Abstract
Tumor necrosis factor (TNF)-α is a proinflammatory cytokine active in the brain. Etanercept, the TNF decoy receptor (TNFR), does not cross the blood-brain barrier (BBB). The TNFR was re-engineered for BBB penetration as a fusion protein with a chimeric monoclonal antibody (MAb) against the mouse transferrin receptor (TfR), and this fusion protein is designated cTfRMAb-TNFR. The cTfRMAb domain of the fusion protein acts as a molecular Trojan horse and mediates transport via the endogenous BBB TfR. To support future chronic treatment of mouse models of neural disease with daily administration of the cTfRMAb-TNFR fusion protein, a series of pharmacokinetics and brain uptake studies in the mouse was performed. The cTfRMAb-TNFR fusion protein was radiolabeled and injected into mice via the intravenous, intraperitoneal (IP), or subcutaneous (SQ) routes of administration at doses ranging from 0.35 to 10 mg/kg. The distribution of the fusion protein into plasma following the IP or SQ routes was enhanced by increasing the injection dose from to 3–10 mg/kg. The fusion protein demonstrated long circulation times with high metabolic stability following the IP or SQ routes of injection. The IP or SQ routes produced concentrations of the cTfRMAb-TNFR fusion protein in brain that exceed by 20- to 50-fold the concentration of TNFα in pathologic conditions of the brain. The SQ injection is the preferred route of administration, as the level of cTfRMAb fusion protein produced in brain is comparable to that generated with intravenous injection, and at a much lower plasma area under the concentration curve of the fusion protein as compared to IP administration.
Keywords: blood-brain barrier, drug targeting, pharmacokinetics, TNFα inhibitors, monoclonal antibody
Introduction
Etanercept, a tumor necrosis factor (TNF)-α receptor (TNFR):Fc fusion protein, is a biologic tumor necrosis factor inhibitor (TNFI). The biologic TNFIs are widely used in clinical medicine for treatment of inflammation of peripheral organs.1 No biologic TNFIs are used to treat diseases of the brain, because large molecule drugs do not readily cross the blood-brain barrier (BBB). In the case of etanercept, a prior study shows that this biologic TNFI does not cross the BBB.2 However, TNFα is a proinflammatory cytokine in multiple brain disorders, including acute conditions such as traumatic brain injury3 or stroke, 4 or chronic conditions, such as Alzheimer’s disease5 or Parkinson’s disease (PD).6
Biologic TNFIs can be made transportable across the BBB by re-engineering the molecules as fusion proteins with BBB molecular Trojan horses (MTH). The latter undergo receptor-mediated transport across the BBB via transport on endogenous peptide receptors such as the insulin receptor or transferrin receptor.7 A BBB MTH specific for the mouse is a genetically engineered chimeric monoclonal antibody (MAb) against the mouse transferrin receptor (TfR), designated the cTfRMAb.8 A BBB-penetrating form of etanercept was engineered by fusion of the extracellular domain of the human type II TNFR to the carboxyl terminus of the heavy chain of the cTfRMAb, and this fusion protein is designated cTfRMAb-TNFR.9 The cTfRMAb-TNFR fusion protein binds TNFα with the same affinity as etanercept.10 The cTfRMAb-TNFR fusion protein binds the TfR on the mouse BBB, and the brain uptake of the cTfRMAb-TNFR fusion protein is high in the mouse, 2.8 ± 0.5 % of injected dose (ID)/gram brain.9 Intravenous (IV) administration of the cTfRMAb-TNFR fusion protein is neuroprotective in both acute brain disorders, such as stroke,11 or chronic neural conditions, such as experimental PD.10 Conversely, IV etanercept has no therapeutic effect in either stroke11 or PD10 because etanercept does not cross the BBB.2
Treatment of a chronic neural disease such as PD with the cTfRMAb-TNFR fusion protein in the mouse employed twice weekly IV administration.10 However, the cTfRMAb-TNFR fusion protein is rapidly removed from plasma after IV administration with a mean residence time of 105 ± 12 minutes.9 Therefore, a more sustained therapeutic effect may be achieved for chronic neural disease with daily treatment of the fusion protein. A preferred route of administration for daily treatment of a neural disease with the IgG fusion protein is via subcutaneous (SQ) or intraperitoneal (IP) injection. However, the systemic bioavailability, plasma stability, and brain uptake of an IgG fusion protein following either the SQ or IP route of administration has not been previously evaluated. The purpose of the present investigation was to determine the plasma pharmacokinetics, fusion protein stability in plasma, and brain uptake of the cTfRMAb-TNFR fusion protein administered at dose levels ranging from 0.3 to 10 mg/kg via the IV, SQ, and IP routes of administration in the mouse.
Experimental Section
Radiolabeling of Fusion Protein
The cTfRMAb-TNFR was expressed by stably transfected Chinese hamster ovary cells grown in serum free medium, and purified by protein G affinity chromatography as described previously.9 The fusion protein is a bi-functional molecule that binds the mouse TfR with high affinity, and binds TNFα with the same affinity as etanercept.9,10 The fusion protein was radiolabeled with [3H]-N-succinimidyl propionate (American Radiolabeled Chemicals, St. Louis, MO) as described previously.9 The radiolabeled protein was purified with a 1.0 × 30 cm Sephadex G-25 column with an elution buffer of 0.01 M sodium acetate/0.14 M NaCl, pH 5.5 (acetate buffered saline or ABS). The cTfRMAb-TNFR was radiolabeled to a specific activity of 0.6 μCi/μg and a trichloroacetic acid (TCA) precipitability of 95%. The [3H]-cTfRMAb-TNFR fusion protein was buffer exchanged in ABS using an Ultra-15 microconcentrator (Millipore, Bedford, MA), which resulted in a final TCA precipitability of >99%.
Systemic Injection and Brain Uptake in the Mouse
Adult male C57BL/6J mice weighing 25–27 g were obtained from The Jackson Laboratory (Bar Harbor, ME). All procedures were carried out in accordance with the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996) as adopted and promulgated by the National Institutes of Health. The [3H]-cTfRMAb-TNFR fusion protein was injected in different experiments via the intravenous (IV), the intraperitoneal (IP), or subcutaneous (SQ) route in volumes of 60–100 uL per mouse. The mice received 10 uCi of [3H]-cTfRMAb-TNFR fusion protein per injection, which was co-mixed with unlabeled cTfRMAb-TNFR fusion protein to adjust the dose of fusion protein to 0.35, 1, 3, or 10 mg/kg for the IV injections, or 0.7, 3, or 10 mg/kg for the IP or SQ injections. Each treatment group was comprised of 3 mice for each injection dose. For each injection, mice were briefly anesthetized using 2% isoflurane in 30% oxygen and a 50 μl aliquot of venous blood was collected via the retro-orbital sinus. For the IV injections, blood was collected at 0.25, 2, 5, 15, 30, and 60 min after injection. For the IP or SQ injections, blood was collected at 60, 180, 360, and 1440 min following injection. Blood samples were centrifuged at 9,600g for 2 min and 10 μl of the resulting plasma was used to determine 3H radioactivity. For the IV injections, mice were euthanized at 1 hour after injection. For the IP or SQ injections, mice were euthanized either at 6h or 24h after injection. Following euthanasia, cerebral hemispheres and major organs (heart, liver, kidney, spleen and lung) were removed, weighed and processed for determination of 3H radioactivity. Each of the plasma and tissue samples was solubilized with Soulene-350 (PerkinElmer, Waltham, MA) at 60°C for 2 hours and counted for 3H radioactivity with Opti-Fluor O (PerkinElmer) using a Tri-Carb 2100TR liquid scintillation counter (PerkinElmer, Downers Grove, IL).
Pharmacokinetics and Organ Uptake
Organ uptake data were expressed as (a) volume of distribution (VD, units=uL/gram), (b) percent of injected dose (ID)/gram, or (c) organ concentration (Cb, units= μg of fusion protein per gram organ). The organ VD is the ratio of the terminal (6 h or 24 h) organ radioactivity [disintegrations per minute (DPM) per gram] divided by the terminal (6 h or 24 h) plasma radioactivity (DPM/μL). The organ concentration of fusion protein (μg fusion protein/gram organ) was computed from the organ VD, the organ plasma volume (Vo), and the terminal plasma concentration [(Cp(T)] as follows:
The organ plasma volumes in the mouse are 152 ± 35, 58 ± 12, 27 ± 3, 62 ± 14, 94 ± 8, and 6.8 ± 2.5 uL/gram, for spleen, liver, lung, kidney, heart, or brain, respectively.12 The plasma radioactivity (DPM/mL) was alternatively expressed as %ID/mL or μg/mL. Plasma radioactivity that was precipitable with ice-cold 10% TCA was determined at each time point. Plasma fusion protein concentration, in μg/mL, was computed as follows: plasma concentration = (plasma radioactivity in %ID/mL) × (μg fusion protein injected)/100.
The plasma area under the concentration curve (AUC) following IV administration was computed with a 2-compartment model as described previously.9 The plasma AUC following IP or SQ administration was determined with the trapezoid method as described previously.9 The AUC was alternatively expressed as % ID·min/mL or μg·min/mL. The plasma AUC was computed from plasma radioactivity that was TCA-precipitable.
Western Blot of Mouse Plasma
The concentration of the cTfRMAb-TNFR fusion protein in plasma was sufficiently high to detect with Western blotting of small volumes (0.5 uL/lane) of mouse plasma obtained at 6 hours following the IP administration of 10 mg/kg of fusion protein. Mouse plasma (3 uL) was diluted in 90 uL of reducing sodium dodecylsulfate (SDS) polyacrylamide gel electrophoresis (PAGE) sample buffer, and 15 uL was loaded per lane of 8.6×6.7×0.1 cm 4–15% Mini-PROTEAN® TGX™ Precast Gel (Biorad, Richmond, CA). Volumes of mouse plasma >0.5 uL/lane overloaded the gel owing to the high concentration of plasma proteins. Following SDS-PAGE and electroblotting to nitrocellulose (0.45 um pore size, Biorad), the filter was probed with a goat antibody to the type II human TNFR (R&D Systems, Minneapolis, MN) at a concentration of 0.4 ug/mL for 60 min at room temperature, followed by detection with a biotinylated horse anti-goat IgG secondary antibody (Vector Labs, Burlingame, CA) at a concentration of 0.3 ug/mL for 60 min at room temperature. The detection system used the Vectastain ABC kit with diaminobenzidine (Vector Labs). The purified cTfRMAb-TNFR fusion protein (120 ng/lane) was applied to parallel lanes as a positive control, along with control mouse plasma as a negative control.
Results
Intravenous administration and brain uptake
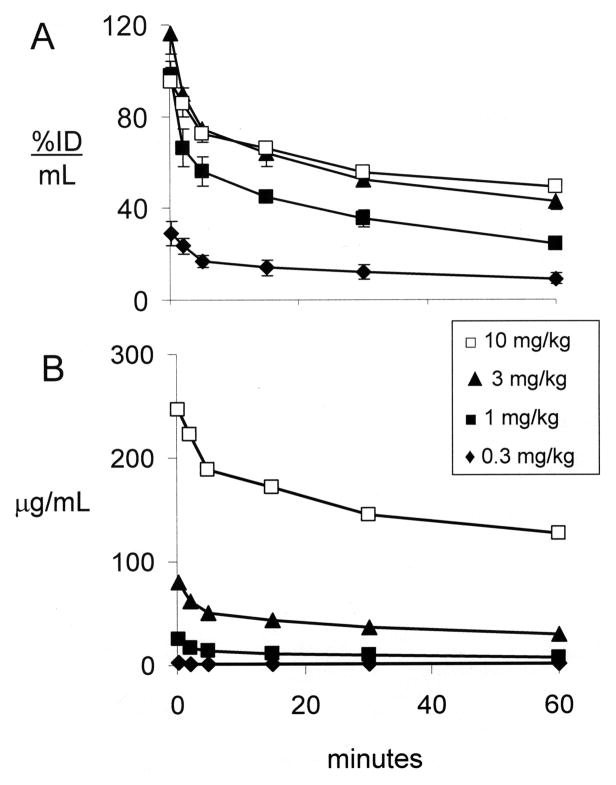
The plasma concentration of the cTfRMAb-TNFR fusion protein following IV injection is expressed as either %ID/mL (Figure 1A) or ug/mL (Figure 1B) at 4 injection doses (0.35, 1, 3, and 10 mg/kg). The plasma AUC, expressed as either %ID·min/mL or ug·min/mL, increases with ID (Table 1). The plasma TCA precipitability, which is a measure of fusion protein stability, is 84 ± 2% after injection of the low dose, 0.35 mg/kg, but is 97–99% at 1 hour after injection of 1–10 mg/kg (Table 1). The brain VD, or the brain uptake (%ID/g), is inversely related to ID (Table 2). However, the brain concentration of the cTfRMAb-TNFR fusion protein is directly related to the ID, and peaks at 0.64 ± 0.14 ug fusion protein/gram brain at 1 hour after an IV injection of 10 mg/kg (Table 2).
Figure 1.
Plasma concentration of cTfRMAb-TNFR fusion protein, expressed either as % ID/mL (A) or ug/mL (B) is plotted vs time after a single IV injection of 0.3–10 mg/kg of fusion protein. Mean ± SE (n=3 mice per time point).
Table 1.
Plasma AUC of cTfRMAb-TNFR fusion proteina
| Route of administration | ID (mg/kg) | Time (hours) | Plasma TCA (%) | AUC (%ID·min/mL) | AUC (ug•min/mL) |
|---|---|---|---|---|---|
| IV | 0.35 | 1 | 84.2 ± 2.0 | 778 ± 13 | 82 ± 1 |
| 1 | 1 | 97.3 ± 0.3 | 2,341 ± 46 | 690 ± 14 | |
| 3 | 1 | 98.5 ± 0.5 | 3,426 ± 64 | 3,060 ± 57 | |
| 10 | 1 | 98.2 ± 0.1 | 3,583 ± 75 | 10,800 ± 226 | |
| IP | 0.7 | 6 | 71.4 ± 4.4 | 492 ± 114 | 106 ± 22 |
| 0.7 | 24 | 34.3 ± 4.2 | 1,382 ± 332 | 290 ± 73 | |
| 3 | 6 | 95.5 ± 0.6 | 4,157 ± 365 | 4,368 ± 351 | |
| 3 | 24 | 81.7 ± 2.8 | 19,568 ± 701 | 23,786 ± 1,020 | |
| 10 | 6 | 98.2 ± 0.3 | 8,467 ± 203 | 25,158 ± 1,139 | |
| 10 | 24 | 94.6 ± 0.7 | 30,534 ± 1,260 | 94,521 ± 7,982 | |
| SQ | 0.7 | 3 | 65.3 ± 6.8 | 19 ± 3 | 2.8 ± 0.6 |
| 0.7 | 24 | 31.5 ± 2.0 | 241 ± 36 | 28 ± 4 | |
| 3 | 6 | 91.4 ± 1.9 | 451 ± 37 | 501 ± 52 | |
| 3 | 24 | 89.9 ± 1.8 | 3,226 ± 246 | 3,600 ± 538 | |
| 10 | 6 | 95.1 ± 0.4 | 1,205 ± 148 | 2,939 ± 353 | |
| 10 | 24 | 85.6 ± 1.3 | 5,990 ± 294 | 17,233 ± 1112 |
Mean ± SE (n = 3 mice per time point). The AUC values were determined over the respective time period listed above.
Table 2.
Brain concentration of cTfRMAb-TNFR fusion proteina
| Route of administration | ID (mg/kg) | Time (hours) | Brain VD (uL/gram) | Cb (% ID/gram) | Cb (ug/gram) |
|---|---|---|---|---|---|
| IV | 0.35 | 1 | 294 ± 40 | 2.78 ± 0.52 | 0.23 ± 0.04 |
| 1 | 1 | 65 ± 6 | 1.56 ± 0.06 | 0.37 ± 0.02 | |
| 3 | 1 | 22 ± 2 | 0.91 ± 0.02 | 0.43 ± 0.01 | |
| 10 | 1 | 12 ± 3 | 0.59 ± 0.13 | 0.64 ± 0.14 | |
| IP | 0.7 | 6 | 6,034 ± 199 | 0.60 ± 0.08 | 0.14 ± 0.02 |
| 0.7 | 24 | 2,506 ± 320 | 0.51 ± 0.11 | 0.11 ± 0.02 | |
| 3 | 6 | 160 ± 16 | 1.19 ± 0.07 | 1.25 ± 0.09 | |
| 3 | 24 | 560 ± 115 | 0.58 ± 0.04 | 0.70 ± 0.04 | |
| 10 | 6 | 39 ± 11 | 0.51± 0.08 | 1.52 ± 0.20 | |
| 10 | 24 | 62 ± 6 | 0.37 ± 0.04 | 1.17 ± 0.11 | |
| SQ | 0.7 | 3 | 440 ± 68 | 0.082 ± 0.013 | 0.012 ± 0.002 |
| 0.7 | 24 | 2,308 ± 230 | 0.38 ± 0.10 | 0.042 ± 0.010 | |
| 3 | 6 | 206 ± 20 | 0.54 ± 0.07 | 0.60 ± 0.09 | |
| 3 | 24 | 257 ± 74 | 0.64 ± 0.13 | 0.68 ± 0.08 | |
| 10 | 6 | 126 ± 11 | 0.81 ± 0.06 | 1.98 ± 0.14 | |
| 10 | 24 | 297 ± 21 | 0.70 ± 0.03 | 2.02 ± 0.08 |
Mean ± SE (n = 3 mice per time point).
Intraperitoneal administration and brain uptake
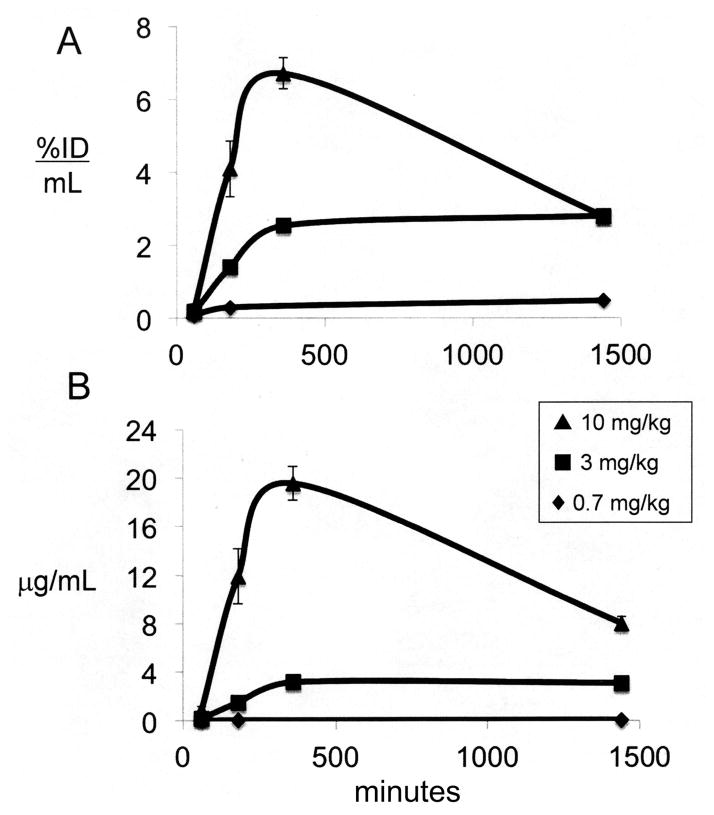
The plasma concentration of the cTfRMAb-TNFR fusion protein following IP injection is expressed as either %ID/mL (Figure 2A) or ug/mL (Figure 2B) at 3 injection doses (0.7, 3, and 10 mg/kg). The maximal fusion protein plasma concentration (Cmax) is 120 ug/mL at 6 hours after IP administration of 10 mg/kg (Figure 2B), and this value is about half of the Cmax observed immediately following an IV injection of 10 mg/kg of the fusion protein (Figure 1B). The plasma AUC, expressed as either %ID·min/mL or ug·min/mL, increases with ID (Table 1). The plasma TCA precipitability of the fusion protein is low, 71 ± 4% at 6 hours after an IP injection of the low dose, 0.7 mg/kg of fusion protein, but is 96–98% at 6 hours after the IP injection of higher doses, 3–10 mg/kg of fusion protein (Table 1). The brain VD, or the brain uptake (%ID/g), is inversely related to ID following IP administration (Table 2). However, the brain concentration of the cTfRMAb-TNFR fusion protein is directly related to the ID, and peaks at 1.52 ± 0.20 ug fusion protein/gram brain at 6 hours after an IP injection of 10 mg/kg (Table 2).
Figure 2.
Plasma concentration of cTfRMAb-TNFR fusion protein, expressed either as % ID/mL (A) or ug/mL (B) is plotted vs time after a single IP injection of 0.7–10 mg/kg of fusion protein. Mean ± SE (n=3 mice per time point).
Subcutaneous administration and brain uptake
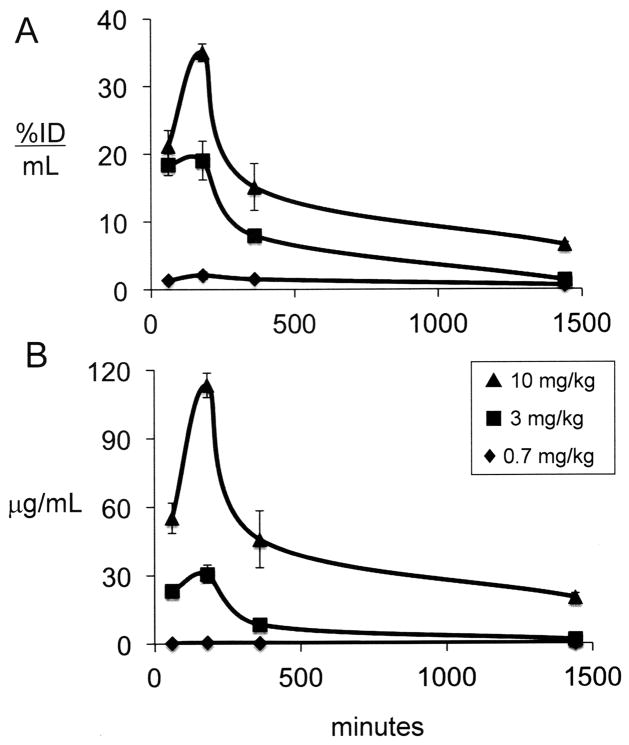
The plasma concentration of the cTfRMAb-TNFR fusion protein following SQ injection is expressed as either %ID/mL (Figure 3A) or ug/mL (Figure 3B) at 3 injection doses (0.7, 3, and 10 mg/kg). The plasma Cmax is 20 ug/mL at 6 hours after SQ administration of 10 mg/kg (Figure 3B), and this value is about 10% of the Cmax observed immediately following an IV injection of 10 mg/kg of the fusion protein (Figure 1B), and is nearly 6-fold lower than the Cmax at 6 hours after IP injection of 10 mg/kg (Figure 2B). The plasma AUC, expressed as either %ID·min/mL or ug·min/mL, increases with ID (Table 1). The plasma TCA precipitability of the fusion protein is low, 65 ± 7% at 3 hours after an SQ injection of the low dose, 0.7 mg/kg of fusion protein, but is 91–95% at 6 hours after the SQ injection of higher doses, 3–10 mg/kg of fusion protein (Table 1). The brain VD, or the brain uptake (%ID/g), is inversely related to ID following IP administration (Table 2). However, the brain concentration of the cTfRMAb-TNFR fusion protein is directly related to the ID, and peaks at 1.98 ± 0.14 ug fusion protein/gram brain at 6 hours after an SQ injection of 10 mg/kg (Table 2).
Figure 3.
Plasma concentration of cTfRMAb-TNFR fusion protein, expressed either as % ID/mL (A) or ug/mL (B) is plotted vs time after a single SQ injection of 0.7–10 mg/kg of fusion protein. Mean ± SE (n=3 mice per time point).
Peripheral organ uptake
The organ concentration of the cTfRMAb-TNFR fusion protein after IP or SQ administration is listed in Table 3 at 6 and 24 hours after injection doses of 3 or 10 mg/kg. The liver and the spleen show the lowest and highest levels of uptake by peripheral organs, respectively (Table 3). There is about a 4-fold higher uptake in heart and lung after IP injection as compared to SQ injection, which parallels the higher plasma AUC after IP injection (Table 1). The organ uptake is dose dependent in heart, kidney, and lung, but not in spleen (Table 3).
Table 3.
Concentration of cTfRMAb-TNFR fusion protein in peripheral organsa
| Route | ID (mg/kg) | Time (hours) | Heart (ug/gram) | Liver (ug/gram) | Kidney (ug/gram) | Spleen (ug/gram) | Lung (ug/gram) |
|---|---|---|---|---|---|---|---|
| IP | 3 | 6 | 0.61 ± 0.09 | 0.64 ± 0.27 | 3.5 ± 0.3 | 23.3 ± 2.4 | 3.1 ± 0.5 |
| 10 | 6 | 4.3 ± 2.9 | 0 | 13.8 ± 4.9 | 19.6 ± 1.5 | 16.9 ± 2.8 | |
| SQ | 3 | 6 | 0.20 ± 0.02 | 0.19 ± 0.06 | 2.5 ± 0.2 | 18.1 ± 2.1 | 1.2 ± 0.1 |
| 10 | 6 | 0.72 ± 0.03 | 1.4 ± 0.6 | 8.6 ± 0.5 | 25.5 ± 2.9 | 4.5 ± 0.5 | |
| IP | 3 | 24 | 1.0 ± 0.2 | 0.048± 0.020 | 1.3 ± 0.2 | 11.0 ± 1.3 | 0.97 ± 0.09 |
| 10 | 24 | 2.4 ± 0.5 | 0 | 6.3 ± 0.5 | 5.6 ± 0.8 | 6.0 ± 0.5 | |
| SQ | 3 | 24 | 0.72 ± 0.06 | 0.051 ± 0.09 | 1.3 ± 0.2 | 4.7 ± 1.3 | 1.0 ± 0.1 |
| 10 | 24 | 6.0 ± 0.2 | 0 | 7.2 ± 0.2 | 4.4 ± 0.5 | 3.6 ± 0.2 |
Mean ± SE (n = 3 mice per time point).
Western blotting
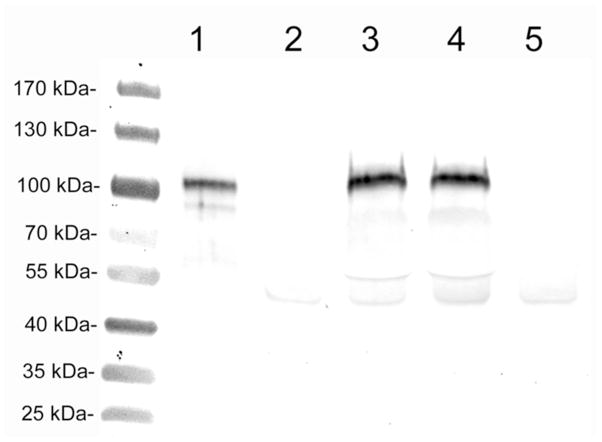
The cTfRMAb-TNFR fusion protein was intact in plasma at 6 hours after the IP injection of 10 mg/kg, as shown by Western blotting with a primary antibody against the human type II TNFR (Figure 4). This antibody reacts only with the heavy chain of the purified cTfRMAb-TNFR fusion protein, which has a molecular weight of about 100 kDa (lane 1, Figure 4). The size of the immunoreactive fusion protein heavy chain in plasma of injected mice is unchanged from the control fusion protein (lanes 3–4, Figure 4). The anti-TNFR antibody shows no specific reactivity with plasma from uninjected mice (lanes 2 and 5, Figure 4).
Figure 4.
Western blot with a primary antibody against the human type II TNFR. Lane 1 is purified cTfRMAb-TNFR fusion protein standard (120 ng/lane); lanes 2 and 5 are control mouse plasma (0.5 uL plasma/lane); lanes 3 and 4 are mouse plasma (0.5 uL plasma/lane) obtained 6 hours after the IP injection of 10 mg/kg fusion protein.
Discussion
The results of these studies are consistent with the following conclusions. First, the plasma Cmax of the cTfRMAb-TNFR fusion protein follows the rank order of IV > IP > SQ for a given injection dose (Figures 1–3). Second, the plasma concentration of the fusion protein is very low following either the IP or SQ injection of a low dose, 0.7 mg/kg, but increases greatly following the IP or SQ injection of higher doses of fusion protein, 3–10 mg/kg (Figures 2–3), and this is attributed to saturation of local TfR binding sites by the higher doses. Third, the plasma AUC of the cTfRMAb-TNFR fusion protein is up to 10-fold higher at 6 or 24 hours after IP injection, as compared to SQ administration (Table 1). Fourth, despite the much higher plasma concentrations of fusion protein after IP injection, as compared to SQ injection, the fusion protein concentration in brain is comparable with either route of administration at a given injection dose of 3–10 mg/kg (Table 2). Fifth, the fusion protein in plasma is rapidly degraded following the administration of the low dose, 0.3–0.7 mg/kg, but is stable in plasma following the injection of higher doses of fusion protein, 3–10 mg/kg, based on plasma TCA precipitability (Table 1). The stability of the fusion protein at 6 hours after IP injection is corroborated by the Western blot study of mouse plasma (Figure 4). Sixth, with respect to uptake by peripheral organs, there is minimal uptake by liver and maximal uptake by spleen (Table 3), and this pattern parallels the tissue-specific expression of transferrin receptors.
There are 2 TfR isoforms, TfR1 and TfR2, in either the mouse13 or human.14 The cTfRMAb domain of the cTfRMAb-TNFR fusion protein is directed against the TfR1 isoform.8 The principal TfR at the BBB was shown by BBB genomics studies to be TfR1.15 Similarly, the principal TfR isoform in the spleen of the mouse is TfR1,16 which explains the high uptake of fusion protein by spleen (Table 3). In contrast, the major TfR isoform in mouse liver is TfR2,13,16 which is consistent with the low uptake of the fusion protein by liver (Table 3). The cTfRMAb-TNFR fusion protein is immediately exposed to local TfR1 following either an IP or SQ injection in the mouse, and this local TfR1 may bind and sequester the fusion protein at local depot sites. However, such local receptors may be saturated following the administration of higher doses. The saturation of local receptors following IP or SQ injection explains the much higher distribution of the fusion protein into the plasma compartment following the IP or SQ injection of 3–10 mg/kg of fusion protein (Figures 2 and 3). There is greater absorption of the fusion protein after IP administration, as compared to SQ injection, which explains the 5- to 10-fold higher plasma AUC following IP injection, as compared to SQ administration for the 3 or 10 mg/kg injection doses of fusion protein (Table 1).
The present study determines the plasma AUC following 3 different routes of administration of the fusion protein, IV, SQ, or IP, at injection doses ranging from 0.3 to 10 mg/kg. The plasma AUC determined for the IV route may be under-estimates (Table 1), since the plasma drug level was determined for only the first 60 min after injection (Figure 1). However, the main purpose of the present study was to compare the plasma profile of the fusion protein for the SQ and IP routes, and these profiles are extended to 24 hours after administration (Figures 2–3). The plasma AUC is up to 10-fold higher after the IP route, as compared to the SQ route (Table 2). Despite the much higher plasma AUC following IP administration, the concentration of the cTfRMAb-TNFR fusion protein in brain is comparable with either the IP or SQ routes of administration (Table 2). The enhanced uptake by brain following SQ injection is explained by examination of the ratio of fusion protein concentration in brain (Cb) (Table 2), relative to the plasma AUC for a given injection dose (Table 1). The Cb/AUC ratio, 1.2 uL/min/g, at 6 hours after SQ injection is 4-fold higher than the Cb/AUC ratio, 0.29 uL/min/g, at 6 hours after IP injection at a dose of 3 mg/kg. The difference is even greater following the administration of 10 mg/kg of fusion protein. The Cb/AUC ratio, 0.67 uL/min/g, at 6 hours after SQ injection is 10-fold higher than the Cb/AUC ratio, 0.06 uL/min/g, at 6 hours after IP injection at a dose of 10 mg/kg. The Cb/AUC ratio is proportional to the BBB permeability-surface area product, which is a measure of the saturation of the BBB TfR1. The higher plasma concentration of fusion protein produced following IP administration causes greater saturation of the BBB TfR1.
This IP or SQ administration of 3–10 mg/kg doses of fusion protein also saturates the uptake of the fusion protein by peripheral tissues, and this leads to a greater metabolic stability of the fusion protein in plasma. The plasma TCA precipitability of the fusion protein is reduced to 34% at 24 hours after the IP injection of a low dose, 0.7 mg/kg, of fusion protein (Table 1). However, the plasma TCA precipitability is 95% for 24 hours after a single IP injection of 10 mg/kg of fusion protein (Table 1). The stability of the fusion protein in plasma is also shown by Western blotting using a primary antibody against the human type II TNFR (Figure 4). The plasma TCA precipitability of the fusion protein is 95% and 86% at 6 and 24 hours after the SQ administration of 10 mg/kg of fusion protein (Table 1). Therefore, a sustained nearly 24-hour exposure of brain to fusion protein in plasma is possible with a once-a-day administration of the cTfRMAb-TNFR fusion protein at SQ injection doses of 3–10 mg/kg. The concentration of the cTfRMAb-TNFR fusion protein in brain peaks at 1.25 ug/gram at 6 hours after the IP injection of 3 mg/kg (Table 2). The molecular weight of the cTfRMAb-TNFR fusion protein hetero-tetramer of 2 heavy chains and 2 light chains is 210,000 Da.9 Therefore, a brain level of fusion protein of 1.25 ug/gram corresponds to a brain concentration of 9 nM, which is many-fold greater than the cerebral concentration of TNFα in pathologic conditions. The brain TNFα concentration under normal conditions is <10 pM.17,18,19 In acute brain injury or acute meningitis, the cerebral TNFα concentration increases to 0.5 nM and 0.1 nM, respectively.3,19 Therefore, a single SQ injection of 3 mg/kg produces a concentration of cTfRMAb-TNFR fusion protein in brain that is 20- to 50-fold higher than the cerebral concentration of TNFα in acute pathologic conditions.
The inactivation of cerebral TNFα by the fusion protein relates to the pharmacodynamics of the therapeutic in brain. While pharmacodynamic studies have not yet been performed following the IP or SQ routes of administration, therapeutic effects in brain have been reported for the cTfRMAb-TNFR fusion protein following the IV route of administration in mouse models of neural disease. A delayed single IV injection of the cTfRMAb-TNFR fusion protein reduces stroke volume and neural deficit in mice subjected to an acute occlusion of the middle cerebral artery.11 Twice-weekly IV injections of the fusion protein to mice with experimental Parkinson’s disease causes an increase in striatal tyrosine hydroxylase activity and an improvement in neurobehavior.10 These studies provide evidence for the retention of biological activity of the fusion protein following drug injection into the blood and transport through the BBB.
The present study shows that therapeutic levels of cTfRMAb-TNFR fusion protein are generated in brain following the IV, SQ, or IP, routes of administration of a given ID, 3 mg/kg, of the fusion protein. For the treatment of acute disorders of the brain, such as stroke or brain trauma, the IV injection is the preferred route of administration. However, for the treatment of chronic brain disorders, such as neurodegeneration, where daily drug administration is required, it is advantageous to administer the drug daily by either the IP or SQ route. The present study shows that either the SQ or the IP route of administration produces a comparable brain concentration of the fusion protein at a given ID (Table 2). However, the SQ route is deemed preferable over the IP route, because the plasma AUC is up to 10-fold lower with the SQ route (Table 1). Therefore, the exposure of the fusion protein to peripheral tissues, which may determine the toxicity profile of the drug, is much lower with the SQ route as compared to the IP route of administration of the fusion protein. With respect to potential toxicity of TfRMAb fusion proteins administered over the long term, a fusion protein of the cTfRMAb and glial derived neurotrophic factor was injected twice/week for 12 weeks at a dose of 4 mg/kg/week in mice.20 Chronic fusion protein treatment caused no toxicity, and no clinical, biochemical, or histopathological effects were observed. There was no change in the serum iron or transferrin levels.20 The lack of effect of chronic administration of the cTfRMAb-based fusion protein on serum iron or transferrin is consistent with the lack of interference of TfRMAb’s on transferrin binding at the TfR,21 unless very high doses of the TfRMAb are administered.22
In summary, the present study shows that once-daily SQ injections of BBB-penetrating cTfRMAb-derived fusion proteins produce sustained distribution of stable fusion protein in plasma over many hours, and may enable future chronic treatment of mouse models of neural disease.
Acknowledgments
This work was supported by a grant from the National Institutes of Health National Institute of Aging [R01-AG032244]. Winnie Tai and Phuong Tram provided expert technical assistance.
References
- 1.Tansey MG, Szymkowski DE. The TNF superfamily in 2009: new pathways, new indications, and new drugs. Drug Discov Today. 2009;14:1082–1088. doi: 10.1016/j.drudis.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 2.Boado RJ, Hui EK, Lu JZ, Zhou QH, Pardridge WM. Selective targeting of a TNFR decoy receptor pharmaceutical to the primate brain as a receptor-specific IgG fusion protein. J Biotechnol. 2010;146:84–91. doi: 10.1016/j.jbiotec.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shohami E, Novikov M, Bass R, Yamin A, Gallily R. Closed head injury triggers early production of TNF alpha and IL-6 by brain tissue. J Cereb Blood Flow Metab. 1994;14:615–619. doi: 10.1038/jcbfm.1994.76. [DOI] [PubMed] [Google Scholar]
- 4.Barone FC, Arvin B, White RF, Miller A, Webb CL, Willette RN, Lysko PG, Feuerstein GZ. Tumor necrosis factor-alpha. A mediator of focal ischemic brain injury. Stroke. 1997;28:1233–1244. doi: 10.1161/01.str.28.6.1233. [DOI] [PubMed] [Google Scholar]
- 5.Janelsins MC, Mastrangelo MA, Park KM, Sudol KL, Narrow WC, Oddo S, LaFerla FM, Callahan LM, Federoff HJ, Bowers WJ. Chronic neuron-specific tumor necrosis factor-alpha expression enhances the local inflammatory environment ultimately leading to neuronal death in 3xTg-AD mice. Am J Pathol. 2008;173:1768–1782. doi: 10.2353/ajpath.2008.080528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCoy MK, Martinez TN, Ruhn KA, Szymkowski DE, Smith CG, Botterman BR, Tansey KE, Tansey MG. Blocking soluble tumor necrosis factor signaling with dominant-negative tumor necrosis factor inhibitor attenuates loss of dopaminergic neurons in models of Parkinson’s disease. J Neurosci. 2006;26:9365–9375. doi: 10.1523/JNEUROSCI.1504-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pardridge WM, Boado RJ. Reengineering biopharmaceuticals for targeted delivery across the blood-brain barrier. Methods Enzymol. 2012;503:269–292. doi: 10.1016/B978-0-12-396962-0.00011-2. [DOI] [PubMed] [Google Scholar]
- 8.Boado RJ, Zhang Y, Wang Y, Pardridge WM. Engineering and expression of a chimeric transferrin receptor monoclonal antibody for blood-brain barrier delivery in the mouse. Biotechnol Bioeng. 2009;102:1251–1258. doi: 10.1002/bit.22135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou QH, Boado RJ, Hui EK, Lu JZ, Pardridge WM. Brain-penetrating tumor necrosis factor decoy receptor in the mouse. Drug Metab Dispos. 2011a;39:71–76. doi: 10.1124/dmd.110.036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou QH, Sumbria R, Hui EKW, Lu JZ, Boado RJ, Pardridge WM. Neuroprotection with a brain-penetrating biologic tumor necrosis factor inhibitor. J Pharmacol Exp Ther. 2011b;339:618–623. doi: 10.1124/jpet.111.185876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sumbria RK, Boado RJ, Pardridge WM. Brain protection from stroke with intravenous TNFalpha decoy receptor-Trojan horse fusion protein. J Cereb Blood Flow Metab. 2012;32:1933–1938. doi: 10.1038/jcbfm.2012.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Skarlatos S, Pardridge WM. Targeting of an anti-CR3 (CD11b/CD18) monoclonal antibody to spleen but not brain, in vivo in mice. J Drug Target. 1995;3:9–14. doi: 10.3109/10611869509015927. [DOI] [PubMed] [Google Scholar]
- 13.Kawabata H, Germain RS, Ikezoe T, Tong X, Green EM, Gombart AF, Koeffler HP. Regulation of expression of murine transferrin receptor 2. Blood. 2001;98:1949–1954. doi: 10.1182/blood.v98.6.1949. [DOI] [PubMed] [Google Scholar]
- 14.Deaglio S, Capobianco A, Cali A, Bellora F, Alberti F, Righi L, Sapino A, Camaschella C, Malavasi F. Structural, functional, and tissue distribution analysis of human transferrin receptor-2 by murine monoclonal antibodies and a polyclonal antiserum. Blood. 2002;100:3782–3789. doi: 10.1182/blood-2002-01-0076. [DOI] [PubMed] [Google Scholar]
- 15.Li JY, Boado RJ, Pardridge WM. Blood-brain barrier genomics. J Cereb Blood Flow Metab. 2001;21:61–68. doi: 10.1097/00004647-200101000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Wallace DF, Summerville L, Lusby PE, Subramaniam VN. First phenotypic description of transferrin receptor 2 knockout mouse, and the role of hepcidin. Gut. 2005;54:980–986. doi: 10.1136/gut.2004.062018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao C, Ling Z, Newman MB, Bhatia A, Carvey PM. TNF-alpha knockout and minocycline treatment attenuates blood-brain barrier leakage in MPTP-treated mice. Neurobiol Dis. 2007;26:36–46. doi: 10.1016/j.nbd.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu N, Chen R, Du H, Wang J, Zhang Y, Wen J. Expression of IL-10 and TNF-alpha in rats with cerebral infarction after transplantation with mesenchymal stem cells. Cell Mol Immunol. 2009;6:207–213. doi: 10.1038/cmi.2009.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barichello T, dos Santos I, Savi GD, Florentino AF, Silvestre C, Comim CM, Feier G, Sachs D, Teixeira MM, Teixeira AL, Quevedo J. Tumor necrosis factor alpha (TNF-alpha) levels in the brain and cerebrospinal fluid after meningitis induced by Streptococcus pneumoniae. Neurosci Lett. 2009;467:217–219. doi: 10.1016/j.neulet.2009.10.039. [DOI] [PubMed] [Google Scholar]
- 20.Zhou QH, Boado RJ, Lu JZ, Hui EKW, Pardridge WM. Chronic dosing of mice with a transferrin receptor monoclonal antibody-GDNF fusion protein. Drug Metab Dispos. 2011;39:1149–1154. doi: 10.1124/dmd.111.038349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daniels TR, Delgado T, Rodriguez JA, Heiguera G, Penichet ML. The transferrin receptor part I: Biology and targeting with cytotoxic antibodies for the treatment of cancer. Clin Immunol. 2006;121:144–158. doi: 10.1016/j.clim.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 22.Ueda F, Raja KB, Simpson RJ, Trowbridge IS, Bradbury MWB. Rate of 59Fe uptake into brain and cerebrospinal fluid and the influence thereon of antibodies against the transferrin receptor. J Neurochem. 1993;60:106–113. doi: 10.1111/j.1471-4159.1993.tb05828.x. [DOI] [PubMed] [Google Scholar]