Summary
Cells regulate adhesion in response to internally-generated and externally-applied forces. Integrins connect the extracellular matrix to the cytoskeleton and provide cells with mechanical anchorages and signaling platforms. Here we show that cyclic forces applied to a fibronectin–integrin α5β1 bond switch the bond from a short-lived state with 1-s lifetime to a long-lived state with 100-s lifetime. We term this phenomenon “cyclic mechanical reinforcement” as the bond strength remembers the history of force application, accumulates over repeated cycles, but does not require force to be sustained. Cyclic mechanical reinforcement strengthens the fibronectin–integrin α5β1 bond through the RGD binding site of the ligand with the synergy binding site greatly facilitating the process. A flexible integrin hybrid domain is also important for cyclic mechanical reinforcement. Our results reveal a mechanical regulation of receptor–ligand interactions and identify a molecular mechanism for cell adhesion strengthening by cyclic forces.
Introduction
Mechanical forces are ubiquitous inside and outside of the cell (Chan and Odde, 2008; Chen, 2008; Gardel et al., 2008; Giannone et al., 2007; Ji et al., 2008). Cells change their behavior in response to force, and this may eventually change their fate. As major cell adhesion molecules, integrins bear force through binding to ligands in the extracellular matrix or on another cell and anchoring to the cytoskeleton. Through these integral connections, integrins contribute to transducing force-dependent mechanical signals to enzyme-dependent chemical signals inside the cell. Integrin engagement and signaling occur focally and are mediated by specialized multimolecular complexes. Consistent with their roles in force sensing, the ligand–integrin–cytoskeleton complex contains major mechano-sensing machineries for the cell. Several intracellular molecules in this complex have been shown to exhibit tension-dependent conformational changes that alter either kinase activities (Sawada et al., 2006) or localization of other signaling molecules (del Rio et al., 2009; Galbraith et al., 2002). Moreover, force can strengthen ligand–integrin interactions (Chen et al., 2010; Friedland et al., 2009; Kong et al., 2009). Forces exerted to and experienced by cells via these molecules are often dynamic and cyclic. For example, cells exert dynamic tractions to the substrate in cycles of edge protrusion, adhesion and retraction to generate motility (Balaban et al., 2001; Giannone et al., 2007), which transmits myosin-generated forces via the retrograde flow of actin filaments to ligand-bound integrins in a repetitive loading-unloading fashion (Chan and Odde, 2008; Gardel et al., 2008). However, how these molecular nano-machines work under dynamic forces is unclear. Here, we analyze the responses an integrin–ligand bond to cyclic forces using atomic force microscopy (AFM) and a biomembrane force probe (BFP).
Results
Observing cyclic mechanical reinforcement of recombinant α5β1-Fc
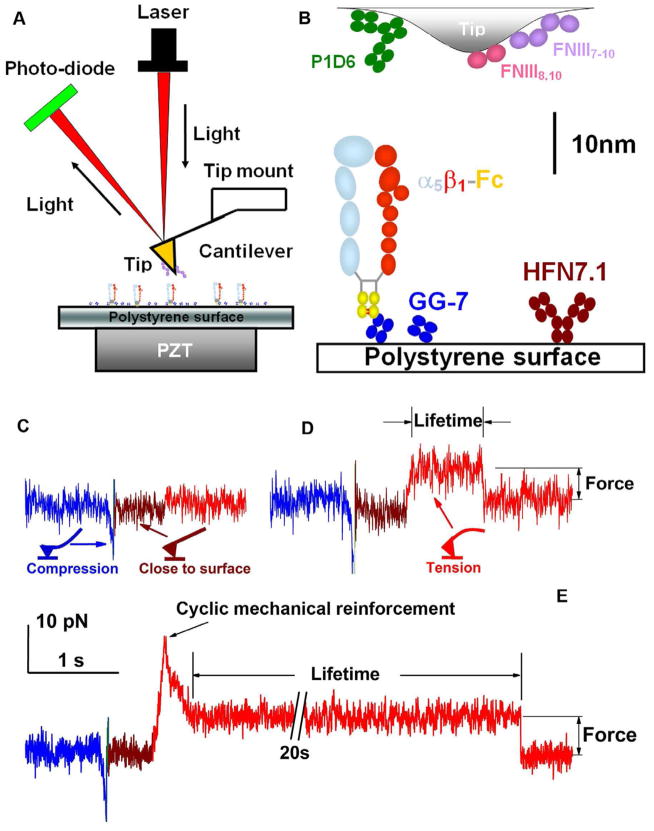
We first studied the interaction between a chimeric integrin α5β1-Fc (Coe et al., 2001) and fibronectin (FN) fragments (Petrie et al., 2006) to cyclic forces using AFM (Figure 1A). Integrins captured by anti-Fc (GG-7) preadsorbed on a polystyrene surface were driven to touch FN adsorbed on a cantilever tip (Figure 1B), held within a close distance (~ 2 nm) to allow bond formation, and retracted to determine the presence of a bond (Figure 1C and D).
Figure 1. AFM experiments.
A, AFM schematic. B, AFM functionalization. A composite of all molecules used is depicted. C, Force-scan trace without adhesion. D, A FN–α5β1-Fc–GG-7 serial bond was loaded to 5 pN and lifetime measured at that force. E, A FN–α5β1-Fc–GG-7 serial bond was first loaded to 25 pN, then unloaded to 5 pN, and held at 5 pN for lifetime measurement.
When loaded by a linear ramp to a 5-pN constant force, FN–α5β1 bonds lasted 1 s on average (Figures 1D and 2A). This value was lengthened to 3 s when the constant force was increased to 25 pN, exhibiting the catch bond (whose lifetime is prolonged by force, Figure S1) behavior as recently observed (Kong et al., 2009). However, molecular bonds often experience dynamic forces that change over time. We therefore asked whether a FN–α5β1 bond would retain its ability to live longer, acquired at a higher force, after force removal. To address this question, we applied a single cycle of force by first loading individual FN–α5β1 bonds to 25 pN and then unloading to 5 pN for lifetime measurements (Figure 1E). Strikingly, mean lifetimes were not kept at 3 s or returned to 1 s; instead, they were substantially prolonged to 30 s (Figures 1E and 2A). We term this phenomenon cyclic mechanical reinforcement (CMR) as it is distinct from the catch bond behavior (Figure S1). By comparison, chemical activation, such as changing the divalent cation composition of the buffer from a physiological mixture of Ca2+/Mg2+ to Mg2+/EGTA or Mn2+ (Figure 2A), greatly increased the on-rate for FN–α5β1 association, but had little effect on off-rate (reciprocal average lifetimes) (Kong et al., 2009). Furthermore, Mg2+/EGTA or Mn2+ did not negate the ability of FN–α5β1 bond lifetimes to be prolonged by cyclic forces (Figure 2A), demonstrating that chemical activation and cyclic mechanical reinforcement are two distinct regulatory mechanisms.
Figure 2. Cyclic mechanical reinforcement prolongs FN–α5β1 bond lifetimes.
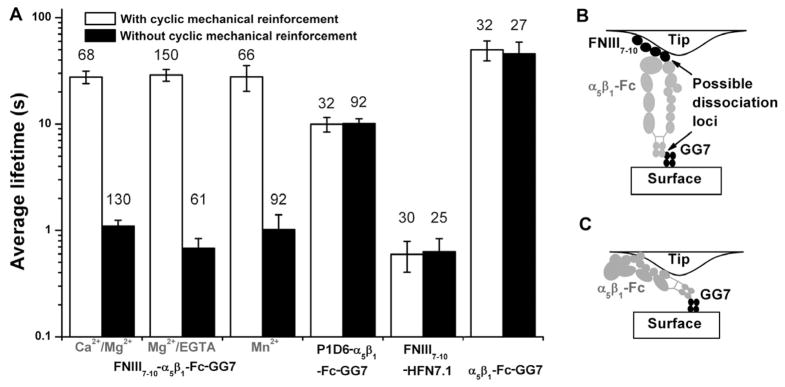
A, Mean ± s.e.m. of indicated numbers (atop each bar) of FN–α5β1-Fc–GG-7, P1D6–α5β1-Fc–GG-7, FN–HFN7.1, and α5β1-Fc–GG-7 bond lifetimes measured at ~5 pN without (closed bars) and with (open bars) cyclic mechanical reinforcement with a ~25 pN peak force in the indicated conditions. B, Schematic of a FN–α5β1-Fc–GG-7 serial bond indicating possible dissociation loci between FN and α5β1 or between Fc and GG-7. C, Schematic of a possible molecular arrangement for experiments that measured the lifetimes of the α5β1-Fc/GG-7 capture bond. See also Figure S1.
Cyclic mechanical reinforcement is specific between integrin and physiological ligands. Replacing either FN by an anti-α5β1 (P1D6) or α5β1-Fc by an anti-FN (HFN7.1) abolished the phenomenon (Figure 2A). Furthermore, α5β1-Fc–GG-7 bonds showed no cyclic mechanical reinforcement (Figure 2A), locating the effect at the FN–α5β1-Fc bond in the FN–α5β1-Fc–GG-7 serial bonds (i.e. FN–α5β1-Fc and α5β1-Fc–GG7 bonds in series). Moreover, lower the unloading rate by 10 folds had no effect on the post-reinforcement lifetime (Figure S1E), indicating that the rate of unloading in the 5–50 pN/s range does not impact its ability to reinforce.
In the cyclic mechanical reinforcement experiments described earlier, unloading was incomplete as the force was held at 5–10 pN but did not return to zero. This was to identify bond dissociation from the abrupt force drop for lifetime measurements. To investigate if the FN–α5β1 would remain in the long-lived state without any tensile force, we unloaded the force to zero after loading to a 25-pN peak force and kept the force at zero for a certain period (holding time) before separating the cantilever from the surface again to detect if the adhesion remained (Figure S2A). Almost 100% of the bonds remained associated for all holding times tested, even after 30 s (Figure S2B, blank bar). Furthermore, the force-molecular extension curves overlapped for two loading phases: 1) first pull for mechanical reinforcement and 2) second pull for detection of the presence of a bond (Figure S2C). This suggests that it was the same bond that was being pulled in both cases. To rule out the possibility that the adhesion detected in the second pull was due to dissociation and re-binding of the same bond, we measured adhesion events by rupturing the bond in the first pull, then bringing the surface and the cantilever back to contact at the same location, and holding at zero force for a similar time period to allow for rebinding (Figure S2B). This time, the chance of detecting an adhesion in the second pull is much lower (Figure S2B, hatched bar), demonstrating that the close-to-100% adhesion detected in the second pull after cyclic mechanical reinforcement is unlikely due to dissociation and re-engagement of the bond.
Observing cyclic mechanical reinforcement of cell surface integrins
To confirm that the cyclic mechanical reinforcement phenomenon also existed for native integrins in addition to the recombinant chimeric α5β1-Fc construct, we studied this effect on living cells using a biomembrane force probe (BFP, Figure 3A and B) (Chen et al., 2008). We measured specific interactions (Figure 3C, left open bars) between FN-coated beads and α5β1-expressing K562 cells in Mg2+/EGTA. Without cyclic mechanical reinforcement, FN–α5β1 bonds dissociated within 1 s at 5 pN. Upon cyclic mechanical reinforcement (with a 20-pN peak force), the FN–α5β1 bond lifetimes were extended to an average of 14 s (Figure 3D, left open bars). A similar effect was observed for specific bonds (Figure 3C, right closed bars) between intercellular adhesion molecule-1 (ICAM-1)-coated beads and integrin αLβ2-expressing Jurkat cells (Figure 3D, right closed bars), suggesting that cyclic mechanical reinforcement may be a common property for ligand–integrin bonds.
Figure 3. Cyclic mechanical reinforcement of cell surface integrins.
A, Experimental setup of BFP. B, Representative force-time traces: i, 1) Approaching phase before contact (with zero force), 2) contact phase with a compressive force, 3) retracting phase without adhesion (the force returned to zero). ii, Similar to (i) except that adhesion was detected in phase (3) by a tensile force. The PZT retraction stopped to hold the force constant for lifetime measurement. iii, Similar to (ii) except that lifetime measurement was taken following a force cycle of loading to a high force then unloading to the low force. After a 10-s holding phase for lifetime measurement the PZT retracted again to rupture the bond because force drift was often observed during measurements of exceedingly long lifetimes. C, Left, adhesion frequency between FN-coated beads and α5β1-expressing K562 cells in 2 mM Mg2+/EGTA was reduced by >2 folds by 10 μg/ml of anti-FN blocking mAb (HFN7.1) and by not coating FN on the beads, indicating adhesion specificity. Right, adhesion frequency between ICAM-1-coated beads and αLβ2-expressing Jurkat cells in 2 mM Mg2+/EGTA was reduced by >4 folds by not coating ICAM-1 on the beads, indicating adhesion specificity. Data are presented as mean ± s.e.m. of ≥5 cell-bead pairs each contacting 100 times. D, Average lifetimes derived from using the two-state model to fit the lifetime distributions of FN–α5β1 (left) or ICAM1-αLβ2 (right) bonds measured at 5 pN with or without cyclic mechanical reinforcement (20 pN peak force). Data are presented as fitted mean ± s.e.m..
Characterizing single-cycled mechanical reinforcement
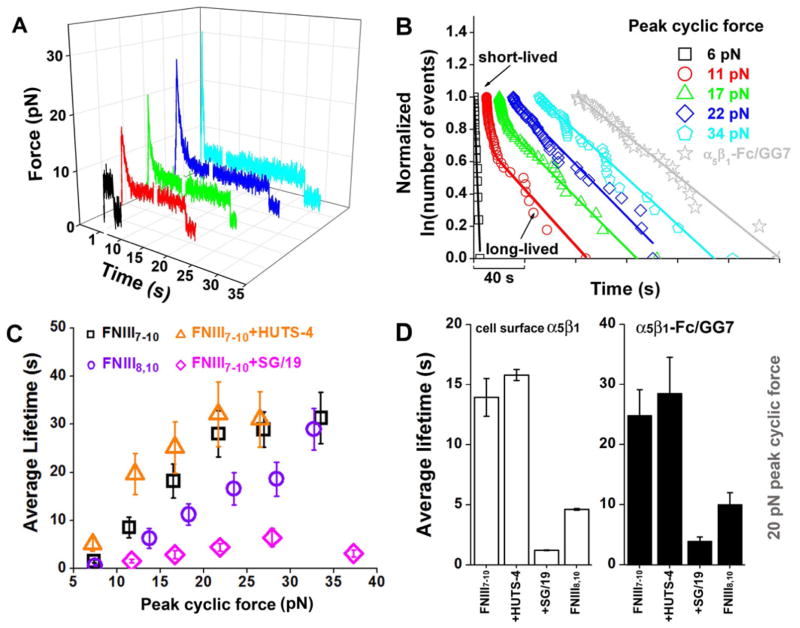
To characterize the mechanical reinforcement phenomenon further, we analyzed FN–α5β1-Fc–GG-7 bond lifetimes at 5 pN after a single cycle of force with a peak ranging from 7–40 pN in Mg2+/EGTA (Figure 4A). As the peak force increased, the average lifetime increased from 1 s to >30 s and reached a plateau at 22 pN (Figure 4C). Without the cyclic force, the lifetime distribution at 5 pN decayed as a single exponential, suggesting a single bond-state (Figure 4B). With cyclic forces at peaks ranging from 11–17 pN, the lifetime distributions displayed multi-exponential decays, indicating multiple bond-states. Beyond 22 pN, the lifetime distributions became single exponentials again. The distributions can be fitted accurately by a two-state model that includes mixed populations of two bond-states: a short-lived state with the same average lifetime as the FN–α5β1-Fc–GG-7 serial bond at 5 pN without cyclic mechanical reinforcement and a long-lived state with the same average lifetime as the Fc–GG-7 capturing bond at 5 pN (Kong et al., 2009). As the peak force increased, the long-lived state fraction increased at the expense of the short-lived state fraction, indicating that more and more FN–α5β1 bonds were switched from the short- to long-lived state.
Figure 4. Effects of peak force of single-cycled mechanical reinforcement on FN–α5β1 bond lifetimes.
A, Representative force-time traces. B, Distributions of FNIII7–10–α5β1-Fc–GG-7 bond lifetimes at ~5 pN with indicated peak forces (points) and their theoretical fits by the two-state model (curves). The natural log of the number of events with a lifetime ≥ t normalized by the natural log of the total number of events is plotted vs. t. Data for different peak forces (indicated) are separated for the sake of clarity. C, Mean ± s.e.m. of FNIII7–10–α5β1-Fc–GG-7 serial bond lifetimes (black squares) increased with increasing peak force before being limited by the Fc–GG-7 capture bond lifetime at >22 pN forces. Also shown are mean ± s.e.m. lifetimes of FNIII8,10–α5β1-Fc–GG-7 bonds (purple circles) and FNIII7–10–α5β1-Fc–GG-7 bonds in the presence of 10 μg/ml HUTS-4 (orange triangles) or SG/19 (pink diamonds) vs. peak forces. D. Effects of FN synergy domain deletion and integrin hybrid domain constraining antibodies on the cell surface α5β1 (left, open bars) are comparable to those on the α5β1-Fc chimera (right, closed bars). See also Figure S2 and S4. Data are presented as mean ± s.e.m. for α5β1-Fc (right figure) and fitted mean ± s.e.m. for cell surface α5β1 (left figure).
Importantly, cyclic force was much more efficient at strengthening FN–α5β1 bonds than a linear ramp to constant force (Figure S1A–D). Indeed, after loading and unloading, the average lifetime of the Fc–GG-7 bond was only slightly longer than that of the FN–α5β1-Fc–GG-7 serial bonds (Figs. 2A and 4B), indicating that the FN–α5β1 bond became significantly longer-lived than the Fc–GG-7 bond (Figure 2B) and that dissociation primarily occurred at the Fc–GG-7 interface (Kong et al., 2009). By comparison, when both bonds were measured at 25 pN after a simple ramp without unloading to 5 pN, the FN–α5β1-Fc–GG-7 serial-bond lifetime was only half of the Fc–GG-7 bond lifetime, indicating significant dissociation from the FN–α5β1 interface (Figure S1A–D).
The differences between cyclic force and linear ramp to constant force suggest that the peak force is not the sole determinant of the rate of switching FN–α5β1 bonds from short- to long-lived state. Indeed, a full cycle of loading to a peak force of 22 pN and then unloading to 5 pN switched 100% FN–α5β1 bonds to the long-lived state. In contrast, loading to 22 pN without unloading (i.e., the first half of the cycle) switched <30% of bonds to the long-lived state (Figure S1C). These data show that in addition to the loading half, the unloading half of the forcing cycle is also an important part of cyclic mechanical reinforcement.
Characterizing multi-cycled mechanical reinforcement
The fact that a full cycle of force has a higher rate of switching FN–α5β1 bonds from the short-lived to long-lived state than half a cycle of force prompted us to examine whether increasing the number of cycles (Figure 5A) would further enhance the switching rate. For a 10-pN half-cycle force, all FN–α5β1-Fc bonds were in the short-lived state but 40% were switched to the long-lived state following a single cycle of unloading and reloading (Figure 5B). Addition of two and three cycles switched 90% of bonds to the long-lived state. Importantly, the increased switching rate was not due to higher dissociation of short-lived bonds during the repeated cycles as the survival rates for bonds that experienced different cycles were indistinguishable (Figure S3). These data demonstrate an accumulation effect of repeated forcing cycles on the switching rate from the short- to long-lived state.
Figure 5. Effects of repetitive force cycles on FN–α5β1 bond lifetimes.

A, Representative force-time traces showing lifetime measurements at 10 pN with 0.5, 1.5, 2.5, and 3.5 cycles of loading-unloading with a 10 pN peak force. Complete unloading to zero force was used. B, Lifetime distributions of the measurements shown in A. Data for different numbers of cycles (indicated) are separated for the sake of clarity. C, Mean ± s.e.m. lifetimes of FNIII7–10–α5β1-Fc–GG-7 (black squares) and FNIII8,10–α5β1-Fc–GG-7 (purple circles) bonds and of FNIII7–10–α5β1-Fc–GG-7 bonds in the presence of HUTS-4 (orange triangles) and SG/19 (pink diamonds) vs. number of repetitive loading-unloading cycles.
The accumulation effect was also observed for FN interacting with cell surface α5β1. With a 20 pN peak force, the average lifetime of FN–α5β1 bonds increased from <1 s to ~30 s as number of force cycles increased (Figure S3C, black squares). Note that the accumulation effect was not observed at 10 pN and 15 pN peak force (Figure S3C, green triangle and red circle), suggesting that cyclic force with a higher peak was needed to generate the accumulation effect with cell surface α5β1 than the α5β1-Fc fusion protein.
The actual lifetime of the long-lived state of the FN–α5β1-Fc bond could not be determined using GG-7 to capture the integrin because dissociation occurred primarily at the Fc–GG-7 interface (Figure 2B). To circumvent this difficulty, we covalently cross-linked α5β1-Fc to a substrate (see Methods) and measured lifetimes of specific FN–α5β1-Fc bonds after one cycle of reinforcement with a 20-pN peak force or multiple cycles with a 10-pN peak force (Figure 6). In both cases, a large fraction of FN–α5β1 bonds were switched to the long-lived state with a 100-s average lifetime (Figure 6D, E).
Figure 6. Cyclic mechanical reinforcement of integrin α5β1 covalently linked to surface.
A, Schematic of covalent tethering of α5β1-Fc to mixed SAMs of alkanthiols using standard NHS/EDC chemistry. Compared to capturing α5β1-Fc by an anti-Fc (GG-7), covalently attaching α5β1-Fc to the substrate via chemical crosslinking prevented the integrin from dissociating from the substrate. B, Binding specificity. Data are presented as mean ± s.e.m.. C, Distributions of FN–α5β1 bond lifetimes measured at 5 pN without (black) or with (magenta) a cyclic force of 20-pN peak (points) and their model fits (curves). D, Distributions of FN–α5β1 bond lifetimes measured at 10 pN with 0.5 (black), 1.5 (red), 2.5 (green), and 3.5 (blue) force cycles (points) and their model fits (curves). E, Lifetimes of the longest-lived state deduced from fitting the lifetime distributions in C (pink) and D (matched colors) with a three-state model.
Structural basis of cyclic mechanical reinforcement
FN binds integrin α5β1 through the RGD and synergy binding sites located in the 10th and 9th domain of FNIII, respectively (Mould et al., 1997) (Figure 7, Left panels). Different roles have been proposed for the synergy site, including a role in direct receptor contact and a role in electrostatic steering (Mould et al., 2003b). It has also been proposed that the synergy site does not contact α5β1 when the bond is tension-free (Takagi, 2003) but is required for a “tensioned-state” (Friedland et al., 2009). To elucidate the molecular mechanism of cyclic mechanical reinforcement at the single-molecule level, we tested a domain 9 deletion construct lacking the synergy site (FNIII8,10) that bound α5β1 specifically via the RGD site (Figure S2D). When single-cycled forces were applied with increasing peak values, FNIII8,10–α5β1 bonds were similarly reinforced as FNIII7–10–α5β1 bonds (Figure 4C). However, the rate to switch FNIII8,10–α5β1 bonds from short- to long-lived states was severely reduced, especially at peak forces from 15–30 pN. Consequently, the peak force at which lifetimes of the long-lived FNIII8,10–α5β1-Fc–GG-7 serial bonds were limited by the Fc–GG-7 capturing bonds was right-shifted to 35 pN (Figure 4C). When cyclic forces with a low peak-level were applied with increasing number of cycles, FNIII8,10–α5β1 bonds were much shorter-lived compared to FNIII7–10–α5β1 bonds (Figure 5C). More importantly, the accumulation effect was diminished as the average lifetimes did not increase with increasing cycle number after one cycle (Figure 5C), demonstrating the requirement for both the RGD and synergy sites for this effect.
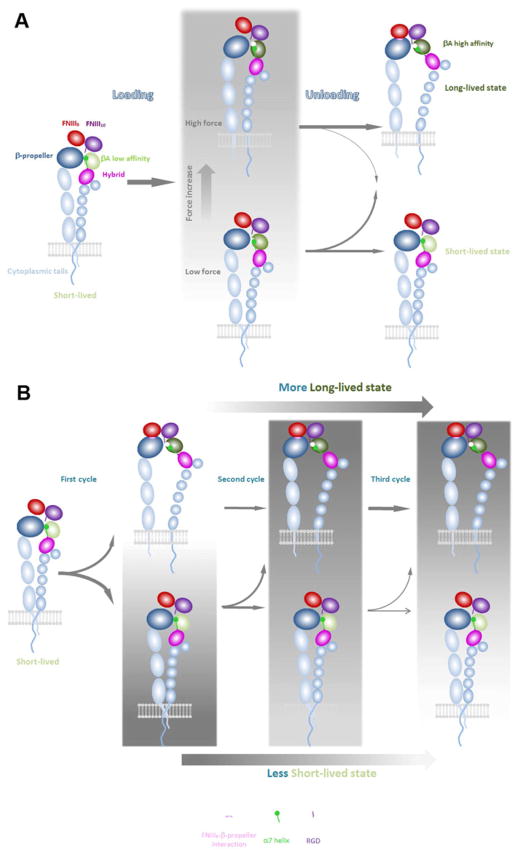
Figure 7. Model of cyclic mechanical reinforcement.
A, Reinforcing FN–α5β1 bond by a single-cycled force. Left: Liganded integrin in the short-lived state. Key α5β1 domains are indicated along with the RGD and synergy binding sites on the FNIII 10 and 9 domains, respectively. Middle: Force applied via the ligand primes the integrin for conformational change in the headpiece. Higher peak force (Top) better primes the integrin than lower peak force (Bottom). Right: During unloading, the FN–α5β1 bond may be switched to the long-lived state (Top) or may return to the short-lived state (Bottom). A bond previously primed by a higher peak force is more likely to be switched to the long lived state than a bond primed by a lower peak force, as indicated by the sizes of the arrows. B, Reinforcing FN–α5β1 bond by multi-cycled small forces. Far-left: FN bound α5β1 in the short-lived state as that in A Left. Mid-left: A loading-unloading cycle has a certain probability to switch the FN–α5β1 bond to the long-lived state (Top) but also a probability not to change the short-lived state (Bottom). Mid-right: After another loading-unloading cycle, the bond that is already in the long-lived state stays in the long-lived state (Top). However, the bond that remains in the short-lived state has a certain probability to switch to the long-lived state (Bottom). Far-right: As the number of cycles increases, the FN–α5β1 bond is more and more likely to be switched to the long-lived state (Top) and less and less likely to remain in the short-lived state (Bottom), resulting in an accumulating effect.
Changes in ligand-binding affinity of integrins are usually accompanied by conformational changes, e.g., in the hinge angle between the hybrid domain and the βA domain (Luo et al., 2004). To examine the impact of the hybrid domain orientation on cyclic mechanical reinforcement, we tested two monoclonal antibodies (mAb), one (HUTS-4) that promotes (Figure S4A) and the other (SG/19) that impedes (Figure S4B) hybrid domain swing-out (Luo et al., 2007; Mould et al., 2003a). Remarkably, the rate for single-cycled forces to switch FNIII7–10–α5β1 bonds from short- to long-lived states was increased by HUTS-4 and decreased by SG/19 (Figure 4C). Interestingly, while HUTS-4 increased and SG/19 decreased the average lifetime of the FN–α5β1 bond subjected to a half-cycle loading to 10 pN, both mAbs eliminated the accumulation effect, as in their presence the average lifetimes did not change with increasing cycle number (Figure 5C). These data indicate that a change to the swing-out conformation of the hybrid domain is required for the single-cycled cyclic mechanical reinforcement and the ease with which such reversible conformational change occurs is important for the reinforcement to accumulate over multiple forcing cycles.
The effects of FN mutant and anti-β1 antibodies were also tested using native α5β1 expressed on K562 cells. Upon a single-cycled (with a 20-pN peak force) mechanical reinforcement, the average lifetimes of the FN7–10-α5β1 bond were compared to those obtained by replacing FN7–10 with the synergy domain-deletion mutant FNIII8,10 and by adding HUTS-4 or SG/19 to lock the hybrid domain in the respective swing-out or closed-in position. The patterns of the effects of the FN mutant and antibodies were found indistinguishable for the native α5β1 and the α5β1-Fc (Figures 4D), indicating that the same structural mechanisms underlying the cyclic mechanical reinforcement for both the native α5β1 and the α5β1-Fc chimera.
Discussion
Force-induced strengthening of cell adhesion has been observed (Balaban et al., 2001; Choquet et al., 1997; Galbraith et al., 2002) and explained in terms of recruitment of integrins and cytoskeletal proteins (Puklin-Faucher and Sheetz, 2009) and/or ligand–integrin catch bonds (Friedland et al., 2009; Kong et al., 2009; Chen et al., 2010; Chen et al., 2012). Cyclic mechanical reinforcement offers a regulatory mechanism with cyclic forces, which is far more effective than the traditional catch bond (Dembo et al., 1988), as it prolongs bond lifetimes to a much greater extent than catch bonds (Figure S1). Catch bonds are assumed to be strengthened under tension but weakened when tension is released (Dembo et al., 1988; Friedland et al., 2009). By comparison, mechanically-reinforced bonds stay long-lived after force removal. Bond lifetimes are prolonged by increasing peak force and repeated cycles. Thus, not only does the loading half, but also the unloading half, of the forcing cycle unidirectionally switch bonds from short- to long-lived states. Furthermore, HUTS-4 augmented the ability of the peak force of single-cycled mechanical reinforcement to prolong the lifetime (Figure 4C and D) but converted the ability of the half-cycled ramping force from prolonging to shortening the lifetime of the FN–α5β1 bond, i.e., changed the catch bond to a slip bond (whose lifetime is shortened by force) (Figure S4C). By comparison, SG/19 suppressed the peak force of a single-cycled mechanical reinforcement to prolong the lifetime (Figure 4C and D) but the FN–α5β1 catch bond remains (Figure S4). Given the opposite effects of the two mAbs on the hybrid domain conformation (Luo et al., 2004; Mould et al., 2003a) (Figure S4A and B), these results indicate that cyclic mechanical reinforcement and catch bond formation are based on distinct structural mechanisms.
Our findings can be summarized by a model of integrin conformational changes that switch α5β1 from short-lived to long-lived bond-states by cyclic force applied via an engaged FN, including single-cycled (Figure 7A) and multi-cycled (Figure 7B) mechanical reinforcements. In this model, loading the FN–α5β1 bond by a ramp force perturbs its short-lived state conformation and primes it for subsequent changes (Figure 7A, left to middle panels). Immediately clamping the force may switch the conformation to a long-lived state, resulting in catch bond. Unloading may also switch the conformation to the long-lived state along a distinct and perhaps more effective pathway, resulting in cyclic mechanical reinforcement (Figure 7A, middle to right panels). However, there is still a chance for the perturbed FN–α5β1 bond to return to the short-lived state conformation. This chance is decreased by increasing the peak force of the loading-unloading cycle. Upon switching to the long-lived state, the integrin conformation is stable after force removal and would not be affected by the next application of cyclic force. But if the FN–α5β1 bond remains in the short-lived state after one loading-unloading cycle, it can still be primed and switched to the long-lived state by the cycle of force application (Figure 7B). Both the RGD and synergy binding sites on FN contribute to cyclic mechanical reinforcement (Figure 7). The synergy site significantly increases the rate of cyclic forces to switch FN–α5β1 bonds from short- to long-lived states. Moreover, to accumulate the reinforcement effects over multiple cycles of small repetitive forces requires both the synergy and RGD binding sites.
The stability of the long-lived state may allow the cell to “remember” where forces have been applied previously. Moreover, since the reinforcement effects are cumulative over small repetitive forces, this ability to integrate may allow the cell to “count” the force cycles and therefore sense its physical microenvironment.
The loading-unloading cycles used in our study mimic a range of physiological situations where cyclic forces are experienced by ligand–integrin bonds (cf. Introduction). The forces tested in our study (<50 pN) are also well within the physiological range. For example, a single myosin can apply >10 pN forces (Takagi et al., 2006). For nascent adhesions at the leading edge of migrating cells, stresses applied by the cell are often beyond 1 nN/μm2 (Balaban et al., 2001; Beningo et al., 2001; Giannone et al., 2007), which are distributed among multiple clusters of ligand–integrin bonds. The 1-s average lifetime of the short-lived bonds may allow the cell to rapidly explore its environment. Indeed, unliganded β1 integrins at lamellipodia of migrating cells have been suggested to probe new attachment sites (Galbraith et al., 2007) by engaging FN to form nascent adhesions with fast turnover (Choi et al., 2008). On the other hand, the 100-s average lifetime of the long-lived bonds may be critical to adhesion maturation and downstream signaling, which take tens of seconds to minutes (Galbraith et al., 2002). Switching ligand–integrin bonds from the short- to long-lived state by cyclic mechanical reinforcement may enable nascent adhesions that undergo fast turnover to be stabilized by myosin-generated contractile forces (Choi et al., 2008). Thus, the ability for ligand–integrin bonds to be mechanically reinforced has broad implications to many cellular functions.
Experimental procedures
AFM experiments
Our AFM and reagents have been described (Kong et al., 2009). The anti-human integrin β1 subunit mAbs SG/19 (Miyake et al., 1992) and HUTS-4 (Mould et al., 2003a) have also been described. Briefly, α5β1-Fc was captured by anti-Fc mAb GG-7 preadsorbed on a polystyrene dish and the FN construct was adsorbed on the AFM cantilever tip. The dish containing buffer of the desired cation composition with or without anti-β1 was mounted on a three-dimensional PZT (P-363, Physik Instrumente, Karlsrube Germany) and brought to contact the cantilever tip at a preset x-y location, immediately retracted slightly and held closely to the tip for 0.5 s to allow bond formation, and retracted along the z direction at a speed of 200 nm/s. The absence (Figure 1C) and presence (Figure 1D and E) of binding was detected from the force-time curves. When a bond was present, force was applied to it via a combination of PZT retraction, approach and holding along one of the three programmed paths: 1) loading to a given force and holding at that force for lifetime measurement (i.e., half-cycle force, same as the traditional path used previously to observe catch bonds (Kong et al., 2009), Figure 1E); 2) loading to a peak force, unloading to a lower force and holding at that force for lifetime measurement (i.e., single-cycle force, Figure 1E); and 3) loading to a given force, unloading to zero force and reloading to the same force 1, 2 or 3 times and holding at that force for lifetime measurement (i.e., multi-cycle force, Figure 6A). Lifetime was measured from the instant when the PZT began holding to the instant of bond dissociation. After each lifetime measurement, the PZT randomly moved the dish to a different x-y location and repeated the above procedure. Experiments were conducted at room temperature with <1 nm/s system drift.
BFP experiments
Our BFP system has also been described (Chen et al., 2008; Chen et al., 2010). K562 cells constitutively expressing α5β1 and Jurkat cells constitutively expressing αLβ2 were from ATCC and cultured in RPMI medium. Red blood cells (RBCs) were isolated from whole blood of healthy volunteers according to a protocol approved by the Institutional Review Board of Georgia Institute of Technology. To prepare force probes, RBCs were coated with 3500 D NHS-PEG-Biotin polymer (Jenkim Technologies) by incubation in a 6-mg/ml polymer/carbonate-bicarbonate buffer (pH = 8.4) for 30 min. Biotinylated RBCs were washed and re-suspended in a 150-mOsm HEPES solution. Silanized glass beads (2 μm diameter) were coated with streptavidin (Sigma Aldrich) by incubation in a 1.33-μM streptavidin-malemide/phosphate buffer solution overnight. Streptavidinylated beads were incubated with 2 ng/ml FNIII7–10 or FNIII8,10 with a biotin tag at N-terminus or 2 ng/ml biotinylated ICAM-1 (R&D) for 30 min, washed, and attached to the apex of a micropipette-pressurized RBC to form a pico-force transducer. Experiments were performed in Mg2+/EGTA (2 mM each) with or without HUTS-4 or SG/19 in the solution. Lifetimes of integrin–ligand bonds were measured at 5 pN without or with a prior force cycle of 20-pN peak, using programs similar to that used in the AFM experiments (paths 1–3) to drive the micropipetteaspirated integrin-expressing cell to approach to, contact with, retract from, and hold near the ligand-bearing force probe.
Lifetime analysis
Lifetimes were analyzed by semi-log ln(number of events with a lifetime >t) vs. t plots to linearize their exponential distributions and normalized by ln(total number of events). Each piecewise-linear data set was fitted by a two- or three-state model (Chen et al., 2010). The two-state model assumes: Normalized ln(number of events) = 1 + ln[CSexp(− koff St) + CLexp(− koff Lt)] where CS (CL) and koff S (koff L) are, respectively, the fraction and off-rate of the short-lived (long-lived) state. Different sets of post-reinforcement lifetimes with different peak forces or cycles but measured at the same force (Figures 4B and 5B as well as Figure S1C and S2E) were fitted using distinct values for the two fractions (constrained by CS + CL = 1). But all data sets in each figure panel were fitted using the same values for koff S and koff L because they were assumed to be functions of only the force at which lifetimes were measured but independent of the prior history of how force was loaded to the current level. The three-state model adds an intermediate state to the two-model. It was used to analyze the lifetime distributions of mechanically reinforced bonds of FN with α5β1-Fc covalently linked to surface (Figure 6C and D) instead of the two-state model for the following reasons: 1) the three-state model fits the distributions better, i.e., with smaller chi-squares and 2) Histograms of rupture forces after mechanical gripping shows three peaks, indicating at least three states.
Covalent tethering of α5β1-Fc chimera to self-assembled monolayers
α5β1-Fc chimera was covalently tethered to self-assembled monolayers (SAMs) of mixed alkanethiols on gold to present integrins with a well-ordered, non-adhesive background. Au-coated substrates were created by cleaning 9 x 9 mm2 glass coverslips in a custom-made oxygen plasma etcher for 4 min, followed by sequential deposition of titanium (100 Å) and gold (200 Å) films at a rate of 2 Å/s via an electron beam evaporator (Thermionics Laboratories, Hayward CA) at a pressure of 2 x 10−6 Torr. Au-coated coverslips were stored desiccated under vacuum for a maximum of two weeks. Two alkanethiols were used to produce SAMs: tri(ethyleneglycol)-terminated alkanethiol (HS-(CH2)11-(OCH2CH2)3-OH; EG3) and carboxylic acid-terminated alkanethiol (HS-(CH2)11-(OCH2CH2)6-OCH2COOH; EG6-COOH), obtained from Prochimia (Sopot, Poland). 1 mM EG3 and 1 mM EG6 solutions in 200 proof ethanol were mixed to produce 0.75 mM EG3 and 0.25 mM EG6, which were incubated overnight on Au-coated substrates. The following day, α5β1-Fc chimera was tethered using standard peptide chemistry (Lahiri et al., 1999). Briefly, after thorough wash in 200 proof ethanol and ultra-pure H20, SAMs were activated for 25 min in 200 mM N-(3-dimethylaminopropyl)-N-ethylcarbodiimide hydrochloride (EDC) and 100 mM N-hydroxysuccinimide (NHS) in 0.1 M 2-(N-morpho)-ethanesulfonic acid and 0.5 M NaCl (pH 6.0). After a rapid DPBS rinse, 5 μg of α5β1-Fc resuspended in 20 μl of DPBS were tethered to activated surfaces for ≥2 h. Following integrin tethering, unreacted NHS esters were quench with blocking in 0.1%-HD BSA for 30 min. Samples were washed in DPBS and tested.
Supplementary Material
Highlights.
Cyclic force reinforces integrin–ligand bond to prolong its lifetime.
Cyclic mechanical reinforcement (CMR) persists after force removal and accumulates.
Both synergy and RDG binding sites of fibronectin contribute to CMR.
CMR is regulated through integrin hybrid domain conformation and flexibility.
Acknowledgments
We thank R. P. McEver for reading the manuscript. We thank J. Takagi for providing mAb SG/19. This work was supported by NIH grants AI44902 (to C.Z.) and GM065918 (to A.J.G.) and Wellcome Trust grants 045225, 074941 and 092015 (to M.J.H).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Balaban NQ, et al. Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nat Cell Biol. 2001;3:466–472. doi: 10.1038/35074532. [DOI] [PubMed] [Google Scholar]
- Beningo KA, et al. Nascent focal adhesions are responsible for the generation of strong propulsive forces in migrating fibroblasts. J Cell Biol. 2001;153:881–8. doi: 10.1083/jcb.153.4.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CE, Odde DJ. Traction dynamics of filopodia on compliant substrates. Science. 2008;322:1687–91. doi: 10.1126/science.1163595. [DOI] [PubMed] [Google Scholar]
- Chen CS. Mechanotransduction - a field pulling together? J Cell Sci. 2008;121:3285–92. doi: 10.1242/jcs.023507. [DOI] [PubMed] [Google Scholar]
- Chen W, et al. Monitoring receptor-ligand interactions between surfaces by thermal fluctuations. Biophy J. 2008;94:694–701. doi: 10.1529/biophysj.107.117895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, et al. Forcing switch from short- to intermediate- and long-lived states of the αA domain generates LFA-1/ICAM-1 catch bonds. J Biol Chem. 2010;285:35967–35978. doi: 10.1074/jbc.M110.155770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, et al. Observing force-regulated conformational changes and ligand dissociation from a single integrin on cells. J Cell Biol. 2012;199(3):497–512. doi: 10.1083/jcb.201201091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi CK, et al. Actin and α-actinin orchestrate the assembly and maturation of nascent adhesions in a myosin II motor-independent manner. Nat Cell Biol. 2008;10:1039–U36. doi: 10.1038/ncb1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choquet D, et al. Extracellular matrix rigidity causes strengthening of integrin-cytoskeleton linkages. Cell. 1997;88:39–48. doi: 10.1016/s0092-8674(00)81856-5. [DOI] [PubMed] [Google Scholar]
- Coe APF, et al. Generation of a minimal α5β1 integrin-Fc fragment. J Biol Chem. 2001;276:35854–35866. doi: 10.1074/jbc.M103639200. [DOI] [PubMed] [Google Scholar]
- del Rio A, et al. Stretching single talin rod molecules activates vinculin binding. Science. 2009;323:638–641. doi: 10.1126/science.1162912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dembo M, et al. The reaction-limited kinetics of membrane-to-surface adhesion and detachment. Proc Royal Soc London B. 1988;234:55–83. doi: 10.1098/rspb.1988.0038. [DOI] [PubMed] [Google Scholar]
- Friedland JC, et al. Mechanically activated integrin switch controls α5β1 function. Science. 2009;323:642–644. doi: 10.1126/science.1168441. [DOI] [PubMed] [Google Scholar]
- Galbraith CG, et al. Polymerizing actin fibers position integrins primed to probe for adhesion sites. Science. 2007;315:992–995. doi: 10.1126/science.1137904. [DOI] [PubMed] [Google Scholar]
- Galbraith CG, et al. The relationship between force and focal complex development. J Cell Biol. 2002;159:695–705. doi: 10.1083/jcb.200204153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardel ML, et al. Traction stress in focal adhesions correlates biphasically with actin retrograde flow speed. J Cell Biol. 2008;183:999–1005. doi: 10.1083/jcb.200810060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannone G, et al. Lamellipodial actin mechanically links myosin activity with adhesionsite formation. Cell. 2007;128:561–575. doi: 10.1016/j.cell.2006.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji L, et al. Fluctuations of intracellular forces during cell protrusion. Nat Cell Biol. 2008;10:1393–U38. doi: 10.1038/ncb1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junichi Takagi KS, et al. Structure of integrin α5β1 in complex with fibronectin. EMBO J. 2003;22:4607–4615. doi: 10.1093/emboj/cdg445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong F, et al. Demonstration of catch bonds between an integrin and its ligand. J Cell Biol. 2009;185:1275–1284. doi: 10.1083/jcb.200810002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahiri J, et al. A strategy for the generation of surfaces presenting ligands for studies of binding based on an active ester as a common reactive intermediate: A surface plasmon resonance study. Analy Chem. 1999;71:777–790. doi: 10.1021/ac980959t. [DOI] [PubMed] [Google Scholar]
- Luo BH, et al. Structural basis of integrin regulation and signaling. Annu Rev Immunol. 2007;25:619–647. doi: 10.1146/annurev.immunol.25.022106.141618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo BH, et al. Allosteric β1 integrin antibodies that stabilize the low affinity state by preventing the swing-out of the hybrid domain. J Biol Chem. 2004;279:27466–27471. doi: 10.1074/jbc.M404354200. [DOI] [PubMed] [Google Scholar]
- Miyake K, et al. Requirement for VLA-4 and VLA-5 integrins in lymphoma cells binding to and migration beneath stromal cells in culture. J Cell Biol. 1992;119:653–662. doi: 10.1083/jcb.119.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mould AP, et al. Defining the topology of integrin α5β1-fibronectin interactions using inhibitory anti-α5 and anti-β1 monoclonal antibodies. Evidence that the synergy sequence of fibronectin is reconginzed by the amino-terminal repeats of the α5 subunit. J Biol Chem. 1997;272:17283–17292. doi: 10.1074/jbc.272.28.17283. [DOI] [PubMed] [Google Scholar]
- Mould AP, et al. Conformational changes in the integrin βA domain provide a mechanism for signal transduction via hybrid domain movement. J Biol Chem. 2003a;278:17028–17035. [Google Scholar]
- Mould AP, et al. Structure of an integrin-ligand complex deduced from solution X-ray scattering and site-directed mutagenesis. J Biol Chem. 2003b;278:39993–39999. doi: 10.1074/jbc.M304627200. [DOI] [PubMed] [Google Scholar]
- Petrie TA, et al. Integrin specificity and enhanced cellular activities associated with surfaces presenting a recombinant fibronectin fragment compared to RGD supports. Biomaterials. 2006;27:5459–5470. doi: 10.1016/j.biomaterials.2006.06.027. [DOI] [PubMed] [Google Scholar]
- Puklin-Faucher E, Sheetz MP. The mechanical integrin cycle. J Cell Sci. 2009;122:179–86. doi: 10.1242/jcs.042127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada Y, et al. Force sensing by mechanical extension of the Src family kinase substrate p130Cas. Cell. 2006;127:1015–1026. doi: 10.1016/j.cell.2006.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi Y, et al. Force generation in single conventional actomyosin complexes under high dynamic load. Biophy J. 2006;90:1295–1307.26. doi: 10.1529/biophysj.105.068429. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.