Abstract
Stroke is one of the most frequent causes of death and disability worldwide. Cerebral ischemia is the major insult of stroke and induces acute inflammation by triggering excessive production of proinflammatory cytokines, leading to the exacerbation of primary brain damage. Toll-like receptor (TLR)- and nitric oxide-mediated signaling pathways have been identified under ischemic stress. However, the interaction between these two pathways in controlling proinflammatory cytokines has not been well addressed during cerebral ischemia. Adult male C57BL/6 mice were subjected to middle cerebral artery occlusion (MCAO) for stroke induction. The MCAO procedure resulted in a significant infarct in the brain after 24 h. The infarcted side of the brain had marked elevation of TNF-α gene and protein expression, compared to the sham brain. The expression of CD14, a co-receptor of TLR4, was induced by MCAO, while the expression of TLR4 remained unchanged. The levels of inducible nitric oxide synthase (iNOS) and nitrotyrosine were also upregulated in the infracted side of the brain. Correspondingly, exposing murine microglial BV2 cells to hypoxia (1% O2) for 20 h resulted in an increased expression of TNF-α, CD14, iNOS, and nitrotyrosine. When BV2 cells were treated with L-canavanine, an iNOS selective inhibitor, the elevation of TNF-α and CD14 induced by hypoxia was inhibited. This inhibition was associated with an increase of IκB. These results suggest that the upregulation of TNF-α production in ischemic stroke is partially through increasing iNOS, and then CD14 expression leading to the activation of the NF-κB pathway in microglia.
Keywords: Cerebral ischemia, iNOS, CD14, TNF-α, microglia, L-canavanine
1. INTRODUCTION
Stroke is the third leading cause of death in industrialized countries killing nearly 145,000 people each year and affecting 7 million over the age of 20 in the United States alone (Lo et al., 2003; Donnan et al., 2008; Roger et al., 2012). Even patients surviving from stroke often require long-term rehabilitations, which cause very high socioeconomic impact (Donnan et al., 2008). Ischemic stroke accounts for 87% of stroke cases, which is caused by the occlusion of a blood vessel from a thrombus or embolism and results in an immediate loss of oxygen and nutrients delivered to the cerebral tissue (Roger et al., 2012). In the center of the ischemic territory, where the blood flow is almost ceased, cerebral tissue becomes severely damaged by rapid necrotic cell death. In the surrounding region, marked reduction of cerebral blood flow occurs and leads to elevations of intracellular cytosolic calcium concentration, release of excitatory amino acids, acidosis and production of toxic free-radicals that induce neuronal death within minutes and hours (Siesjo, 1992a, 1992b). Deterioration of brain tissue surrounding the ischemic core further progresses in days after the stroke (Stoll et al., 1998). A better understanding of the molecular mechanisms of pathogenic progression after ischemia is needed to develop therapeutic strategies for reducing further brain damage after stroke.
In the progression of ischemic stroke, an inflammatory response significantly contributes to the pathogenesis of brain injury by stimulating various cellular and molecular events, leading to neuron damage (Muir et al., 2007; Lakhan et al., 2009). Microglia are resident macrophages within the central nervous system (CNS) and are primarily involved in immune surveillance, but have macrophage-like capabilities including phagocytosis, inflammatory cytokine production, and antigen presentation after activation (Jordan et al., 2008; Lakhan et al., 2009). Toll-like receptors (TLRs)-mediated signaling pathway has a central role in the initiation of innate immunity. The ligands originally identified to interact with TLRs are invading microbial pathogens. Recently, it has been shown that TLRs also detect some endogenous ligands, such as degradation products of macromolecules, products of proteolytic cascades, intracellular components of ruptured cells and products of genes (Johnson et al., 2003; Lorne et al., 2010). Functional TLR signal transduction is complex and relies on receptor dimerization as well as the presence of accessory proteins and co-receptors (Okun et al., 2009; Zhang and Ghosh, 2000). After engaging with the receptors, several signaling pathways are activated. Among them, NF-κB pathway is the most distinctive, leading to the production of various inflammatory mediators, including cytokines, chemokines, and vasoactive peptides (Zhang and Ghosh, 2000).
Induction of oxidative stress and nitric oxide (NO) are the early events of stroke and play an important role in promoting the inflammatory response (Samdani et al., 1997; Wang et al., 2007). NO is generated catalytically in tissues by three isoforms of nitric oxide synthase (NOS). The constitutive form, present in neurons (nNOS) and endothelial cells (eNOS), is a calcium-dependent enzyme (Jordan et al., 2008; Samdani et al., 1997). The inducible form (iNOS), expressed in various cell types including microglia to respond to a wide variety of stimuli, is regulated mainly at the transcriptional level and does not require calcium for its activity (Galea et al., 1992). While the regulation of iNOS by inflammatory cytokines or lipopolysaccharide (LPS) has received considerable attention (Saha and Pahan, 2006; Galea et al., 1992), NO modulation of cytokine expression has yet to be fully explored.
In order to examine the correlation between TLR- and NO-mediated signaling pathways in regulating proinflammatory cytokine release during stroke, we applied an animal model of middle cerebral artery occlusion (MCAO). We first determined the expression levels of tumor necrosis factor-α (TNF-α), TLR4 and its co-receptor CD14, iNOS and nitrotyrosine in the ischemic brain. We then used murine microglial BV2 cells exposed to hypoxia to validate the observation in animals and further elucidate the interaction between CD14 and iNOS in the activation of NF-κB to regulate cytokine expression by a pharmacological agent.
2. RESULTS
2.1. Focal cerebral ischemia stimulates an inflammatory response in the brain
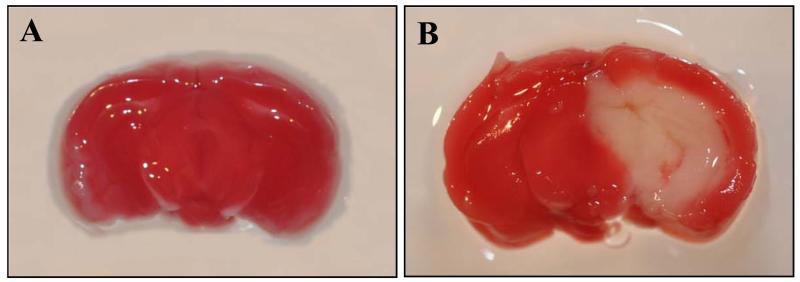
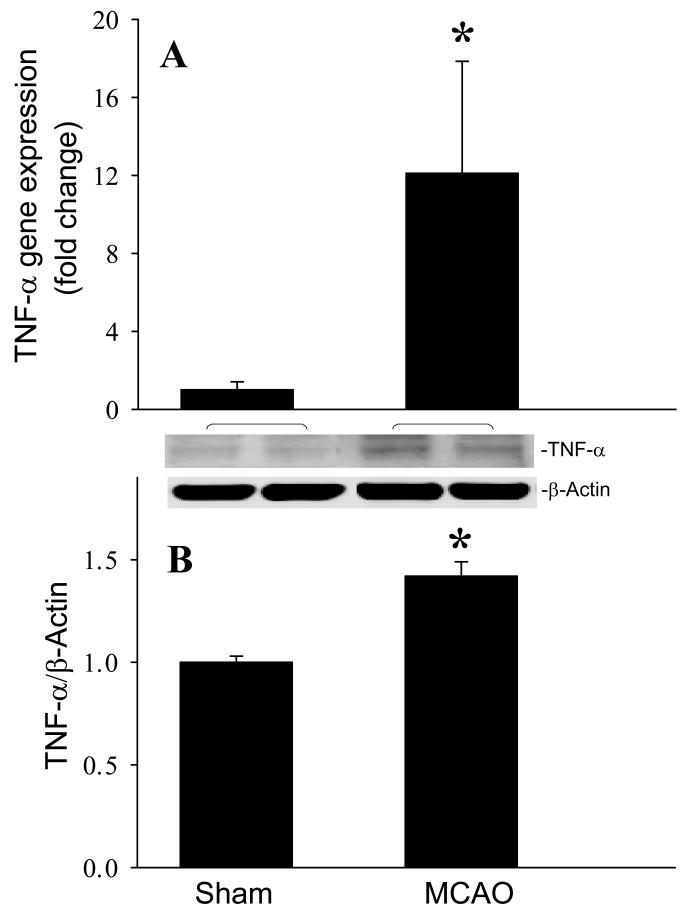
To confirm the induction of brain injury in the mouse after MCAO, we sectioned the whole brain tissue and stained with triphenyl tetrazolium chloride (TTC). The infarct area appeared pale after staining with TTC, while the viable brain tissue exhibited a plum red color. As the representative image shown in Fig. 1, the center section of the injured brain has an average of 31.0 ± 0.9% infarct area at 24 h after MCAO (n=5). We then examined the effect of cerebral ischemia on inflammation. At 24 h after MCAO, the gene and protein expression levels of TNF-α, a proinflammatory cytokine, were increased 12- and 1.4-fold, respectively, compared to sham-operated animals (P < 0.05, Fig. 2).
Fig. 1.
Induction of brain injury in the mouse after MCAO. Coronal slices of fresh brain tissue were stained with triphenyl tetrazolium chloride. Infarct area of the brain appears as pale staining on the slice and viable brain area shows plum red color. A representative brain slice from (A) sham-operated animals and (B) 24 h after subjecting to MCAO.
Fig. 2.
Induction of TNF-α production in the mouse brain after MCAO. Infracted side of the brain at 24 h after MCAO or sham-operated brain tissues were harvested for the analysis. (A) TNF-α gene expression level was determined by real-time RT-PCR analysis. (B) Intracellular TNF-α protein level was determined by Western blotting measurements. Representative blots against TNF-α (26 kDa) and β-actin (43 kDa) are shown. Blots were scanned and quantified with densitometry. The levels of gene and protein expression in the sham sample are designated as 1 for comparison purposes. Data are expressed as means ± SE (n = 4-5) and compared by Student-t test. *P < 0.05 vs. Sham.
2.2. Focal cerebral ischemia upregulates the CD14 receptor in the brain
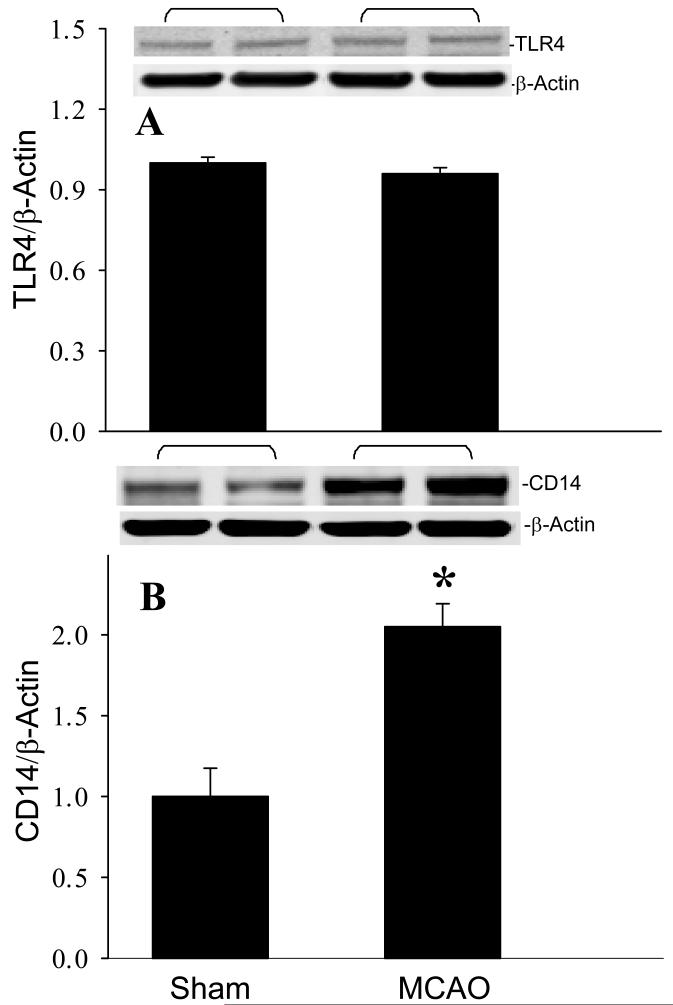
To identify the involvement of TLR in mediating the inflammatory response, we examined the effect of cerebral ischemia on the expression of TLR4 and its co-receptor, CD14 (Lorne et al., 2010). At 24 h after MCAO, there was no change in TLR4 protein expression (Fig. 3A). In contrast, the protein levels of CD14 in the ischemic brain tissue were 2.1-fold higher than those in the sham group (Fig. 3B). This result suggested that CD14 may play a role in regulating the inflammatory response during stroke.
Fig. 3.
Effect of MCAO operation on TLR4 and CD14 expression in the mouse brain. Infracted side of the brain at 24 h after MCAO or sham-operated brain tissues were harvested for the analysis. (A) TLR4 and (B) CD14 protein levels were determined by Western blotting measurements. Representative blots against TLR4 (110 kDa), CD14 (55 kDa), and β-actin are shown. Blots were scanned and quantified with densitometry. The levels of protein expression in the sham sample are designated as 1 for comparison purposes. Data are expressed as means ± SE (n = 4-5) and compared by Student-t test. *P < 0.05 vs. Sham.
2.3. Focal cerebral ischemia activates the NO generation pathway in the brain
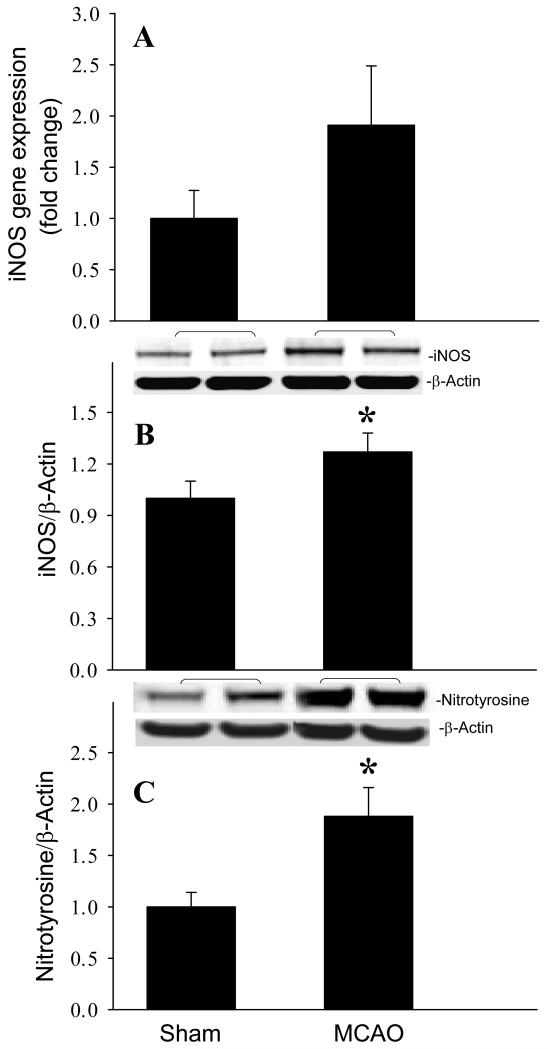
To determine the activation of NO production after cerebral ischemia, we first measured iNOS expression in the brain 24 h after MCAO. As shown in Fig. 4A and B, the gene and protein expression levels of iNOS were increased 1.9- and 1.3- fold, respectively, compared to the sham group. Nitrotyrosine is a product of tyrosine nitration mediated by reactive nitrogen species such as peroxynitrite anion and NO. We then examined the production of nitrotyrosine by Western blotting. Nitrotyrosine levels were elevated 1.9-fold in the brain 24 h after MCAO (Fig. 4C, P < 0.05), which corresponds to the upregulation of iNOS.
Fig. 4.
Induction of iNOS and nitrotyrosine expression in the mouse brain after MCAO. Infracted side of the brain at 24 h after MCAO or sham-operated brain tissues were harvested for the analysis. (A) iNOS gene expression level was determined by real-time RT-PCR analysis. (B) iNOS and (C) nitrotyrosine protein levels were determined by Western blotting measurements. Representative blots against iNOS (130 kDa), nitrotyrosine (65 kDa), and β-actin are shown. Blots were scanned and quantified with densitometry. The levels of gene and protein expression in the sham sample are designated as 1 for comparison purposes. Data are expressed as means ± SE (n = 4-5) and compared by Student-t test. *P < 0.05 vs. Sham.
2.4. Hypoxia induces cellular changes are related to the inflammatory response in microglia
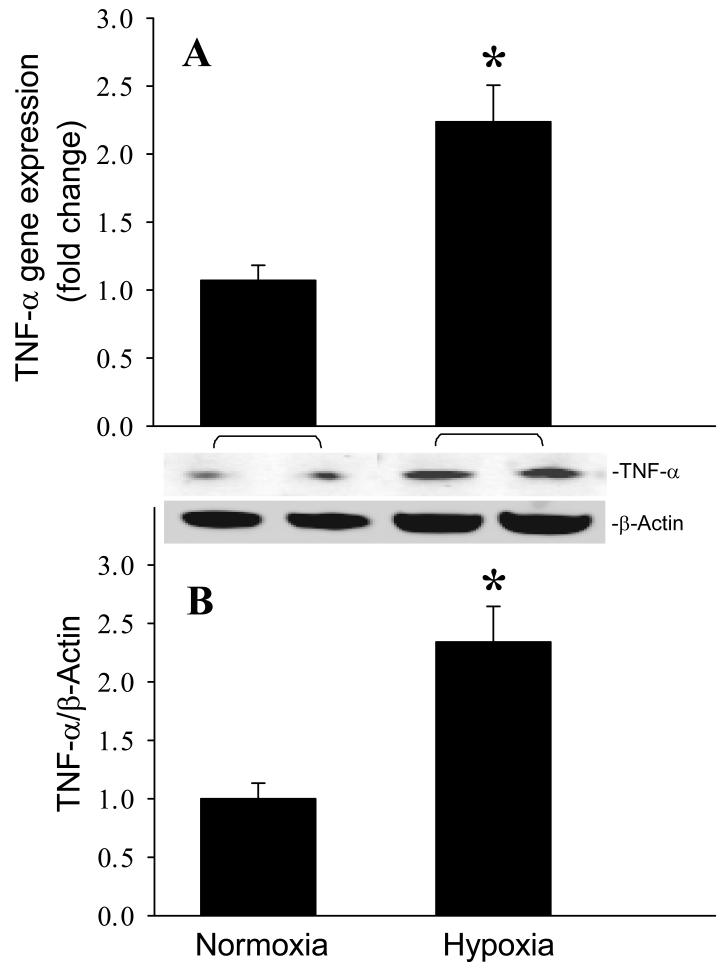
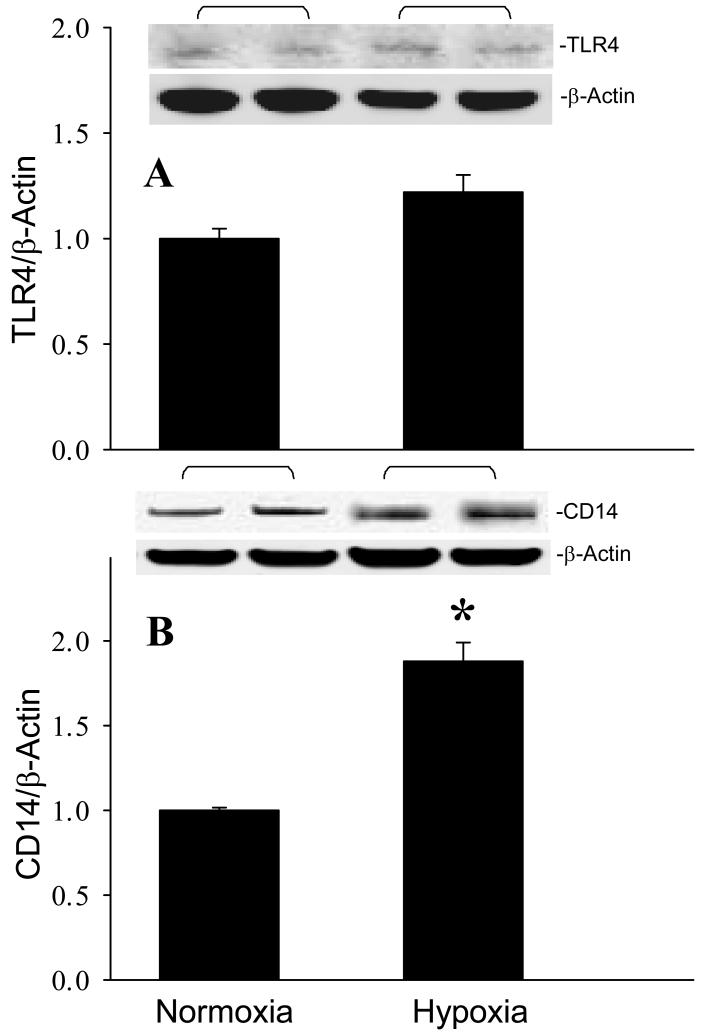
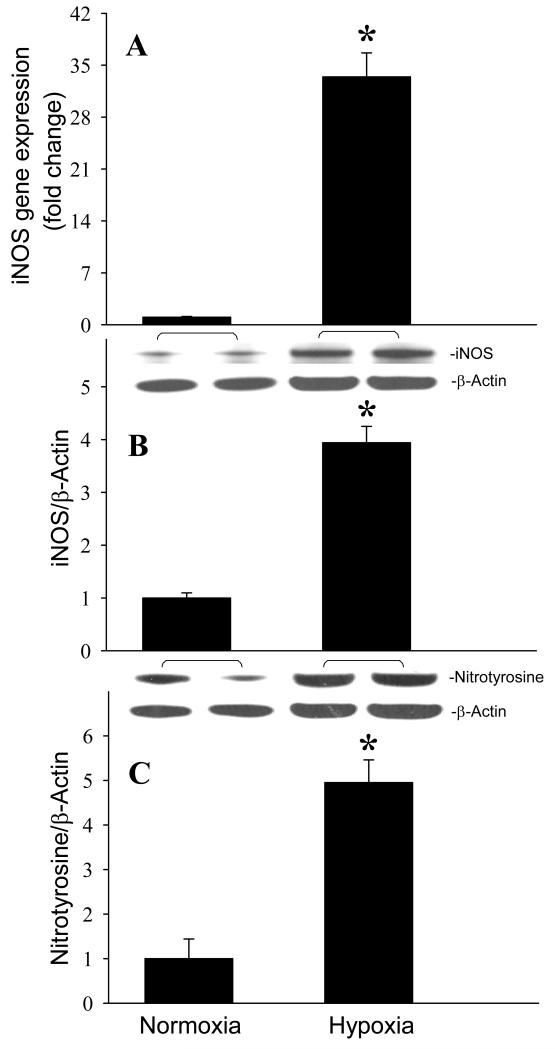
After observing the responses of the mouse brain to MCAO, we then investigated whether microglia were responsible for these changes. Cultured microglial BV2 cells were exposed to hypoxia (1% O2) for 20 h to mimic an ischemic condition. The gene and protein expression levels of TNF-α were increased 2.2- and 2.3-fold, respectively, in BV2 cells exposed to hypoxia in comparison to the cells cultured under normoxia (Fig. 5, P < 0.05). We then examined the involvement of the TLR-mediated pathway in activation of cytokine expression by measuring the expression of TLR4 and CD14 in BV2 cells. Similar to the in vivo observation, hypoxia didn’t alter TLR4 levels in BV2 cells (Fig. 6A). However, the CD14 protein expression was increased 1.9-fold in BV2 cells after exposure to hypoxia (Fig. 6B). Furthermore, the gene and protein expression levels of iNOS were increased 33.4- and 3.9-fold, respectively, in BV2 cells exposed to hypoxia in comparison to the normoxia (Fig. 7A and B, P < 0.05). We also found that nitrotyrosine levels were elevated 5.0-fold after hypoxia (Fig. 7C, P < 0.05).
Fig. 5.
Induction of TNF-α production in microglia after exposure to hypoxia. BV2 cells cultured in normoxia or exposed to hypoxia for 20 h were harvested for analysis. (A) TNF-α gene expression level was determined by real-time RT-PCR analysis. (B) Intracellular TNF-α protein level was determined by Western blotting measurements. Representative blots against TNF-α and β-actin are shown. Blots were scanned and quantified with densitometry. The levels of gene and protein expression in normoxia are designated as 1 for comparison purposes. Data are expressed as means ± SE (n = 4-5) and compared by Student-t test. *P < 0.05 vs. Normoxia.
Fig. 6.
Effect of hypoxia on TLR4 and CD14 expression in microglia. BV2 cells cultured in normoxia or exposed to hypoxia for 20 h were harvested for analysis. (A) TLR4 and (B) CD14 protein levels were determined by Western blotting measurements. Representative blots against TLR4, CD14, and β-actin are shown. Blots were scanned and quantified with densitometry. The levels of protein expression in normoxia are designated as 1 for comparison purposes. Data are expressed as means ± SE (n = 4-5) and compared by Student-t test. *P < 0.05 vs. Normoxia.
Fig. 7.
Induction of iNOS and nitrotyrosine expression in microglia after exposure to hypoxia. BV2 cells cultured in normoxia or exposed to hypoxia for 20 h were harvested for analysis. (A) iNOS gene expression level was determined by real-time RT-PCR analysis. (B) iNOS and (C) nitrotyrosine protein levels were determined by Western blotting measurements. Representative blots against iNOS, nitrotyrosine, and β-actin are shown. Blots were scanned and quantified with densitometry. The levels of gene and protein expression in normoxia are designated as 1 for comparison purposes. Data are expressed as means ± SE (n = 4-5) and compared by Student-t test. *P < 0.05 vs. Normoxia.
2.5. Hypoxia-induced inflammation is mediated by activation of iNOS in microglia
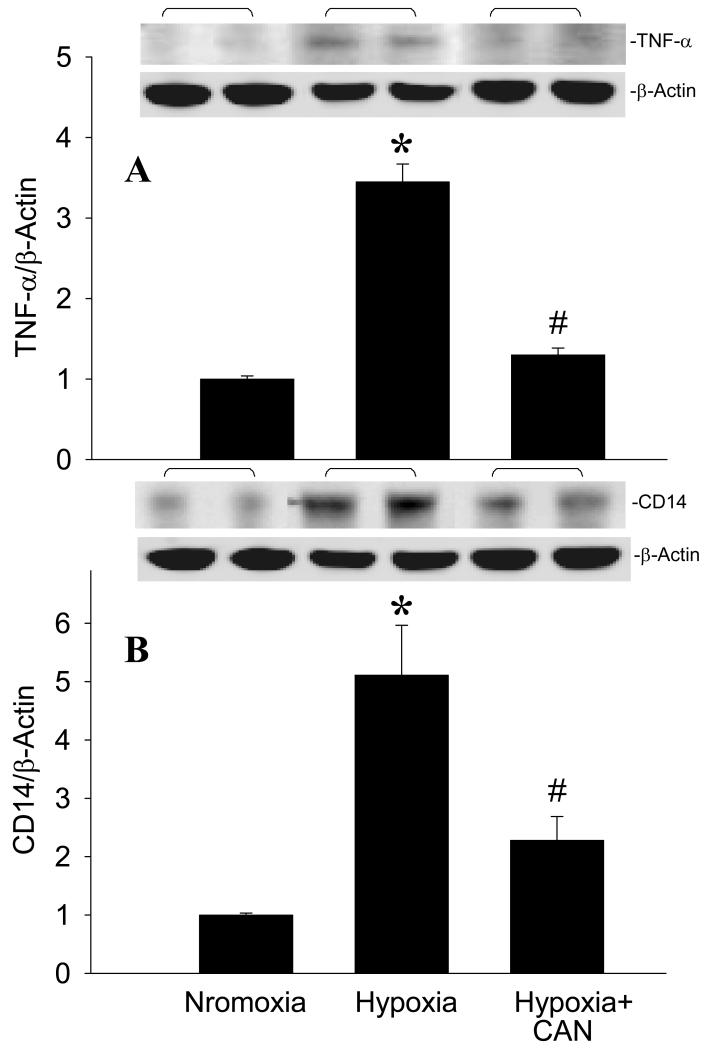
The generation of NO occurs very early in the ischemic attack. To identify the placement of iNOS in the regulatory cascade of hypoxia-induced inflammation, we applied L-canavanine (CAN), an iNOS selective inhibitor, to BV2 cells before exposure to hypoxia. As shown in Fig. 8A, an increase of TNF-α protein expression by hypoxia was diminished in the presence of CAN. As indicated earlier, CD14 expression was upregulated under ischemic or hypoxic conditions. We then examined the effect of CAN on CD14 protein expression in BV2 cells exposed to hypoxia. We also observed a significant reduction of CD14 expression in BV2 cells treated with CAN in comparison to the cells exposed to hypoxia alone (Fig. 8B).
Fig. 8.
Effect of an iNOS inhibitor on TNF-α and CD14 expression in microglia after exposure to hypoxia. BV2 cells cultured in normoxia or exposed to hypoxia in the absence or presence of L-canavanine (CAN, 50 μM) for 20 h were harvested for analysis. (A) TNF-α and (B) CD14 protein levels were determined by Western blotting measurements. Representative blots against TNF-α, CD14, and β-actin are shown. Blots were scanned and quantified with densitometry. The levels of protein expression in the normoxia are designated as 1 for comparison purposes. Data are expressed as means ± SE (n = 4-5) and compared by one-way ANOVA and SNK test. *P < 0.05 vs. Normoxia; #P < 0.05 vs. Hypoxia.
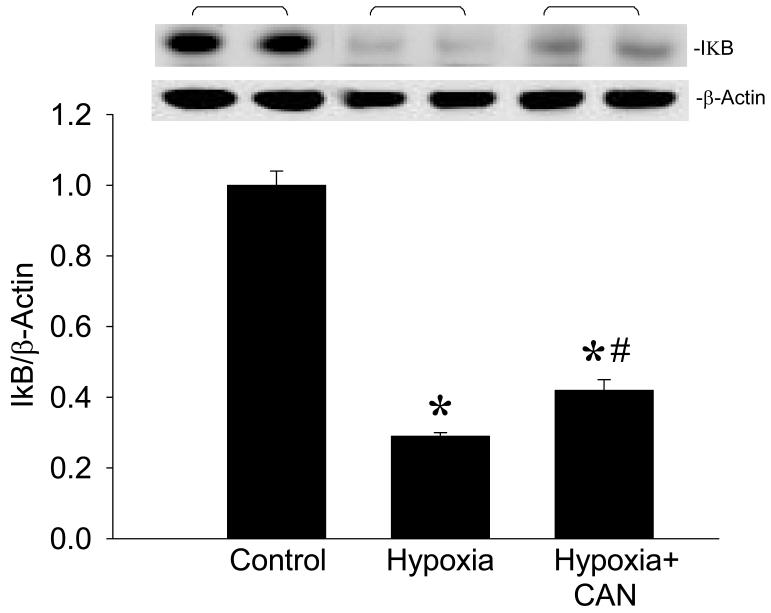
Among the TLR-mediated signaling pathways in regulating proinflammatory cytokine expression, NF-κB is the most distinctive. To determine the effect of CAN on the activation of NF-κB, we measured the cellular levels of IκB. Under non-stress conditions, NF-κB is stabilized by IκB in the cytoplasm. Upon activation, it triggers the degradation of IκB to free NF-κB for transport into the nucleus. After hypoxia, cellular IκB levels were decreased by 71% in the BV2 cells, while their levels became 60% reduced in the presence of CAN, compared to normoxia (Fig. 9). Taken together, inhibition of iNOS resulted in a reduction of TNF-α production that is correlated with the downregulation of CD14 expression and partially mediated through inhibiting the activation of NF-κB pathway in BV2 cells exposed to hypoxia.
Fig. 9.
Effect of an iNOS inhibitor on IκB expression in microglia after exposure to hypoxia. BV2 cells cultured in normoxia or exposed to hypoxia in the absence or presence of L-canavanine (CAN, 50 μM) for 20 h were harvested for analysis. IκB protein levels were determined by Western blotting measurements. Representative blots against IκB (37 kDa) and β-actin are shown. Blots were scanned and quantified with densitometry. The levels of protein expression in the normoxia are designated as 1 for comparison purposes. Data are expressed as means ± SE (n = 4-5) and compared by one-way ANOVA and SNK test. *P < 0.05 vs. Normoxia; #P < 0.05 vs. Hypoxia.
3. DISCUSSION
Although different mechanisms are involved in the pathogenesis of stroke, there is increasing evidence showing that inflammation plays a critical role in the disease progression. In this study, we used a mouse MCAO model to examine the regulation of the inflammatory response during stroke. In our model, an average of 31% infarct area was generated in the brain 24 h after the engagement and resulted in an increase of proinflammatory cytokine TNF-α expression at the gene and protein levels. The elevation of TNF-α in the ischemic cortex was also observed within a few hours up to day 5 following permanent MCAO in other studies (Liu et al., 1994; Wang et al., 2007). The deteriorated effect of TNF-α during cerebral ischemia has been demonstrated by an exacerbated infarct size of the brain through intracerebroventricular injection of TNF-α 24 h prior MCAO procedure (Arvin et al., 1996; Barone et al., 1997). Consistently, administration of anti-TNF-α antibodies can alleviate the brain damage induced by MCAO (Meistrell et al., 1997; Barone et al., 1997). Thus, induction of TNF-α may prime the brain for subsequent damage after occurrence of stroke.
To identify the source of TNF-α production during ischemic stroke, we subjected cultured microglial BV2 cells to a hypoxic chamber and observed an increase of TNF-α gene and protein expression 20 h after hypoxia. This observation corresponds to our detection of upregulated TNF-α expression in the mouse brain tissue after MCAO. Microglia are resident brain macrophage and they become virtually indistinguishable under pathological conditions, including stroke (Perry et al., 1993). TNF-α immunoreactivity has been reported to be primarily associated with macrophages located within the infarcted tissue (Stoll et al, 1998). Moreover, TNF-α can trigger neuronal apoptosis, leading to brain lesions (Jurewicz et al., 2005; Fehsel et al., 1991).
To further identify the receptor-mediated pathway in upregulating TNF-α expression during stroke, we found the expression of CD14, but not TLR-4, was increased in the mouse brain tissue subjected to permanent MCAO and BV2 cells after hypoxia. In contrast, the number of TLR4-positive cells, determined by immunehistochemistry, gradually increased from 1 h after MCAO to 22 h after ischemia-reperfusion in mice subjected to transient MCAO for 2 h (Hyakkoku et al., 2010). Similarly, upregulation of TLR4 expression is detected in rats subjected to either permanent or transient MCAO (Wang et al., 2010; Ye et al., 2011). It has been indicated that several factors, such as rodent species, size, age, sex, and suture size, can affect the outcome in the MCAO model (Liu and McCullough, 2011). In additional, the results are also affected by the duration of MCAO, with or without reperfusion, and the time points of collecting brain tissue after MCAO. Thus, it requires further study to identify the major factors contributing to the discrepancy in TLR4 expression levels between this and other studies.
Membrane-anchored CD14 acts as a co-receptor with TLR4 during the inflammatory response and it alone doesn’t initiate intracellular signaling cascades (Milatovic et al., 2004). The CD14/TLR4 complex functions as the signal-transduction receptor for LPS, which is a major component of the outer membrane of Gram-negative bacteria. The involvement of CD14 and TLR4 has also been demonstrated in ischemia-reperfusion injury and trauma where Gram-negative or Gram-positive bacteria are not detectible in the circulation or local organ sites (Lorne et al., 2010; Kaczorowski et al., 2009; Johnson et al., 2003). Accumulating evidence indicates that endogenous agonists, such as reactive oxygen species (ROS), heat shock proteins, proinflammatory mediators, and degradation products of macromolecules, can also activate and CD14/TLR4 signaling (Lorne et al., 2010; Johnson et al., 2003).
Microglia are the major cells in the CNS that express CD14 and TLR4 (Lehnardt et al., 2003). Cortical neurons do not express these receptors (Lehnardt et al., 2003). The significant contribution of CD14 expression in monocytes and activated microglia has been identified in ischemic brain lesions (Beschorner et al., 2002a). The involvement of CD14 has also been reported in other CNS diseases. For example, CD14 expression is increased in the human brain after traumatic brain injury and its early expression is essential for the acute inflammatory response following brain trauma (Beschorner et al., 2002b). In Alzheimer’s, CD14 with TLR4 are required for microglia to respond to fibrillar β-amyloid and result in microglial activation and toxicity (Fassbender et al., 2004; Reed-Geaghan et al., 2009). In addition, CD14-mediated cerebral pathology has been reported in glioblastoma (Haghparast et al., 2011). CD14 may significantly contribute to the overall neuroinflammatory response in the brain in disease condition. In the BV2 cell study, we identified that CD14 is involved in regulating TNF-α levels under hypoxic stress. It has been demonstrated that CD14 knockout mice in response to sepsis and burn injury have less production of proinflammatory cytokines, including, TNF-α, IL-1β, and IL-6, in comparison to wild-type mice (Ebong et al., 2001; Barber et al., 2008). We will further investigate whether similar phenomena occurs in the CD14 knockout mice subjected to MCAO.
NO biosynthesis is another key factor in the pathophysiological response of the brain to ischemia/hypoxia. We demonstrated that iNOS was induced in gene and protein levels in the mouse brain tissue after MCAO and BV2 cells after hypoxia. In the healthy brain, microglia do not express iNOS, while reactive microglia express iNOS following ischemic, traumatic, neurotoxic, or inflammatory damage (Moro et al., 2004; Galea et al., 1992; Saha and Pahan, 2006). In the rat brain, iNOS protein and catalytic activity are detectable 12 h after cerebral ischemia, peak at 48 h (Samdani et al., 1997). The expression of iNOS has been linked to the damage induced by focal ischemia through the dysfunction of the mitochondria that leads to energy depletion and cell death (Samdani et al., 1997). Consistently, mice lacking the iNOS gene or inhibition of its activity by pharmacological agents has shown to have significantly reduced infarct volumes in ischemic brain injury (Zhao et al., 2000; Zhang et al, 1996). It has been reported that administration of N(G)-nitro-L-arginine methyl (L-NAME), another inhibitor of NOS, reduces infarct volume in the rat pup that underwent MCAO for 4 h (Ashwal et.al., 1995). In a rat model of hemorrhagic shock, CAN has a better activity than L-NAME in treating neurological deficits (Ng et al., 2003). Thus, it will be interesting to compare the efficacy of CAN and other NOS inhibitors in protecting neurons from cerebral ischemic damage.
Furthermore, the induction of iNOS in activated microglia can trigger a high output production of NO to generate reactive radicals, such as peroxynitrite, and cause neuronal death during ischemia (Wang et al., 2007; Bolanos and Almeida, 1999). In more detail, the death mode of neuronal cells in responding to peroxynitrite is dependent on its exposure duration and intensity. Under exposure at short time or low concentration, peroxynitritet induces a delayed form of neurotoxicity predominated by apoptotic features. In contrast, intense exposure to high levels of peroxynitrite induces necrotic cell damage, which cannot be ameliorated by superoxide dismutase and catalase (Bonfoco et al., 1995).
Finally, we examined the interaction between the CD14/TLR4 receptor complex and iNOS in regulating cytokine expression under hypoxic stress. Increasing evidence has shown that NO is involved in the upregulation of proinflammatory cytokines (Magrinat et al., 1992; Kallmann et al., 1999). But little is known how NO mediates cytokine production. CAN, an iNOS selective inhibitor, is a naturally occurring L-amino acid that interferes in iNOS to catalyze the production of NO from L-arginine (Knowles and Moncada, 1994). By applying CAN, we identified that upregulation of CD14 in BV2 cells was inhibited after exposure to hypoxia, which is associated with a reduction of TNF-α expression. Our data suggested that NO could through activation of the CD14 receptor to induce TNF-α production. It has been reported that the CD14/TLR4 receptor complex increases proinflammatory cytokines production through activation of the NF-κB pathway (da Silveira Cruz-Machado et al., 2010; Nadeau and Rivest, 2000). Correspondingly, we observed that CAN treatment partially recovered the IκB protein levels in hypoxia-exposed BV2 cells. The activation of the NF-κB is regulated by its cytoplasmic inhibitor, IκBα, through phosphorylation of serine at residues 32 and 36, which targets it for degradation by the proteosome and releases NF-κB to migrate to the nucleus and activate the promoter of target genes (Baeuerle and Baltimore, 1988). Thus, the activation of NF-κB by hypoxia in BV2 cells was partially blocked by CAN. The above results suggested that iNOS can activate microglial CD14 to contribute to neuroinflammation in cerebral ischemia.
NF-κB, a transcription factor, not only plays a crucial role in regulating inflammatory response but also has multiple physiological and pathological functions. Activation of NF-κB has been considered a survival pathway under some stress conditions; however, it has also been demonstrated that its activation causes neuronal apoptosis (Camandola and Mattson, 2007; Qin et al., 2007). Due to the complexity of the NF-κB pathway, the mechanism by which NF-κB induces apoptosis is not yet clear, but its involvement in regulating pro-apoptotic genes, such as p53, c-Myc, cyclin D1, and Bcl-Xs, has been indicated (Qin et al., 2007). In ischemic brain injury, mice lacking the p50 subunit of NF-κB develop significantly smaller infarcts after transient focal ischemia (Schneider et al., 1999; Nurmi et al., 2004). The role of NF-κB in neuronal death was further suggested by a neuroprotective effect of several antioxidants with an inhibition of NF-κB activation to reduce infarct volume and improve behavior deficits (Qin et al., 2007). Therefore, it is a strong rationale for use of NF-κB inhibitors in alleviating ischemic brain injury.
In summary, we have demonstrated that upregulation of CD14 and iNOS is associated with an elevation of TNF-α expression in mouse brain tissue after ischemic stroke. In parallel, microglia exposed to hypoxia also exhibited an induction of CD14, iNOS, and TNF-α expression, indicating that microglia are the major cell population in the brain to provoke inflammation in ischemic lesions. Furthermore, the upregulation of TNF-α production induced by hypoxic stress in microglia is partially through increasing iNOS, and then CD14 expression to activate the NF-κB pathway.
4. EXPERIMENTAL PROCEDURE
4.1. Experimental animals
Male C57BL/6 mice (20 – 25 g) purchased from Taconic, Albany, NY were housed in a temperature controlled room on a 12 h light-to-dark cycle and fed a standard laboratory diet. The animals were allowed at least 5 days to acclimate under these conditions before being used for experiments. All animal experiments were carried out in accordance with the NIH guidelines for the use of experimental animals. The project was approved by the Institutional Animal Care and Use Committee (IACUC) of the Feinstein Institute for Medical Research.
4.2. Animal model of cerebral ischemia
Mice were fasted overnight but had access to water ad libitum before induction of cerebral ischemia. Permanent focal cerebral ischemia was induced by MCAO as previously described by Belayev et al (Belayev et al., 1996), with some modifications. Briefly, anesthesia was induced by isoflurane inhalation and subsequently maintained by intravenous boluses of pentobarbital (15 mg/kg BW). Body temperature was maintained at 37°C using a heating pad with a homoeothermic monitor (Harvard Apparatus, Holliston, MA). The right common carotid artery (CCA) was exposed through a ventral midline neck incision and was carefully dissected free from the vagus nerve and fascia, from its bifurcation to the base of the skull. The distal branches of the external carotid artery (ECA) were then dissected, ligated and divided to create an ECA stump. The internal carotid artery (ICA) was isolated and separated from the adjacent vagus nerve and the pterygopalatine artery was dissected and ligated close to its origin. Then, a 1-cm length of 7-0 poly-L-Lysine coated monofilament nylon suture was inserted through the proximal ECA into the ICA and advanced to the middle cerebral artery (MCA) origin to occlude it. The silk suture around the ECA stump was tightened around the intraluminal nylon suture to prevent bleeding. Occlusion of the MCA was ascertained by inserting the suture to a pre-determined length of 8-10 mm from the carotid biburcation and feeling for resistance as the suture tip approaches the proximal anterior cerebral artery. The cervical wound was then closed in layers and mice were allowed to recover from anesthesia. The intraluminal suture was left in-situ and mice were allowed unrestricted access to food and water. Sham-operated animals underwent the same surgical procedures without the performance of MCAO for comparison. At 24 h post-operation, the animals were sacrificed and brain tissues were collected for various analysis. To confirm the brain injury after conduction of MCAO, the coronally sectioned brain (2 mm-thickness) was stained with 1.5% triphenyl tetrazolium chloride (TTC) at 37°C for 30 min and then immersed in 10% formalin overnight.
4.3. Cell culture and hypoxia
Microglial BV2 cells have been frequently used as a reliable substitute for studying the biology of primary microglia (Henn et al., 2009). Murine microglial BV2 cells were kindly provided by Dr. Philippe Marambaud at the Feinstein Institute for Medical Research. BV2 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Invitrogen, Carlsbad, CA) containing 2 mM L-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin and 10% heat inactivated fetal bovine serum in a 37 °C incubator with 5% CO2. To simulate the in vivo hypoxic condition, BV2 cells were cultured in a sealed chamber containing 1% O2, 5% CO2 and 94% N2 for 20 h. To inhibit iNOS activity, the iNOS selective inhibitor, L-canavanine (50 μM, Sigma, St.Louis, MO), was administed just before hypoxia.
4.4. Determination of gene expression by real-time polymerase chain reaction (PCR)
Total RNA was extracted from the mouse brain and BV2 cells by using a TRIzol reagent (Invitrogen, Carlsbad, CA). Real-time PCR was carried out on cDNA samples which were reversely transcribed from 2 μg RNA by using murine leukemia virus reverse-transcriptase (Applied Biosystems, Foster City, CA). A PCR reaction was carried out in a 24 μl final volume containing 0.08 μmol of each forward and reverse primer, 2 μl cDNA, 9.2 μl H2O and 12 μl SYBR Green PCR Master Mix (Applied Biosystems). Amplification was conducted in an Applied Biosystems 7300 real-time PCR machine under the thermal profile of 50°C for 2 min, 95°C for 10 min, followed by 40 cycles of 95°C for 15 sec and 60°C for 1 min. The levels of β-actin mRNA were used for normalization and each specific mRNA measurement was conducted in duplicate. Relative expression of mRNA was calculated by the 2−ΔΔCt method and results were expressed as fold change in comparison to the control group. The sequence of primers for this study is listed as follow: TNF-α (X02611), forward (AGACCCTCACACTCAGATCATCTTC) and reverse (TTGCTACGACGTGGGCTACA); iNOS (NM_007705), forward (GGCAAACCCAAGGTCTACGTT) and reverse (GAGCACGCTGAGTACCTCATTG); and β-actin (NM_007393), forward (CGTGAAAAGATGACCCAGATCA) and reverse (TGGTACGACCAGAGGCATACAG).
4.5. Determination of protein levels by Western blot analysis
Mouse brain and harvested BV2 cells were homogenized in lysis buffer (0.1 M Tris-HCl pH 7.5, 120 mM NaCl, and 1% Triton X-100) with a protease inhibitor cocktail (Roche Applied Science; Indianapolis, IN). The supernatants were obtained after centrifugation at 10,000 rpm for 10 min. Protein concentration of the lysate was determined by the DC protein assay kit (Bio-Rad, Hercules, CA). Equal amounts of protein were fractionated on a 4-12% Bis-Tris gel (Invitrogen) and transferred to a 0.2-μm nitrocellulose membrane (Invitrogen). The membrane was blocked with 0.1% casein in 10 mM phosphate buffer with 0.1% Tween-20, pH 7.5 (PBST) and incubated overnight at 4°C with primary antibodies against TNF-α, iNOS, nitrotyrosine, CD14, IκB, β-Actin (Santa Cruz Biotechnology, Santa Cruz, CA), or TLR4 (Cell Signaling Technology, Danvers, MA). After washing, the membrane was incubated in the dark with infrared dye-linked secondary antibodies (LI-COR Biosciences, Lincoln, NE) for 1 h and then subjected to the Odyssey infrared image system (LI-COR Biosciences) for the quantification. β-actin served as loading control. The expression levels of each measured protein were normalized to β-actin. The value of the control group is designated as 1 for comparison.
4.6. Statistical analysis
All data are expressed as means ± SE and compared by Student-t test or one-way analysis of variance (ANOVA) and Student-Newman-Keuls (SNK) test. Differences in values were considered significant if P < 0.05.
Highlights.
Proinflammatory cytokine levels were elevated in mouse brain after stroke.
Expression of CD14 and iNOS was upregulated in association with neuroinflammation.
Cellular responses of microglia to hypoxia in vitro corresponded to the stroked brain.
Signaling pathway iNOS->CD14->NF-κB mediated inflammatory response during stroke.
ACKNOWLEDGMENTS
The authors sincerely thank Dr. Youxin Ji for his contribution on cerebral ischemic stroke animal model. We also thank Ms. Lana Corbo for critically reading of the manuscript and helpful comments. This study was supported by the National Institutes of Health grants R01HL076179, R01GM053008, and R01GM057468 (P. Wang)
Abbreviations
- MCAO
middle cerebral artery occlusion
- CAN
L-canavanine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Arvin B, Neville LF, Barone FC, Feuerstein GZ. The role of inflammation and cytokines in brain injury. Neurosci. Biobehav. Rev. 1996;20:445–452. doi: 10.1016/0149-7634(95)00026-7. [DOI] [PubMed] [Google Scholar]
- Ashwal S, Cole DJ, Osborne S, Osborne TN, Pearce WJ. L-NAME reduces infarct volume in a filament model of transient middle cerebral artery occlusion in the rat pup. Pediatr. Res. 1995;38:652–656. doi: 10.1203/00006450-199511000-00004. [DOI] [PubMed] [Google Scholar]
- Baeuerle PA, Baltimore D. Ikappa B: a specific inhibitor of the NF-kappa B transcription factor. Science. 1988;242:540–546. doi: 10.1126/science.3140380. [DOI] [PubMed] [Google Scholar]
- Barber RC, Maass DL, White DJ, Chang LY, Horton JW. Molecular or pharmacologic inhibition of the CD14 signaling pathway protects against burn-related myocardial inflammation and dysfunction. Shock. 2008;30:705–713. doi: 10.1097/SHK.0b013e31816f6caa. [DOI] [PubMed] [Google Scholar]
- Barone FC, Arvin B, White RF, Miller A, Webb CL, Willette RN, Lysko PG, Feuerstein GZ. Tumor necrosis factor-alpha. A mediator of focal ischemic brain injury. Stroke. 1997;28:1233–1244. doi: 10.1161/01.str.28.6.1233. [DOI] [PubMed] [Google Scholar]
- Belayev L, Alonso OF, Busto R, Zhao W, Ginsberg MD. Middle cerebral artery occlusion in the rat by intraluminal suture. Neurological and pathological evaluation of an improved model. Stroke. 1996;27:1616–1622. doi: 10.1161/01.str.27.9.1616. [DOI] [PubMed] [Google Scholar]
- Beschorner R, Schluesener HJ, Gozalan F, Meyermann R, Schwab JM. Infiltrating CD14+ monocytes and expression of CD14 by activated parenchymal microglia/macrophages contribute to the pool of CD14+ cells in ischemic brain lesions. J. Neuroimmunol. 2002a;126:107–115. doi: 10.1016/s0165-5728(02)00046-2. [DOI] [PubMed] [Google Scholar]
- Beschorner R, Nguyen TD, Gozalan F, Pedal I, Mattern R, Schluesener HJ, Meyermann R, Schwab JM. CD14 expression by activated parenchymal microglia/macrophages and infiltrating monocytes following human traumatic brain injury. Acta Neuropathol. 2002b;103:541–549. doi: 10.1007/s00401-001-0503-7. [DOI] [PubMed] [Google Scholar]
- Bolanos JP, Almeida A. Roles of nitric oxide in brain hypoxia-ischemia. Biochim. Biophys. Acta. 1999;1411:415–436. doi: 10.1016/s0005-2728(99)00030-4. [DOI] [PubMed] [Google Scholar]
- Bonfoco E, Krainc D, Ankarcrona M, Nicotera P, Lipton SA. Apoptosis and necrosis: two distinct events induced, respectively, by mild and intense insults with N-methyl-D-aspartate or nitric oxide/superoxide in cortical cell cultures. Proc. Natl. Acad. Sci. USA. 1995;92:7162–7166. doi: 10.1073/pnas.92.16.7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camandola S, Mattson MP. NF-kappa B as a therapeutic target in neurodegenerative diseases. Expert Opin. Ther. Targets. 2007;11:123–132. doi: 10.1517/14728222.11.2.123. [DOI] [PubMed] [Google Scholar]
- da Silveira Cruz-Machado S, Carvalho-Sousa CE, Tamura EK, Pinato L, Cecon E, Fernandes PA, de Avellar MC, Ferreira ZS, Markus RP. TLR4 and CD14 receptors expressed in rat pineal gland trigger NFKB pathway. J. Pineal Res. 2010;49:183–192. doi: 10.1111/j.1600-079X.2010.00785.x. [DOI] [PubMed] [Google Scholar]
- Donnan GA, Fisher M, Macleod M, Davis SM. Stroke. Lancet. 2008;371:1612–1623. doi: 10.1016/S0140-6736(08)60694-7. [DOI] [PubMed] [Google Scholar]
- Ebong SJ, Goyert SM, Nemzek JA, Kim J, Bolgos GL, Remick DG. Critical role of CD14 for production of proinflammatory cytokines and cytokine inhibitors during sepsis with failure to alter morbidity or mortality. Infect. Immun. 2001;69:2099–2106. doi: 10.1128/IAI.69.4.2099-2106.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassbender K, Walter S, Kuhl S, Landmann R, Ishii K, Bertsch T, Stalder AK, Muehlhauser F, Liu Y, Ulmer AJ, Rivest S, Lentschat A, Gulbins E, Jucker M, Staufenbiel M, Brechtel K, Walter J, Multhaup G, Penke B, Adachi Y, Hartmann T, Beyreuther K. The LPS receptor (CD14) links innate immunity with Alzheimer’s disease. FASEB J. 2004;18:203–205. doi: 10.1096/fj.03-0364fje. [DOI] [PubMed] [Google Scholar]
- Fehsel K, Kolb-Bachofen V, Kolb H. Analysis of TNF alpha-induced DNA strand breaks at the single cell level. Am. J. Pathol. 1991;139:251–254. [PMC free article] [PubMed] [Google Scholar]
- Galea E, Feinstein DL, Reis DJ. Induction of calcium-independent nitric oxide synthase activity in primary rat glial cultures. Proc. Natl. Acad. Sci. USA. 1992;89:10945–10949. doi: 10.1073/pnas.89.22.10945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haghparast A, Heidari KM, Malvandi AM. Down-regulation of CD14 transcripts in human glioblastoma cell line U87 MG. Iran J. Immunol. 2011;8:111–119. [PubMed] [Google Scholar]
- Henn A, Lund S, Hedtjarn M, Schrattenholz A, Porzgen P, Leist M. The suitability of BV2 cells as alternative model system for primary microglia cultures or for animal experiments examining brain inflammation. ALTEX. 2009;26:83–94. doi: 10.14573/altex.2009.2.83. [DOI] [PubMed] [Google Scholar]
- Hyakkoku K, Hamanaka J, Tsuruma K, Shimazawa M, Tanaka H, Uematsu S, Akira S, Inagaki N, Nagai H, Hara H. Toll-like receptor 4 (TLR4), but not TLR3 or TLR9 knock-out mice have neuroprotective effects against focal cerebral ischemia. Neuroscience. 2010;171:258–267. doi: 10.1016/j.neuroscience.2010.08.054. [DOI] [PubMed] [Google Scholar]
- Johnson GB, Brunn GJ, Platt JL. Activation of mammalian Toll-like receptors by endogenous agonists. Crit. Rev. Immunol. 2003;23:15–44. doi: 10.1615/critrevimmunol.v23.i12.20. [DOI] [PubMed] [Google Scholar]
- Jordan J, Segura T, Brea D, Galindo MF, Castillo J. Inflammation as therapeutic objective in stroke. Curr. Pharm. Des. 2008;14:3549–3564. doi: 10.2174/138161208786848766. [DOI] [PubMed] [Google Scholar]
- Jurewicz A, Matysiak M, Tybor K, Kilianek L, Raine CS, Selmaj K. Tumour necrosis factor-induced death of adult human oligodendrocytes is mediated by apoptosis inducing factor. Brain. 2005;128:2675–2688. doi: 10.1093/brain/awh627. [DOI] [PubMed] [Google Scholar]
- Kaczorowski DJ, Nakao A, Vallabhaneni R, Mollen KP, Sugimoto R, Kohmoto J, Zuckerbraun BS, McCurry KR, Billiar TR. Mechanisms of Toll-like receptor 4 (TLR4)-mediated inflammation after cold ischemia/reperfusion in the heart. Transplantation. 2009;87:1455–1463. doi: 10.1097/TP.0b013e3181a36e5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallmann BA, Malzkorn R, Kolb H. Exogenous nitric oxide modulates cytokine production in human leukocytes. J. Life Sci. 1999;65:1787–1794. doi: 10.1016/s0024-3205(99)00431-2. [DOI] [PubMed] [Google Scholar]
- Knowles RG, Moncada S. Nitric oxide synthases in mammals. Biochem. J. 1994;298(Pt 2):249–258. doi: 10.1042/bj2980249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakhan SE, Kirchgessner A, Hofer M. Inflammatory mechanisms in ischemic stroke: therapeutic approaches. J. Transl. Med. 2009;7:97. doi: 10.1186/1479-5876-7-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehnardt S, Massillon L, Follett P, Jensen FE, Ratan R, Rosenberg PA, Volpe JJ, Vartanian T. Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4-dependent pathway. Proc. Natl. Acad. Sci. USA. 2003;100:8514–8519. doi: 10.1073/pnas.1432609100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, McCullough LD. Middle cerebral artery occlusion model in rodents: methods and potential pitfalls. J. Biomed. Biotechnol. 2011;2011:464701. doi: 10.1155/2011/464701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Clark RK, McDonnell PC, Young PR, White RF, Barone FC, Feuerstein GZ. Tumor necrosis factor-alpha expression in ischemic neurons. Stroke. 1994;25:1481–1488. doi: 10.1161/01.str.25.7.1481. [DOI] [PubMed] [Google Scholar]
- Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat. Rev. Neurosci. 2003;4:399–415. doi: 10.1038/nrn1106. [DOI] [PubMed] [Google Scholar]
- Lorne E, Dupont H, Abraham E. Toll-like receptors 2 and 4: initiators of non-septic inflammation in critical care medicine? Intensive Care Med. 2010;36:1826–1835. doi: 10.1007/s00134-010-1983-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magrinat G, Mason SN, Shami PJ, Weinberg JB. Nitric oxide modulation of human leukemia cell differentiation and gene expression. Blood. 1992;80:1880–1884. [PubMed] [Google Scholar]
- Meistrell ME, 3rd, Botchkina GI, Wang H, Di SE, Cockroft KM, Bloom O, Vishnubhakat JM, Ghezzi P, Tracey KJ. Tumor necrosis factor is a brain damaging cytokine in cerebral ischemia. Shock. 1997;8:341–348. [PubMed] [Google Scholar]
- Milatovic D, Zaja-Milatovic S, Montine KS, Shie FS, Montine TJ. Neuronal oxidative damage and dendritic degeneration following activation of CD14-dependent innate immune response in vivo. J. Neuroinflammation. 2004;1:20. doi: 10.1186/1742-2094-1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moro MA, Cardenas A, Hurtado O, Leza JC, Lizasoain I. Role of nitric oxide after brain ischaemia. Cell Calcium. 2004;36:265–275. doi: 10.1016/j.ceca.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Muir KW, Tyrrell P, Sattar N, Warburton E. Inflammation and ischaemic stroke. Curr. Opin. Neurol. 2007;20:334–342. doi: 10.1097/WCO.0b013e32813ba151. [DOI] [PubMed] [Google Scholar]
- Nadeau S, Rivest S. Role of microglial-derived tumor necrosis factor in mediating CD14 transcription and nuclear factor kappa B activity in the brain during endotoxemia. J. Neurosci. 2000;20:3456–3468. doi: 10.1523/JNEUROSCI.20-09-03456.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng KC, Moochhala SM, Md S, Yap EL, Low SY, Lu J. Preservation of neurological functions by nitric oxide synthase inhibitors following hemorrhagic shock. Neuropharmacology. 2003;44:244–252. doi: 10.1016/s0028-3908(02)00368-4. [DOI] [PubMed] [Google Scholar]
- Nurmi A, Lindsberg PJ, Koistinaho M, Zhang W, Juettler E, Karjalainen-Lindsberg ML, Weih F, Frank N, Schwaninger M, Koistinaho J. Nuclear factor-kappaB contributes to infarction after permanent focal ischemia. Stroke. 2004;35:987–991. doi: 10.1161/01.STR.0000120732.45951.26. [DOI] [PubMed] [Google Scholar]
- Okun E, Griffioen KJ, Lathia JD, Tang SC, Mattson MP, Arumugam TV. Toll-like receptors in neurodegeneration. Brain Res. Rev. 2009;59:278–292. doi: 10.1016/j.brainresrev.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry VH, Andersson PB, Gordon S. Macrophages and inflammation in the central nervous system. Trends Neurosci. 1993;16:268–273. doi: 10.1016/0166-2236(93)90180-t. [DOI] [PubMed] [Google Scholar]
- Qin ZH, Tao LY, Chen X. Dual roles of NF-kappaB in cell survival and implications of NF-kappaB inhibitors in neuroprotective therapy. Acta. Pharmacol. Sin. 2007;28:1859–1872. doi: 10.1111/j.1745-7254.2007.00741.x. [DOI] [PubMed] [Google Scholar]
- Reed-Geaghan EG, Savage JC, Hise AG, Landreth GE. CD14 and toll-like receptors 2 and 4 are required for fibrillar A{beta}-stimulated microglial activation. J. Neurosci. 2009;29:11982–11992. doi: 10.1523/JNEUROSCI.3158-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha RN, Pahan K. Regulation of inducible nitric oxide synthase gene in glial cells. Antioxid. Redox. Signal. 2006;8:929–947. doi: 10.1089/ars.2006.8.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samdani AF, Dawson TM, Dawson VL. Nitric oxide synthase in models of focal ischemia. Stroke. 1997;28:1283–1288. doi: 10.1161/01.str.28.6.1283. [DOI] [PubMed] [Google Scholar]
- Schneider A, Martin-Villalba A, Wieh F, Vogel J, Wirth T, Schwaninger M. NF-κB is activated and promotes cell death in focal cerebral ischemia. Nat. Med. 1999;5:554–559. doi: 10.1038/8432. [DOI] [PubMed] [Google Scholar]
- Siesjo BK. Pathophysiology and treatment of focal cerebral ischemia. Part I: Pathophysiology. J. Neurosurg. 1992a;77:169–184. doi: 10.3171/jns.1992.77.2.0169. [DOI] [PubMed] [Google Scholar]
- Siesjo BK. Pathophysiology and treatment of focal cerebral ischemia. Part II: Mechanisms of damage and treatment. J. Neurosurg. 1992b;77:337–354. doi: 10.3171/jns.1992.77.3.0337. [DOI] [PubMed] [Google Scholar]
- Stoll G, Jander S, Schroeter M. Inflammation and glial responses in ischemic brain lesions. Prog. Neurobiol. 1998;56:149–171. doi: 10.1016/s0301-0082(98)00034-3. [DOI] [PubMed] [Google Scholar]
- Wang L, Zhang X, Liu L, Yang R, Cui L, Li M. Atorvastatin protects rat brains against permanent focal ischemia and downregulates HMGB1, HMGB1 receptors (RAGE and TLR4), NF-kappaB expression. Neurosci. Lett. 2010;471:152–156. doi: 10.1016/j.neulet.2010.01.030. [DOI] [PubMed] [Google Scholar]
- Wang Q, Tang XN, Yenari MA. The inflammatory response in stroke. J. Neuroimmunol. 2007;184:53–68. doi: 10.1016/j.jneuroim.2006.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Chopp M, Liu X, Zacharek A, Cui X, Yan T, Roberts C, Chen J. Niaspan reduces high-mobility group box 1/receptor for advanced glycation endproducts after stroke in type-1 diabetic rats. Neuroscience. 2011;190:339–345. doi: 10.1016/j.neuroscience.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Casey RM, Ross ME, Iadecola C. Aminoguanidine ameliorates and L-arginine worsens brain damage from intraluminal middle cerebral artery occlusion. Stroke. 1996;27:317–323. doi: 10.1161/01.str.27.2.317. [DOI] [PubMed] [Google Scholar]
- Zhang G, Ghosh S. Molecular mechanisms of NF-kappaB activation induced by bacterial lipopolysaccharide through Toll-like receptors. J. Endotoxin. Res. 2000;6:453–457. doi: 10.1179/096805100101532414. [DOI] [PubMed] [Google Scholar]
- Zhao X, Haensel C, Araki E, Ross ME, Iadecola C. Gene-dosing effect and persistence of reduction in ischemic brain injury in mice lacking inducible nitric oxide synthase. Brain Res. 2000;872:215–218. doi: 10.1016/s0006-8993(00)02459-8. [DOI] [PubMed] [Google Scholar]