky, 2004; Carrera & Bogousslavsky, 2006). Circuits that link these thalamic nuclei with the prefrontal cortices are thought to have a role in language function (Petrovici, 1980; Nadeau & Crosson, 1997).
The connections of the above thalamic nuclei with the prefrontal cortex are unequal in density (Fig. 4D, pie charts) and show variation in their laminar distribution in the prefrontal cortex, as elaborated below. Quantitative analysis has shown that limbic prefrontal cortices, situated on the medial surface in the ACC, and the posterior orbitofrontal cortex, have the most distributed thalamic connections. Lateral prefrontal cortices also have connections with many thalamic nuclei, but they receive input from a higher proportion of projection neurons from MD, and especially its parvicellular (MDpc) and multiform (MDmf) divisions (Barbas, Henion, & Dermon, 1991; Dermon & Barbas, 1994; Xiao, Zikopoulos, & Barbas, 2009).
The thalamic VA nucleus is a common link for prefrontal and premotor cortices
The VA nucleus is of particular interest because in primates it has dense common connections with prefrontal as well as premotor cortices (Fig. 5), including areas that are associated with language processes. Consequently, the VA is considered here in more detail, but the laminar patterns of connections extend to other motor-related nuclei (subdivisions of VL and VPLo), as well as MD. Classic studies have established that all prefrontal cortices are connected with the VA [e.g., (Kievit & Kuypers, 1977; Kunzle, 1978b; Ilinsky, Jouandet, & Goldman-Rakic, 1985; Preuss & Goldman-Rakic, 1987; Yeterian & Pandya, 1988; Chiba, Kayahara, & Nakano, 2001; McFarland & Haber, 2002); reviewed in (Cavada, Company, Tejedor, Cruz-Rizzolo, & Reinoso-Suarez, 2000)]. Moreover, there is a degree of topographic specificity in the connections, which involve mostly VAmc (Dermon & Barbas, 1994), with preferential projection of the ventral sector of VA to orbitofrontal cortices, and the dorsal sector to dorsolateral prefrontal areas (Barbas, Henion, & Dermon, 1991). More recent findings indicate that the prefrontal projections to the VA are more extensive than previously thought.
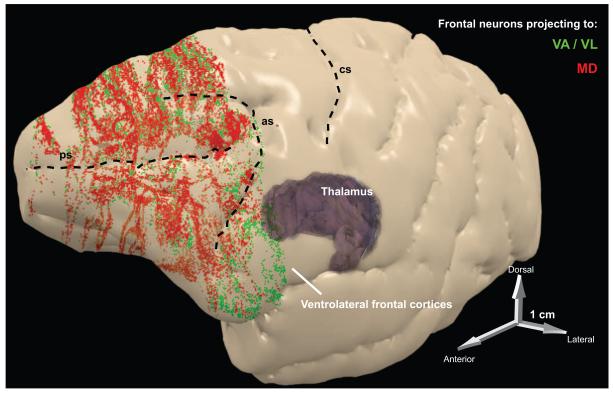
Figure 5.
Summary of frontal projections to thalamic nuclei associated with language. Three-dimensional reconstruction of the left hemisphere of the rhesus monkey cortex, which was rendered semi-transparent to show frontal neurons in layers 5 and 6 that project to MD (red) or to VA/VL (green). The projection zones include prefrontal areas (in front of the arcuate sulcus), as well as premotor areas situated behind the upper and lower limbs of the arcuate sulcus (as). Abbreviations: as, arcuate sulcus; cs, central sulcus; MD, mediodorsal nucleus; ps, principal sulcus; VA, ventral anterior nucleus; VL, ventrolateral nucleus.
Importantly, dorsolateral prefrontal cortices (areas 8 and 9) and the ACC (areas 24 and 32) have the most robust connections with the VA (Xiao & Barbas, 2004; Xiao, Zikopoulos, & Barbas, 2009). The ACC has a key role in vocalization and emotional communication [reviewed in (Vogt & Barbas, 1988)], exemplified by separation calls emitted when infant monkeys become separated from their mother (Barbas, 2000b). Humans with damage to the ACC exhibit the akinetic mute syndrome, characterized by inability to initiate speech even though they can speak (Devinsky, Morrell, & Vogt, 1995), which resembles the classical neglect syndrome within the motor domain (Xiao & Barbas, 2004). Based on its robust connections with all prefrontal as well as premotor cortices, the VA may be a central link for structures that underlie cognition, attention, emotions and action.
The connections between thalamus and frontal cortex have a specific laminar organization
The above discussion highlights the topographic specificity of pathways linking thalamic nuclei with the frontal cortex. However, the laminar relationships of these connections are also important for understanding thalamo-cortical recruitment in behavi or. The classical circuit that links the thalamus with the cortex involves a projection from the thalamus that terminates in the middle layers (Fig. 6). In the primary visual cortex, the thalamic projection from the lateral geniculate nucleus terminates exclusively within the highly specialized layer 4. However, in all other cortices, the classical thalamo-cortical projection terminates in layer 4, as well as the lower part of layer 3 and the upper part of layer 5. We refer to this projection as termination in the middle layers. The reciprocal projection from the cortex to the thalamus arises from layer 6 in all cortical systems [reviewed in (Steriade, Jones, & McCormick, 1997; Jones, 2007)].
Figure 6.
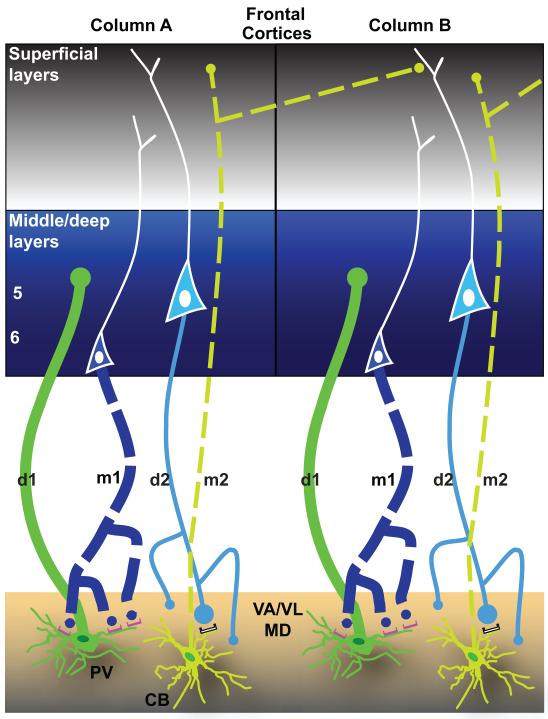
Two parallel systems link the thalamus with the cortex. Schematic diagram summarizes the features of reciprocal driving and modulatory corticothalamic pathways linking the frontal cortex with VA/VL thalamic nucleus. The thickness of the lines indicates the strength of the projection. The size of the dot denotes the size of the terminals. Solid lines (d1 and d2) represent driving pathways, and dotted lines (m1 and m2) represent modulatory pathways. Dark green represents PV, and light green CB thalamic projection neurons. There are two parallel circuits between prefrontal cortex and thalamus. One originates mostly from PV thalamic projection neurons (dark green, bottom panels), and terminates focally with large terminals in the middle layers (3b – 5a, center blue panels) of PFC (d1). In turn, layer 6 neurons project (m1) and terminate as numerous small terminals that form synapses mainly with PV thalamic projection neurons (dark green, bottom panels), through metabotropic receptors (mGluR1a, magenta). The other circuit originates in layer 5 (d2) and forms synapses through a mix of large and small terminals mainly on CB thalamic projection neurons (light green) with ionotropic receptors, white). The CB thalamic neurons, in turn, send widespread projections (m2) mainly to the superficial layers (1 – 3a) of frontal cortex (grey), and terminate mostly as small terminals that cross borders of neighboring regions and are intermingled with the apical dendrites of neighboring layer 5 neurons. All depicted pathways are excitatory and glutamatergic. Adapted from: Zikopoulos and Barbas, 2007b.
However, there is a parallel circuit that links the thalamus with the cortex. This frequently overlooked pathway has a complementary laminar distribution to the circuit described above (Fig. 6). In this circuit, a pathway from the thalamus terminates in layer 1, and in several prefrontal and premotor areas it also innervates layers 2 and the upper part of layer 3; we refer to this pathway as a projection to the upper layers. Projections to the thalamus from prefrontal cortices, in general, originate from cortical layer 5 as well as layer 6. The contribution of layer 5 to the thalamic projection varies depending on thalamic destination. For example, projection neurons from layer 5 directed to the thalamic MD or to anterior nuclei constitute about 20% of all prefrontal projection neurons, with the rest found in layer 6 (Xiao, Zikopoulos, & Barbas, 2009). Frontal projection neurons to motor thalamic nuclei have a different laminar distribution: they originate nearly as frequently from layer 5 as they do from layer 6 (Xiao & Barbas, 2004). In addition, frontal projection neurons that innervate VA/VL occupy primarily the upper part of layer 5, whereas projection neurons that innervate MD occupy the deep part of layer 5, showing a distinct sublaminar organization (Xiao, Zikopoulos, & Barbas, 2009).
The significance of this pattern is based on the fact that cortical layer 5 neurons project to other motor-related subcortical structures as well (Guillery, 1995). In frontal cortices the upper part of layer 5 includes the majority of projection neurons directed to the striatum in several species including primates (Selemon & Goldman-Rakic, 1985; Arikuni & Kubota, 1986; Goldman-Rakic & Selemon, 1986; Tanaka, Jr., 1987; Yeterian & Pandya, 1994; Thomson & Bannister, 2003). On the other hand, neurons in the deep part of layer 5 of frontal cortices project to several thalamic nuclei, such as the intralaminar, which then project widely to several cortical areas (Catsman-Berrevoets & Kuypers, 1978; Thomson & Bannister, 2003). This evidence suggests specificity in layer 5 projection neurons directed to VA/VL, on one hand, and to MD, on the other hand, which may be differentially recruited in behavior.
The highest proportion of layer 5 corticothalamic projection neurons in prefrontal cortices is found in dorsolateral areas (e.g., areas 9 and 46) and the ACC (e.g., areas 24 and 32). In non-human primates, VA and VL are strongly interconnected with ventrolateral prefrontal areas, spanning ventral area 46, ventral area 8, and area 12 (Fig. 5) (Xiao, Zikopoulos, & Barbas, 2009). The homologue of human area 45 in rhesus monkeys is situated between these regions (Petrides & Pandya, 2002; Schenker et al., 2008; Petrides & Pandya, 2009). All of these areas are robustly interconnected in primates, participating in circuits involved in cognitive and attentional functions. Area 9 is situated at the cusp of premotor and prefrontal areas and has a role in planning sequential tasks requiring response monitoring and attentional control (Goldman-Rakic, 1987; Fuster, 1993; Petrides, 1995; Petrides, 1996; Hikosaka et al., 1999; Sakai et al., 1999; Levy & Goldman-Rakic, 2000). Area 9 is connected with premotor and ACC areas, which are engaged when cognitive demands are high, such as monitoring performance during conflicting events (Devinsky, Morrell, & Vogt, 1995; Botvinick, Nystrom, Fissell, Carter, & Cohen, 1999; Carter et al., 2000; Miller & Cohen, 2001; Paus, 2001; Milham et al., 2001; Walton, Croxson, Behrens, Kennerley, & Rushworth, 2007; Brown & Braver, 2008). The high proportion of projection neurons in layer 5 in dorsolateral and medial prefrontal areas may have a role in processing sequential events for action, mechanisms that are essential for the motor and cognitive aspects of language.
The significance of the above observations is also based on the laminar specialization of the cortex at the level of cortical columns. For example, the thalamo-cortical projection to the middle layers is focal. On the other hand, the parallel projection to the upper layers is widespread (Fig. 6). Moreover, the upper part of cortical layer 5 includes mostly large pyramidal neurons which have long apical dendrites that reach layer 1; they also have long horizontal dendrites that extend laterally over several millimeters within layer 5 (Mountcastle, 1997; Tardif, Probst, & Clarke, 2007), and are thought to have a role in binding information across cortical columns (Wiesel & Gilbert, 1989; Stettler, Das, Bennett, & Gilbert, 2002). Computational models predict that the middle-deep layers of prefrontal cortex store information temporarily, whereas the upper layers group items represented in deep layers, facilitating sequential processing in working memory (Grossberg & Pearson, 2008).
The two parallel circuits between thalamus and cortex have distinct func tional and neurochemical features
Functional and neurochemical features distinguish the two parallel circuits that link the thalamus with the cortex. In primates, each circuit contains a driving and a modulatory limb in mirror-like arrangement [reviewed in (Jones, 2007)]. A driving pathway has features suggesting assured transmission of a signal to the postsynaptic neuron. Driving pathways include those that carry sensory signals from the periphery to the thalamus, or motor commands from subcortical movement-related centers, and consist of focally projecting large synaptic terminals, which interact mostly with fast ionotropic glutamate receptors. A modulatory pathway modifies the overall activity of the recipient neuron without necessarily eliciting an action potential. Modulatory pathways are more abundant and widespread, and contain small boutons that predominantly interact with metabotropic glutamate receptors to modify neuronal activity (Guillery, 1995; Sherman & Guillery, 1998; Jones, 1998a; Jones, 1998b; Rouiller & Welker, 2000; Guillery & Sherman, 2002; Reichova & Sherman, 2004; Casagrande, Guillery, & Sherman, 2005).
Complementary loops are also found in the reciprocal pathways linking frontal cortices with their associated thalamic nuclei, including the VA and MD (Zikopoulos & Barbas, 2007b; Xiao, Zikopoulos, & Barbas, 2009). The driving limb of the first circuit (or loop) that links the thalamus with the prefrontal cortex, originates mainly from core neurons of the thalamus, which express the calcium-binding protein parvalbumin (PV). Thalamic PV neurons, which are excitatory, give rise to a pathway that projects focally through mostly large boutons, which innervate the middle layers of the cortex (Fig. 6, d1). PV thalamic neurons, in turn, are innervated by pyramidal neurons in layer 6 of the cortex, through small boutons that interact postsynaptically with metabotropic glutamate receptors (Fig. 6, m1). This feedback corticothalamic projection has a modulatory role and completes the first circuit.
The parallel circuit that originates from pyramidal neurons in cortical layer 5 innervates through large and small terminals thalamic projection neurons that mainly express the calcium-binding protein calbindin (CB). This cortical pathway interacts postsynaptically mostly with ionotropic glutamate receptors (Fig. 6, d2), driving activity of CB thalamic neurons. Thalamic CB neurons, which are also excitatory, constitute the ascending modulatory projection that innervates mainly the superficial layers of the cortex (Fig. 6, m2). This projection terminates expansively and mostly through small boutons in the superficial cortical layers, and especially layer 1, where it impinges on the apical dendrites of layer 5 pyramidal neurons from neighboring sites (von Stein, Chiang, & Konig, 2000; Larkum, Senn, & Luscher, 2004). This pattern of projection suggests spread of activation beyond the original focus (Haber & McFarland, 2001; McFarland & Haber, 2002; Zikopoulos & Barbas, 2007b). Activation of fast firing driver neurons in layer 5 of the cortex thus provides a mechanism for transmission of signals across several cortical areas (Guillery, 1995; Sherman & Guillery, 1998; Rouiller & Welker, 2000; Guillery & Sherman, 2002). This idea is in agreement with evidence that in prefrontal cortex, activation of layer 5 pyramidal neurons may initiate slow wave oscillations that propagate vertically to other layers (Sanchez-Vives & McCormick, 2000; Ulbert, Heit, Madsen, Karmos, & Halgren, 2004). Through these extensive thalamocortical projections to layer 1 of prefrontal cortices, circuits through the VA may interact with circuits through MD and other thalamocortical systems to process and integrate information used to plan, communicate, and execute flexible behaviors.
The significance of terminal size is based on evidence that large boutons have more mitochondria, more synaptic vesicles, and form larger synapses than small boutons (Pierce & Lewin, 1994; Shepherd & Harris, 1998; Germuska, Saha, Fiala & Barbas, 2006; Zikopoulos & Barbas, 2007b; Medalla & Barbas, 2009; Medalla & Barbas, 2010; Bunce & Barbas, 2011). Consistent with their increased mitochondrial content, which is activity dependent (Thomson, 2000), large boutons may be present in highly active networks. Physiologic and modeling studies have shown that the enlarged axon caliber of large boutons allows fast transmission, and increases the probability that multiple vesicles are released with each action potential (Rosenmund & Stevens, 1996; Murthy, Sejnowski, & Stevens, 1997). These properties suggest that large boutons are more efficient in activating their targets, conveying essential information. In contrast, feedback corticothalamic input from layer 6 neurons modulates thalamic activity through small boutons.
Studies in humans have shown that activation of the superficial layers of Broca’s area can spread over long distances both parallel to the cortical surface to neighboring sites, and also radially within cortical columns (Tardif, Probst, & Clarke, 2007). Disruption of the delicate balance of driving and modulatory frontal cortical and thalamic inputs and outputs can alter the linkage of frontal cortices with MD, VA/VL, the basal ganglia and cerebellum, and consequently disrupt motor and cognitive aspects of language. Such disturbances may include inability to initiate speech, as in Parkinson’s disease, or acquire language, as in a subgroup of autistic individuals (Tager-Flusberg & Caronna, 2007; Stefanatos & Baron, 2011).
The inhibitory TRN has a role in focusing attention through the thalamus and prefrontal cortex
To complete the description of the reciprocal loops linking the thalamus and the cortex it is necessary to take into account the inhibitory thalamic reticular nucleus (TRN), which occupies a strategic position between the cortex and the thalamus. The TRN, which has been implicated in thalamic lesions that disrupt language function [reviewed in (Nadeau & Crosson, 1997)], is a thin sheet of inhibitory neurons that covers the dorsal thalamus like a veil. The TRN receives projections from all cerebral cortices and their associated thalamic nuclei in a topographic manner, but sends inhibitory output only to the thalamus [reviewed in (Zikopoulos & Barbas, 2007a); Fig. 7]. Through this circuitry, the TRN is poised to intercept and gate thalamocortical communication and filter signals through the thalamus at an early stage of processing, enabling focused attention [(Crick, 1984; Montero, 1997; Weese, Phillips, & Brown, 1999; McAlonan, Cavanaugh, & Wurtz, 2008; Petrof & Brown, 2010); reviewed in (Jones, 2007)].
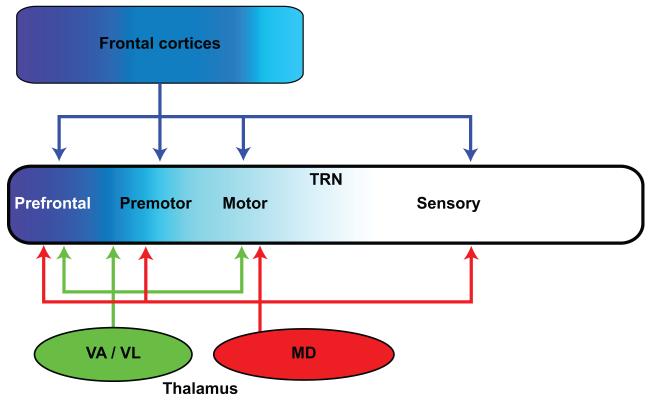
Figure 7.
Frontal cortical and associated thalamic nuclei project to TRN. Frontal cortices and the VA/VL thalamic nuclei predominantly target the frontal sectors of TRN (prefrontal, premotor, and motor; shades of blue). Some dorsolateral prefrontal cortices (areas 9 and 46), and their principal thalamic nucleus, MD, target all TRN sectors, including the posterior sensory-related regions.
Projections from sensory and motor cortices and their associated thalamic nuclei innervate distinct TRN sites, forming topographically-organized, modality-specific sectors [reviewed in (Pinault, 2004)]. These sectors form limbic, motor, visceral and somatosensory, visual and auditory subdivisions, found consecutively along the anteroposterior axis of TRN [reviewed in (Zikopoulos & Barbas, 2007a)]. Projections from sensory and motor cortices to TRN originate from layer 6 and terminate exclusively as small terminals, suggesting a modulatory function [reviewed in (Zikopoulos & Barbas, 2007a)].
Prefrontal cortices and their associated thalamic nuclei, including MD, the anterior medial (AM), and VA/VL strongly project to the frontal/limbic sector of TRN in rhesus monkeys (Zikopoulos & Barbas, 2006). However, dorsolateral prefrontal areas 46 and 9, and their principal MD thalamic nucleus, innervate all sectors of TRN (Fig. 7). This pattern differs from primary sensory, motor, or other association cortices and their thalamic nuclei, whose projections are largely confined to their respective TRN sectors. In addition, prefrontal cortices issue significant driver-like projections to TRN (~10%), which terminate as large boutons and may originate from large layer 5 pyramidal neurons that project to the thalamus (Zikopoulos & Barbas, 2006; Zikopoulos & Barbas, 2007b; Xiao, Zikopoulos, & Barbas, 2009) . These unique prefrontal projections to TRN may allow prefrontal cortices and MD to exert top-down control on sensory and other signals passing through the thalamus, making it possible to shift rapidly attention to relevant stimuli and suppress distracters (Zikopoulos & Barbas, 2006; Barbas & Zikopoulos, 2007; Zikopoulos & Barbas, 2007a). As discussed above, the MD thalamic nucleus is a major recipient of the output of the basal ganglia as well as the cerebellum. In addition, the amygdala, which projects to MD and most robustly to posterior orbitofrontal cortex and the ACC, also projects widely to TRN (Zikopoulos & Barbas, 2012). The strength of this projection suggests a powerful influence in shifting attention to emotional stimuli.
Frontal-related thalamic nuclei are modulated by dopamine
Significant modulatory systems originate from neurotransmitter-specific systems from the brainstem and basal forebrain and innervate the thalamus as well as the frontal and other cortices. A detailed description of the specific innervation by these systems is beyond the scope of this review, with the exception of the dopaminergic system, which represents a specialization in primates and likely affects language function in humans. As discussed above and illustrated in Figure 3, dopamine has significant and distinct roles in the function of the basal ganglia. In addition, in non-human primates thalamic nuclei that are connected with frontal cortices are innervated by dopaminergic axons (Fig. 8).
Figure 8.
Dopaminergic fiber distribution in the primate thalamus. Dark shades represent high, and light shades represent sparser innervation. For list of abbreviations see legend to Figure 4.
The dopaminergic innervation to the thalamus can be divided into pathways that express the dopamine transporter (DAT) and those that do not. The VA, VL and MD are among thalamic nuclei that receive the densest innervation from DAT-expressing axons, whose origin has been traced mostly to mesencephalic dopaminergic groups of the ventral tegmental area. The thalamic CnMd-Pf nucleus receives a moderate innervation of DAT expressing axons. In contrast, while midline nuclei receive a dense dopaminergic innervation, it is mediated through axons that are either devoid or sparsely labeled for DAT, and originate mostly from hypothalamic dopaminergic cell groups (Sanchez-Gonzalez, Garcia-Cabezas, Rico, & Cavada, 2005). In the human thalamus, VA, VL and MD are also innervated by DAT-expressing axons, whose density is even higher than in the monkey in several sites (Garcia-Cabezas, Rico, Sanchez-Gonzalez, & Cavada, 2007). In both the monkey and human thalamus the sectors of TRN that are connected with VA, VL and MD have dense plexuses of DAT-expressing axons (Sanchez-Gonzalez, Garcia-Cabezas, Rico, & Cavada, 2005; Garcia-Cabezas, Rico, Sanchez-Gonzalez, & Cavada, 2007). The significance of this pattern is based on evidence that DAT controls the spatial extent and time that dopamine remains in the extracellular space (Cragg & Rice, 2004). This evidence suggests that dopamine signaling in VA, VL, MD and TRN may be more spatially and temporally restricted than in the midline nuclei.
Interestingly, the dopaminergic innervation of the rat thalamus is scant and restricted to small groups of axons in MD and the midline paraventricular nucleus (Groenewegen, 1988; Garcia-Cabezas, Martinez-Sanchez, Sanchez-Gonzalez, Garzon, & Cavada, 2009). Thus, there seems to be an expansion in the extent and density of the dopaminergic innervation in the primate dorsal thalamus, which prominently involves the set of thalamic nuclei associated with language functions (Garcia-Cabezas, Rico, Sanchez-Gonzalez, & Cavada, 2007). The expansion of the dopaminergic innervation in primates parallels the expansion of dopaminergic innervation of the cerebral cortex (Berger, Gaspar, & Verney, 1991). Thus, the densely innervated thalamic territories, like VL, VA, MD, and midline nuclei, are connected with prefrontal cortices that are also innervated by dopaminergic fibers (Williams & Goldman-Rakic, 1993; Ciliax et al., 1999; Lewis et al., 2001), as well as the cortical motor/premotor system which has the densest dopaminergic innervation in the frontal cortex (Lewis, Campbell, Foote, Goldstein, & Morrison, 1987).
Concluding remarks on the thalamic hub that links the basal ganglia, cerebellum and frontal cortices associated with language
The motor related thalamic nuclei (subdivisions of VL, VA and VPLo) and MD link the output of the basal ganglia and cerebellum with the frontal cortex, in circuits that are engaged for motor and cognitive aspects of language. For example, inner speech and retrieval of appropriate words for fluent speech activate right language-related cerebellar sites as well as left dorsolateral prefrontal areas associated with working memory (Canales et al., 2002; Strick, Dum, & Fiez, 2009; Marvel & Desmond, 2010; Murdoch, 2010). Functions associated with the basal ganglia, such as sequential processing and behavioral switching, are also necessary for fluent speech as they are for motor functions with which they have been classically associated. The basal ganglia have an integral role in learning and plasticity, so that routines of every day life become habitual (Graybiel, 2005; Graybiel, 2008), a process that may extend to the acquisition of language in children (Friederici, 2006).
The basal ganglia as well as the thalamus develop early in ontogeny relative to the cerebral cortex (Darlington, Dunlop, & Finlay, 1999), and especially Broca’s area, which shows a slow developmental course until past age 7 (Friederici, 2012). The cerebellum has a protracted developmental course, with an early start in embryogenesis and continued development until 1-2 years after birth in humans [reviewed in (ten Donkelaar, Lammens, Wesseling, Thijssen, & Renier, 2003)].
The relative contribution of the basal ganglia and the cerebellum in language is not well understood. In the context of circuits the two structures differ in an important way: the output of the cerebellum to the thalamus is excitatory, but for the basal ganglia it is inhibitory. Moreover, the basal ganglia have either disinhibitory effects on the thalamo-cortical system via the direct loop, or inhibitory effects via the indirect loop, as shown in Figure 3. This circuitry suggests that whereas the cerebellar output has a direct excitatory influence on the thalamo-cortical system, the output of the basal ganglia has a permissive effect on the thalamus, allowing communication with the frontal cortex and initiation of movement. In addition, the basal ganglia have a direct inhibitory effect on the thalamus, in a circuit that is thought to prevent inappropriate movements (Fig. 3).
Interestingly, the ‘projection’ of the cerebellum through the dentate nucleus to the thalamus and then to prefrontal cortex appears to be restricted to dorsolateral prefrontal cortices which are associated with working memory (Middleton & Strick, 2001). The restricted prefrontal-cerebellar projections differ from the ubiquitous ‘projection’ of the output of the basal ganglia through the thalamus to the frontal cortex. The strong association of the basal ganglia with prefrontal as well as motor and premotor cortices suggests control of movements such as speech articulation, which involves the centrally located mouth and larynx that must be controlled by both hemispheres. Haploinsufficiency of the FOXP2 gene disrupts structures that have a role in language processes, including the basal ganglia and the cerebellum, by affecting the coordination of movements associated with speech production [reviewed in (Bolhuis, Okanoya, & Scharff, 2010; Liegeois, Morgan, Connelly, & Vargha-Khadem, 2011; Enard, 2011)]. On the other hand, linguistic tasks that involve working memory engage the right lateral cerebellum and the left prefrontal cortex, consistent with the cerebellar projection to the dominant hemisphere for language (Gebhart, Petersen, & Thach, 2002). Interestingly, the volumes of the neocortex and cerebellum increase concomitantly and disproportionately with increase of brain size (Yopak et al., 2010). In ontogeny the cerebellum and dorsolateral prefrontal cortices develop/and or myelinate late (Flechsig, 1901; von Bonin, 1950; Yakovlev & Lecours, 1967), which may help explain their shared engagement in cognitive aspects of language. The most recently evolved cerebellar structures appear to link seamlessly and efficiently cognitive processes for the selection of language to motor processes for speech (Leiner, 2010).
Interestingly, the output of the cerebellum projects via the thalamus to the putamen, an input stage of the basal ganglia, as well as to the external segment of the globus pallidus (GPe), via a trisynaptic projection (Hoshi, Tremblay, Feger, Carras, & Strick, 2005), suggesting some degree of collaboration between these structures for a variety of functions [see also (Doyon, Penhune, & Ungerleider, 2003)]. Partial segregation as well as partial overlap of the output of the cerebellum and basal ganglia in the thalamus is also consistent with the idea that the two structures have complementary as well as overlapping functions in language. Working together through a set of thalamic nuclei and their frontal connections, the basal ganglia and the cerebellum make it possible to link smoothly streams of thought to effector systems for fluent articulation of language with the same finesse achieved for highly rehearsed movements.
Highlights.
Language-related thalamic nuclei link frontal areas, basal ganglia and cerebellum
Thalamo-frontal connections are organized in two parallel laminar-specific systems
The inhibitory thalamic reticular nucleus gates thalamo-cortical communication
Frontal language-related areas and thalamic nuclei are modulated by dopamine
Acknowledgements
Supported by grants from NIH (NINDS RO1 NS024760 and NIMH MH057414) and NSF (CELEST). M.Á. García-Cabezas received a fellowship (Grant for Research in Foreign Universities and Centers from Fundación Alfonso Martín Escudero (Spain).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander GE, Delong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annual Review of Neuroscience. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Anderson ME. Pallidal and cortical detriments of thalamic activity. In: Kultas-Ilinsky K, Ilinsky IA, editors. Basal ganglia and thalamus in health and movement disorders. Kluwer Academic/ Plenum Publishers; New York: 2001. pp. 93–104. [Google Scholar]
- Arikuni T, Kubota K. The organization of prefrontocaudate projections and their laminar origin in the Macaque monkey: A retrograde study using HRP-Gel. Journal of Comparative Neurology. 1986;244:492–510. doi: 10.1002/cne.902440407. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Li BM. Neurobiology of executive functions: catecholamine influences on prefrontal cortical functions. Biological Psychiatry. 2005;57:1377–1384. doi: 10.1016/j.biopsych.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Bachevalier J, Meunier M, Lu MX, Ungerleider LG. Thalamic and temporal cortex input to medial prefrontal cortex in rhesus monkeys. Experimental Brain Research. 1997;115:430–444. doi: 10.1007/pl00005713. [DOI] [PubMed] [Google Scholar]
- Barbas H. Architecture and cortical connections of the prefrontal cortex in the rhesus monkey. Advances in Neurology. 1992;57:91–115. [PubMed] [Google Scholar]
- Barbas H. Complementary role of prefrontal cortical regions in cognition, memory and emotion in primates. Advances in Neurology. 2000a;84:87–110. [PubMed] [Google Scholar]
- Barbas H. Connections underlying the synthesis of cognition, memory, and emotion in primate prefrontal cortices. Brain Research Bulletin. 2000b;52:319–330. doi: 10.1016/s0361-9230(99)00245-2. [DOI] [PubMed] [Google Scholar]
- Barbas H, Ghashghaei H, Rempel-Clower N, Xiao D. Anatomic basis of functional specialization in prefrontal cortices in primates. In: Grafman J, editor. Handbook of Neuropsychology. 2 ed Vol. 7: The Frontal Lobes. Elsevier Science B.V; Amsterdam: 2002. pp. 1–27. [Google Scholar]
- Barbas H, Henion TH, Dermon CR. Diverse thalamic projections to the prefrontal cortex in the rhesus monkey. Journal of Comparative Neurology. 1991;313:65–94. doi: 10.1002/cne.903130106. [DOI] [PubMed] [Google Scholar]
- Barbas H, Zikopoulos B. The prefrontal cortex and flexible behavior. Neuroscientist. 2007;13:532–545. doi: 10.1177/1073858407301369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett MR, Hacker PM. Language and cortical function: conceptual developments. Progress in Neurobiology. 2006;80:20–52. doi: 10.1016/j.pneurobio.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Berger B, Gaspar P, Verney C. Dopaminergic innervation of the cerebral cortex: Unexpected differences between rodents and primates. Trends in Neuroscience. 1991;14:21–27. doi: 10.1016/0166-2236(91)90179-x. [DOI] [PubMed] [Google Scholar]
- Bolhuis JJ, Okanoya K, Scharff C. Twitter evolution: converging mechanisms in birdsong and human speech. Nature Reviews Neuroscience. 2010;11:747–759. doi: 10.1038/nrn2931. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;402:179–181. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- Brodmann K. Beitrage zur histologischen lokalisation der Grosshirnrinde. III. Mitteilung: Die Rindenfelde r der niederen Affen. Journal of Psychology and Neurology. 1905;4:177–266. [Google Scholar]
- Brown JW, Braver TS. A computational model of risk, conflict, and individual difference effects in the anterior cingulate cortex. Brain Research. 2008;1202:99–108. doi: 10.1016/j.brainres.2007.06.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunce JG, Barbas H. Prefrontal pathways target excitatory and inhibitory systems in memory-related medial temporal cortices. Neuroimage. 2011;55:1461–1474. doi: 10.1016/j.neuroimage.2011.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canales JJ, Capper-Loup C, Hu D, Choe ES, Upadhyay U, Graybiel AM. Shifts in striatal responsivity evoked by chronic stimulation of dopamine and glutamate systems. Brain. 2002;125:2353–2363. doi: 10.1093/brain/awf239. [DOI] [PubMed] [Google Scholar]
- Carrera E, Bogousslavsky J. The thalamus and behavior - Effects of anatomically distinct strokes. Neurology. 2006;66:1817–1823. doi: 10.1212/01.wnl.0000219679.95223.4c. [DOI] [PubMed] [Google Scholar]
- Carrera E, Michel P, Bogousslavsky J. Anteromedian, central, and posterolateral infarcts of the thalamus - Three variant types. Stroke. 2004;35:2826–2831. doi: 10.1161/01.STR.0000147039.49252.2f. [DOI] [PubMed] [Google Scholar]
- Carter CS, Macdonald AM, Botvinick M, Ross LL, Stenger VA, Noll D, Cohen JD. Parsing executive processes: strategic vs. evaluative functions of the anterior cingulate cortex. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:1944–1948. doi: 10.1073/pnas.97.4.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casagrande VA, Guillery RW, Sherman SM. Progress in Brain Research. Vol. 149. Elsevier; Amsterdam: 2005. Cortical function: A view from the thalamus. [Google Scholar]
- Catsman-Berrevoets CE, Kuypers HGJM. Differential laminar distribution of corticothalamic neurons projecting to the VL and the center median. An HRP study in the cynomolgus monkey. Brain Research. 1978;154:359–365. doi: 10.1016/0006-8993(78)90706-0. [DOI] [PubMed] [Google Scholar]
- Cavada C, Company T, Tejedor J, Cruz-Rizzolo RJ, Reinoso-Suarez F. The anatomical connections of the macaque monkey orbitofrontal cortex. A review. Cerebral Cortex. 2000;10:220–242. doi: 10.1093/cercor/10.3.220. [DOI] [PubMed] [Google Scholar]
- Chan SH, Ryan L, Bever TG. Role of the striatum in language: Syntactic and conceptual sequencing. Brain and Language. 2011 doi: 10.1016/j.bandl.2011.11.005. EPub Dec. 24. [DOI] [PubMed] [Google Scholar]
- Chiba T, Kayahara T, Nakano K. Efferent projections of infralimbic and prelimbic areas of the medial prefrontal cortex in the Japanese monkey, Macaca fuscata. Brain Research. 2001;888:83–101. doi: 10.1016/s0006-8993(00)03013-4. [DOI] [PubMed] [Google Scholar]
- Chouinard PA, Paus T. The primary motor and premotor areas of the human cerebral cortex. Neuroscientist. 2006;12:143–152. doi: 10.1177/1073858405284255. [DOI] [PubMed] [Google Scholar]
- Ciliax BJ, Drash GW, Staley JK, Haber S, Mobley CJ, Miller GW, Mufson EJ, Mash DC, Levey AI. Immunocytochemical localization of the dopamine transporter in human brain. Journal of Comparative Neurology. 1999;409:38–56. doi: 10.1002/(sici)1096-9861(19990621)409:1<38::aid-cne4>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Coull JT, Cheng RK, Meck WH. Neuroanatomical and neurochemical substrates of timing. Neuropsychopharmacology. 2011;36:3–25. doi: 10.1038/npp.2010.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragg SJ, Rice ME. DAncing past the DAT at a DA synapse. Trends in Neurosciences. 2004;27:270–277. doi: 10.1016/j.tins.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Crick F. Function of the thalamic reticular complex: the searchlight hypothesis. Proceedings of the National Academy of Sciences of the United States of America. 1984;81:4586–4590. doi: 10.1073/pnas.81.14.4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosson B. Subcortical mechanisms in language: lexical-semantic mechanisms and the thalamus. Brain and Cognition. 1999;40:414–438. doi: 10.1006/brcg.1999.1088. [DOI] [PubMed] [Google Scholar]
- Darlington RB, Dunlop SA, Finlay BL. Neural development in metatherian and eutherian mammals: variation and constraint. Journal of Comparative Neurology. 1999;411:359–368. [PubMed] [Google Scholar]
- Dermon CR, Barbas H. Contralateral thalamic projections predominantly reach transitional cortices in the rhesus monkey. Journal of Comparative Neurology. 1994;344:508–531. doi: 10.1002/cne.903440403. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118:279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- Doyon J, Penhune V, Ungerleider LG. Distinct contribution of the cortico-striatal and cortico-cerebellar systems to motor skill learning. Neuropsychologia. 2003;41:252–262. doi: 10.1016/s0028-3932(02)00158-6. [DOI] [PubMed] [Google Scholar]
- Dum RP, Strick PL. Motor areas in the frontal lobe of the primate. Physiology and Behavior. 2002;77:677–682. doi: 10.1016/s0031-9384(02)00929-0. [DOI] [PubMed] [Google Scholar]
- Enard W. FOXP2 and the role of cortico-basal ganglia circuits in speech and language evolution. Current Opinion in Neurobiology. 2011;21:415–424. doi: 10.1016/j.conb.2011.04.008. [DOI] [PubMed] [Google Scholar]
- Erickson SL, Melchitzky DS, Lewis DA. Subcorti cal afferents to the lateral mediodorsal thalamus in cynomolgus monkeys. Neuroscience. 2004;129:675–690. doi: 10.1016/j.neuroscience.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Flechsig P. Developmental (myelogenetic) localisation of the cerebral cortex in the human subject. The Lancet. 1901:1027–1029. [Google Scholar]
- Francois C, Tande D, Yelnik J, Hirsch EC. Distribution and morphology of nigral axons projecting to the thalamus in primates. Journal of Comparative Neurology. 2002;447:249–260. doi: 10.1002/cne.10227. [DOI] [PubMed] [Google Scholar]
- Friederici AD. What’s in control of language? Nature Neuroscience. 2006;9:991–992. doi: 10.1038/nn0806-991. [DOI] [PubMed] [Google Scholar]
- Friederici AD. Language development and the ontogeny of the dorsal pathway. Frontiers in Evolutionary Neuroscience. 2012;4:3. doi: 10.3389/fnevo.2012.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman L, Kenny JT, Wise AL, Wu D, Stuve TA, Miller DA, Jesberger JA, Lewin JS. Brain activation during silent word generation evaluated with functional MRI. Brain and Language. 1998;64:231–256. doi: 10.1006/brln.1998.1953. [DOI] [PubMed] [Google Scholar]
- Fuster JM. Frontal lobes. Current Opinion in Neurobiology. 1993;3:160–165. doi: 10.1016/0959-4388(93)90204-c. [DOI] [PubMed] [Google Scholar]
- Galaburda AM, LeMay M, Kemper TL, Geschwind N. Right-left asymmetries in the brain. Science. 1978;199:852–856. doi: 10.1126/science.341314. [DOI] [PubMed] [Google Scholar]
- Garcia-Cabezas MA, Martinez-Sanchez P, Sanchez-Gonzalez MA, Garzon M, Cavada C. Dopamine innervation in the thalamus: monkey versus rat. Cerebral Cortex. 2009;19:424–434. doi: 10.1093/cercor/bhn093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Cabezas MA, Rico B, Sanchez-Gonzalez MA, Cavada C. Distribution of the dopamine innervation in the macaque and human thalamus. Neuroimage. 2007;34:965–984. doi: 10.1016/j.neuroimage.2006.07.032. [DOI] [PubMed] [Google Scholar]
- Gebhart AL, Petersen SE, Thach WT. Role of the posterolateral cerebellum in language. Annals of the New York Academy of Sciences. 2002;978:318–333. doi: 10.1111/j.1749-6632.2002.tb07577.x. [DOI] [PubMed] [Google Scholar]
- Germuska M, Saha S, Fiala J, Barbas H. Synaptic distinction of laminar-specific prefrontal-temporal pathways in primates. Cerebral Cortex. 2006;16:865–75. doi: 10.1093/cercor/bhj030. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Motor control function of the prefrontal cortex. CIBA Foundation Symposium. 1987;132:187–200. doi: 10.1002/9780470513545.ch12. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Bates JF, Chafee MV. The prefrontal cortex and internally generated motor acts. Current Opinion in Neurobiology. 1992;2:830–835. doi: 10.1016/0959-4388(92)90141-7. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Porrino LJ. The primate mediodorsal (MD) nucleus and its projection to the frontal lobe. Journal of Comparative Neurology. 1985;242:535–560. doi: 10.1002/cne.902420406. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Selemon LD. Topography of corticostriatal projections in nonhuman primates and implications for functional parcellation of the neostiatum. In: Jones EG, Peters A, editors. Cerebral Cortex. Plenum Press; New York and London: 1986. pp. 447–466. [Google Scholar]
- Grafton ST, Fadiga L, Arbib MA, Rizzolatti G. Premotor cortex activation during observation and naming of familiar tools. Neuroimage. 1997;6:231–236. doi: 10.1006/nimg.1997.0293. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. The basal ganglia. Current Biology. 2000;10:R509–R511. doi: 10.1016/s0960-9822(00)00593-5. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. The basal ganglia: learning new tricks and loving it. Current Opinion in Neurobiology. 2005;15:638–644. doi: 10.1016/j.conb.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. Habits, rituals, and the evaluative brain. Annual Review of Neuroscience. 2008;31:359–387. doi: 10.1146/annurev.neuro.29.051605.112851. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ. Organization of the afferent connections of the mediodorsal thalamic nucleus in the rat, related to the mediodorsal-prefrontal topography. Neuroscience. 1988;24:379–431. doi: 10.1016/0306-4522(88)90339-9. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ. The basal ganglia and motor control. Neural Plasticity. 2003;10:107–120. doi: 10.1155/NP.2003.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewegen HJ, Berendse HW, Wolters JG, Lohman AH. The anatomical relationship of the prefrontal cortex with the striatopallidal system, the thalamus and the amygdala: evidence for a parallel organization. Progress in Brain Research. 1990;85:95–116. doi: 10.1016/s0079-6123(08)62677-1. [DOI] [PubMed] [Google Scholar]
- Grossberg S, Pearson LR. Laminar cortical dynamics of cognitive and motor working memory, sequence learning and performance: toward a unified theory of how the cerebral cortex works. Psychological Reviews. 2008;115:677–732. doi: 10.1037/a0012618. [DOI] [PubMed] [Google Scholar]
- Grossman M. Sentence processing in Parkinson’s disease. Brain and Cognition. 1999;40:387–413. doi: 10.1006/brcg.1999.1087. [DOI] [PubMed] [Google Scholar]
- Guillery RW. Anatomical evidence concerning the role of the thalamus in corticocortical communication: a brief review. Journal of Anatomy. 1995;187(Pt 3):583–592. [PMC free article] [PubMed] [Google Scholar]
- Guillery RW, Sherman SM. Thalamic relay functions and their role in corticocortical communication: generalizations from the visual system. Neuron. 2002;33:163–175. doi: 10.1016/s0896-6273(01)00582-7. [DOI] [PubMed] [Google Scholar]
- Haber S, McFarland NR. The place of the thalamus in frontal cortical-basal ganglia circuits. Neuroscientist. 2001;7:315–324. doi: 10.1177/107385840100700408. [DOI] [PubMed] [Google Scholar]
- Haber SN. The primate basal ganglia: parallel and integrative networks. Journal of Chemical Neuroanatomy. 2003;26:317–330. doi: 10.1016/j.jchemneu.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. Dorsal and ventral streams: a framework for understanding aspects of the functional anatomy of language. Cognition. 2004;92:67–99. doi: 10.1016/j.cognition.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. Opinion - The cortical organization of speech processing. Nature Reviews Neuroscience. 2007;8:393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Nakahara H, Rand MK, Sakai K, Lu X, Nakamura K, Miyachi S, Doya K. Parallel neural networks for learning sequential procedures. Trends in Neuroscience. 1999;22:464–471. doi: 10.1016/s0166-2236(99)01439-3. [DOI] [PubMed] [Google Scholar]
- Hirai T, Jones EG. A new parcellation of the human thalamus on the basis of histochemical staining. Brain Research, Brain Research Reviews. 1989;14:1–34. doi: 10.1016/0165-0173(89)90007-6. [DOI] [PubMed] [Google Scholar]
- Hoshi E, Tremblay L, Feger J, Carras PL, Strick PL. The cerebellum communicates with the basal ganglia. Nature Neuroscience. 2005;8:1491–1493. doi: 10.1038/nn1544. [DOI] [PubMed] [Google Scholar]
- Ilinsky IA, Jouandet ML, Goldman-Rakic PS. Organization of the nigrothalamocortical system in the rhesus monkey. Journal of Comparative Neurology. 1985;236:315–330. doi: 10.1002/cne.902360304. [DOI] [PubMed] [Google Scholar]
- Joel D, Weiner I. The organization of the basal ganglia-thalamocortical circuits: open interconnected rather than closed segregated. Neuroscience. 1994;63(2):363–379. doi: 10.1016/0306-4522(94)90536-3. [DOI] [PubMed] [Google Scholar]
- Johnson MD, Ojemann GA. The role of the human thalamus in language and memory: Evidence from electrophysiological studies. Brain and Cognition. 2000;42:218–230. doi: 10.1006/brcg.1999.1101. [DOI] [PubMed] [Google Scholar]
- Jones EG. A new view of specific and nonspecific thalamocortical connections. Advances in Neurology. 1998a;77:49–71. [PubMed] [Google Scholar]
- Jones EG. Viewpoint: the core and matrix of thalamic organization. Neuroscience. 1998b;85:331–345. doi: 10.1016/s0306-4522(97)00581-2. [DOI] [PubMed] [Google Scholar]
- Jones EG. The Thalamus. Cambridge University Press; New York: 2007. [Google Scholar]
- Jurgens U. The efferent and afferent connections of the supplementary motor area. Brain Research. 1984;300:63–81. doi: 10.1016/0006-8993(84)91341-6. [DOI] [PubMed] [Google Scholar]
- Kievit J, Kuypers HGJM. Subcortical afferents to the frontal lobe in the rhesus monkey studied by means of retrograde horseradish peroxidase transport. Brain Research. 1975;85:261–266. doi: 10.1016/0006-8993(75)90079-7. [DOI] [PubMed] [Google Scholar]
- Kievit J, Kuypers HGJM. Organization of the thalamo-cortical connexions to the frontal lobe in the rhesus monkey. Experimental Brain Research. 1977;29:299–322. doi: 10.1007/BF00236173. [DOI] [PubMed] [Google Scholar]
- Kultas-Ilinsky K, Sivan-Loukianova E, Ilinsky IA. Reevaluation of the primary motor cortex connections with the thalamus in primates. Journal of Comparative Neurology. 2003;457:133–158. doi: 10.1002/cne.10539. [DOI] [PubMed] [Google Scholar]
- Kunzle H. An autoradiographic analysis of the efferent connections from premotor and adjacent prefrontal regions (Areas 6 and 9) in Macaca fascicularis. Brain Behavior and Evolution. 1978a;15:185–234. doi: 10.1159/000123779. [DOI] [PubMed] [Google Scholar]
- Kunzle H. Cortico-cortical efferents of primary motor and somatosensory regions of the cerebral cortex in Macaca fascicularis. Neuroscience. 1978b;3:25–39. [Google Scholar]
- Larkum ME, Senn W, Luscher HR. Top-down dendritic input increases the gain of layer 5 pyramidal neurons. Cerebral Cortex. 2004;14:1059–1070. doi: 10.1093/cercor/bhh065. [DOI] [PubMed] [Google Scholar]
- Leiner HC. Solving the mystery of the human cerebellum. Neuropsychology Review. 2010;20:229–235. doi: 10.1007/s11065-010-9140-z. [DOI] [PubMed] [Google Scholar]
- Levy R, Goldman-Rakic PS. Segregation of working memory functions within the dorsolateral prefrontal cortex. Experimental Brain Research. 2000;133:23–32. doi: 10.1007/s002210000397. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Campbell MJ, Foote SL, Goldstein M, Morrison JH. The distribution of tyrosine hydroxylase-immunoreactive fibers in primate neocortex is widespread but regionally specific. Journal of Neuroscience. 1987;7:279–290. doi: 10.1523/JNEUROSCI.07-01-00279.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Melchitzky DS, Sesack SR, Whitehead RE, Auh S, Sampson A. Dopamine transporter immunoreactivity in monkey cerebral cortex: Regional, laminar, and ultrastructural localization. Journal of Comparative Neurology. 2001;432:119–136. doi: 10.1002/cne.1092. [DOI] [PubMed] [Google Scholar]
- Lieberman P. On the nature and evolution of the neural bases of human language. American Journal of Physical Anthropology. 2002;(Suppl 35):36–62. doi: 10.1002/ajpa.10171. [DOI] [PubMed] [Google Scholar]
- Liegeois F, Morgan AT, Connelly A, Vargha-Khadem F. Endophenotypes of FOXP2: Dysfunction with in the human articulatory network. European Journal of Paediatric Neurology. 2011;15:283–288. doi: 10.1016/j.ejpn.2011.04.006. [DOI] [PubMed] [Google Scholar]
- Luebke J, Barbas H, Peters A. Effects of normal aging on prefrontal area 46 in the rhesus monkey. Brain Research Reviews. 2010;62:212–232. doi: 10.1016/j.brainresrev.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvel CL, Desmond JE. Functional topography of the cerebellum in verbal working memory. Neuropsychology Review. 2010;20:271–279. doi: 10.1007/s11065-010-9137-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlonan K, Cavanaugh J, Wurtz RH. Guarding the gateway to cortex with attention in visual thalamus. Nature. 2008;456:391–394. doi: 10.1038/nature07382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland NR, Haber SN. Thalamic relay nuclei of the basal ganglia form both reciprocal and nonreciprocal cortical connections, linking multiple frontal cortical areas. Journal of Neuroscience. 2002;22:8117–8132. doi: 10.1523/JNEUROSCI.22-18-08117.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medalla M, Barbas H. Synapses with inhibitory neurons differentiate anterior cingulate from dorsolateral prefrontal pathways associated with cognitive control. Neuron. 2009;61:609–620. doi: 10.1016/j.neuron.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medalla M, Barbas H. Anterior cingulate synapses in prefrontal areas 10 and 46 suggest differential influence in cognitive control. Journal of Neuroscience. 2010;30:16068–16081. doi: 10.1523/JNEUROSCI.1773-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Anatomical evidence for cerebellar and basal ganglia involvement in higher cognitive function. Science. 1994;266:458–461. doi: 10.1126/science.7939688. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Research, Brain Research Reviews. 2000;31:236–250. doi: 10.1016/s0165-0173(99)00040-5. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Cerebellar Projections to the Prefrontal Cortex of the Primate. Journal of Neuroscience. 2001;21:700–712. doi: 10.1523/JNEUROSCI.21-02-00700.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Basal-ganglia ‘projections’ to the prefrontal cortex of the primate. Cerebral Cortex. 2002;12:926–935. doi: 10.1093/cercor/12.9.926. [DOI] [PubMed] [Google Scholar]
- Milham MP, Banich MT, Webb A, Barad V, Cohen NJ, Wszalek T, Kramer AF. The relative involvement of anterior cingulate and prefrontal cortex in attentional control depends on nature of conflict. Brain Research, Cognitive Brain Research. 2001;12:467–473. doi: 10.1016/s0926-6410(01)00076-3. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Mitchell IJ, Cooper AJ, Griffiths MR. The selective vulnerability of striatopallidal neurons. Progress in Neurobiology. 1999;59:691–719. doi: 10.1016/s0301-0082(99)00019-2. [DOI] [PubMed] [Google Scholar]
- Miyata M, Sasaki K. Horseradish peroxidase studies on thalamic and striatal connections of the mesial part of area 6 in the monkey. Neuroscience Letters. 1984;49:127–133. doi: 10.1016/0304-3940(84)90148-4. [DOI] [PubMed] [Google Scholar]
- Montero VM. c-fos induction in sensory pathways of rats exploring a novel complex environment: shifts of active thalamic reticular sectors by predominant sensory cues. Neuroscience. 1997;76:1069–1081. doi: 10.1016/s0306-4522(96)00417-4. [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, Geula C, Mesulam MM. Cytoarchitecture and neural afferents of orbitofrontal cortex in the brain of the monkey. Journal of Comparative Neurology. 1992;323:341–358. doi: 10.1002/cne.903230304. [DOI] [PubMed] [Google Scholar]
- Mountcastle VB. The columnar organization of the neocortex. Brain. 1997;120(Pt 4):701–722. doi: 10.1093/brain/120.4.701. [DOI] [PubMed] [Google Scholar]
- Murdoch BE. The cerebellum and language: historical perspective and review. Cortex. 2010;46:858–868. doi: 10.1016/j.cortex.2009.07.018. [DOI] [PubMed] [Google Scholar]
- Murthy VN, Sejnowski TJ, Stevens CF. Heterogeneous release properties of visualized individual hippocampal synapses. Neuron. 1997;18:599–612. doi: 10.1016/s0896-6273(00)80301-3. [DOI] [PubMed] [Google Scholar]
- Nadeau SE, Crosson B. Subcortical aphasia. Brain and Language. 1997;58:355–402. doi: 10.1006/brln.1997.1707. [DOI] [PubMed] [Google Scholar]
- Olszewski J. The Thalamus of the Macaca mulatta . An Atlas for Use with the Stereotaxic Instrument. Karger,S; Basel, Switzerland: 1952. [Google Scholar]
- Parent M, Levesque M, Parent A. Two types of projection neurons in the internal pallidum of primates: single-axon tracing and three-dimensional reconstruction. Journal of Comparative Neurology. 2001;439:162–175. doi: 10.1002/cne.1340. [DOI] [PubMed] [Google Scholar]
- Paus T. Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nature Reviews Neuroscience. 2001;2:417–424. doi: 10.1038/35077500. [DOI] [PubMed] [Google Scholar]
- Petrides M. Functional organization of the human fr ontal cortex for mnemonic processing. Evidence from neuroimaging studies. Annals of the New York Academy of Sciences. 1995;769:85–96. doi: 10.1111/j.1749-6632.1995.tb38133.x. [DOI] [PubMed] [Google Scholar]
- Petrides M. Lateral frontal cortical contribution to memory. Seminars in the Neurosciences. 1996;8:57–63. [Google Scholar]
- Petrides M, Pandya DN. Comparative cytoarchitectonic analysis of the human and the macaque ventrolateral prefrontal cortex and corticocortical connection patterns in the monkey. European Jouranl of Neuroscience. 2002;16:291–310. doi: 10.1046/j.1460-9568.2001.02090.x. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Distinct parietal and temporal pathways to the homologues of Broca’s area in the monkey1. PLoS Biology. 2009;7:e1000170. doi: 10.1371/journal.pbio.1000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrof I, Brown VJ. Attention to visual, but not tactile, properties of a stimulus results in activation of FOS protein in the visual thalamic reticular nucleus of rats. Behavioural Brain Research. 2010;211:248–252. doi: 10.1016/j.bbr.2010.03.045. [DOI] [PubMed] [Google Scholar]
- Petrovici JN. Speech Disturbances Following Stereotaxic Surgery in Ventrolateral Thalamus. Neurosurgery Review. 1980;3:189–195. doi: 10.1007/BF01647128. [DOI] [PubMed] [Google Scholar]
- Pierce JP, Lewin GR. An ultrastructural size principle. Neuroscience. 1994;58:441–446. doi: 10.1016/0306-4522(94)90071-x. [DOI] [PubMed] [Google Scholar]
- Pinault D. The thalamic reticular nucleus: structure, function and concept. Brain Research, Brain Research Reviews. 2004;46:1–31. doi: 10.1016/j.brainresrev.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Preuss TM, Goldman-Rakic PS. Crossed corticothalamic and thalamocortical connections of macaque prefrontal cortex. Journal of Comparative Neurology. 1987;257:269–281. doi: 10.1002/cne.902570211. [DOI] [PubMed] [Google Scholar]
- Pribram KH. The role of cortico-cortical connections. In: Lepore F, Ptito M, Jasper HH, editors. Two hemispheres, one brain: functions of the corpus callosum. Liss; New York: 1986. pp. 523–540. [Google Scholar]
- Price CJ. The anatomy of language: a review of 100 fMRI studies published in 2009. Cognitive Neuroscience. 2010;1191:62–88. doi: 10.1111/j.1749-6632.2010.05444.x. 2010. [DOI] [PubMed] [Google Scholar]
- Radanovic M, Scaff M. Speech and language disturbances due to subcortical lesions. Brain and Language. 2003;84:337–352. doi: 10.1016/s0093-934x(02)00554-0. [DOI] [PubMed] [Google Scholar]
- Reichova I, Sherman SM. Somatosensory corticothalamic projections: distinguishing drivers from modulators. Journal of Neurophysiology. 2004;92:2185–2197. doi: 10.1152/jn.00322.2004. [DOI] [PubMed] [Google Scholar]
- Rosenmund C, Stevens CF. Definition of the readily releasable pool of vesicles at hippocampal synapses. Neuron. 1996;16:1197–1207. doi: 10.1016/s0896-6273(00)80146-4. [DOI] [PubMed] [Google Scholar]
- Rosvold HE. The frontal lobe system: cortical-subcortical interrelationships. Acta Neurobiologiae Experimentalis (Warszaw) 1972;32:439–460. [PubMed] [Google Scholar]
- Rouiller EM, Liang F, Babalian A, Moret V, Wiesendanger M. Cerebellothalamocortical and pallidothalamocortical projections to the primary and supplementary motor cortical areas: a multiple tracing study in macaque monkeys. Journal of Comparative Neurology. 1994;345:185–213. doi: 10.1002/cne.903450204. [DOI] [PubMed] [Google Scholar]
- Rouiller EM, Welker E. A comparative analysis of the morphology of corticothalamic projections in mammals. Brain Research Bulletin. 2000;53:727–741. doi: 10.1016/s0361-9230(00)00364-6. [DOI] [PubMed] [Google Scholar]
- Sakai K, Hikosaka O, Miyauchi S, Sasaki Y, Fujimaki N, Putz B. Presupplementary motor area activation during sequence learning reflects visuo-motor association. Journal of Neuroscience. 1999;19:RC1. doi: 10.1523/JNEUROSCI.19-10-j0002.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai ST, Inase M, Tanji J. Comparison of cerebellothalamic and pallidothalamic projections in the monkey (Macaca fuscata): a double anterograde labeling study. Journal of Comparative Neurology. 1996;368:215–228. doi: 10.1002/(SICI)1096-9861(19960429)368:2<215::AID-CNE4>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Sakai ST, Stepniewska I, Qi HX, Kaas JH. Pallidal and cerebellar afferents to pre-supplementary motor area thalamocortical neurons in the owl monkey: a multiple labeling study. Journal of Comparative Neurology. 2000;417:164–180. [PubMed] [Google Scholar]
- Sanchez-Gonzalez MA, Garcia-Cabezas MA, Rico B, Cavada C. The primate thalamus is a key target for brain dopamine. Journal of Neuroscience. 2005;25:6076–6083. doi: 10.1523/JNEUROSCI.0968-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Vives MV, McCormick DA. Cellular and network mechanisms of rhythmic recurrent activity in neocortex. Nature Neuroscience. 2000;3:1027–1034. doi: 10.1038/79848. [DOI] [PubMed] [Google Scholar]
- Saur D, Kreher BW, Schnell S, Kummerer D, Kellmeyer P, Vry MS, Umarova R, Musso M, Glauche V, Abel S, Huber W, Rijntjes M, Hennig J, Weiller C. Ventral and dorsal pathways for language. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:18035–18040. doi: 10.1073/pnas.0805234105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenker NM, Buxhoeveden DP, Blackmon WL, Amunts K, Zilles K, Semendeferi K. A comparative quantit ative analysis of cytoarchitecture and minicolumnar organization in Broca’s area in humans and great apes. Journal of Comparative Neurology. 2008;510:117–128. doi: 10.1002/cne.21792. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD. Vascular syndromes of the thalamus. Stroke. 2003;34:2264–2278. doi: 10.1161/01.STR.0000087786.38997.9E. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Goldman-Rakic PS. Longitudinal topography and interdigitation of corticostriatal projections in the rhesus monkey. Journal of Neuroscience. 1985;5:776–794. doi: 10.1523/JNEUROSCI.05-03-00776.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd GM, Harris KM. Three-dimensional structure and composition of CA3-->CA1 axons in rat hippocampal slices: implications for presynaptic connectivity and compartmentalization. Journal of Neuroscience. 1998;18:8300–8310. doi: 10.1523/JNEUROSCI.18-20-08300.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SM, Guillery RW. On the actions that one nerve cell can have on another: distinguishing “drivers” from “modulators”. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:7121–7126. doi: 10.1073/pnas.95.12.7121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidibe M, Pare JF, Smith Y. Nigral and pallidal inputs to functionally segregated thalamostriatal neurons in the centromedian/parafascicular intralaminar nuclear complex in monkey. Journal of Comparative Neurology. 2002;447:286–299. doi: 10.1002/cne.10247. [DOI] [PubMed] [Google Scholar]
- Stefanatos GA, Baron IS. The Ontogenesis of Language Impairment in Autism: A Neuropsychological Perspective. Neuropsychology Review. 2011;21:252–270. doi: 10.1007/s11065-011-9178-6. [DOI] [PubMed] [Google Scholar]
- Steriade M, Jones EG, McCormick DA. Thalamus - Organisation and function. Elsevier Science; Oxford: 1997. [Google Scholar]
- Stettler DD, Das A, Bennett J, Gilbert CD. Lateral connectivity and contextual interactions in macaque primary visual cortex. Neuron. 2002;36:739–750. doi: 10.1016/s0896-6273(02)01029-2. [DOI] [PubMed] [Google Scholar]
- Stoodley CJ, Schmahmann JD. Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex. 2010;46:831–844. doi: 10.1016/j.cortex.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowe LA, Haverkort M, Zwarts F. Rethinking the neurological basis of language. Lingua. 2005;115:997–1042. [Google Scholar]
- Strick PL, Dum RP, Fiez JA. Cerebellum and nonmotor function. Annual Review of Neuroscience. 2009;32:413–434. doi: 10.1146/annurev.neuro.31.060407.125606. [DOI] [PubMed] [Google Scholar]
- Tager-Flusberg H, Caronna E. Language disorders: Autism and other pervasive developmental disorders. Pediatric Clinics of North America. 2007;54:469. doi: 10.1016/j.pcl.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Tanaka D., Jr. Differential laminar distribution of corticostriatal neurons in the prefrontal and pericruciate gyri of the dog. Journal of Neuroscience. 1987;7:4095–4106. doi: 10.1523/JNEUROSCI.07-12-04095.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardif E, Probst A, Clarke S. Laminar specificity of intrinsic connections in Broca’s area. Cerebral Cortex. 2007;17:2949–2960. doi: 10.1093/cercor/bhm021. [DOI] [PubMed] [Google Scholar]
- ten Donkelaar HJ, Lammens M, Wesseling P, Thijssen HO, Renier WO. Development and developmental disorders of the human cerebellum. Journal of Neurology. 2003;250:1025–1036. doi: 10.1007/s00415-003-0199-9. [DOI] [PubMed] [Google Scholar]
- Thomson AM. Molecular frequency filters at central synapses. Progress in Neurobiology. 2000;62:159–196. doi: 10.1016/s0301-0082(00)00008-3. [DOI] [PubMed] [Google Scholar]
- Thomson AM, Bannister AP. Interlaminar connections in the neocortex. Cerebral Cortex. 2003;13:5–14. doi: 10.1093/cercor/13.1.5. [DOI] [PubMed] [Google Scholar]
- Toga AW, Thompson PM. Mapping brain asymmetry. Nature Reviews Neuroscience. 2003;4:37–48. doi: 10.1038/nrn1009. [DOI] [PubMed] [Google Scholar]
- Ulbert I, Heit G, Madsen J, Karmos G, Halgren E. Laminar analysis of human neocortical interictal spike generation and propagation: current source density and multiunit analysis in vivo. Epilepsia. 2004;45:Suppl–4. doi: 10.1111/j.0013-9580.2004.04011.x. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Barbas H. Structure and connections of the cingulate vocalization region in the rhesus monkey. In: Newman JD, editor. The Physiological Control of Mammalian Vocalization. Plenum Publ. Corp; New York: 1988. pp. 203–225. [Google Scholar]
- von Bonin G. Essay on the cerebral cortex. Thomas,C.C.; Springfield,Illinois: 1950. [Google Scholar]
- von Stein A, Chiang C, Konig P. Top-down processing mediated by interareal synchronization. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:14748–14753. doi: 10.1073/pnas.97.26.14748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton ME, Croxson PL, Behrens TE, Kennerley SW, Rushworth MF. Adaptive decision making and value in the anterior cingulate cortex. Neuroimage. 2007;36(Suppl 2):T142–T154. doi: 10.1016/j.neuroimage.2007.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward AA, Jr., McCulloch WS. The projection of the frontal lobe on the hypothalamus. Journal of Neurophysiology. 1947;10:309–314. doi: 10.1152/jn.1947.10.4.309. [DOI] [PubMed] [Google Scholar]
- Weese GD, Phillips JM, Brown VJ. Attentional orienting is impaired by unilateral lesions of the thalamic reticular nucleus in the rat. Journal of Neuroscience. 1999;19:10135–10139. doi: 10.1523/JNEUROSCI.19-22-10135.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesel TN, Gilbert CD. Columnar specificity of intrinsic horizontal and corticocortical connections in cat visual cortex. Journal of Neuroscience. 1989;9:2432–2442. doi: 10.1523/JNEUROSCI.09-07-02432.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesendanger R, Wiesendanger M. The thalamic connections with medial area 6 (supplementary motor cortex) in the monkey (Macaca fascicularis) Experimental Brain Research. 1985;59:91–104. doi: 10.1007/BF00237670. [DOI] [PubMed] [Google Scholar]
- Williams SM, Goldman-Rakic PS. Characterization of the Dopaminergic Innervation of the Primate Frontal-Cortex Using A Dopamine-Specific Antibody. Cerebral Cortex. 1993;3:199–222. doi: 10.1093/cercor/3.3.199. [DOI] [PubMed] [Google Scholar]
- Xiao D, Barbas H. Pathways for emotions and memory I. input and output zones linking the anterior thalamic nuclei with prefontal cortices in the rhesus monkey. Thalamus and Related Systems. 2002a;2:21–32. [Google Scholar]
- Xiao D, Barbas H. Pathways for emotions and memory II. afferent input to the anterior thalamic nuclei from prefrontal, temporal, hypothalamic areas and the basal ganglia in the rhesus monkey. Thalamus and Related Systems. 2002b;2:33–48. [Google Scholar]
- Xiao D, Barbas H. Circuits through prefrontal cortex, basal ganglia, and ventral anterior nucleus map pathways beyond motor control. Thalamus & Related Systems. 2004;2:325–343. [Google Scholar]
- Xiao D, Zikopoulos B, Barbas H. Laminar and modular organization of prefrontal projections to multiple thalamic nuclei. Neuroscience. 2009;161:1067–1081. doi: 10.1016/j.neuroscience.2009.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakovlev PI, Lecours AR. The myelogenetic cycles of regional maturation of the brain. In: Minowski A, editor. Regional Development of the Brain in Early Life. Blackwell Scientific Publications; Oxford and Edinburgh: 1967. pp. 3–70. [Google Scholar]
- Yeterian EH, Pandya DN. Corticothalamic connections of paralimbic regions in the rhesus monkey. Journal of Comparative Neurology. 1988;269:130–146. doi: 10.1002/cne.902690111. [DOI] [PubMed] [Google Scholar]
- Yeterian EH, Pandya DN. Laminar origin of striatal and thalamic projections of the prefrontal cortex in rhesus monkeys. Experimental Brain Research. 1994;99:383–398. doi: 10.1007/BF00228975. [DOI] [PubMed] [Google Scholar]
- Yopak KE, Lisney TJ, Darlington RB, Collin SP, Montgomery JC, Finlay BL. A conserved pattern of brain scaling from sharks to primates. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:12946–12951. doi: 10.1073/pnas.1002195107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zikopoulos B, Barbas H. Prefrontal projections to the thalamic reticular nucleus form a unique circuit for attentional mechanisms. Journal of Neuroscience. 2006;26:7348–7361. doi: 10.1523/JNEUROSCI.5511-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zikopoulos B, Barbas H. Circuits for multisensory integration and attentional modulation through the prefrontal cortex and the thalamic reticular nucleus in primates. Reviews in the Neurosciences. 2007a;18:417–438. doi: 10.1515/revneuro.2007.18.6.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zikopoulos B, Barbas H. Parallel driving and modulatory pathways link the prefrontal cortex and thalamus. PLoS One. 2007b;2:e848. doi: 10.1371/journal.pone.0000848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zikopoulos B, Barbas H. Pathways for emotions and attention converge on the thalamic reticular nucleus in primates. Journal of Neuroscience. 2012;32:5338–5350. doi: 10.1523/JNEUROSCI.4793-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]