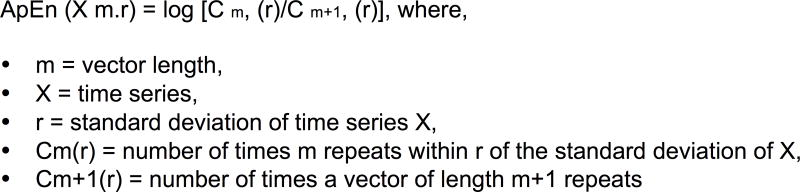Abstract
Background
The objective of this study was to determine movement variability in the more-affected upper-extremity in chronic stroke survivors. We investigated two hypotheses: (1) individuals with stroke will have increased amount of variability and altered structure of variability in upper-extremity joint movement patterns as compared to age-matched controls; and (2) the degree of motor impairment and joint kinematics will be correlated with the temporal structure of variability.
Methods
Sixteen participants with chronic stroke and nine age-matched controls performed three trials of functional reach-to-grasp. The amount of variability was quantified by computing the standard deviation of shoulder, elbow, wrist and index finger flexion/extension joint angles. The temporal structure of variability was determined by calculating approximate entropy in shoulder, elbow, wrist and index finger flexion/extension joint angles.
Findings
Individuals with stroke demonstrated greater standard deviations and significantly reduced approximate entropy values as compared to controls. Furthermore, motor impairments and kinematics demonstrated moderate to strong correlations with temporal structure of variability.
Interpretation
Changes in the temporal structure of variability in upper-extremity joint angles suggest that movement patterns used by stroke survivors are less adaptable. This knowledge may yield additional insights into the impaired motor system and suggest better interventions that can enhance upper-extremity movement adaptability.
Keywords: Time-dependent structure, Motor skills, Complexity, Kinematics, Upper extremity
1. Introduction
Stroke is a leading cause of disability in the United States affecting over 795,000 individuals every year (American Heart Association, 2010). Up to 85% of individuals with stroke exhibit hemiparesis resulting in upper-extremity (UE) impairments (Olsen, 1990). Unfortunately, despite the development of various rehabilitation techniques, residual UE impairments remain (Duncan et al., 2000; Nakayama et al., 1994). Thus, a more thorough understanding of UE impairments is needed to develop effective treatments maximizing motor ability post-stroke.
Among the constellation of UE impairments, individuals with post-stroke hemiparesis often exhibit atypical movement patterns characterized by mass and whole limb movements with limited dissociation between joints (Cirstea and Levin, 2000). These aberrant movement patterns exhibit high variability in terms of increased standard deviation (SD) and/or coefficient of variation (CV) in several kinematic measures: UE joint range of motion, peak velocity, movement time and trajectory accuracy as compared to healthy controls (Cirstea and Levin, 2000; Woodbury et al., 2009). SD and CV are linear measures of variability and quantify the amount of variability, or movement error, around a central point (Newell, 1976); however, they cannot capture the fine adjustments of the limbs that occur during the course of motor performance (Harbourne and Stergiou, 2009). UE movements involve continuous adjustments to successfully reach and grasp objects of various sizes and shapes. For instance, individuals make continuous fine adjustments to maintain their grip on a glass, if they perceive that the glass may slip from their hands. These fine adjustments or variations made during continuous movements over time are referred as temporal structure of variability (Harbourne and Stergiou, 2009). Temporal structure of variability allows individuals to adapt their movement patterns to overcome perturbations encountered during daily tasks. Temporal structure of variability can be quantified using nonlinear measures such as approximate entropy (ApEn) (Harbourne and Stergiou, 2009). Unlike linear measures of variability, which compute variability around the mean of a movement parameter, ApEn examines the variability by evaluating all values of a movement parameter over the entire time series. Nonlinear measures capture the temporal structure of variability that occurs over time reflecting the adaptability of the motor system. There is limited evidence of the application of non-linear measures in UE motor impairments post stroke. Therefore, the application of non-linear measures to characterize the temporal structure of variability in UE movement may yield additional insights into impaired motor control post-stroke.
Stergiou, Harbourne and Cavanaugh (2006) proposed that an optimal state of variability is associated with a healthy motor system. This model suggests that healthy states are associated with optimal movement variability and this variability reflects the adaptability of the underlying control system. The principle of optimality is demonstrated by an inverted U-shape relationship exhibited between complexity and predictability. At an optimal state of movement variability, the largest complexity lies in the intermediate region between maximum predictability and no predictability and is representative of a “healthy” state. For a detailed description of the optimal variability model refer to figure 2 in Stergiou, Harbourne and Cavanaugh (2006). Complexity signifies the presence of chaotic temporal variations in the steady state output of a healthy biological system and represents the underlying physiologic capability to adapt to everyday stresses placed on the human body (Lipsitz and Goldberger, 1992; Lipsitz, 2002). Decrease or loss of the optimal state of variability renders the system more predictable and rigid exhibiting a robotic type of motor behavior. For example, individuals with stroke often exhibit UE movements with limited dissociation between joints resulting in predictable or stereotypical movements referred as abnormal synergies. Conversely, increases beyond optimal variability render the system more noisy and unpredictable. For instance, individuals with movement disorders such as ataxia or athetosis, often demonstrate jerky, uncontrolled and less predictable movements of extremities. Both situations reveal decreased complexity, flexibility and adaptability to perturbations and are associated with impairments in ability to engage UE in meaningful tasks.
Movement adaptability is an innate and fundamental feature of a healthy nervous system (Lipsitz and Goldberger, 1992; Stergiou, Harbourne and Cavanaugh, 2006). Everyday functional tasks involve continuous adaptations of reach and grasp movements to meet the dynamic demands of the tasks. Temporal structure of variability allows individuals to adapt their movement patterns to overcome perturbations encountered during daily tasks. Several changes associated with stroke, including spasticity, decreased range of motion (Cirstea and Levin, 2000), difficulty dealing with the interaction torques produced by muscle contractions, and abnormal motor recruitment patterns, (Dewald et al., 1995) might alter the temporal structure of variability in UE joints. Consequently, altered temporal structure of variability should be reflected in the altered adaptability of UE movement.
Examining variability in reaching movements post-stroke provides a window to understand the impaired motor system and suggest better interventions that enhance UE movement adaptability. Therefore, the primary aim of this study was to compare the amount and the temporal structure of variability of the shoulder, elbow, wrist and proximal interphalangeal (PIP of index finger) flexion/extension joint angles during reach-to-grasp movements between healthy individuals and individuals with stroke. We hypothesized that the amount of variability of shoulder, elbow, wrist, and PIP angles would be significantly greater and the temporal structure of variability of shoulder, elbow, wrist and PIP joint angle movement patterns would be significantly reduced in individuals post- stroke as compared to in healthy individuals.
2. Methods
2.1. Participants
The participants were 16 individuals diagnosed with stroke and nine healthy controls. The mean years of age for the participants with stroke was 67.6 (SD 8.1) and for the healthy controls 57.2 (SD 6.7). Demographic information as well as lesion location and severity of stroke based upon the UE Fugl-Meyer subscale for individuals with stroke are presented in Table 1. The participants were part of a larger study investigating upper-extremity motor rehabilitation. Participants were included if they: (1) were between the ages of 18–90 years; (2) had a single ischemic stroke at least 6 months prior to enrollment; (3) were able to follow two-step commands; (4) had no history of more than minor head trauma, subarachnoid hemorrhage, dementia or other neural disorder/dysfunction, drug or alcohol abuse, schizophrenia, serious medical illness, or refractory depression. A sample of convenience comprised of eight right hand dominant females and one left hand dominant male were recruited from the staff of the Brain Rehabilitation Research Center to serve as healthy age-matched controls.
Table 1.
Participants demographics: individuals with stroke
| Participant | Gender | Age | Affected Side | UE_FM | Lesion Location | Months after CVA |
|---|---|---|---|---|---|---|
| 1 | M | 76 | L | 41 | Right middle cerebral artery | 102 |
| 2 | M | 62 | L | 46 | Right M1, middle cerebral artery | 48 |
| 3 | F | 70 | L | 44 | Right Striatoscapular infarct | 131 |
| 4 | F | 66 | R | 58 | Left Midlle/Posterior cerebellar artery | 102 |
| 5 | M | 73 | R | 45 | Left medullary/brainstem infarct | 103 |
| 6 | M | 76 | R | 53 | Left middle cerebral artery | 174 |
| 7 | M | 55 | L | 41 | Right medial medullary infarct | 43 |
| 8 | M | 66 | L | 43 | Right Striatoscapular infarct | 105 |
| 9 | F | 47 | L | 35 | Right basal ganglia | 7 |
| 10 | M | 62 | L | 27 | Right middle cerebral artery | 118 |
| 11 | F | 64 | L | 31 | Right lacunar infarct | 67 |
| 12 | F | 62 | L | 27 | Posterior ventricular white matter | 19 |
| 13 | M | 72 | R | 38 | Left lacunar infarct | 24 |
| 14 | M | 77 | R | 38 | Left pontine infarct | 34 |
| 15 | M | 72 | L | 30 | Right middle cerebral artery | 48 |
| 16 | M | 68 | R | 31 | Left middle cerebral artery | 16 |
2.2. Procedures
Eligible participants provided written informed consent approved by the University of Florida Institutional Review Board and North Florida/South Georgia Veterans Health System’s Research and Development Committee. Each participant was evaluated once at the Human Motor Performance Laboratory located within the Brain Rehabilitation Research Center.
Individuals with stroke reached to grasp a soda can (56 mm in diameter; 208 mm circumference) with the paretic UE. Healthy controls reached with their non-dominant hand. Sixty-seven reflective markers were secured to various landmarks of the upper body as illustrated in Figure 1. Marker placements were determined using a marker set described by the Plug-In-UE marker set defined by our laboratory (Patterson et al., 2011). All participants wore dark colored sleeveless shirts and were seated on an adjustable, backless bench with knees bent at 90° flexion and feet flat on the floor. The hands were placed palm down on a table in front of them and supported in 90° of elbow flexion by arm rests positioned flush with the table. This position was the starting position for all the trials.
Figure 1.
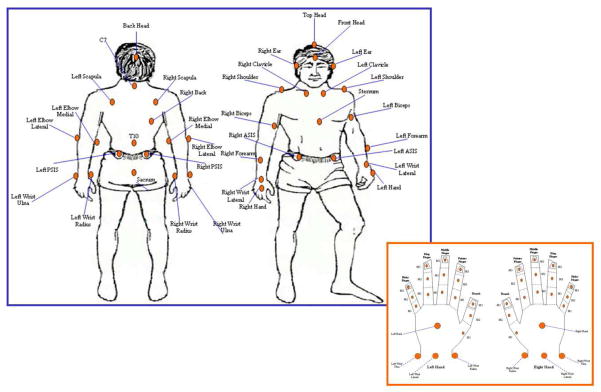
Upper extremity marker set.
A soda can was placed at 80% arm’s length (Michaelsen et al., 2004) on the table directly in front of the respective shoulder of the participant. This distance has been referred to as the “critical boundary” (Mark et al., 1997). Healthy individuals use UE joints alone to reach for objects within this workspace; to obtain objects beyond this boundary; they might involve the trunk by leaning forward (Mark et al., 1997). All participants were instructed to reach for the can, lift it off the table, and put it back down as fast as possible and return to the starting position. All participants performed four trials with the first serving as a practice trial. Each trial was cued with a “go” command.
2.3. Data analysis
Kinematics of reaching were recorded using two different 12-camera VICON motion capture systems (Vicon 612; Oxford Metrics In., Oxford, UK). All controls and 11 individuals post-stroke were tested using a 12MX camera system and Vicon Workstation v4.6 software at a sampling frequency of 100Hz. The remaining five individuals post-stroke were tested using 12 T40 Vicon cameras and Vicon Nexus 1.5.2 software with data sampled at 200 Hz. Data collected using VICON Nexus were down sampled from 200 to 100Hz to construct comparable time series and enable appropriate comparisons.
Data analysis was performed on the last three trials. The data were the 3D positional coordinates of each marker with respect to a laboratory coordinate system throughout the movement series. The data were manually labeled and reconstructed using Vicon software, and then modeled using SIMM (4.2, Santa Rosa, CA) to calculate the shoulder, elbow, wrist and PIP angles. The start of reach was identified as the time point at which the velocity of the index finger marker exceeded 5% peak velocity and the termination of reach as the time point at which velocity of this marker fell below 5% peak velocity. One degree of freedom in the sagittal plane (flexion/extension) was used to determine shoulder, elbow, wrist and PIP joint angle. To retain the inherent temporal structure of the variability present, the kinematic data were not filtered prior to analysis (Rapp, Albano, Schmah, and Farwell, 1993).
2.4 Variability of UE kinematics
To measure the amount of variability, SDs of three trials of the shoulder, elbow, wrist and PIP joint angle range of motion were computed. The temporal structure of variability of shoulder, elbow, wrist and PIP joint angle time series was determined by computing approximate entropy (ApEn) with the MATLAB code (R2009a, Natick, MA) developed by Kaplan and Staffin (1996) utilizing the algorithm provided by Pincus, Gladstone, and Ehrenkranz (1991). Each joint angle time series was analyzed from the start of the reach through the entire length of the respective time series including the pauses between the three trials. This approach was adopted because ApEn is effectively a measure of probability, developed to identify whether small patterns of a time series repeat later in the entire time series. These small patterns might not be repeated in a single trial of reach-to-grasp movement. Overall, four time series were obtained (one for each joint). The most common method employed in the computation of ApEn is to identify repeating vectors of length m across the entire time series (figure 2). Biomechanical data analysis conventionally utilizes r = 0.2 times the standard deviation of the time series, lag =1 and m = 2 (Slifkin and Newell, 1999). Because the length of the data could affect ApEn values, we normalized the ApEn values of each participant to the length of their time series and then multiplied the ratio with a constant equal to 100. A more detailed description of the computation of ApEn can be reviewed in the Appendix of Slifkin and Newell (1999). Generally, a vector of shorter length repeats more often than a longer one within a time series, thus the lowest possible ApEn value can be the natural logarithm of 1, which is 0. ApEn values range from 0 to 2. In a highly periodic time series, values of Cm(r) can be similar to Cm+1(r) producing ApEn = 0. Hence, smaller values characterize a more regular time series where similar patterns are more likely to follow one another. In contrast, high ApEn values, suggest a highly irregular time series, where the predictability of subsequent patterns is low and ApEn could be close to 2 (Stergiou et al., 2004).
Figure 2.
Approximate entropy (ApEn) equation
We also computed the percentage contribution of each joint to the total ApEn of UE. Total ApEn was computed by adding the ApEn from shoulder, elbow, wrist and PIP for each participant. Thereafter, the percentage contribution from each joint was obtained by multiplying the ratio of the individual joint ApEn to total ApEn by 100. Such analyses would reveal the distribution of ApEn across UE joints.
2.6. Surrogate analysis
A surrogation procedure was applied prior to computing ApEn utilizing the Theiler et al. (1992) first algorithm. Surrogation procedure is a critical step to perform prior to computing ApEn to verify whether the kinematic data were deterministic in nature and not a source of noise. Theiler’s first algorithm (1992) utilizes a phase randomization technique which removes the deterministic structure from the original shoulder, elbow, wrist and PIP joint angle time series creating 20 surrogate time series of each trial with the same mean, variance, and power spectrum as the original time series. ApEn was then computed on the original as well as each of the 20 surrogate time series. Significant differences in ApEn between the original and 19 of 20 surrogate time series confirm the deterministic nature of the original data.
2.7 Statistical Analysis
Dependent one-tailed t-tests were conducted to compare ApEn shoulder, elbow, wrist and PIP values between the original and surrogate time series using SPSS (17.0, Chicago, IL). For the remaining analyses non-parametric statistics were employed due to the violation of assumptions of normality using SPSS (17.0, Chicago,IL). Mann-Whitney U tests were employed to investigate the differences in SD and ApEn shoulder, elbow, wrist and PIP between individuals with stroke and healthy controls. Mann-Whitney U tests were also employed to compare the percent contribution of each joint’s ApEn to total ApEn between healthy controls and individuals with stroke. Data were analyzed with statistical significance set at P<0.05. Holm’s step-down procedure was used to correct for multiple comparisons (Holm, 1979).
3. Results
3.1 Determinism in joint angle time series using surrogate analysis
Determinism in the joint angle time series was confirmed in both control (P = 0.001) and stroke (P = 0.000) groups which revealed significantly greater shoulder, elbow, wrist and PIP ApEn values in surrogate time series. These findings suggest that the data were deterministic in nature and not a source of noise.
3.2 Amount of variability in joint angle time series
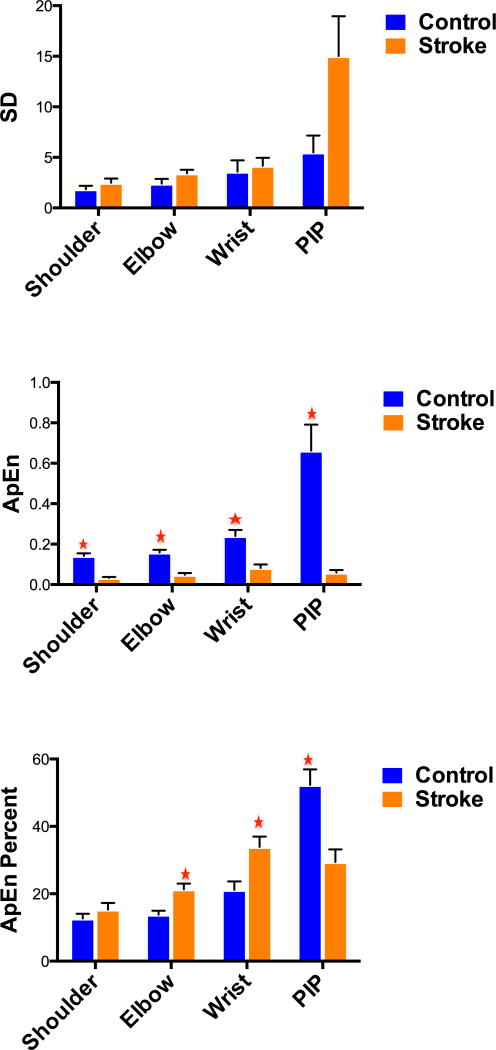
Individuals with stroke had larger SDs for shoulder, elbow, wrist and PIP angles than for healthy controls. However, these differences did not reach statistical significance (P>0.05) (Table 3).
3.3 Temporal structure of variability in joint angle time series
Individuals with stroke exhibited significantly less (P<0.05) ApEn values across all UE joints than controls (Table 2). Additionally, the contribution of ApEn of movement at each joint to the total ApEn differed between the groups. The percent contribution of ApEn PIP joint to total ApEn was significantly greater (P = 0.002) for controls than for individuals with stroke (Table 2). In contrast, individuals with stroke demonstrated a significantly greater percent contribution of ApEn elbow (P = 0.002) and wrist (P = 0.014) joints to total ApEn than controls (Table 3). However, the difference in percent contribution of ApEn shoulder joint to total ApEn was not significantly different (P = 0.803) between controls and individuals with stroke (Table 2).
Table 2.
Standard deviation, approximate entropy and approximate entropy percentage of upper extremity joints between controls and individuals with stroke
| Median SD | Mann-Whitney U (P value) | Median ApEn | Mann-Whitney U (P value) | Median % of total ApEn | Mann-Whitney U (P value) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Controls | Stroke | Controls | Stroke | Controls | Stroke | ||||
| Shoulder | 1.81 | 1.79 | 60 (P = 0.52) | .14 | .03 | 2 (P<0.016)* | 13 | 15 | 67 (P>0.05) |
| Elbow | 2.27 | 2.90 | 47 (P = 0.16) | .16 | .05 | 3 (P<0.025)* | 21 | 14 | 19 (P<0.016)* |
| Wrist | 2.86 | 3.48 | 63 (P = 0.63) | .24 | .08 | 9 (P<0.05)* | 21 | 34 | 29 (P<0.025)* |
| PIP | 6.34 | 7.42 | 50 (P = 0.22) | .66 | .06 | 1 (P<0.012)* | 52 | 29 | 20 (P<0.012)* |
SD-Standard deviation; ApEn-Approximate entropy; ApEn%-Approximate entropy percentage;
= Significant
4. Discussion
The primary purpose of the study was to compare the differences between the amount and temporal structure of variability in UE movements between individual’s post-stroke and healthy controls. Although not statistically significant, SD values were lower across all joints in healthy controls than individuals post-stroke. In contrast, ApEn values across all joints were significantly greater in healthy controls than individuals post-stroke. Based upon the optimal variability model, healthy controls exhibit an optimal nervous system, which may demonstrate chaotic temporal variations revealing optimum adaptability to meet the demands of everyday stresses placed on the human body. Deviance from the optimal variability model may suggest the presence of pathology; less than optimal variability may be representative of a more rigid, less adaptable system limiting the repertoire of movement strategies (Harbourne and Stergiou, 2009; Scholz, 1990). The results of this study suggest that temporal structure of variability is reduced in individuals post stroke, which potentially could alter the adaptability in their reach to grasp movements.
In healthy controls, ApEn was significantly greater in the index finger PIP joint than the shoulder, elbow and wrist joints. Lower ApEn values characterize a more stable or regular time series whereas; high ApEn values suggest an unstable or irregular time series. Hence, lower shoulder ApEn values suggest that shoulder is utilized primarily for stabilization of the arm during reach-to-grasp. Alternatively, the PIP joint might have produced greater adjustments essential in manipulating the grasp around the can during the reach-to-grasp task. Greater ApEn values at the PIP compared to more proximal joints in the healthy controls are consistent with the current literature, which supports the versatile nature of hand (Lemon, 1993; Tallis, 2003). The advanced ability of the hand to grasp and manipulate objects of various sizes, shapes and textures is one of the key features of the human motor system (Begliomini et al., 2008).
In contrast to healthy controls, participants post-stroke demonstrated a significantly greater percent contribution from the wrist and elbow joints to total ApEn. Individuals post-stroke possibly made significantly greater adjustments with the wrist and elbow than with the PIP joint implicating an alternative compensatory strategy for accomplishing the reach-to-grasp task. The significant reduction in the percentage contribution of PIP joint ApEn values post- stroke could be due to the fact that motor neuron pools of distal UE segments are primarily innervated by the corticospinal tract, which is frequently compromised in stroke (Colebatch and Gandevia, 1989). Furthermore, Raghavan et al. (2010) also observed alternative movement strategies, where individuals with stroke compensated PIP joint flexion by increased flexion at the metacarpophalangeal joint during grasping of concave and convex shaped objects. Understanding how multiple effectors coordinate to produce a goal directed movement still remains a challenge to motor control researchers (Diedrichsen et al., 2009). Commonly referred to as the degrees of freedom problem (Bernstein, 1967), motor coordination is concerned with how work is distributed across multiple effectors (muscles, joints) when multiple options exist to perform a task. Optimal control theory suggests that an optimization process might be a potential solution to the degree of freedom problem of motor control (Diedrichsen et al., 2009). Optimal control theory proposes that the selection of effectors for a particular task is the consequence of an optimization process based upon the cost function made up of the goal and the effort required to accomplish the goal. Stroke might change the cost function for a particular movement. For individuals with moderate UE deficits post-stroke, manipulating the index finger PIP joint around the soda can might require too much effort. Thus, the compensation strategy involving the wrist and elbow joints might involve re-optimization in setting up the new cost function and redistributing work across effectors. In fact, using the wrist may have made it easier to open and close the fingers due to the biomechanical properties of the long flexors (e.g., flexor digitorum superficialis), which cross both the wrist, and fingers.
We acknowledge certain limitations of this study. Given the heterogeneity observed in stroke, this sample size was relatively small, thus the lack of significant differences between groups in shoulder and elbow SD might reflect a lack of statistical power. The findings of this study are also limited to seated unimanual, discrete reach-to-grasp tasks. Further research is necessary to understand specific neurological mechanisms contributing to the changes in variability in UE joints post-stroke compared to other kinematic and functional variables. In particular, the effects of location and size of brain lesion, severity of the lesion, integrity of the descending motor pathways, individual degree of spontaneous recovery, and the duration of stroke onset upon temporal structural of variability of UE joints needs to be explored. Additionally, future research is warranted to determine whether or not constraining the trunk might affect the temporal structure of variability. There is also a need to determine the effects of intervention on these variables.
5. Conclusion and Implications for Rehabilitation
Our findings reveal that the temporal structure of variability in reach-to-grasp movements is significantly reduced post-stroke. A measure of the temporal structure of variability seems to capture differences between the groups; even with a small cohort of individuals post-stroke we were able to significantly differentiate between healthy controls and individuals with stroke utilizing ApEn. In contrast, employing linear measures, such as the standard deviation, we failed to detect differences between healthy controls and individuals with stroke. Analyzing temporal structure of variability in UE movements provides a novel perspective on understanding motor impairments in individuals living with stroke. ApEn could potentially be utilized to measure the efficacy of UE rehabilitation intervention. Future research is warranted to establish the psychometric properties of ApEn prior to its use as an outcome measure.
Supplementary Material
Figure 3.
a. Standard deviation (SD) of various UE joints between healthy controls and individuals with stroke
b. Approximate Entropy (ApEn) of various UE joints between healthy controls and individuals with stroke (* = significant)
c. Approximate entropy (ApEn) percent of each joint to total ApEn in healthy controls and individuals with stroke (* = significant)
Acknowledgments
This material is based upon work supported by the following grants from the Office of Rehabilitation Research and Development, Department of Veterans Affairs and the Office of Academic Affairs, Department of Veterans Affairs: Rehabilitation Research and Development Center, grant F2182C (Leslie J. Gonzalez Rothi), Career Development Award II B4789W (Lorie Richards), B3964R (Carolynn Patten) and NIH grant 1 R03 (HD051624-01A1) (Lorie Richards). The sponsors had no role in the design, methods, subject recruitment, data collections, analysis, or preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Heart Association. Heart disease and stroke statistics. Dallas, TX: 2010. [Accessed July 31, 2010]. http://www.americanheart.org/downloadable/heart/1265665152970DS-3241%20HeartStrokeUpdate_2010.pdf. [Google Scholar]
- Baud-Bovy G, Soechting JF. Factors influencing variability of load forces in a tripod grasp. Exp Brain Res. 2002;143:57–66. doi: 10.1007/s00221-001-0966-8. [DOI] [PubMed] [Google Scholar]
- Begliomini C, Nelini C, Caria A, Grodd W, Castiello U. Cortical activations in humans grasp-related areas depend on hand used and handedness. PLoS One. 2008;3 (10):e3388. doi: 10.1371/journal.pone.0003388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirstea MC, Levin MF. Compensatory strategies for reaching in stroke. Brain. 2000;123 (Pt 5):940–953. doi: 10.1093/brain/123.5.940. [DOI] [PubMed] [Google Scholar]
- Colebatch JG, Gandevia SC. The distribution of muscular weakness in upper motor neuron lesions affecting the arm. Brain. 1989;112 (Pt 3):749–763. doi: 10.1093/brain/112.3.749. [DOI] [PubMed] [Google Scholar]
- Deffeyes JE, Harbourne RT, DeJong SL, Kyvelidou A, Stuberg WA, Stergiou N. Use of information entropy measures of sitting postural sway to quantify developmental delay in infants. J Neuroengineering Rehabil. 2009;6:34. doi: 10.1186/1743-0003-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deffeyes JE, Harbourne RT, Kyvelidou A, Stuberg WA, Stergiou N. Nonlinear analysis of sitting postural sway indicates developmental delay in infants. Clin Biomech. 2009;24 (7):564–570. doi: 10.1016/j.clinbiomech.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Dewald JP, Pope PS, Given JD, Buchanan TS, Rymer WZ. Abnormal muscle coactivation patterns during isometric torque generation at the elbow and shoulder in hemiparetic subjects. Brain. 1995;118 (2):495–510. doi: 10.1093/brain/118.2.495. [DOI] [PubMed] [Google Scholar]
- Diedrichsen J, Shadmehr R, Ivry RB. The coordination of movement: optimal feedback control and beyond. Trends Cogn Sci. 2009;14 (1):31–39. doi: 10.1016/j.tics.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein N. Coordination and regulation of movement. New York: Pergamon Press; 1967. [Google Scholar]
- Duncan PW, Lai SM, Keighley J. Defining post-stroke recovery: implications for design and interpretation of drug trials. Neuropharmacology. 2000;39 (5):835–841. doi: 10.1016/s0028-3908(00)00003-4. [DOI] [PubMed] [Google Scholar]
- Harbourne RT, Stergiou N. Movement Variability and the Use of Nonlinear Tools: Principles to Guide Physical Therapist Practice Response. Phys Ther. 2009;89 (3):284–285. doi: 10.2522/ptj.20080130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6:65–70. [Google Scholar]
- Kamm K, Thelen E, Jensen JL. A dynamical systems approach to motor development. Phys Ther. 1990;70 (12):763–775. doi: 10.1093/ptj/70.12.763. [DOI] [PubMed] [Google Scholar]
- Kaplan D, Staffin P. [Accessed December 21, 2009];1996 http://www.macalester.edu/~kaplan/hrv/doc/
- Kurz MJ, Stergiou N. The aging human neuromuscular system expresses less certainty for selecting joint kinematics during gait. Neurosci Lett. 2003;348 (3):155–158. doi: 10.1016/s0304-3940(03)00736-5. [DOI] [PubMed] [Google Scholar]
- Lacquaniti F, Soechting JF, Terzuolo SA. Path constraints on point-to point arm movements in three-dimensional space. Neuroscience. 1986;17 (2):313–324. doi: 10.1016/0306-4522(86)90249-6. [DOI] [PubMed] [Google Scholar]
- Lemon RN. Cortical control of the primate hand. Exp Physiol. 1993;78:263–301. doi: 10.1113/expphysiol.1993.sp003686. [DOI] [PubMed] [Google Scholar]
- Lindberg P, Ody C, Feydy A, Maier MA. Precision in isometric precision grip force is reduced in middle-aged adults. Exp Brain Res. 2009;193 (2):213–224. doi: 10.1007/s00221-008-1613-4. [DOI] [PubMed] [Google Scholar]
- Lipsitz LA, Goldberger AL. Loss of ‘complexity’ and aging. Potential applications of fractals and chaos theory to senescence. JAMA. 1992;267 (13):1806–1809. [PubMed] [Google Scholar]
- Mark LS, Nemeth K, Gardner D, Dainoff MJ, Paasche J, Duffy M, et al. Postural dynamics and the preferred critical boundary for visually guided reaching. J Exp Psychol Hum Percept Perform. 1997;23 (5):1365–1379. doi: 10.1037//0096-1523.23.5.1365. [DOI] [PubMed] [Google Scholar]
- Michaelsen SM, Jacobs S, Roby-Brami A, Levin MF. Compensation for distal impairments of grasping in adults with hemiparesis. Exp Brain Res. 2004;157 (2):162–173. doi: 10.1007/s00221-004-1829-x. [DOI] [PubMed] [Google Scholar]
- Nakayama H, Jorgensen HS, Raaschou HO, Olsen TS. Recovery of upper extremity function in stroke patients: the Copenhagen Stroke Study. Arch Phys Med Rehabil. 1994;75 (4):394–398. doi: 10.1016/0003-9993(94)90161-9. [DOI] [PubMed] [Google Scholar]
- Newell KM. More on Absolute Error, Etc. J Mot Behav. 1976;8 (2):139–142. doi: 10.1080/00222895.1976.10735064. [DOI] [PubMed] [Google Scholar]
- Olsen TS. Arm and leg paresis as outcome predictors in stroke rehabilitation. Stroke. 1990;21 (2):247–251. doi: 10.1161/01.str.21.2.247. [DOI] [PubMed] [Google Scholar]
- Patterson T, Bishop MD, McGuirk TS, Sethi A, Richards L. Reliability of kinematics while performing different tasks in individuals post stroke. J Mot Behav. 2011;43 (2):121–30. doi: 10.1080/00222895.2010.548422. [DOI] [PubMed] [Google Scholar]
- Pincus SM, Gladstone IM, Ehrenkranz RA. A Regularity Statistic for Medical Data-Analysis. J Clin Monit. 1991;7 (4):335–345. doi: 10.1007/BF01619355. [DOI] [PubMed] [Google Scholar]
- Raghavan P, Santello M, Gordon AM, Krakauer JW. Compensatory motor control after stroke: an alternative joint strategy for object-dependent shaping of hand posture. J Neurophysiol. 2010;103 (6):3034–3043. doi: 10.1152/jn.00936.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp PE, Albano AM, Schmah TI, Farwell LA. Filtered noise can mimic low-dimensional chaotic attractors. Phys Rev E Stat Phys Plasmas Fluids Relat Interdiscip Topics. 1993;47 (4):2289–2297. doi: 10.1103/physreve.47.2289. [DOI] [PubMed] [Google Scholar]
- Reisman DS, Scholz JP. Workspace location influences joint coordination during reaching in post-stroke hemiparesis. Exp Brain Res. 2006;170 (2):265–276. doi: 10.1007/s00221-005-0209-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz JP. Dynamic pattern theory--some implications for therapeutics. Phys Ther. 1990;70 (12):827–843. doi: 10.1093/ptj/70.12.827. [DOI] [PubMed] [Google Scholar]
- Shadmehr R, Mussa-Ivaldi FA. Adaptive representation of dynamics during learning of a motor task. J Neurosci. 1994;14 (5 Pt 2):3208–3224. doi: 10.1523/JNEUROSCI.14-05-03208.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slifkin AB, Newell KM. Noise, information transmission, and force variability. J Exp Psychol Hum Percept Perform. 1999;25 (3):837–851. doi: 10.1037//0096-1523.25.3.837. [DOI] [PubMed] [Google Scholar]
- Stergiou N, Buzzi UG, Kurz MS, Heidel J. Nonlinear tools in human movement. In: Stergiou, editor. Innovative analysis of human movement. Champaign, IL: Human Kinetics; 2004. pp. 63–90. [Google Scholar]
- Stergiou N, Harbourne R, Cavanaugh J. Optimal movement variability: a new theoretical perspective for neurologic physical therapy. J Neurol Phys Ther. 2006;30 (3):120–129. doi: 10.1097/01.npt.0000281949.48193.d9. [DOI] [PubMed] [Google Scholar]
- Tallis R. The hand: a philosophical inquiry in human being. Edinburg, UK: Edinburg University Press; 2003. [Google Scholar]
- Thach WT, Goodkin HP, Keating JG. The cerebellum and the adaptive coordination of movement. Annu Rev Neurosci. 1992;15:403–442. doi: 10.1146/annurev.ne.15.030192.002155. [DOI] [PubMed] [Google Scholar]
- Theiler J, Eubank S, Longtin A, Galdrikian B, Farmer JD. Testing for nonlinearity in time series: the method of surrogate data. Physica D. 1992;58 (1–4):77–94. [Google Scholar]
- Todorov E, Shadmehr R, Bizzi E. Augmented Feedback Presented in a Virtual Environment Accelerates Learning of a Difficult Motor Task. J Mot Behav. 1997;29 (2):147–158. doi: 10.1080/00222899709600829. [DOI] [PubMed] [Google Scholar]
- vanEmmerik RE, Sprague RL, Newell KM. Quantification of postural sway patterns in tardive dyskinesia. Mov Disord. 1993;8 (3):305–314. doi: 10.1002/mds.870080309. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Ghahramani Z, Jordan MI. Are arm trajectories planned in kinematic or dynamic coordinates? An adaptation study. Exp Brain Res. 1995;103 (3):460–470. doi: 10.1007/BF00241505. [DOI] [PubMed] [Google Scholar]
- Woodbury ML, Howland DR, McGuirk TE, Davis SB, Senesac CR, Kautz S, et al. Effects of Trunk Restraint Combined With Intensive Task Practice on Post stroke Upper Extremity Reach and Function: A Pilot Study. Neurorehabil Neural Repair. 2009;23 (1):78–91. doi: 10.1177/1545968308318836. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.