Abstract
Background & Aims
Little is known about the effects of geographic factors, such as rural vs urban residence and travel time to colonoscopy providers, on risk-appropriate use of colorectal cancer (CRC) screening in the general population. We evaluated the effects of geographic factors on adherence to CRC screening and differences in screening use among familial risk groups.
Methods
We analyzed data from the 2010 Utah Behavior Risk Factor Surveillance System, which included state-added questions on familial CRC. Using multiple logistic regression models, we assessed the effects of rural vs urban residence, travel time to the nearest colonoscopy provider, and spatial accessibility of providers on adherence to risk-appropriate screening guidelines. Study participants (n=4260) were respondents 50–75 years old.
Results
Sixty-six percent of the sample adhered to risk-appropriate CRC screening guidelines, with significant differences between urban and rural residents (68% vs 57%, respectively; P<.001) across all familial risk groups. Rural residents were less likely than urban dwellers to be up-to-date with screening guidelines (multivariate odds ratio=0.65; 95% confidence interval [CI], 0.53–0.79). In the unadjusted analysis, rural vs urban residence (P<.001), travel time to the nearest colonoscopy provider (P=.003), and spatial accessibility of providers (P=.012) were significantly associated with adherence to screening guidelines. However, rural vs urban residence (P<.001) was the only geographic variable independently associated with screening adherence in the adjusted analyses.
Conclusion
There are marked disparities in use of risk-appropriate CRC screening between rural and urban residents in Utah. Differences in travel time to the nearest colonoscopy provider and spatial accessibility of providers did not account for the geographic variations observed in screening adherence.
Keywords: travel time, health disparities, geography
Introduction
Colorectal cancer (CRC), the third most frequently diagnosed cancer and second leading cause of cancer-related mortality in the United States,1 is highly preventable through appropriate use of screening tests, like colonoscopy.2 Overall CRC screening rates in the United States increased from 52% in 2002 to 64% in 2010; but despite recent increases in screening uptake, only one-third of CRCs are diagnosed at early stages (stages I–II).3
Previous studies identified differences in CRC screening utilization among sub-populations in the United States, like age, race, and income groups;4, 5 but, fewer studies examined CRC screening utilization in rural populations and none have examined geographic correlates by familial risk groups. Geographic factors, including travel time and spatial accessibility to CRC screening providers, may influence adherence to risk-appropriate screening.6 For example, lengthy or costly travel to screening services or long wait times due to service shortages may discourage patients from receiving the recommended cancer screenings.7 Rural populations are particularly vulnerable to access barriers, resulting in possible geographic disparities in health services utilization.
Available evidence indicates that rural residents are less likely than urban residents to receive CRC screening and to be up-to-date with CRC screening guidelines.8, 9 Geographic proximity to cancer screening providers may explain differences in screening utilization between rural and urban groups. Studies of mammography use in rural Kansas and the United Kingdom found women with shorter travel distances to screening centers were more likely to receive a mammogram than those with longer travel distances.10, 11 Yet, comparable studies in California and Colorado revealed no association between mammography use and travel distance.12, 13 To our knowledge, no studies in the United States have explored the relationship between travel distance to the nearest CRC screening facility and screening utilization. Understanding the geographic mechanisms behind risk-appropriate CRC screening will help inform interventions targeted at both urban and rural residents.
Consensus-approved risk-appropriate CRC screening guidelines stratify patients by a familial history of the disease recommending earlier and more frequent testing for those with a positive family history of CRC;14, 15 specifically, individuals with a first-degree relative (FDR) diagnosed with CRC less than 60 years of age should begin screening at age 40 receiving a colonoscopy every five years.14 Individuals with a positive family history of CRC are significantly more likely to receive screening, but not necessarily consistent with risk-appropriate screening guidelines.16, 17 Existing studies only assessed screening practices among familial risk groups, not adherence to risk-appropriate guidelines which are more aggressive than recommendations for the general population.
This is the first study to address risk-appropriate CRC screening uptake among urban and rural populations by examining the influence of geographic proximity to CRC screening providers on utilization of risk-appropriate screening, differences in screening adherence among familial risk groups, and geographic patterns of risk-appropriate screening uptake.
Methods
Study Sample
The Behavioral Risk Factor Surveillance System (BRFSS), coordinated by the Centers for Disease Control and Prevention in conjunction with state health departments, is a set of cross-sectional telephone surveys of the non-institutionalized population 18 years and older in the United States.18 Questions about CRC screening history are routinely asked on BRFSS questionnaires, but not information on familial CRC history. We collaborated with the Utah Department of Health to add the following three questions about familial CRC history to the 2010 BRFSS:
“Have any of your nearest blood relatives, that is parents, siblings, or children, ever been told by a doctor or other health professional that he or she had colon or rectal cancer?”
“How many or your nearest blood relatives, that is parents, siblings, or children, have been diagnosed with colon or rectal cancer?”
“Were any of your nearest blood relatives that is parents, siblings, or children, less than 60 years of age when they were diagnosed with colon or rectal cancer?”
The BRFSS uses a disproportionate stratified sampling design to select household telephone numbers from Utah’s 12 health districts and samples rural health districts at a higher rate.19 The sample size for the 2010 questionnaire was 10,173 with a 64.61% response rate. For the purposes of this study, we only included respondents from 50 to 75 years who answered the state-added familial CRC questions, reported on CRC screening history, and provided a current Zip code resulting in a final study sample size of 4,260 men and women.
Analytic Variables
Respondents were classified into three familial risk groups average, increased, and high risk—based on their self-reported familial CRC history and American Cancer Society guidelines.20 Those reporting no family history of the disease were classified as average risk. Respondents with any FDR diagnosed with CRC at age 60 or older were classified as increased risk. Those with CRC in any FDR diagnosed before age 60 or in two or more FDRs at any age were classified as high risk. We then determined respondents’ adherence to risk-appropriate CRC screening using American Cancer Society guidelines. Both average and increased risk respondents from 50 to 75 years of age were considered adherent to screening recommendations if they received a blood stool test using a home kit within the past year, a sigmoidoscopy in the previous five years, or a colonoscopy in the previous 10 years. Those classified as high risk were considered adherent if they reported having a colonoscopy in the previous five years.
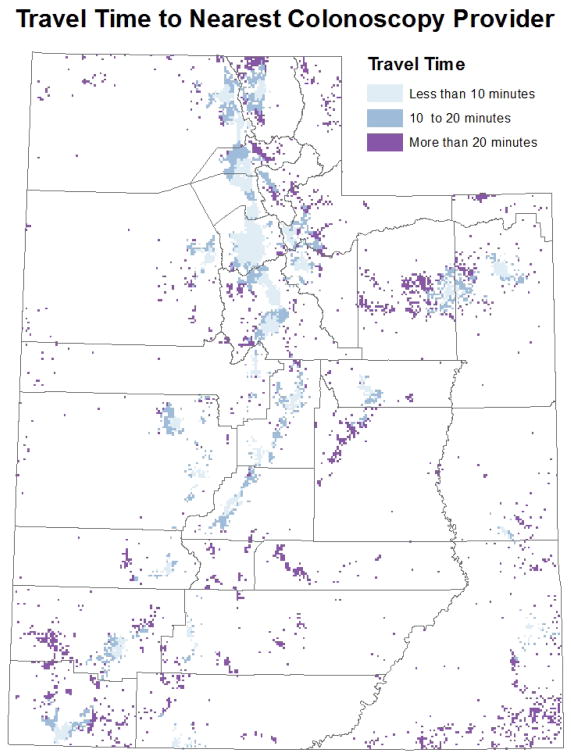
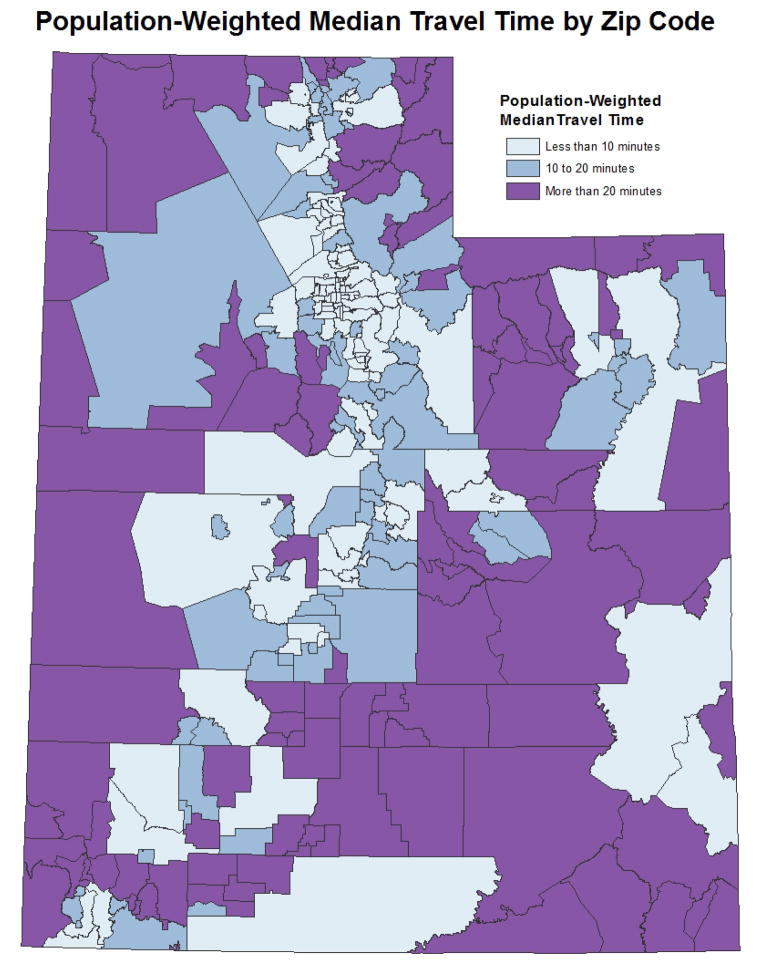
Geographic proximity to CRC screening facilities was defined as the population-weighted median travel time to the nearest colonoscopy provider from respondents’ Zip codes. To calculate geographic proximity by Zip code, we first created a one mile grid for the state of Utah and for each grid cell populated with individuals 50 or older we calculated the actual travel time to nearest colonoscopy provider (Figure 1). Populations were based on 2010 Census block tabulations. Using all of the travel times within a Zip code we calculated a population-weighted median travel time by Zip code (Figure 2). Travel times were calculated using the North American Association of Central Cancer Registries Shortest Path Tool20 and grouped into three categories: less than 10 minutes, 10 to 20 minutes, and more than 20 minutes. Colonoscopy providers were identified using comprehensive Internet searches and current lists of gastroenterologists provided by the American Medical Association and National Provider Identifier file.21, 22 We contacted each facility to verify colonoscopy providers practicing from 2000–2010 to account for changes in providers over time.
Figure 1.
Actual travel time to the nearest colonoscopy provider for each populated one mile grid cell in Utah
Figure 2.
Population-weighted median travel time to the nearest colonoscopy provider for each Utah Zip code
Travel time measures geographic proximity to CRC screening sites, but does not account for spatial accessibility, or the ratio of providers to the population in need of services. We used the two-step floating catchment area (2SFCA) method to measure accessibility to colonoscopy providers which calculates the ratio of providers to the population within a specified service catchment area, in this case 30 minute driving time.23 Since the majority of providers divided their practice between multiple locations, we summed the full-time equivalent of providers for each health facility. Methodology for the 2SFCA is documented elsewhere.23 We collapsed spatial accessibility into quintiles. Rural/urban residence was based on Rural-Urban Computing Area (RUCA) codes at the Zip code level. RUCA codes were developed using standard U.S. Bureau of Census urbanized area and urban cluster definitions classified by Census tracts and later by Zip code. The 33 RUCA categories were aggregated into urban and rural as recommended by the Washington, Wyoming, Alaska, Montana, and Idaho Rural Health Research Center.24 Additional measures included gender, age, self-reported income, marital status, education, race/ethnicity, health insurance status, and access to a personal healthcare provider.
Statistical Analyses
Descriptive analysis examined the demographic characteristics of the sample. Bivariate analysis estimated the proportion of respondents who were up-to-date with risk-appropriate screening guidelines by each independent variable. Differences were tested using Wald chi-square tests. Crude odds ratios (OR) and 95% confidence intervals (CI) were also calculated to measure each variable’s association with screening adherence.
Multiple logistic regression models were calculated to identify factors associated with risk-appropriate CRC screening utilization. The full model included all independent variables mentioned above while the restricted models included only those variables found to be significantly associated with screening adherence in the full model. We used three restricted models, the first with rural/urban residence, the second with travel time, and the third with spatial accessibility to account for collinearity between geographical measures. Data were weighted to compensate for the disproportionate stratified sampling design used in the survey. All analyses were conducted using SAS 9.2 (SAS Institute Inc., Carry, NC, 2001).
Results
Characteristics of the sample are presented in Table 1. Respondents were generally non-Hispanic white (91.8%), married (81.0%), urban residents (85.3%), covered by health insurance (91.7%), and had a personal healthcare provider (89.0%). A large majority of participants (90.1%) were at average risk for CRC with no family history of the disease. Rural residents (14.7%) were more likely to report lower household incomes, be less educated, travel more than 10 minutes to the nearest colonoscopy provider, and live in Zip codes with less spatial accessibility to providers. Over 66% of the sample was adherent to risk-appropriate CRC screening guidelines with significant differences in adherence between urban (68.3%) and rural (56.8%) populations. Screening adherence differed significantly by familial risk group with 65.9% of average risk, 78.6 % of increased risk, and 65.5% of high risk participants being up-to-date with screening guidelines. The percentage of respondents that were adherent to screening guidelines by rural/urban residence is presented in Table 2. We identified a significant inverse relationship between travel time and adherence to risk-appropriate screening guidelines with higher percentages of adherence seen in individuals living closer to screening providers.
Table 1.
Demographic characteristics of the sample by rural/urban residence
| Total | % Total | Urban | % Urban | Rural | % Rural | P-Value | |
|---|---|---|---|---|---|---|---|
| Total observations | 4260 | 100.00 | 3082 | 85.34 | 1178 | 14.66 | |
| Race | <.001 | ||||||
| Non-Hispanic White | 3949 | 91.80 | 2848 | 91.70 | 1101 | 92.42 | |
| Hispanic | 152 | 4.49 | 117 | 4.64 | 35 | 3.62 | |
| Other | 127 | 3.09 | 98 | 3.14 | 29 | 2.78 | |
| Missing/unknown | 32 | 0.61 | 19 | 0.52 | 13 | 1.18 | |
| Sex | <.001 | ||||||
| Male | 1809 | 49.45 | 1306 | 49.16 | 503 | 51.14 | |
| Female | 2451 | 50.55 | 1776 | 50.84 | 675 | 48.86 | |
| Age group | <.001 | ||||||
| 50–54 | 961 | 28.82 | 706 | 28.85 | 255 | 28.67 | |
| 55–59 | 999 | 24.38 | 715 | 24.63 | 284 | 22.96 | |
| 60–64 | 870 | 19.21 | 631 | 19.01 | 239 | 20.35 | |
| 65–69 | 738 | 14.19 | 524 | 14.03 | 214 | 15.13 | |
| 70–75 | 692 | 13.39 | 506 | 13.48 | 186 | 12.88 | |
| Education | <.001 | ||||||
| Less than high school | 167 | 3.66 | 106 | 3.38 | 61 | 5.25 | |
| High school graduate | 1132 | 25.21 | 762 | 24.05 | 370 | 31.97 | |
| Some college | 1376 | 32.62 | 997 | 32.37 | 379 | 34.06 | |
| College graduate | 1581 | 38.38 | 1213 | 40.03 | 368 | 28.72 | |
| Income | <.001 | ||||||
| Less than $25,000 | 725 | 13.63 | 507 | 13.30 | 218 | 15.57 | |
| $25,000 to $50,000 | 1046 | 23.35 | 722 | 22.13 | 324 | 30.46 | |
| $50,000 to $75,000 | 690 | 16.79 | 489 | 16.17 | 201 | 20.44 | |
| More than $75,000 | 1341 | 35.79 | 1065 | 38.00 | 306 | 22.96 | |
| Missing/unknown | 458 | 10.43 | 329 | 10.41 | 129 | 10.58 | |
| Marital status | <.001 | ||||||
| Married | 3064 | 81.00 | 2190 | 80.71 | 874 | 82.74 | |
| Not married | 1151 | 18.05 | 860 | 18.38 | 291 | 16.14 | |
| Missing/unknown | 45 | 0.94 | 32 | 0.91 | 13 | 1.13 | |
| Health insurance | <.001 | ||||||
| Yes | 3936 | 91.69 | 2861 | 91.87 | 1075 | 90.61 | |
| No | 319 | 8.23 | 219 | 8.08 | 100 | 9.10 | |
| Missing/unknown | 5 | 0.08 | 2 | 0.05 | 3 | 0.29 | |
| Personal provider | <.001 | ||||||
| Yes | 3790 | 88.96 | 2762 | 89.24 | 1028 | 87.34 | |
| No | 460 | 10.86 | 313 | 10.58 | 147 | 12.47 | |
| Risk | <.001 | ||||||
| Average risk | 3843 | 90.05 | 2781 | 90.14 | 1062 | 89.51 | |
| Increased risk | 245 | 5.91 | 184 | 6.03 | 61 | 5.23 | |
| High risk | 172 | 4.04 | 117 | 3.83 | 55 | 5.26 | |
| Median travel time | <.001 | ||||||
| <10 minutes | 3388 | 81.34 | 2581 | 84.17 | 807 | 64.85 | |
| 10 – <20 minutes | 673 | 15.26 | 456 | 14.59 | 217 | 19.13 | |
| ≥20 | 199 | 3.40 | 45 | 1.24 | 154 | 16.02 | |
| Spatial accessibility per 1,000 people | <.001 | ||||||
| <0.002 | 54 | 0.92 | 2 | 0.12 | 52 | 5.59 | |
| 0.002–<0.182 | 544 | 9.50 | 340 | 6.98 | 204 | 37.34 | |
| 0.182–<0.319 | 952 | 17.27 | 436 | 15.16 | 516 | 25.06 | |
| 0.319–<0.542 | 1146 | 30.76 | 895 | 32.46 | 261 | 9.92 | |
| ≥0.542 | 1554 | 41.55 | 1409 | 45.28 | 145 | 7.01 |
All percentage values are weighted to reflect 2010 Utah population
Table 2.
Percentage of respondents adherent to risk-appropriate colorectal cancer screening by rural/urban residence for selected factors
| Count Urban Adherent | % Urban Adherent | Count Rural Adherent | % Rural Adherent | |
|---|---|---|---|---|
| Total observations | 2151 | 68.34 | 693 | 56.84** |
| Race | ||||
| Non-Hispanic White | 2003 | 69.15 | 655 | 57.54** |
| Hispanic | 70 | 58.78 | 17 | 47.16** |
| Other | 64 | 56.92 | 15 | 45.55** |
| Missing/unknown | 14 | 79.91 | 6 | 58.06 |
| Sex | ||||
| Male | 925 | 69.44 | 308 | 58.76** |
| Female | 1226 | 67.28 | 385 | 54.83** |
| Age group | ||||
| 50–54 | 372 | 52.70 | 107 | 41.94** |
| 55–59 | 496 | 69.55 | 172 | 59.66** |
| 60–64 | 475 | 75.53 | 148 | 62.29** |
| 65–69 | 408 | 78.48 | 146 | 69.73** |
| 70–75 | 400 | 78.92 | 120 | 61.23** |
| Education | ||||
| Less than high school | 55 | 46.72 | 30 | 43.98* |
| High school graduate | 502 | 64.51 | 189 | 49.57** |
| Some college | 666 | 64.01 | 229 | 58.77** |
| College graduate | 926 | 76.05 | 245 | 65.01** |
| Income | ||||
| Less than $25,000 | 303 | 57.52 | 110 | 49.78** |
| $25,000 to $50,000 | 511 | 69.12 | 182 | 53.42** |
| $50,000 to $75,000 | 340 | 67.90 | 121 | 57.71** |
| More than $75,000 | 771 | 72.78 | 208 | 66.01** |
| Missing/unknown | 226 | 65.04 | 72 | 55.49** |
| Marital status | ||||
| Married | 1577 | 70.42 | 536 | 59.40** |
| Not married | 555 | 60.25 | 154 | 47.04** |
| Health insurance | ||||
| Yes | 2051 | 70.39 | 665 | 60.14** |
| No | 99 | 45.27 | 26 | 23.23** |
| Personal provider | ||||
| Yes | 2012 | 71.48 | 633 | 59.22** |
| No | 135 | 41.72 | 59 | 40.76 |
| Median travel time | ||||
| <10 minutes | 1810 | 68.83 | 489 | 58.96** |
| 10 - <20 minutes | 313 | 66.21 | 124 | 54.12** |
| ≥20 | 28 | 60.09 | 80 | 51.52** |
| Risk | ||||
| Average risk | 1923 | 67.61 | 612 | 56.07** |
| Slightly increased risk | 151 | 79.34 | 45 | 73.36** |
| High risk | 77 | 68.31 | 36 | 53.56** |
All percentage are values weighted to reflect 2010 Utah population
p<.05
p<.001
Crude and adjusted odds ratios for adherence to risk-appropriate CRC screening are presented in Table 3. In the crude analyses, rural residents were less likely than urban dwellers to be up-to-date with screening guidelines (OR=0.61, 95% CI 0.51–0.73). Similarly, those traveling more than 20 minutes to the nearest colonoscopy provider were significantly less likely to be adherent with CRC screening than those traveling less than 10 minutes (OR=0.57, 95% CI 0.40–0.80). Compared with respondents at average risk for CRC, those with an increased familial risk for the disease were more likely to report adherence to CRC screening (OR=1.89, 95% CI 1.31–2.75), while those at high risk were not more likely to be up-to-date with screening guidelines. Respondents with the lowest spatial accessibility to colonoscopy providers were less likely to be adherent with screening guidelines than those with the greatest spatial accessibility (OR=0.37, 95% CI 0.19–0.72).
Table 3.
Crude odds ratios, model-adjusted odds ratios, and 95% confidence intervals for adherence to risk-appropriate colorectal cancer screening
| Crude OR (95% CI) | P-Value | Full Model (95% CI) | P-Value | Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Adjusted OR (95% CI) | P-Value | Adjusted OR (95% CI) | P-Value | Adjusted OR (95% CI) | P-Value | |||||
| Race | .020 | .318 | ||||||||
| Non-Hispanic White | 1.00 (Reference) | 1.00 (Reference) | ||||||||
| Hispanic | 0.65 (0.43, 0.98) | 1.21 (0.75, 1.97) | ||||||||
| Other | 0.60 (0.38, 0.93) | 0.68 (0.42, 1.09) | ||||||||
| Missing/unknown | 1.36 (0.58, 3.18) | 1.21 (0.53, 2.80) | ||||||||
| Gender | .197 | .519 | ||||||||
| Male | 1.00 (Reference) | 1.00 (Reference) | ||||||||
| Female | 0.90 (0.77, 1.06) | 0.94 (0.79, 1.13) | ||||||||
| Age group | <.001 | <.001 | <.001 | <.001 | <.001 | |||||
| 50–54 | 1.00(Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | |||||
| 55–59 | 2.05 (1.64, 2.56) | 2.21 (1.74, 2.80) | 2.19 (1.73, 2.78) | 2.19 (1.73, 2.77) | 2.20 (1.74, 2.78) | |||||
| 60–64 | 2.65 (2.10, 3.34) | 3.00 (2.34, 3.84) | 2.98 (2.32, 3.82) | 2.97 (2.32, 3.81) | 2.97 (2.31, 3.80) | |||||
| 65–69 | 3.22 (2.49, 4.16) | 3.39 (2.57, 4.48) | 3.38 (2.57, 4.46) | 3.35 (2.56, 4.41) | 3.36 (2.55, 4.42) | |||||
| 70–75 | 3.10 (2.41, 3.99) | 3.45 (2.61, 4.57) | 3.44 (2.60, 4.54) | 3.45 (2.61, 4.55) | 3.46 (2.62, 4.56) | |||||
| Education | <.001 | <.001 | <.001 | <.001 | <.001 | |||||
| Less than high school | 0.29 (0.19, 0.43) | 0.37 (0.24, 0.57) | 0.38 (0.24, 0.58) | 0.37 (0.24, 0.57) | 0.37 (0.24, 0.56) | |||||
| High school graduate | 0.54 (0.44, 0.67) | 0.65 (0.52, 0.83) | 0.65 (0.51, 0.81) | 0.64 (0.51, 0.80) | 0.64 (0.51, 0.81) | |||||
| Some college | 0.58 (0.48, 0.70) | 0.64 (0.52, 0.79) | 0.63 (0.51, 0.78) | 0.63 (0.51, 0.77) | 0.63 (0.51. 0.78) | |||||
| College graduate | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | |||||
| Missing/unknown | 0.33 (0.04, 2.43) | 0.19 (0.03, 1.38) | 0.18 (0.03, 1.25) | 0.19 (0.30, 1.29) | 0.19 (0.03, 1.22) | |||||
| Annual household income | <.001 | .017 | .012 | .007 | .008 | |||||
| Less than $25,000 | 0.50 (0.39, 0.63) | 0.64 (0.47, 0.87) | 0.63 (0.46, 0.85) | 0.62 (0.46, 0.84) | 0.62 (0.46, 0.84) | |||||
| $25,000 to $50,000 | 0.75 (0.61, 0.93) | 0.73 (0.57, 0.94) | 0.73 (0.57, 0.93) | 0.71 (0.55, 0.91) | 0.71 (0.56, 0.91) | |||||
| $50,000 to $75,000 | 0.75 (0.59, 0.96) | 0.77 (0.59, 0.99) | 0.76 (0.58, 0.98) | 0.74 (0.57, 0.96) | 0.74 (0.58, 0.97) | |||||
| More than $75,000 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | |||||
| Missing/unknown | 0.68 (0.51, 0.89) | 0.65 (0.47, 0.88) | 0.65 (0.47, 0.87) | 0.63 (0.46, 0.86) | 0.64 (0.47, 0.87) | |||||
| Marital status | <.001 | .002 | .002 | .002 | .002 | |||||
| Married | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | |||||
| Not married | 0.64 (0.54, 0.76) | 0.78 (0.63, 0.97) | 0.78 (0.63, 0.96) | 0.79 (0.64, 0.97) | 0.79 (0.64, 0.98) | |||||
| Missing/unknown | 0.31 (0.16, 0.62) | 0.34 (0.17, 0.71) | 0.34 (0.17, 0.70) | 0.35 (0.17, 0.71) | 0.35 (0.17, 0.70) | |||||
| Risk | .003 | .005 | .006 | .005 | .006 | |||||
| Average risk | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | |||||
| Increased risk | 1.89 (1.31, 2.75) | 1.91 (1.29, 2.84) | 1.88 (1.27, 2.78) | 1.91 (1.29, 2.82) | 1.89 (1.27, 2.80) | |||||
| High risk | 0.98 (0.66, 1.46) | 1.16 (0.76, 1.79) | 1.17 (0.76, 1.80) | 1.15 (0.75, 1.77) | 1.16 (0.75, 1.79) | |||||
| Health insurance | <.001 | .011 | .012 | .014 | .019 | |||||
| Yes | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | |||||
| No | 0.32 (0.24, 0.43) | 0.59 (0.42, 0.83) | 0.59 (0.42, 0.84) | 0.60 (0.42, 0.85) | 0.61 (0.43, 0.86) | |||||
| Missing/unknown | 0.60 (0.08, 4.38) | 0.78 (0.11, 5.33) | 0.85 (0.13, 5.48) | 0.72 (0.12, 4.45) | 0.71 (0.11, 4.52) | |||||
| Personal provider | <.001 | <.001 | <.001 | <.001 | <.001 | |||||
| Yes | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | |||||
| No | 0.31 (0.24, 0.40) | 0.38 (0.29, 0.50) | 0.39 (0.30, 0.51) | 0.39 (0.30, 0.51) | 0.38 (0.29, 0.50) | |||||
| Missing/unknown | 0.90 (0.23, 3.57) | 0.68 (0.17, 2.74) | 0.64 (0.16, 2.56) | 0.63 (0.15, 2.60) | 0.65 (0.16, 2.71) | |||||
| Rural/urban residence | <.001 | <.001 | <.001 | |||||||
| Urban | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | |||||||
| Rural | 0.61 (0.51, 0.73) | 0.67 (0.53, 0.82) | 0.65 (0.53, 0.79) | |||||||
| Median travel time | .003 | .779 | .086 | |||||||
| <10 minutes | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | |||||||
| 10 - <20 minutes | 0.85 (0.68, 1.01) | 0.93 (0.72, 1.21) | 0.91 (0.71, 1.15) | |||||||
| ≥20 | 0.57 (0.40, 0.80) | 0.87 (0.53, 1.44) | 0.64 (0.43, 0.97) | |||||||
| Spatial accessibility per 1,000 people | .012 | .634 | .191 | |||||||
| <0.002 | 0.37 (0.19, 0.72) | 0.72 (0.28, 1.83) | 0.45 (0.19, 1.03) | |||||||
| 0.002–<0.182 | 0.77 (0.59, 0.99) | 0.96 (0.70, 1.32) | 0.83 (0.62, 1.12) | |||||||
| 0.182–<0.319 | 0.87 (0.70, 1.08) | 0.99 (0.76, 1.29) | 0.89 (0.70, 1.12) | |||||||
| 0.319–<0.542 | 0.82 (0.68, 0.99) | 0.86 (0.73, 1.06) | 0.85 (0.69, 1.04) | |||||||
| ≥0.542 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | |||||||
All values are weighted to reflect 2010 Utah population
Multiple logistic regression analyses were used to assess whether rural/urban residence, travel time, and spatial accessibility were independently associated with CRC screening adherence. In the full model, median travel time (P=.779) and spatial accessibility (P=.634) did not remain significantly associated with screening adherence; however, the lower odds of screening adherence for rural residents persisted (OR=0.67, 94% CI 0.53–0.82). Because rural/urban residence, median travel time, and spatial accessibility may measure similar constructs (rural residents likely travel longer distances to colonoscopy providers), we ran three restricted models separating the geographic variables. In Model 1, rural residents remained significantly less likely to be adherent with screening guidelines than urban dwellers (OR=0.65, 95% CI 0.53–0.79). Only respondents with an increased familial risk had significantly higher odds of screening adherence compared with those at average risk (OR=1.88, 95% CI 1.27–2.78). In Model 2, travel time did not remain significantly associated with CRC screening adherence (P=.086); however, respondents traveling more than 20 minutes to the nearest colonoscopy provider had significantly lower odds of adherence to CRC screening guidelines compared to those living less than 10 minutes from a provider (OR=0.64, 95% CI 0.43–0.97). We also collapsed travel time into two groups of more or less than 30 minutes. The results showed that residents living farther than 30 minutes from the nearest provider had lower odds of screening adherence than those living closer than 30 minutes (OR=0.68, 95% CI 0.34–1.37). These results were not significant likely due to small numbers (only 72 of 4260 respondents lived more than 30 minutes from the nearest provider). Spatial accessibility was not independently associated with screening adherence in Model 3 (P=.191).
Age group, education, income, marriage, health insurance, access to a personal healthcare provider, and familial risk were significant indicators of screening adherence in all three restricted models. We tested for potential interactions between each geographic variable and having a personal healthcare provider. No meaningful interactions were identified. A subgroup analysis revealed that rural residents with a personal health care provider remained less likely (OR=0.61, 95% CI 0.50–0.75) to be up-to-date with risk-appropriate screening guidelines than urban dwellers with a healthcare provider. No significant differences existed between rural and urban residents without a healthcare provider.
Discussion
This is the first study to demonstrate geographic disparities in risk-appropriate CRC screening according to rural/urban residence. Rural residents were less likely to be adherent with screening guidelines than urban dwellers, consistent with previous research on CRC screening behaviors in average-risk populations.8, 9 Travel time and spatial accessibility did not further elucidate differences in screening utilization between rural and urban populations. Only rural/urban residence remained independently associated with screening adherence in the multivariable analyses. Although median travel time did not significantly contribute to the adjusted model, residents living more than 20 minutes from the nearest colonoscopy provider were significantly less likely to be up-to-date with risk-appropriate screening than those living less than 10 minutes from the nearest provider. One might expect to see a reduction in utilization of CRC screening as the distance from colonoscopy providers increases because of the added costs, time, and effort needed to travel longer distances, a concept known as “distance decay”. 23 The effects of longer travel times coupled with limited public transportation in rural communities, may contribute to the lower likelihood of CRC screening adherence seen in this group.
Access to a personal healthcare provider and provider recommendation are powerful predictors of CRC screening in the general population.4, 9 We did not observe any meaningful geographic differences in access to personal healthcare providers albeit rural communities commonly experience primary care shortages.25 In a subgroup analysis, rural residents with a personal health care provider were still less likely to be up-to-date with risk-appropriate screening than urban dwellers with a healthcare provider. Thus, differential access to personal providers does not explain the observed geographic disparities. However, patterns of physician recommendation may vary across rural/urban groups. One study suggests that inadequate patient-provider communication about CRC risk is the primary barrier to screening in rural regions; sixty-one percent of patients reported insufficient time to discuss CRC screening with their physicians or no discussion at all.26 Also, providers may not incorporate familial risk assessment and risk-appropriate screening guidelines into patient-provider discussions resulting in sub-optimal risk communication and lower levels of screening adherence in high risk patients.27 Subsequent research should consider patterns in provider recommendations as plausible reasons for geographical disparities in CRC screening adherence across risk groups.
Provisions in the Affordable Care Act aim to improve rural health outcomes, including cancer screenings, by increasing rural primary care providers, expanding tele-healthcare services for specialty care, and ensuring coverage for preventive services.28 Expansion of health care coverage and services presents a prime opportunity to address risk-appropriate CRC cancer screening in rural populations; however, the Affordable Care Act alone may not increase screening rates. Comprehensive coverage for preventive services will increase demand. In turn, rural health care systems will need to respond with an increase in supply. Spatial accessibility was not independently associated with CRC screening adherence suggesting that colonoscopy services are well-distributed over both urban and rural Utah. However, this may change in 2014 when health care coverage is expanded to 42 million Americans. Rural communities often rely on specialty care physicians who travel from urban medical practices a few times a month, further reducing accessibility. Training non-physician providers in endoscopy may present a feasible, cost-effective strategy to enhancing CRC screening services in underserved populations. The Alaska Tribal Health System launched a three-tiered CRC screening program that expanded endoscopy services by training midlevel healthcare providers, contacted first-degree relatives of CRC patients, and hired patient navigators to guide average-risk patients through the screening process. Screening uptake significantly increased by 14% during the study period.29 Multifaceted interventions, like those in Alaska, targeting personal, behavioral, social, and environmental barriers to CRC screening in rural populations will complement provisions in the Affordable Care Act. Screening interventions should also capitalize on additional rural health care services by encouraging primary care providers to collect family health histories, training providers in health behavior counseling, and advocating provider recommendation of risk-appropriate screening.
Limitations of this study warrant discussion. Behavioral Risk Factor Surveillance System data is collected through telephone surveys, excluding households without telephones. Household telephone coverage differs by sub-population with lower coverage in young, poor, and minority groups.30 The Utah BRFSS used post-stratification methods to adjust for noncoverage and nonresponse and ensure the total number of respondents is equal to the population estimates for each geographic region. Ideally, post-stratification methods and the large sample size should reduce sampling error. Participants with missing information on familial CRC history, screening history, and residential Zip code were excluded from our analysis introducing possible bias. Respondents to the above questions may be inherently different from nonrespondents. Self-reported family health histories in the BRFSS are subject to recall bias and misreporting by survey respondents; self-reported cancer screening histories may disagree with hospital or clinic records with over-reporting of screening tests and under-reporting of time lapse since last screening.31
The BRFSS only collects data on residential Zip codes, restricting our travel time analysis to the Zip code level. Without residential addresses, we could only calculate a population-weighted median travel time to the nearest colonoscopy provider. This allowed us to exclude unpopulated areas in each Zip code from our calculations for a more accurate measure of respondents’ travel time. However, a population-weighted median travel time is still less precise than actual travel time. Changes in colonoscopy providers over time, especially in rural Zip codes, may also bias our travel time and spatial accessibility estimates. We contacted each health care facility for information on providers practicing from 2000–2010 to account for temporal changes. Not all facilities offered data on providers over the 10-year period causing some discrepancy in our analysis.
Finally, the 2010 BRFSS only asked respondents about their personal cancer history on one of three waves of questionnaires. Without data on all participants, we could not exclude prior cancer cases from our analysis. Respondents with a previous cancer diagnosis are more likely to be up-to-date with risk-appropriate cancer screening guidelines.32 However, we assume a relatively small percentage of previous CRC cases were in the sample.33
Conclusion
Significant disparities in risk-appropriate CRC screening uptake were identified between urban and rural populations in Utah. Differences in travel time to the nearest colonoscopy provider and spatial accessibility of providers did not explain geographical variation in screening adherence. Such differences in rural/urban screening utilization underscore the need for interventions targeted at rural residents in all familial risk groups.
Acknowledgments
Financial Support: Huntsman Cancer Foundation; we acknowledge the use of shared resources supported by P30 CA042014 awarded to Huntsman Cancer Institute.
Abbreviations used in this paper
- BRFSS
Behavioral Risk Factor Surveillance System
- CI
confidence interval
- CRC
colorectal cancer
- FDR
first-degree relative
- FOBT
fecal occult blood test
- OR
odds ratio
- RUCA
Rural-Urban Commuting Area
- 2SFCA
Two-Step Floating Catchment Area
Footnotes
Potential conflict of interest: The authors declare no conflict of interest. Part of this work was presented at The American Society of Preventive Oncology 36th Annual Meeting, District of Columbia, March 3–6, 2012.
Author contributions: AEA: study planning, analysis, drafted manuscript. AYK: study planning, oversight of analysis. KAH, RMM: analysis. AEA, KAH, NJS, RMM, AYK: critical revision of manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.US Cancer Statistics Working Group. United States Cancer Statistics (USCS) 1999–2007 incidence and mortality data. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Cancer Institute; 2010. [Accessed January 11, 2012.]. http://www.cdc.gov/uscs. [Google Scholar]
- 2.US Preventive Services Task Force. Screening for colorectal cancer. Rockville, MD: Agency for Healthcare Research and Quality; 2008. [Accessed January 11, 2012]. http://www.uspreventiveservicestaskforce.org/uspstf/uspscolo.htm. [Google Scholar]
- 3.MMWR. Colorectal cancer screening, incidence, and mortality United States, 2002–2010. Mor Mortal Wkly Rep. 2011;60(26):884–889. [PubMed] [Google Scholar]
- 4.Beydoun HA, Beydoun MA. Predictors of colorectal cancer screening behaviors among average-risk older adults in the United States. Cancer Causes Control. 2008;19:339–359. doi: 10.1007/s10552-007-9100-y. [DOI] [PubMed] [Google Scholar]
- 5.Halbert HC, Barg FK, Guerra CE, et al. Cultural, economic, and psychological predictors of colonoscopy in a national sample. J Gen Intern Med. 2011;6(11):1311–1316. doi: 10.1007/s11606-011-1783-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guagliardo MF. Spatial accessibility of primary care: concepts, methods and challenges. Int J Health Geogr. 2004;3(1):3. doi: 10.1186/1476-072X-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Probst JC, Laditka SB, Wang JY, Johnson AO. Effects of residence and race on burden of travel for care: cross sectional analysis of the 2001 US National Household Travel Survey. BMC Health Serv Res. 2007;9(7):40. doi: 10.1186/1472-6963-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bennet KJ, Probst JC, Bellinger JD. Receipt of cancer screening services: surprising results for some rural minorities. J Rural Health. 2012;28:63–72. doi: 10.1111/j.1748-0361.2011.00365.x. [DOI] [PubMed] [Google Scholar]
- 9.Coughlin SS, Thomson TD. Colorectal cancer screening practices among men and women in rural and nonrural areas of the United States. J Rural Health. 2004;20(2):118–184. doi: 10.1111/j.1748-0361.2004.tb00017.x. [DOI] [PubMed] [Google Scholar]
- 10.Engelman KK, Hawley DB, Gazaway R, et al. Impact of geographic barriers on the utilization of mammograms by older rural women. JAGS. 2002;50:62–68. doi: 10.1046/j.1532-5415.2002.50009.x. [DOI] [PubMed] [Google Scholar]
- 11.Maheswaran R, Pearson T, Jordan H, et al. Socioeconomic deprivation, travel distance, location of service, and uptake of breast cancer screening in North, Derbyshire, UK. J Epidemiol Community Health. 2006;60:208–212. doi: 10.1136/jech.200X.038398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rahman S, Price JH, Dignan M, et al. Access to mammography facilities and detection of breast cancer by screening mammography: A GIS approach. Int J Canc Prev. 2009;2(6):403–413. [PMC free article] [PubMed] [Google Scholar]
- 13.Jackson MC, Davis WW, Waldron W, et al. Impact of geography on mammography use in California. Cancer Causes Control. 2009;20:1339–1353. doi: 10.1007/s10552-009-9355-6. [DOI] [PubMed] [Google Scholar]
- 14.American Cancer Society. American Cancer Society recommendations for colorectal cancer early detection. Bethesda, MD: American Cancer Society, Inc; 2011. [Google Scholar]
- 15.Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin. 2008;58:130–60. doi: 10.3322/CA.2007.0018. [DOI] [PubMed] [Google Scholar]
- 16.Christman LK, Abdulla R, Jacobsen PB, et al. Colorectal cancer screening among a sample of community health center attendees. J Health Care Poor Underserved. 2004;15(2):281–293. doi: 10.1353/hpu.2004.0021. [DOI] [PubMed] [Google Scholar]
- 17.Tessaro I, Mangone C, Parkar I, et al. Knowledge, barriers, and predictors of colorectal cancer screening in an Appalachian church population. Prev Chronic Dis. 2006;3(4):A123. [PMC free article] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention. About the BRFSS. Atlanta, GA: Office of Surveillance, Epidemiology, and Laboratory Services; 2008. [Accessed February 15, 2012.]. http://www.cdc.gov/brfss/about.htm. [Google Scholar]
- 19.Utah Department of Health. Utah Behavioral Risk Factor Surveillance System small area report 2001–2005. Salt Lake City, UT: Office of Public Health Assessment Center for Health Data Utah Department of Health; 2007. [Accessed February 15, 2012.]. http://health.utah.gov/opha/publications/brfss/SA2001-2005/SmallArea%20Report-FINAL.pdf. [Google Scholar]
- 20.University of Southern California GIS Research Laboratory. Shortest Path. https://webgis.usc.edu/Sevices/ShortestPath/Default.aspx.
- 21.American Medical Association. [Accessed December 1, 2012.];Doctor Finder. https://extapps.ama-assn.org/doctorfinder/recaptcha.jsp.
- 22.National Plan and Provider Enumeration System (NPPES) NPI Registry. Retrieved from: https://nppes.cms.hhs.gov/NPPES/NPIRegistryHome.do.
- 23.Wang F. Measurement, optimization, and impact of health care accessibility: a methodological review. Annals of the Association of American Geographers. doi: 10.1080/00045608.2012.657146.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rural Health Research Center University of Washington. Rural-Urban Commuting Areas (RUCAs) Seattle, WA: University of Washington; 2007. [Accessed December 1, 2012.]. http://depts.washington.edu/uwruca/index.php. [Google Scholar]
- 25.MacDowell M, Glasser M, Fitts M, et al. A national view of rural health workforce issues in the USA. Rural Remote Health. 2010;10(3):15–31. [PMC free article] [PubMed] [Google Scholar]
- 26.Greiner KA, Engelman KK, Hall MA, et al. Barriers to colorectal cancer screening in rural primary care. Prev Med. 2004;38:269–275. doi: 10.1016/j.ypmed.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 27.Rich EC, Burke W, Heaton CJ, et al. Reconsidering the family history in primary care. J Gen Intern Med. 2004;19:273–280. doi: 10.1111/j.1525-1497.2004.30401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patient Protection and Affordable Care Act, §3022, Pub L No. 111–148, 124 Stat 119 (2010).
- 29.Redwood D, Provost E, Perdue D, et al. The last frontier: innovative efforts to reduce colorectal cancer disparities among the remote Alaska Native population. Gastrointestinal Endoscopy. 2012;75(3):474–80. doi: 10.1016/j.gie.2011.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kempf AM, Remington PL. New challenges for telephone survey research in the Twenty-First Century. Annu Rev of Public Health. 2007;28(1):113–126. doi: 10.1146/annurev.publhealth.28.021406.144059. [DOI] [PubMed] [Google Scholar]
- 31.Montano DE, Phillips WR. Cancer screening by primary care physicians: a comparision of rates obtained from physician self-report, patient survey, and chart audit. Am J Public Health. 1995;85:795–800. doi: 10.2105/ajph.85.6.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taylor DDP, Cannon-Albright LA, Sweeney C, et al. Comparison of compliance for colorectal cancer screening and surveillance by colonoscopy based on risk. Genet Med. 2011;13(8):737–743. doi: 10.1097/GIM.0b013e3182180c71. [DOI] [PubMed] [Google Scholar]
- 33.Stroup AM, Dibble R, Harrell CJ. Cancer incidence and mortality trends in Utah: 1973–2004. Utah’s Health: An Annual Review. 2008;13:25–32. [Google Scholar]