Abstract
Background
Reversibility of aberrant methylation via pharmacological means is an attractive target for therapies through epigenetic reprogramming. To establish that pharmacologic reversal of methylation could result in functional inhibition of angiogenesis, we undertook in vitro and in vivo studies of thrombospondin-1 (TSP1), a known inhibitor of angiogenesis. TSP1 is methylated in several malignancies, and can inhibit angiogenesis in melanoma xenografts. We analyzed effects of 5-Aza-deoxycytidine (5-Aza-dC) on melanoma cells in vitro to confirm reversal of promoter hypermethylation and restoration of TSP1 expression. We then investigated the effects of TSP1 expression on new blood vessel formation and tumor growth in vivo. Finally, to determine potential for clinical translation, the methylation status of TSP1 promoter regions of nevi and melanoma tissues was investigated.
Results
5-Aza-dC reduced DNA (cytosine-5)-methyltransferase 1 (DNMT1) protein, reversed promoter hypermethylation, and restored TSP1 expression in five melanoma cell lines, while having no effect on TSP1 protein levels in normal human melanocytes. In in vivo neovascularization studies, mice were implanted with melanoma cells (A375) either untreated or treated with 5Aza-dC. Vessels at tumor sites were counted by an observer blinded to treatments and the number of tumor vessels was significantly decreased at pretreated tumor sites. This difference occurred before a significant difference in tumor volumes was seen, yet in further studies the average tumor volume in mice treated in vivo with 5-Aza-dC was decreased by 55% compared to untreated controls. Knockdown of TSP1 expression with shRNA enhanced tumor-induced angiogenesis by 68%. Analyses of promoter methylation status of TSP1 in tumors derived from untreated and treated mice identified 67% of tumors from untreated and 17% of tumors from treated mice with partial methylation consistent with the methylation specific PCR analysis of A375 cells. Examination of methylation patterns in the promoter of TSP1 and comparison of aberrantly methylated TSP1 in melanoma with non-malignant nevi identified a significantly higher frequency of promoter methylation in tumor samples from melanoma patients.
Conclusions
Pharmacological reversal of methylation silenced TSP1 had functional biological consequences in enhancing angiogenesis inhibition and inducing antitumor effects to decrease murine melanoma growth. Angiogenesis inhibition is an additional mechanism by which epigenetic modulators can have antitumor effects.
Keywords: 5-Aza-deoxycytidine, thrombospondin-1, promoter hypermethylation, methyltransferase inhibitor, anti-angiogenesis
Introduction
Tumor cells secrete pro-angiogenic proteins such as vascular endothelial growth factor (VEGF) whereas host tissues can cleave secreted proteins to yield angiogenic inhibitors such as angiostatin, endostatin, tumstatin, and thrombospondin-1 (TSP1) (Jain, 2008; Kerbel, 2008; Naumov et al., 2006a). TSP1 is a glycoprotein containing multiple interacting domains that has an important role in cell proliferation, adhesion, migration, angiogenesis, and tumor metastasis through interaction with several different proteins and cell receptors (Silverstein and Febbraio, 2007). Decreased expression of TSP1 can occur in primary tumors. Aberrant methylation of TSP1 has been postulated as associated with angiogenesis in gastric cancer, colorectal cancer, glioblastoma multiforme, and neuroblastoma (Kim et al., 2005; Lee et al., 2004; Li et al., 1999; Yang et al., 2003).
Transcriptional inactivation of inhibitors and overexpression of angiogenic stimulators by tumor cells occurs during tumor progression (Jain, 2008; Kerbel, 2008;Naumov et al., 2006a). Methylation is one of the key mechanisms for epigenetic regulation of gene expression (Herman and Baylin, 2003; Jones and Baylin, 2002). Reversibility of aberrant methylation via pharmacological means is a potential target for therapy through epigenetic reprogramming. DNA methyltransferase 1 (DNMT1) plays a primary role in the maintenance of methylation patterns during DNA replication (Herman and Baylin, 2003). DNA methyltransferase inhibitors can reactivate epigenetically silenced genes and suppress tumor cell growth in vitro and in vivo (Herman and Baylin, 2003; Jones and Baylin, 2002; Robert et al., 2003; Suzuki et al., 2002).
TSP1 is methylated in several malignancies, decreased in melanoma, and can inhibit angiogenesis in melanoma xenografts (Li et al., 1999; Rofstad and Graff, 2001; Rojas et al., 2008; Yang et al., 2003; Zabrenetzky et al., 1994). To extend these studies to establish that pharmacologic reversal of methylation could result in functional inhibition of angiogenesis by TSP-1 and to establish effects in melanoma, we undertook preclinical studies in vitro and in vivo. To determine potential for clinical translation, the methylation status of the TSP1 promoter regions of nevi and melanoma tissues were investigated.
Results
1. 5-Aza-dC reduced DNMT1 protein
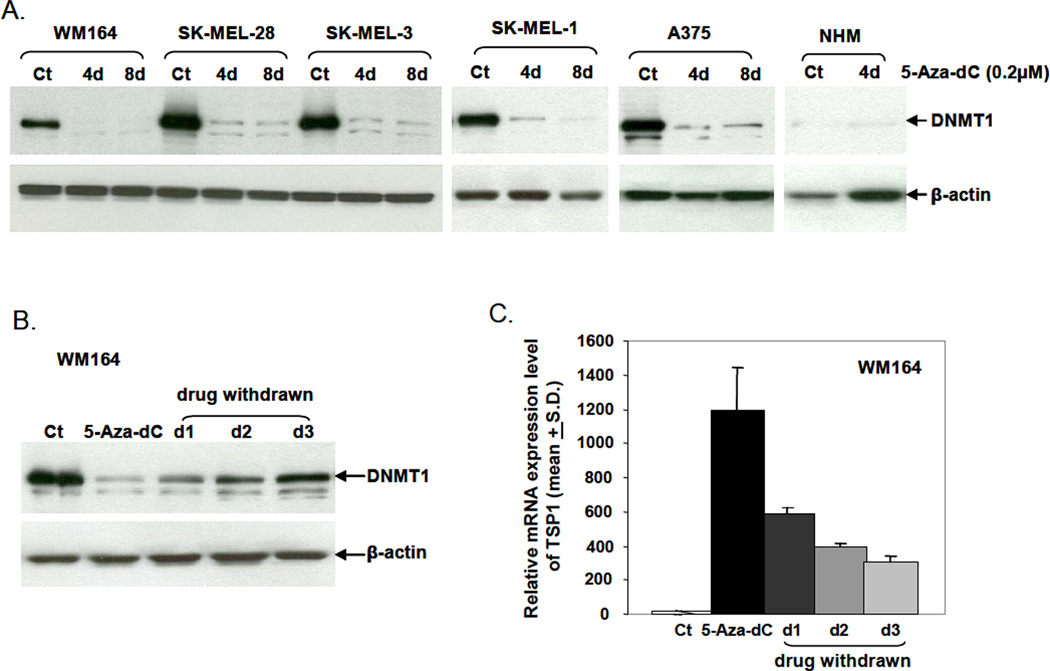
5-Aza-dC, when incorporated into DNA covalently binds DNMT1, resulting in DNMT1 depletion, demethylation, and gene reactivation (Ghoshal et al., 2005; Jung et al., 2007; Momparler, 2005). Prior to probing methylation of TSP1 in melanoma cells, the expression of DNMT1 before and after 5-Aza-dC was assessed. DNMT1 was markedly reduced in cell extracts derived from 5-Aza-dC-treated melanoma cells as compared with control untreated cells (Fig. 1A). Normal melanocytes expressed low basal levels of DNMT1 protein and DNMT1 expression was not affected by 5-Aza-dC treatment.
Figure 1.
Western blot analysis of DNMT1 levels during and after 5-Aza-dC treatment. (A) Cells were treated with 0.2 µM 5-Aza-dC daily for 4 and 8 days with medium and 5-Aza-dC refreshed every day. Whole-cell lysates were prepared from treated and untreated control cells. DNMT1 detection was performed using polyclonal rabbit anti-human DNMT1 antibody. Anti-β-actin antibody served as the loading control. (Ct) is untreated control; (NHM) is normal human melanocytes. (B) WM164 cells were treated with 0.2 µM 5-Aza-dC daily for 8 days, then cells were allowed to grow for an additional three days without treatment. DNMT1 protein levels in WM164 cells before, during, and after 5-Aza-dC treatment were analyzed by Western blotting. (C) The relative mRNA expression of TSP1 in WM164 cells was analyzed using a TaqMan probe. GAPDH served as endogenous control.
To confirm that DNMT1 was required for gene reactivation, WM164 cells were treated with 0.2 µM 5-Aza-dC daily for 8 days. Cells were then allowed to grow for an additional 3 days without treatment, after which time DNMT protein levels were measured. DNMT protein was markedly reduced at the end of 5-Aza-dC treatment but started to recover two days after drug removal and further increased to control levels at day 3 (Fig. 1B). Concomitantly, TSP1 gene expression was decreased by 5-Aza-dC but increased after 5-Aza-dC removal (Fig. 1C).
2. 5-Aza-dC reversed promoter hypermethylation and restored TSP1 expression
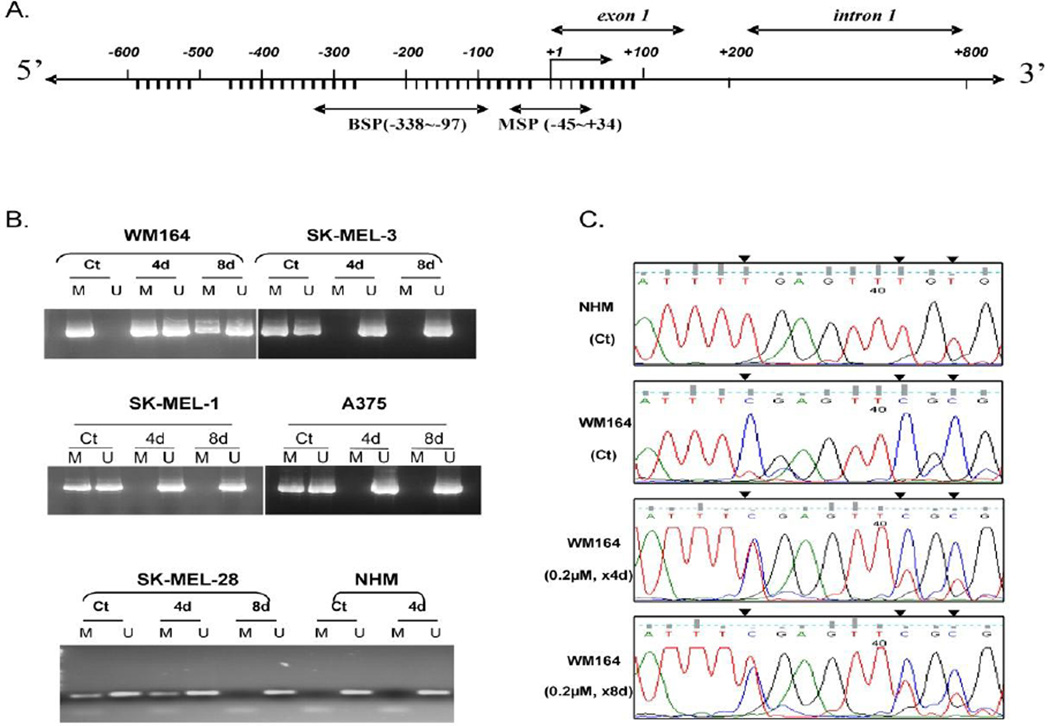
To confirm the effects of 5-Aza-dC on methylation reversal in melanoma cells, five established melanoma cell lines (SK-MEL-1, SK-MEL-3, SK-MEL-28, WM164, and A375) were evaluated. Initially, methylation-specific PCR was used to assess methylation of the TSP1 promoter region (Fig. 2A). In WM164 cells, complete methylation of the promoter region was observed in untreated cells, but after 5-Aza-dC treatment for 4 and 8 days, the promoter region changed from completely methylated to a partially methylated state (Fig. 2B). Similar results were found in SK-MEL-28, SK-MEL-1, SK-MEL-3, and A375 cells, in which the promoter region changed from partially methylated to a completely unmethylated state after treatment with 5-Aza-dC (Fig. 2B).
Figure 2.
Methylation analysis of TSP1 promoter regions. Genomic DNA isolated from 5-Aza-dC treated and untreated melanoma cell lines and normal melanocytes was bisulfite modified. The methylation status was analyzed by MSP. (A) Map of CpG-rich promoter region analyzed by MSP. (B) CpG-rich promoter region (−45 to +34, relative to transcription start site) methylation status. The presence of only the M band indicated complete methylation; the presence of only the U band indicated an unmethylated state; the presence of both M and U bands indicated partial methylated state. (Ct) is DNA from untreated control cells; (4d) is DNA from cells treated with 0.2 µM 5-Aza-dC daily for 4 days; (8d) is DNA from cells treated with 0.2 µM 5-Aza-dC daily for 8 days. (C) Direct bisulfite sequencing (−338 to −97) using bisulfite-modified genomic DNA from normal melanocytes (NHM) and 5-Aza-dC treated and untreated WM164 melanoma cells and. Black arrows indicate CpG sites. Results are representative of three different experiments.
To confirm promoter hypermethylation in melanoma cells, direct bisulfite sequencing was performed. The analysis was carried out using the sequences spanning the −338– to −97-bp region of the TSP1 promoter. In normal melanocytes, all C residues were converted to T residues, indicating an unmethylated promoter region (Fig. 2C, first row). In untreated WM164 cells, the DNA showed retention of all C residues in the CpG sequences (Fig. 2C, second row), indicating promoter hypermethylation in these cells. Partial methylation (both C and T residues in the same CpG sequence) occurred in WM164 cells treated with 5-Aza-dC (Fig. 2C, third row). The signal intensity representing T residues was stronger in these cells treated with 5-Aza-dC for 8 days than that of cells treated for 4 days (Fig. 2C, fourth row), indicating that more cells underwent demethylation after prolonged 5-Aza-dC treatment. These results further confirmed the MSP analysis.
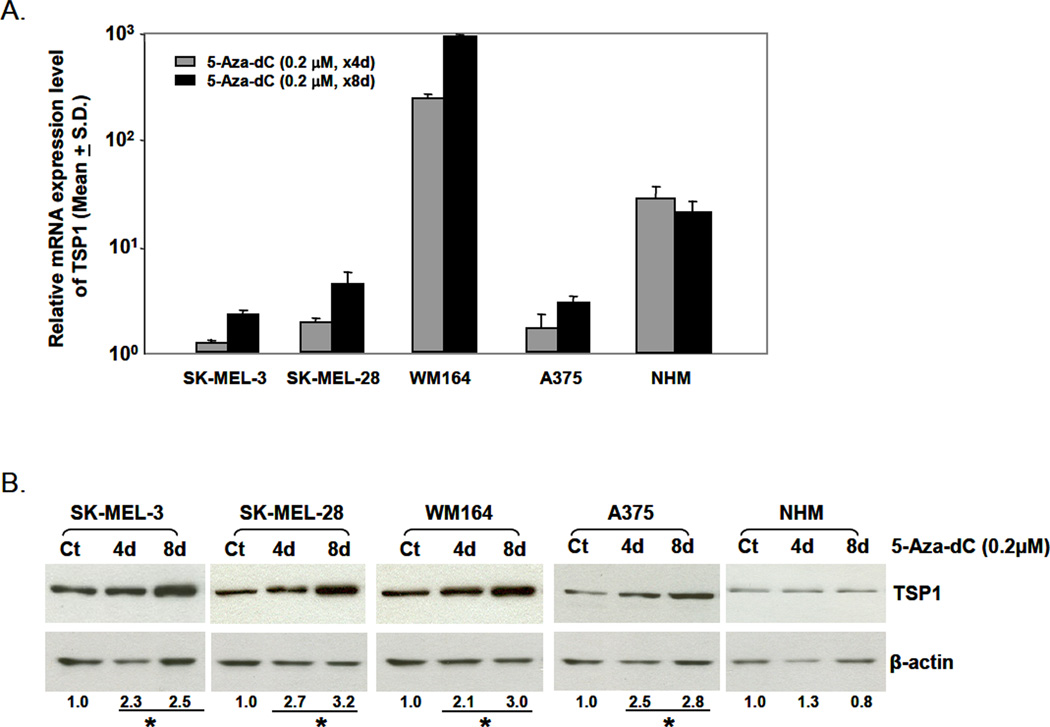
We then evaluated whether the change in promoter methylation was associated with change in expression of TSP1 after 5-Aza-dC treatment using Real-time PCR. The expression of TSP1 was increased in all cell lines after 4 days of 5-Aza-dC treatment compared with that of untreated cells. TSP1 expression was significantly further increased in WM164 cells after 8 days of 5-Aza-dC (P<0.001) compared with expression in the corresponding untreated cells and cells treated with 5-Aza-dC for 4 days (Fig. 3A). This was consistent with changes in the methylation status of TSP1 in WM164 cells and other cell lines. TSP1 protein levels were increased ~2- to 3-fold (P≤0.05) after 5-Aza-dC treatment in all melanoma cell lines; however, 5-Aza-dC had no effect on TSP1 protein levels in normal melanocytes (Fig. 3B), suggesting that the promoter methylation status determined TSP1 gene and protein expression in melanoma cell lines.
Figure 3.
Effect of 5-Aza-dC on TSP1 mRNA and protein expression. (A) Total RNA was isolated from untreated control cells and 5-Aza-dC-treated cells (4 and 8 days). The relative expression of TSP1 mRNA was analyzed using a TaqMan probe. GAPDH served as endogenous control. (B) Western blot analysis. Whole-cell lysates were prepared from untreated control cells (Ct) and 5-Aza-dC-treated cells (4 and 8 days). The expression of TSP1 protein was detected using monoclonal mouse anti-TSP1 antibody. Anti-β-actin antibody served as control. Results are representative of three independent experiments.
3. 5-Aza-dC inhibited new blood vessel formation
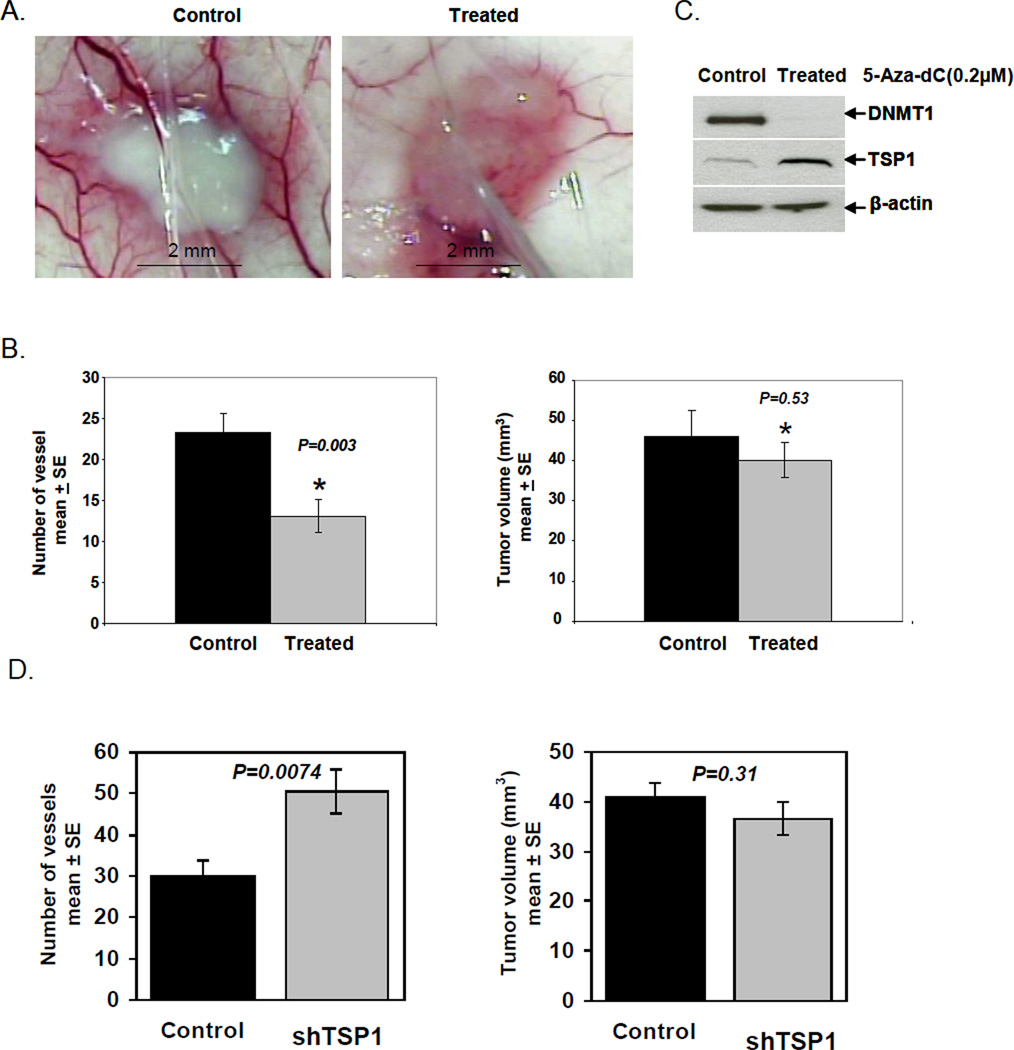
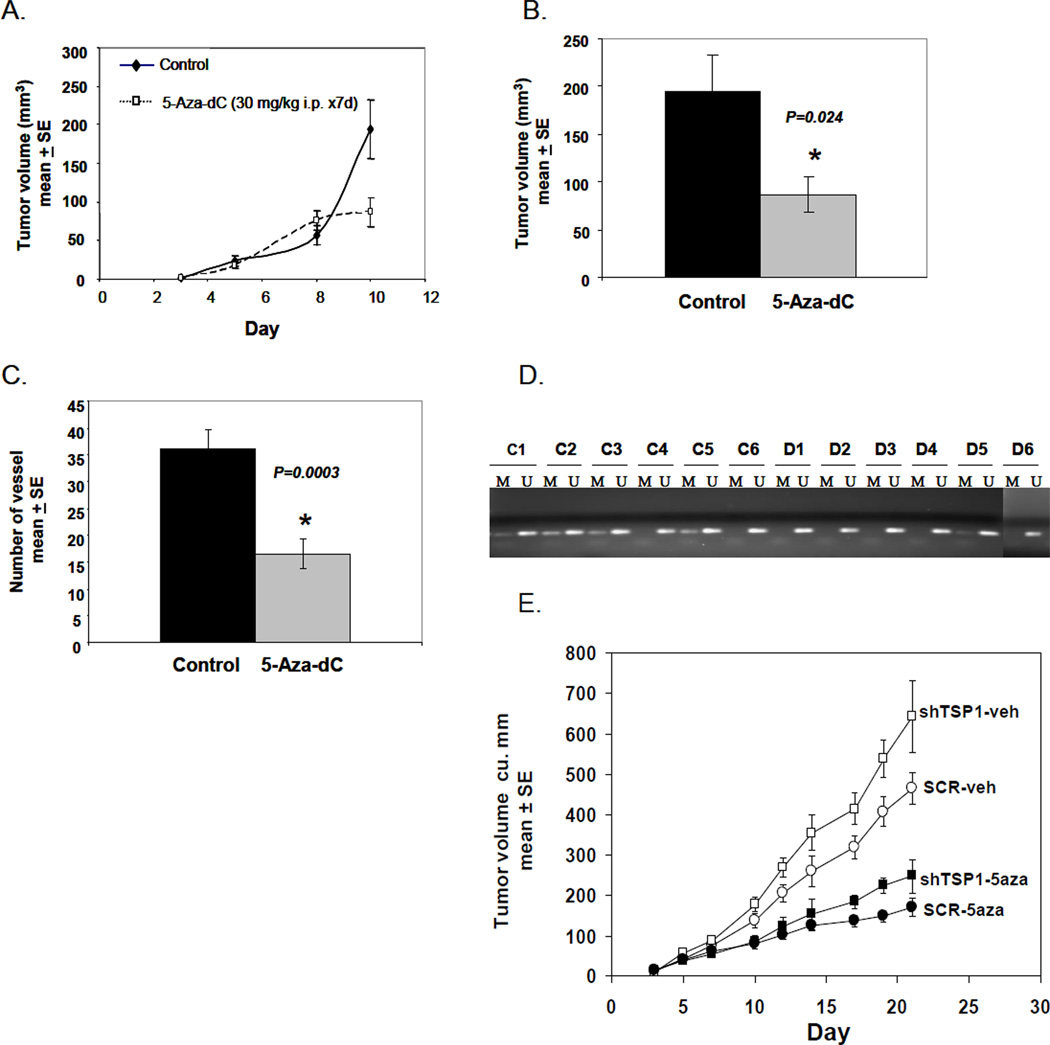
Given the in vitro effect of 5-Aza-dC on melanoma cells, which augmented expression of the TSP1 gene, we hypothesized that 5-Aza-dC would reduce tumor vascularization in vivo. Mice received A375 cells that had been pretreated with 5-Aza-dC in vitro and control mice received untreated A375 cells. Using a murine model for quantitating vascular response to tumor (Sidky and Auerbach, 1976; Sidky et al., 1986;Taylor et al., 2006), three days after inoculation, vessels at the tumor inoculation sites were counted by an observer blinded to treatments. There were more radially-oriented vessels contacting the periphery of the tumors in control mice (Fig. 4A). The number of vessels was significantly decreased (P=0.003, Student’s t-test) in mice with pretreated A375 cells compared with mice that received untreated A375 cells (Fig. 4B, left panel). To assure that decreased angiogenesis was not secondary to inhibition of tumor growth, tumor volume was also quantified. There was no significant difference in tumor volume between treated and control groups (P=0.53) (Fig. 4B, right panel). Thus, inhibition of angiogenesis, mediated by 5-Aza-dC, preceded any effect on tumor volume.
Figure 4.
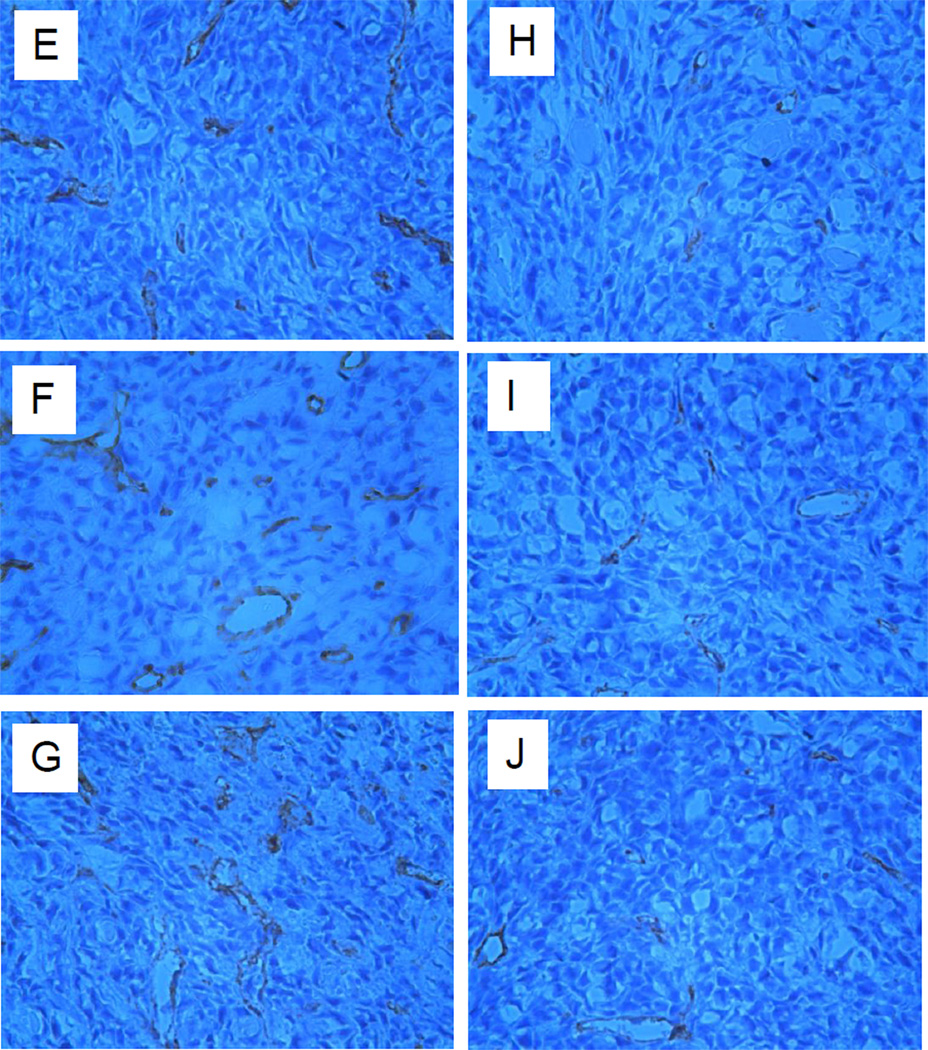
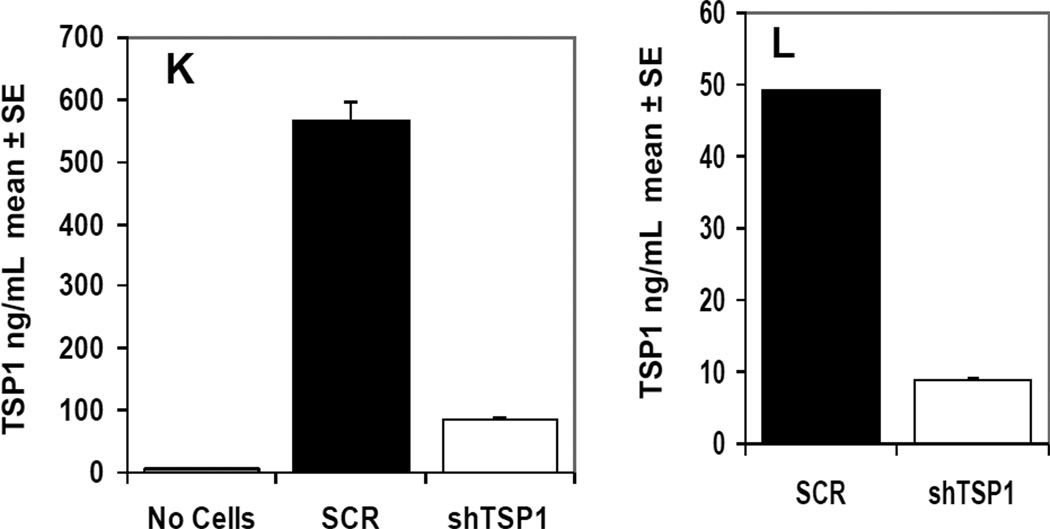
In vivo angiogenesis assay. 5-Aza-dC treated or untreated control A375 cells were inoculated into the dermis of nude mice. (A) Tumor inoculation sites from treated A375 cells had a reduction in vessel counts compared with controls 72 h post inoculation. (B) The number of blood vessels was significantly reduced in tumors established from treated A375 cells compared with those from controls 72 h post inoculation (left panel) (*P=0.003). Tumor vessels were evaluated in a 6×6 mm area centered on the tumor mass. There was no significant difference in tumor volume between treated and control tumors (right panel) (*P=0.53). (C) Western blot analysis indicated that 5-Aza-dC-treated A375 tumors had higher levels of TSP1 and lower levels of DNMT1 than controls. The results represent two independent experiments. (D) The number of blood vessels was significantly increased in tumors generated from shRNA-TSP1 cells (left) (P=0.0074) without an increase in tumor volume (right) (P=0.31). Immunohistochemical detection of tumor micro-vasculature using anti-CD31 antibodies in A375 xenografts expressing shTSP1 RNA (E-G) and scrambled RNA (H-J). TSP1 protein levels were determined in cultured SCR-A375 cells and shTSP1-A375 cells. TSP1 levels were approximately 10- fold higher in conditioned medium (K) compared to cell lysates (L).
TSP1 expression and DNMT1 levels in 5-Aza-dC pretreated and untreated A375 cells were measured and results were consistent with those shown previously (Figs. 1 and 3). TSP1 protein was significantly upregulated in 5-Aza-dC pretreated A375 tumors; moreover, 5-Aza-dC significantly diminished DNMT1 expression in these cells (Fig. 4C). Therefore, increased TSP1 expression induced by 5-Aza-dC was associated with inhibition of angiogenesis.
To demonstrate that cellular TSP1 levels had a direct effect upon the angiogenic response, the murine angiogenesis assay was performed with A375 cells that had been stably transfected with shRNA directed against TSP1. These cells have previously been shown to have suppressed levels of TSP1 (Trapp et al., 2010). Compared to control (scrambled RNA expressing cells, SCR) the shRNA-TSP1 cells induced 68% more vessels in the dermis (P=0.0074) (Fig. 4D, left panel). Enhanced angiogenesis in the shRNA-TSP1 was not due to differences in tumor size (P=0.31) (Fig. 4D, right panel). Repeat experiments confirmed these findings. To compare macroscopic peritumoral vessel formation in the dermis to intratumoral vascularity, microvessel density was assessed. At 3 days growth, sh-TSP1 and SCR tumors were analyzed for CD31 (PECAM-1) expression. A375 tumors expressing sh-TSP1 RNA (Fig. 4E-G) displayed 12.74 ± 1.08 vessels per mm2, whereas SCR expressing tumors (Fig. 4H-J) displayed 4.31 ± 0.72 vessels per mm2 (P=0.0001). Hence, vascularity of xenografts assessed by dermal angiogenesis assay (extratumoral vessels) correlated well with microvascular density (intratumoral vessels) as determined by CD31 staining. Both tumor types exhibited non-productive structures (unstained by CD31) that may represent vasculogenic mimicry. ELISA was utilized to quantitate TSP1 protein in A375 cell lines used to generate these tumors. TSP1 levels were 5–6 fold higher in SCR-A375 cells compared to shTSP1-A375 cells (Fig. 4K-L). The majority of TSP1 appeared to be secreted, as levels were 10-fold higher in conditioned medium (Fig. 4K) compared to cell lysates (Fig. 4L).
4. Tumor growth was suppressed in mice receiving 5-Aza-dC
To further investigate the effect of 5-Aza-dC on tumor growth, mice were inoculated with untreated A375 cells, and tumor volume was assessed. When average tumor volumes were approximately 2 mm3, half the mice received 5-Aza-dC i.p. 30 mg/kg, daily for 7 days. On day 10, the average tumor volume in treated mice was reduced by 55% compared with that of controls (P=0.024) (Fig. 5A and B). The number of blood vessels around the tumors was also significantly decreased (P=0.0003) after 5-Aza-dC treatment (Fig. 5C). To assess the contribution of TSP1 suppression to drug sensitivity, mice were inoculated with shTSP1-A375 cells or SCR-A375 cells and treated with vehicle or 5-Aza-dC as above. A375-shTSP1 tumors grown in mice receiving vehicle were 38% larger by day 21 compared to SCR-TSP1 tumors (P=0.021). Treatment with 5-Aza-dC inhibited the growth rate of both tumor types, A375-shTSP1 by 38%, and SCR-TSP1 by 37%, (P=0.00017, P=0.00032, respectively).
Figure 5.
In vivo anti-tumor activity of 5-Aza-dC. A375 melanoma cells were inoculated into the flanks of nude mice on day 0. Treatment of 5-Aza-dC (30 mg/kg i.p.) was initiated at day 3 and continued daily for 7 days. (A) Tumor volume was monitored every two days after 5-Aza-dC injection. Each data point represents 12 tumors. (B) Tumor volume was significantly reduced after 7 days of 5-Aza-dC treatment (*P=0.024). Tumor vessels were evaluated in a 6×6 mm area centered on the tumor mass. (C) The number of blood vessels around the tumors was significantly decreased (*P=0.0003). (D) MSP analysis of TSP1 promoter region in tumors derived from control (C1-6) and 5-Aza-dC treated (D1-6) A375 xenografts. The results represent three independent experiments. (E) A375 cells stably transfected with SCR (circles) or sh-TSP1 (squares) were inoculated into nude mice that were subsequently treated with vehicle (white squares and circles) or 5-Aza-dC (black squares and circles) as in (A).
We further analyzed the promoter methylation status of TSP1 in tumors derived from control and 5-Aza-dC-treated mice. Partial methylation of the TSP1 gene in the promoter region was present in 4 of 6 (67%) tumors from control mice (consistent with MSP analysis shown in Fig. 2B), whereas only 1 of 6 (17%) tumors from treated mice showed partial methylation (Fig. 5D). Thus, 5-Aza-dC induced TSP1 promoter demethylation in vivo, consistent with MSP analysis of A375 cells (Fig. 2B).
5. Methylation of TSP1 promoter region in nevi and melanoma tissues
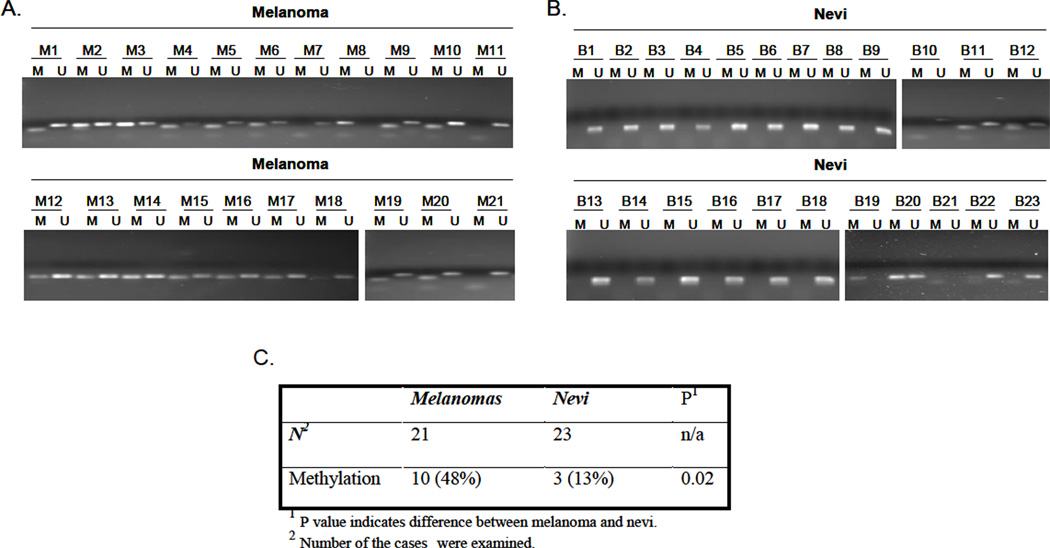
In view of frequent decreases in TSP1 expression in malignant melanoma (Zabrenetzky et al., 1994), we examined methylation patterns in the promoter of TSP1 and compared the frequency of aberrantly methylated TSP1 in malignant and benign nevi tissues. The results of MSP analysis showed that 1 of 21 melanomas (5%) and 1 of 23 nevi (4.3%) were fully methylated while, 9 of 21 melanomas (43%) and 2 of 23 nevi (8.7%) were partially methylated in this region (Fig. 6A and B). The frequency of promoter methylation in melanomas was significantly higher than that in the nevi (P=0.02) (Fig. 6C).
Figure 6.
Methylation status of TSP1 promoter region in melanoma and nevi samples. MSP analysis of promoter region in melanomas (A) and nevi samples (B). (C) Summary of hypermethylation frequency in TSP1 promoter region.
Discussion
Aberrant DNA methylation of promoters of genes that mediate tumor suppression and/or apoptosis is an important mechanism of oncogenesis (Baylin and Ohm, 2006;Esteller, 2002; Ghoshal et al., 2005; Jung et al., 2007; Momparler, 2005; Yoo and Jones, 2006). 5-Aza-dC and other methylation inhibitors can reverse DNA methylation in a replication-dependent manner and can reactivate gene expression silenced by hypermethylation. Treatment with 5-Aza-dC and other inhibitors of DNA methylation can result in methylated gene reactivation in both transplantable and xenograft tumor models, as well as improved mouse survival when used alone or, more commonly, in combinations (Alleman et al., 2004; Cantor et al., 2007; Eramo et al., 2005; Guo et al., 2006; Kaminski et al., 2004; Kozar et al., 2003; Laird et al., 1995; Liang et al., 2002;Natsume et al., 2008; Reid et al., 1992; Reu et al., 2006a; Tang et al., 2004; Venturelli et al., 2007; Zorn et al., 2007). Aberrant methylation has prompted studies of methylationspecific PCR for early diagnosis (Baylin and Ohm, 2006; Esteller, 2002; Yoo and Jones, 2006). The diversity of genes in melanoma affected by aberrant methylation, including RASSF1A, XAF1, PTEN, RARβ2, MAGE, and MGMT, suggests that epigenetic aberrations may be involved in the pathogenesis of melanoma (Hoon et al., 2004;Spugnardi et al., 2003). To determine whether promoter hypermethylation might be influencing these events in part through angiogenesis, we undertook studies of a proangiogenic factor TSP1 with CpG islands subject to promoter hypermethylation.
Decreased expression of the TSP1 gene in melanoma occurred secondary to promoter hypermethylation (Fig 4). In addition, 5-Aza-dC resulted in up-regulation of TSP1 and attenuated angiogenesis in vivo (Fig 5), suggesting that pharmacological intervention might rectify epigenetic aberrations in melanoma and might even reverse the course of melanoma progression. The 5-Aza-dC dose and schedule used induced a low frequency of apoptosis in melanoma cells (Reu et al., 2006b); however cell numbers in all treated cell lines increased in vitro 3–20×. DNMT1 was significantly depleted by 5-Aza-dC and resulted in TSP1 demethylation with a concomitant increase in gene and protein expression (Figs. 1–3). DNMT1 protein was returned to pretreatment levels three days after drug withdrawal, consistent with gene expression levels (Fig. 1B and C), suggesting that DNMT1 was critical for maintaining gene silencing. Suggesting selectivity of effect for tumor cells, 5-Aza-dC had no effect on DNMT1 depletion and TSP1 protein level in normal melanocytes.
In vivo angiogenesis assays identified markedly reduced tumor associated vessels in mice inoculated with melanoma cells that had been pretreated with 5-Aza-dC and in mice treated with 5-Aza-dC (Figs. 4B and 5C), suggesting an antiangiogenic effect of methylation reversal. Even though TSP1 was strongly induced by 5-Aza-dC, it was unlikely that TSP1 was mediating the entire antiangiogenic effect in melanoma, since vessel count was reduced by approximately half following 5-Aza-dC, but not eliminated entirely. Tumor growth was significantly suppressed when mice received 5-Aza-dC for a seven days after tumor implantation (Fig. 5A and B). Knockdown of TSP1 expression with shRNA caused a 68% increase in peri-tumoral vessel formation, strongly suggesting that TSP1 itself had a direct effect on suppression of the angiogenic phenotype (Fig. 4D). Importantly, inhibition of angiogenesis occurred prior to any detectable inhibitory effects on tumor volume. Therefore, it is likely that inhibition of angiogenesis led to diminished tumor volume. Consistent with in vitro analysis, TSP1 demethylation occurred in more than 60% of the tumors derived from 5-Aza-dC-treated mice (Fig. 5D). A375 tumors expressing sh-TSP1 displayed increased growth rate compared to SCR-A375 tumors (Fig 5E), suggesting a direct tumor suppressive role for TSP1 in this model. These results confirm the roles of 5-Aza-dC as an inhibitor of both angiogenesis and tumor growth. Furthermore, increased expression of TSP1 in tumor cells might play a major role in angiogenesis suppression in vivo. Our finding further supports other studies that the expression level of endogenous TSP1 is important in the regulation of the onset of tumor angiogenesis (Naumov et al., 2006b; Rastinejad et al., 1989).
Transfection of TSP1 into human breast carcinoma cells decreased angiogenesis, tumor growth, and metastasis in athymic nude mice (Leone et al., 1993; Pratt et al., 1989). Immunohistochemical analysis of human breast cancers has shown increases in the levels of TSP1 in stromal cells near carcinomas in situ but not in normal non adjacent breast tissue (Weidner et al., 1992). These findings are accord with the hypothesis that TSP1 may be a critical inhibitor of the angiogenic switch in tumor progression, since enhanced tumor angiogenesis was associated with decreases in TSP1 (Bocci et al., 2003;Naumov et al., 2006b; Rastinejad et al., 1989). Although we cannot eliminate the influence of other possible methylation-silenced antiangiogenic genes (Hellebrekers et al., 2006), our findings suggest that decreased TSP1 can result in accelerated tumor progression.
In conclusion, epigenetic modification repressed TSP1 expression in melanomas and 5-Aza-dC by inhibiting DNMT1 activity restored TSP-1 expression. In vivo, 5-Aza-dC significantly inhibited both tumor angiogenesis and subsequent tumor growth. This study has thus extended prior studies of methylation of TSP1 by identifying pharmacologic reversal of its silenced gene expression as having therapeutic impact through the functional biological component of the tumorigenic process, angiogenesis. Although our focus was TSP1, these results provide additional rationale for 5-AZA-dC in treating melanoma or other solid tumors—in addition to previously recognized effects on genes influencing immune response and apoptosis (Al-Romaih et al., 2007; Janson et al., 2008; Kaminski et al., 2004; Manning et al., 2008; Reu et al., 2006a; Reu et al., 2006b; Saudemont et al., 2007; Sigalotti et al., 2005; Spugnardi et al., 2003). Although further research is warranted to elucidate the molecular abnormality that maintains DNMT1 overexpression in melanoma cells, these studies confirm methylation of TSP1 in human melanomas and anti-angiogenic effects of an inhibitor of methylation.
Experimental Procedures
Cell line maintenance
Five melanoma cell lines, SK-MEL-1, SK-MEL-3, SK-MEL-28, A375, and WM164 (ATCC, Manassas, VA), were maintained in Dulbecco’s Modified Eagle Medium (Cellgro, Manassas, VA) supplemented with 10% fetal bovine serum (PAA, New Bedford, MA) plus 1% nonessential amino acids and 1 mM sodium pyruvate (Cellgro). Normal human melanocytes (Lonza, Walkersville, MD) were maintained in KBM basal medium plus MGM-4 SingleQuote kits (Lonza). A375 0121.P cells (stable shRNA-TSP1 knockdown) and A375 NS.p cells (vector control cells) were maintained in Advanced MEM (Gibco, Invitrogen, Carlsbad CA) supplemented with 10% fetal bovine serum plus 2 mM L-glutamine (Cellgro); TSP1 knockdown was confirmed by ELISA (Trapp et al., 2010).
Tissue collection
Melanoma and nevi specimens were obtained from patients who underwent surgical resection. These specimens were formalin fixed and embedded in paraffin. Each patient provided written informed consent.
Chemicals and antibodies
5-Aza-dC was purchased from Sigma-Aldrich (St. Louis, MO). Primary mouse anti-TSP1 monoclonal antibody was purchased from Neomarkers (Thermo Fisher Scientific, Fremont, CA), rabbit anti-DNMT1 polyclonal antibody from Abcam (Cambridge, MA), and mouse anti-β-actin monoclonal antibody from Bio-Rad (Hercules, CA). Secondary horseradish peroxidase-conjugated goat anti-rabbit IgG(H+L) and horseradish peroxidase-conjugated goat anti-mouse IgG(H+L) were purchased from Bio-Rad.
5-Aza-dC treatment
Melanoma cell lines were treated with 0.2 µM of 5-Aza-dC daily for 4 and 8 days. The medium and the 5-Aza-dC were refreshed every day. Cells were confluent at time of harvest.
DNA extraction, RNA isolation, and cDNA synthesis
Genomic DNA was extracted from melanoma cell lines and formalin-fixed paraffin-embedded specimens using MasterPure DNA Purification Kit (Epicentre Biotechnologies, Madison, WI). Cells were confluent at time of harvest. Total RNA was isolated from control and 5-Aza-dC-treated melanoma cells using TRIzol (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. The cDNA was synthesized using Superscript III Reverse Transcriptase kit (Invitrogen).
Real-time PCR
To measure mRNA expression levels of TSP1, quantitative real-time PCR was performed. TaqMan primer probes for TSP1, GAPDH, and Universal PCR Master Mix were purchased from Applied Biosystems (Foster City, CA). Real-time PCR was carried out on an ABI 7500 Real Time PCR System (Applied Biosystems). The fold change expression of each gene was represented as relative expression to the corresponding control after normalization to GAPDH.
Methylation-specific polymerase chain reaction (MSP)
Genomic DNA extracted from control and 5-Aza-dC-treated melanoma cell lines was modified with sodium bisulfite following the manufacturer’s instructions (Zymo Research, South San Francisco, CA). Cells were confluent at time of harvest. This modification resulted in a conversion of unmethylated cytosine to thymine, whereas methylated cytosine remained unchanged. Converted DNA was screened at promoter regions of TSP1 by MSP. When CpG sites were methylated, the M (methylation-specific PCR product) band was present. If the sites were unmethylated, the U (unmethylation-specific PCR product) band was present. If the sites were partially methylated, both bands were present. The CpG-rich region of TSP1 promoter, which was located in the 5’ regulatory regions of this gene, was analyzed by MSP. The primers designed for promoter region were as follows: 1) Methylated: sense 5’- TGCGAGCGTTTTTTTAAAAGTGC-3’, and antisense 5’- TAAACTCGCAAACCAACTCG-3’; Unmethylated: sense 5’- GAATGTGAGTGTTTTTTTAAAAGTGTG-3’, and antisense 5’- CCTAAACTCACAAACCAACTCAA-3’.
Bisulfite sequencing
Bisulfite-modified DNA was subjected to PCR amplification and directly sequenced using the ABI 3100 automated sequencing system (Applied Biosystems). The primer for bisulfite sequencing was designed to be complementary to DNA fragment containing no CpG dinucleotides, thus allowing unbiased amplification of methylated and unmethylated alleles. The primers were as follows: sense 5’- GGGTTTTTTTAGGTGGTTTTTTTAG-3’ and antisense 5’- AAACCAATCTAAACTCCTCTCTCC.
Western blot analysis
Western blot analysis was performed to measure protein expression of TSP1, DNMT1, with β-actin as a loading control. Whole-cell lysates were prepared using RIPA lysis buffer (Sigma) containing 1% protease inhibitor cocktail V (Calbiochem, Gibbstown, NJ). Cells were confluent at time of harvest. Total protein concentration was quantified using Protein Assay kit (Bio-Rad). Total protein (50 µg) was separated by electrophoresis on 7% SDS-polyacrylamide gels. Proteins were then transferred onto PVDF membranes (Millipore, Bedford, MA). To inhibit nonspecific binding, membranes were blocked with 5% nonfat dry milk in 1× TBST (Bio-Rad) for 1 h at 25°C. Blots were incubated with primary antibody for 16 h at 4°C. After washing with 1× TBST, membranes were incubated with horseradish peroxidase-conjugated secondary antibody for 2 h at 25°C. Membranes were incubated with enhanced chemiluminescence (ECL) reagent (Perkin-Elmer, Boston, MA) after washing. The signals were normalized to the corresponding endogenous β-actin as control.
TSP-1 Immunoassay
TSP-1 levels were measured using the Quantikine Human Thrombospondin-1 immunoassay kit (R&D Systems, Minneapolis, MN) according to manufacturer’s instructions. Cell culture supernates (conditioned medium) were collected from sub-confluent monolayers of silenced (shTSP1) and non-silenced (SCR) A375 cells, centrifuged, and filtered to remove cellular debris. The respective monolayers were then lysed using RIPA buffer (Sigma, St. Louis, MO), and protein concentrations were determined using the Bio-Rad protein assay (Bio-Rad Laboratories, Inc. Hercules, CA). Cell culture supernates and protein lysates were stored at −80C until analysis. The background level of TSP-1 in culture medium was determined using medium not exposed to cells.
Immunohistochemistry
Staining for intratumoral blood vessels was performed on formalin fixed paraffin embedded sections processed on a Ventana Benchmark automated immunostainer (Ventana Medical Systems, Inc., Tucson, Arizona) utilizing a Ventana iVIEW DAB Detection kit. Primary antibody, rabbit anti-mouse PECAM-1 (CD31) (#sc-1506, Santa Cruz Biotechnology, Inc.) was diluted 1:100. Goat anti-rabbit biotinylated secondary antibody (Vector Laboratories, Burlingame, CA) was used at 1:200. DAB (brown colored) positive vessels were manually counted on a reticule grid and expressed as vessels per mm2.
In vivo angiogenesis assay
A375 melanoma cells were exposed to 0.2 µM 5-Aza-dC in vitro daily for 4 days, with medium and drug refreshed every day. Four million viable cells were inoculated intradermally into the left and right flanks of 6-week-old athymic nude mice (Taconic, Germantown, NY). Three days after inoculation, mice were sacrificed via injection of 0.5 ml of avertin intraperitoneally (i.p.). The radially oriented blood vessels contacting the periphery of the tumor were counted by an observer blinded to treatments, and tumor volumes were measured. Similar analysis was performed for A375 0121.P cells (shTSP1, stable TSP1 knockdown) and A375 NS.p cells (scrambled, SCR, vector control), but these cells were not treated with Aza-dC.
In vivo anti-tumor activity of 5-Aza-dC
One million A375 cells were inoculated intradermally into the flanks of 6-week-old athymic nude mice on day 0. When mean tumor volume was approximately 2 mm3, mice received 30 mg/kg 5-Aza-dC or PBS i.p. daily for 7 days in experiments utilizing WT A375 (Fig. 5A). In experiments utilizing sh-TSP1-A375 and SCR-A375 cells, mice received treatment five days per week for three weeks (Fig. 5E). Tumor volume was measured three times per week. On day 10, mice were killed via injection of 0.5 ml of avertin i.p. The blood vessels at the periphery of the tumor were counted as described above, and tumor volumes were measured by the formula V= (4/3)πab2, where a=major tumor radius, b=minor tumor radius. Statistical significance was determined by Student’s t-test.
Data analysis
The difference in methylation frequency between benign lesions (nevi) and malignant melanoma tissues was evaluated for statistical significance using the Fisher’s exact test. P<0.05 was deemed as statistically significant.
Highlights.
Thrombospondin-1, an inhibitor of angiogenesis, is methylated in several malignancies.> Pharmacologic reversal of methylation can inhibit angiogenesis in malignant melanoma. > Knockdown of TSP-1 enhances melanoma angiogenesis in a xenograft model.
Acknowledgments
We thank Dr. Sun-Wei Guo for his advice on statistical analysis and Dr. Xuerong Shi for critical reading of the paper and Kristin Kraus for editorial assistance. Studies supported in part by Taussig Cancer Institute and NCI grant number P30-CA043703, Case Comprehensive Cancer Center Support Grant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests
The authors declare that they have no competing interests.
Author’s contributions
DL designed angiogenesis experiments and performed the in vivo experiments with RH and analyzed the data, and drafted the manuscript. YW designed and performed demethylation experiments, acquired and interpreted the data, and drafted the manuscript. BJ coordinated human tissue procurement, performed DNA extraction FFPE tissues, TSP1 ELISA, and drafted the manuscript. JF interpreted data. RT was the consulting pathologist for the human malignant melanoma and nevi tissue sections. EB helped to design the study, interpret the data, and draft the manuscript.
References
- Al-Romaih K, Somers GR, Bayani J, Hughes S, Prasad M, Cutz JC, Xue H, Zielenska M, Wang Y, Squire JA. Modulation by decitabine of gene expression and growth of osteosarcoma U2OS cells in vitro and in xenografts: identification of apoptotic genes as targets for demethylation. Cancer Cell Int. 2007;7:14. doi: 10.1186/1475-2867-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alleman WG, Tabios RL, Chandramouli GV, Aprelikova ON, Torres-Cabala C, Mendoza A, Rogers C, Sopko NA, Linehan WM, Vasselli JR. The in vitro and in vivo effects of re-expressing methylated von Hippel-Lindau tumor suppressor gene in clear cell renal carcinoma with 5-aza-2′-deoxycytidine. Clin. Cancer Res. 2004;10:7011–7021. doi: 10.1158/1078-0432.CCR-04-0516. [DOI] [PubMed] [Google Scholar]
- Baylin SB, Ohm JE. Epigenetic gene silencing in cancer - a mechanism for early oncogenic pathway addiction? Nat Rev Cancer. 2006;6:107–116. doi: 10.1038/nrc1799. [DOI] [PubMed] [Google Scholar]
- Bocci G, Francia G, Man S, Lawler J, Kerbel RS. Thrombospondin 1, a mediator of the antiangiogenic effects of low-dose metronomic chemotherapy. Proc Natl Acad Sci U S A. 2003;100:12917–12922. doi: 10.1073/pnas.2135406100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor JP, Iliopoulos D, Rao AS, Druck T, Semba S, Han SY, McCorkell KA, Lakshman TV, Collins JE, Wachsberger P, Friedberg JS, Huebner K. Epigenetic modulation of endogenous tumor suppressor expression in lung cancer xenografts suppresses tumorigenicity. Int J Cancer. 2007;120:24–31. doi: 10.1002/ijc.22073. [DOI] [PubMed] [Google Scholar]
- Eramo A, Pallini R, Lotti F, Sette G, Patti M, Bartucci M, Ricci-Vitiani L, Signore M, Stassi G, Larocca LM, Crino L, Peschle C, De Maria R. Inhibition of DNA methylation sensitizes glioblastoma for tumor necrosis factor-related apoptosis-inducing ligand-mediated destruction. Cancer Res. 2005;65:11469–11477. doi: 10.1158/0008-5472.CAN-05-1724. [DOI] [PubMed] [Google Scholar]
- Esteller M. CpG island hypermethylation and tumor suppressor genes: a booming present, a brighter future. Oncogene. 2002;21:5427–5440. doi: 10.1038/sj.onc.1205600. [DOI] [PubMed] [Google Scholar]
- Ghoshal K, Datta J, Majumder S, Bai S, Kutay H, Motiwala T, Jacob ST. 5-Aza-deoxycytidine induces selective degradation of DNA methyltransferase 1 by a proteasomal pathway that requires the KEN box, bromo-adjacent homology domain, and nuclear localization signal. Mol Cell Biol. 2005;25:4727–4741. doi: 10.1128/MCB.25.11.4727-4741.2005. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Guo ZS, Hong JA, Irvine KR, Chen GA, Spiess PJ, Liu Y, Zeng G, Wunderlich JR, Nguyen DM, Restifo NP, Schrump DS. De novo induction of a cancer/testis antigen by 5-aza-2′-deoxycytidine augments adoptive immunotherapy in a murine tumor model. Cancer Res. 2006;66:1105–1113. doi: 10.1158/0008-5472.CAN-05-3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellebrekers DM, Jair KW, Vire E, Eguchi S, Hoebers NT, Fraga MF, Esteller M, Fuks F, Baylin SB, van Engeland M, Griffioen AW. Angiostatic activity of DNA methyltransferase inhibitors. Mol Cancer Ther. 2006;5:467–475. doi: 10.1158/1535-7163.MCT-05-0417. [DOI] [PubMed] [Google Scholar]
- Herman JG, Baylin SB. Gene silencing in cancer in association with promoter hypermethylation. N Engl J Med. 2003;349:2042–2054. doi: 10.1056/NEJMra023075. [DOI] [PubMed] [Google Scholar]
- Hoon DS, Spugnardi M, Kuo C, Huang SK, Morton DL, Taback B. Profiling epigenetic inactivation of tumor suppressor genes in tumors and plasma from cutaneous melanoma patients. Oncogene. 2004;23:4014–4022. doi: 10.1038/sj.onc.1207505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain RK. Lessons from multidisciplinary translational trials on anti-angiogenic therapy of cancer. Nat Rev Cancer. 2008;8:309–316. doi: 10.1038/nrc2346. [DOI] [PubMed] [Google Scholar]
- Janson PC, Marits P, Thorn M, Ohlsson R, Winqvist O. CpG methylation of the IFNG gene as a mechanism to induce immunosuppression [correction of immunosupression] in tumor-infiltrating lymphocytes. J Immunol. 2008;181:2878–2886. doi: 10.4049/jimmunol.181.4.2878. [DOI] [PubMed] [Google Scholar]
- Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat Rev Genet. 2002;3:415–428. doi: 10.1038/nrg816. [DOI] [PubMed] [Google Scholar]
- Jung Y, Park J, Kim TY, Park JH, Jong HS, Im SA, Robertson KD, Bang YJ, Kim TY. Potential advantages of DNA methyltransferase 1 (DNMT1)-targeted inhibition for cancer therapy. J Mol Med (Berl) 2007;85:1137–1148. doi: 10.1007/s00109-007-0216-z. [DOI] [PubMed] [Google Scholar]
- Kaminski R, Kozar K, Niderla J, Grzela T, Wilczynski G, Skierski JS, Koronkiewicz M, Jakobisiak M, Golab J. Demethylating agent 5-aza-2′-deoxycytidine enhances expression of TNFRI and promotes TNF-mediated apoptosis in vitro and in vivo. Oncol Rep. 2004;12:509–516. [PubMed] [Google Scholar]
- Kerbel RS. Tumor angiogenesis. N Engl J Med. 2008;358:2039–2049. doi: 10.1056/NEJMra0706596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HC, Kim JC, Roh SA, Yu CS, Yook JH, Oh ST, Kim BS, Park KC, Chang R. Aberrant CpG island methylation in early-onset sporadic gastric carcinoma. J Cancer Res Clin Oncol. 2005;131:733–740. doi: 10.1007/s00432-005-0017-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozar K, Kaminski R, Switaj T, Oldak T, Machaj E, Wysocki PJ, Mackiewicz A, Lasek W, Jakobisiak M, Golab J. Interleukin 12-based immunotherapy improves the antitumor effectiveness of a low-dose 5-Aza-2′-deoxycitidine treatment in L1210 leukemia and B16F10 melanoma models in mice. Clin. Cancer Res. 2003;9:3124–3133. [PubMed] [Google Scholar]
- Laird PW, Jackson-Grusby L, Fazeli A, Dickinson SL, Jung WE, Li E, Weinberg RA, Jaenisch R. Suppression of intestinal neoplasia by DNA hypomethylation. Cell. 1995;81:197–205. doi: 10.1016/0092-8674(95)90329-1. [DOI] [PubMed] [Google Scholar]
- Lee S, Hwang KS, Lee HJ, Kim JS, Kang GH. Aberrant CpG island hypermethylation of multiple genes in colorectal neoplasia. Lab Invest. 2004;84:884–893. doi: 10.1038/labinvest.3700108. [DOI] [PubMed] [Google Scholar]
- Leone A, Flatow U, VanHoutte K, Steeg PS. Transfection of human nm23-H1 into the human MDA-MB-435 breast carcinoma cell line: effects on tumor metastatic potential, colonization and enzymatic activity. Oncogene. 1993;8:2325–2333. [PubMed] [Google Scholar]
- Li Q, Ahuja N, Burger PC, Issa JP. Methylation and silencing of the Thrombospondin-1 promoter in human cancer. Oncogene. 1999;18:3284–3289. doi: 10.1038/sj.onc.1202663. [DOI] [PubMed] [Google Scholar]
- Liang G, Gonzales FA, Jones PA, Orntoft TF, Thykjaer T. Analysis of gene induction in human fibroblasts and bladder cancer cells exposed to the methylation inhibitor 5-aza-2′-deoxycytidine. Cancer Res. 2002;62:961–966. [PubMed] [Google Scholar]
- Manning J, Indrova M, Lubyova B, Pribylova H, Bieblova J, Hejnar J, Simova J, Jandlova T, Bubenik J, Reinis M. Induction of MHC class I molecule cell surface expression and epigenetic activation of antigen-processing machinery components in a murine model for human papilloma virus 16-associated tumours. Immunology. 2008;123:218–227. doi: 10.1111/j.1365-2567.2007.02689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momparler RL. Epigenetic therapy of cancer with 5-aza-2′-deoxycytidine (decitabine) Semin Oncol. 2005;32:443–451. doi: 10.1053/j.seminoncol.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Natsume A, Wakabayashi T, Tsujimura K, Shimato S, Ito M, Kuzushima K, Kondo Y, Sekido Y, Kawatsura H, Narita Y, Yoshida J. The DNA demethylating agent 5-aza-2′-deoxycytidine activates NY-ESO-1 antigenicity in orthotopic human glioma. Int J Cancer. 2008;122:2542–2553. doi: 10.1002/ijc.23407. [DOI] [PubMed] [Google Scholar]
- Naumov GN, Akslen LA, Folkman J. Role of angiogenesis in human tumor dormancy: animal models of the angiogenic switch. Cell Cycle. 2006a;5:1779–1787. doi: 10.4161/cc.5.16.3018. [DOI] [PubMed] [Google Scholar]
- Naumov GN, Bender E, Zurakowski D, Kang SY, Sampson D, Flynn E, Watnick RS, Straume O, Akslen LA, Folkman J, Almog N. A model of human tumor dormancy: an angiogenic switch from the nonangiogenic phenotype. J Natl Cancer Inst. 2006b;98:316–325. doi: 10.1093/jnci/djj068. [DOI] [PubMed] [Google Scholar]
- Pratt DA, Miller WR, Dawes J. Thrombospondin in malignant and non-malignant breast tissue. Eur J Cancer Clin Oncol. 1989;25:343–350. doi: 10.1016/0277-5379(89)90028-x. [DOI] [PubMed] [Google Scholar]
- Rastinejad F, Polverini PJ, Bouck NP. Regulation of the activity of a new inhibitor of angiogenesis by a cancer suppressor gene. Cell. 1989;56:345–355. doi: 10.1016/0092-8674(89)90238-9. [DOI] [PubMed] [Google Scholar]
- Reid TR, Merigan TC, Basham TY. Resistance to interferon-alpha in a mouse B-cell lymphoma involves DNA methylation. J Interferon Res. 1992;12:131–137. doi: 10.1089/jir.1992.12.131. [DOI] [PubMed] [Google Scholar]
- Reu FJ, Bae SI, Cherkassky L, Leaman DW, Lindner D, Beaulieu N, Macleod AR, Borden EC. Overcoming Resistance to Interferon-Induced Apoptosis of Renal Carcinoma and Melanoma Cells by DNA Demethylation. J Clin Oncol. 2006a;24:3771–3779. doi: 10.1200/JCO.2005.03.4074. [DOI] [PubMed] [Google Scholar]
- Reu FJ, Leaman DW, Maitra RR, Bae SI, Cherkassky L, Fox MW, Rempinski DR, Beaulieu N, MacLeod AR, Borden EC. Expression of RASSF1A, an epigenetically silenced tumor suppressor, overcomes resistance to apoptosis induction by interferons. Cancer Res. 2006b;66:2785–2793. doi: 10.1158/0008-5472.CAN-05-2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert MF, Morin S, Beaulieu N, Gauthier F, Chute IC, Barsalou A, MacLeod AR. DNMT1 is required to maintain CpG methylation and aberrant gene silencing in human cancer cells. Nat Genet. 2003;33:61–65. doi: 10.1038/ng1068. [DOI] [PubMed] [Google Scholar]
- Rofstad EK, Graff BA. Thrombospondin-1-mediated metastasis suppression by the primary tumor in human melanoma xenografts. J Invest Dermatol. 2001;117:1042–1049. doi: 10.1046/j.0022-202x.2001.01552.x. [DOI] [PubMed] [Google Scholar]
- Rojas A, Meherem S, Kim YH, Washington MK, Willis JE, Markowitz SD, Grady WM. The aberrant methylation of TSP1 suppresses TGF-beta1 activation in colorectal cancer. Int J Cancer. 2008;123:14–21. doi: 10.1002/ijc.23608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saudemont A, Hamrouni A, Marchetti P, Liu J, Jouy N, Hetuin D, Colucci F, Quesnel B. Dormant tumor cells develop cross-resistance to apoptosis induced by CTLs or imatinib mesylate via methylation of suppressor of cytokine signaling 1. Cancer Res. 2007;67:4491–4498. doi: 10.1158/0008-5472.CAN-06-1627. [DOI] [PubMed] [Google Scholar]
- Sidky YA, Auerbach R. Lymphocyte-induced angiogenesis in tumor-bearing mice. Science. 1976;192:1237–1238. doi: 10.1126/science.5775. [DOI] [PubMed] [Google Scholar]
- Sidky YA, Borden EC, Wierenga W, Bryan GT. Inhibitory effects of interferon-inducing pyrimidinones on the growth of transplantable mouse bladder tumors. Cancer Res. 1986;46:3798–3802. [PubMed] [Google Scholar]
- Sigalotti L, Coral S, Fratta E, Lamaj E, Danielli R, Di Giacomo AM, Altomonte M, Maio M. Epigenetic modulation of solid tumors as a novel approach for cancer immunotherapy. Semin Oncol. 2005;32:473–478. doi: 10.1053/j.seminoncol.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Silverstein RL, Febbraio M. CD36-TSP-HRGP interactions in the regulation of angiogenesis. Curr Pharm Des. 2007;13:3559–3567. doi: 10.2174/138161207782794185. [DOI] [PubMed] [Google Scholar]
- Spugnardi M, Tommasi S, Dammann R, Pfeifer GP, Hoon DS. Epigenetic inactivation of RAS association domain family protein 1 (RASSF1A) in malignant cutaneous melanoma. Cancer Res. 2003;63:1639–1643. [PubMed] [Google Scholar]
- Suzuki H, Gabrielson E, Chen W, Anbazhagan R, van Engeland M, Weijenberg MP, Herman JG, Baylin SB. A genomic screen for genes upregulated by demethylation and histone deacetylase inhibition in human colorectal cancer. Nat Genet. 2002;31:141–149. doi: 10.1038/ng892. [DOI] [PubMed] [Google Scholar]
- Tang QB, Sun HW, Zou SQ. Inhibitory effect of methylation inhibitor 5-aza-2-deoxycytidine on bile duct cancer cell line in vivo and in vitro. Hepatobiliary Pancreat Dis Int. 2004;3:124–128. [PubMed] [Google Scholar]
- Taylor KL, Oates RK, Grane RW, Leaman DW, Borden EC, Lindner DJ. Interferon-alpha1,8 inhibits tumor-induced angiogenesis in murine angiosarcomas. J. Interferon & Cytokine Res. 2006;26:353–361. doi: 10.1089/jir.2006.26.353. [DOI] [PubMed] [Google Scholar]
- Trapp V, Parmakhtiar B, Papazian V, Willmott L, Fruehauf JP. Anti-angiogenic effects of resveratrol mediated by decreased VEGF and increased TSP1 expression in melanoma-endothelial cell co-culture. Angiogenesis. 2010;13:305–315. doi: 10.1007/s10456-010-9187-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venturelli S, Armeanu S, Pathil A, Hsieh CJ, Weiss TS, Vonthein R, Wehrmann M, Gregor M, Lauer UM, Bitzer M. Epigenetic combination therapy as a tumor-selective treatment approach for hepatocellular carcinoma. Cancer. 2007;109:2132–2141. doi: 10.1002/cncr.22652. [DOI] [PubMed] [Google Scholar]
- Weidner N, Folkman J, Pozza F, Bevilacqua P, Allred EN, Moore DH, Meli S, Gasparini G. Tumor angiogenesis: a new significant and independent prognostic indicator in early-stage breast carcinoma. J Natl Cancer Inst. 1992;84:1875–1887. doi: 10.1093/jnci/84.24.1875. [DOI] [PubMed] [Google Scholar]
- Yang QW, Liu S, Tian Y, Salwen HR, Chlenski A, Weinstein J, Cohn SL. Methylation-associated silencing of the thrombospondin-1 gene in human neuroblastoma. Cancer Res. 2003;63:6299–6310. [PubMed] [Google Scholar]
- Yoo CB, Jones PA. Epigenetic therapy of cancer: past, present and future. Nat Rev Drug Discov. 2006;5:37–50. doi: 10.1038/nrd1930. [DOI] [PubMed] [Google Scholar]
- Zabrenetzky V, Harris CC, Steeg PS, Roberts DD. Expression of the extracellular matrix molecule thrombospondin inversely correlates with malignant progression in melanoma, lung and breast carcinoma cell lines. Int J Cancer. 1994;59:191–195. doi: 10.1002/ijc.2910590209. [DOI] [PubMed] [Google Scholar]
- Zorn CS, Wojno KJ, McCabe MT, Kuefer R, Gschwend JE, Day ML. 5-aza-2′-deoxycytidine delays androgen-independent disease and improves survival in the transgenic adenocarcinoma of the mouse prostate mouse model of prostate cancer. Clin. Cancer Res. 2007;13:2136–2143. doi: 10.1158/1078-0432.CCR-06-2381. [DOI] [PubMed] [Google Scholar]