Abstract
The mouse eye has physiological and genetic advantages to study conventional outflow function. However, its small size and shallow anterior chamber presents technical challenges to efficient intracameral delivery of genetic material to conventional outflow cells. The goal of this study was to optimize methods to overcome this technical hurdle, without damaging ocular structures or compromising outflow function. Gene targeting was monitored by immunofluorescence microscopy after transduction of adenovirus encoding green fluorescent protein driven by a CMV promoter. Guided by a micromanipulator and stereomicroscope, virus was delivered intracamerally to anesthetized mice by bolus injection using 33 gauge needle attached to Hamilton syringe or infusion with glass micropipette connected to syringe pump. The total number of particles introduced remained constant, while volume of injected virus solution (3–10 µl) was varied for each method and time of infusion (3–40 min) tested. Outflow facility and intraocular pressure were monitored invasively using established techniques. Unlike bolus injections or slow infusions, introduction of virus intracamerally during rapid infusions (3 min) at any volume tested preferentially targeted trabecular meshwork and Schlemm's canal cells, with minimal transduction of neighboring cells. While infusions resulted in transient intraocular pressure spikes (commensurate with volume infused, Δ40–70 mmHg), eyes typically recovered within 60 minutes. Transduced eyes displayed normal outflow facility and tissue morphology 3–6 days after infusions. Taken together, fast infusion of virus solution in small volumes intracamerally is a novel and effective method to selectively deliver agents to conventional outflow cells in living mice.
Keywords: Aqueous Humor, Glaucoma, Trabecular Meshwork, Schlemm’s Canal
Introduction
Ocular hypertension is the primary risk factor for developing a group of diseases called “glaucoma”, the number one cause of irreversible blindness in the world (Boland & Quigley, 2007). Importantly, sustained lowering of intraocular pressure (IOP) in those with glaucoma (whether ocular hypertensive or not) is neuroprotective, slowing down or stopping vision loss (AGIS-Investigators, 2000). Unfortunately, current IOP-lowering medical treatments are not entirely effective in everyone and do not target the diseased tissue, the conventional outflow tract, responsible for ocular hypertension (Grant, 1951; Grant, 1963).
The majority (up to 90% in the elderly) of aqueous humor exiting the eye passes through the conventional outflow tract (Bill & Phillips, 1971; Toris et al, 1999). This pathway is pressure-sensitive, keeping IOP in most people within a couple of millimeters of mercury throughout life (David et al, 1987; Klein et al, 1992). Its unique architecture facilitates the interception of pigment and cell debris from the aqueous humor before reaching the juxtacanalicular tissue (JCT) where resistance is generated and regulated (Bahler et al, 2004; Ethier & Chan, 2001; Grant, 1963; Johnson et al, 1992; Mäepa & Bill, 1992). Increased resistance to outflow is characteristic of people with glaucoma (Grant, 1951). Not surprisingly, the control of resistance in the JCT is complicated and mechano-responsive. For example, elevation in IOP distends the two interacting cell types, trabecular meshwork and Schlemm's canal cells in the JCT, triggering the release and/or activation of numerous signaling molecules (Bradley et al, 2003; Bradley et al, 2001; Li et al, 2011; Luna et al, 2009; Ramos & Stamer, 2008; Ramos et al, 2009; Tumminia et al, 1998).
Among readily available mammalian models, only non-human primates and rodents have a conventional outflow pathway analogous in design to human. The conventional tract of mice is a similar, simpler version of human (Aihara et al, 2003). Anatomically, the mouse conventional tract has a prominent Schlemm's canal and JCT, but fewer uveal meshwork beams. Functionally, (i) the conventional outflow tract is responsible for removing the majority of aqueous humor from the eye, (ii) resistance to outflow of mouse eyes increases linearly with increasing pressure steps, and (iii) mouse eyes do not appear to "wash out" (decreased resistance with time of perfusion) (Lei et al, 2011; Millar et al, 2011; Stamer et al, 2011).
Mice have the advantage of genetic malleability, which is important for mechanistic study of conventional outflow function and IOP regulation. Recently, methods have been developed to examine outflow function in enucleated eyes of mice, including knock out and transgenic, enabling the examination of outflow effects of single somatic gene changes without confounding variables like aqueous humor inflow and episcleral venous pressure (Lei et al, 2011; Millar et al, 2011). For tissue-specific delivery of genetic material to mouse outflow tissues, intraocular injection of viruses has shown promise; although intravitreal injections are preferred to intracameral due to technical issues (Balaggan et al, 2006; Budenz et al, 1995; Millar et al, 2008; Shepard et al, 2007; Shepard et al, 2010; Spencer et al, 2000).
Despite their genetic and physiological advantages mouse eyes present several problems: They are small, and their anterior chamber is shallow, with a total volume of only about 3 µl. These physical limitations present technical difficulties with intracameral injections such as accurately guiding needles between the iris, cornea and lens as well as concerns with the amount of fluid injected. While aqueous flow directs injected agents towards the outflow tissues, the close proximity of tissues in the anterior chamber of mice impacts cells that are transduced with virus. For example, the profile of cells transduced following bolus intercameral injection is different between mouse and rat (Shepard et al, 2010; Spencer et al, 2000). Importantly, intracameral injections into mouse eyes do not specifically target conventional outflow cells like they do in the rat. Instead, the cornea and lens are equally transduced. The purpose of the present study was to evaluate and optimize intracameral injection/infusion methods to selectively deliver genetic material to the conventional outflow tissue of mice.
Material and Methods
Animals
The animals were handled in accordance with animal care and use guidelines of the Animal Care and Use Program of Duke University and in compliance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. CD4 white and C57BL/6 black background mice were purchased from the Jackson Laboratory (Bar Harbor, Maine, USA). Mice were bred and housed in clear cages. Housing rooms were kept at 21°C with a 12 h: 12 h light-dark cycle. Mice were used at ages between 8 and 30 weeks old and anesthetized by intraperitoneal injections of ketamine (100 mg/kg) and xylazine (10 mg/kg). Supplemental doses of anesthetic were administered as needed. One 10 µl drop of topical proparacaine (Proparacaine HCl 0.5%, Akorn, Inc, Buffalo Grove, Illinois, USA) was applied to the cornea prior to all ocular measurements. Anesthetized mice were kept warm throughout the measurements with a mouse heating pad set at 32°C.
Adenovirus
Adenovirus expressing GFP driven by CMV promoter was made in our laboratory using ViraPower Adenoviral Expression System (Invitrogen, Grand Island, NY, USA). The virus was purified and titered using Adenovirus Mini purification ViraKit (VIRAPUR, LLC. San Diego, CA, USA) and Adeno-X Rapid Titer kit (Clonetech Laboratories, Inc. Mountain View, CA, USA).
Intracameral Injection of ad.cmv.gfp
Bolus injection of virus solution into anterior chambers of anesthetized mice was conducted using 33 gauge needle connected to a Hamilton syringe. Four different volumes of virus (3, 5, 7 and 10 µl, total virus particles: 5×105 diluted in PBS) was slowly injected into mouse anterior chamber over ~30 seconds. The needle was then withdrawn and topical erythromycin antibiotic ointment was placed to the injected eyes.
Infusion System
The infusion system was similar to that described previously (Aihara et al, 2003) with slight modifications. Glass microneedles were used to perform all perfusion and infusion experiments. Briefly, glass microneedles were made with a micropipette puller (Narishige Scientific Instrument Lab, Tokyo, Japan) and beveller (Sutter Instrument Co, Novato, California, USA). Each microneedle was placed in a micropipette manipulator (World Precision Instruments), which was attached to a tubing (0.65×19 mm; TERUMO Medical Products Hangzhou Co.LTD., Hangzhou, China) that was connected to a one-ml syringe with a pump (Model 780202, KD Scientific, Holliston, Massachusetts, USA).
Intracameral Infusion of ad.cmv.gfp
One drop of topical proparacaine was placed on the cornea of anesthetized mice. A glass microneedle filled with virus solution and connected to a pump was inserted into the anterior chamber. Four different volumes of virus solution (3, 5, 7 or 10 µl) containing the same number of virus particles (5×105) diluted in Phosphate-Buffered Saline (PBS) was infused into mouse anterior chamber at four different flow rates (1, 1.7, 2.34 or 3.33 µl/min, respectively). Another group of mice were infused over 20 or 40 min at a rate of 0.5 or 0.25 µl/min, respectively. After infusion, the needle was withdrawn and topical erythromycin antibiotic ointment was given to the infused eyes.
IOP Measurements during Intracameral Infusions
Intraocular pressure was measured invasively with the aid of a dissecting microscope. One drop of topical proparacaine was placed onto the cornea of anesthetized mice. The tear film was set as the zero pressure reference point for the system. To record IOP, one microneedle (connected to a pressure transducer and mounted on a micromanipulator) was inserted into the anterior chamber of one eye, entering in parallel with iris just above the limbus. Another microneedle filled with PBS connected to a syringe pump was then inserted into the anterior chamber of the same eye, entering from the opposite side. Four different experiments were conducted: In first group of mice, different volumes of PBS (3, 5, 7 and 10 µl) were infused into anterior chamber at various flow rates (6, 10, 14 and 20 µl/min) over 30 seconds. After perfusion, needle with PBS was withdrawn first and then the needle for monitoring IOP was taken out. In second group of mice, different volumes of PBS (3, 5, 7 and 10 µl) were infused over 3 minutes, giving infusion rates of 1, 1.7, 2.34 and 3.33 µl/min. In third group, the effects of prolonged delivery of virus were evaluated by infusing a total volume of 10 µl of PBS at a rate of 0.5 µl/min for 20 minutes, or 0.25 µl/min for 40 minutes. IOP was monitored continuously during the during the entire time perfusion.
GFP Expression
Three to five days after virus infusion or injection, mice were euthanized by CO2 inhalation and eyes were enucleated and fixed in 0.5% glutaraldehyde and 2% paraformaldehyde in 0.1M cacodylate buffer. The anterior segments were removed from lens and posterior segments and kept in fixative for 3 additional hours at room temperature (RT) and then flat mounted on slides. GFP expression was visualized and captured with a fluorescence microscope (Carl Zeiss microscopy, LLC, San Diego, CA, USA). Wedges of specimens were then embedded in OCT or LR White and processed for cryosection (10 µm), semithin (0.5 µm) or thin sections (65 nm). The cryosections were visualized with a fluorescence microscope (Carl Zeiss microscopy, LLC, San Diego, CA, USA). GFP expression in semi-thin sections were immunostained with anti-GFP IgGs (1:250, Abcam, Cambridge, Massachusetts, USA) and Alex Fluor® 488 goat anti-rabbit (H + L) (1:500, Invitrogen). Nuclei were stained with 4’,6-diamidino-2-phenylindole (DAPI, 0.5 µg/ml) for 10 min. The images were captured with Nikon C90i confocal automated microscope and visualized with EZ-C1.3.10 Nikon confocal software (D-ECLIPSE C1, Nikon). The thin sections were immunostained with specific anti-GFP primary antibody (1:250) and 6 µm immunogold secondary antibody. The images were visualized and captured with an electron microscope (JEM-1400; JEOL USA, Peabody, MA).
Measurement of outflow facility in living mouse eyes
The system for outflow facility measurements was set up as reported previously (Camras et al, 2010) with slight modifications. Briefly, for bilateral measurements two micropipette manipulators were used to place a microneedle in each eye of mouse. Each microneedle was connected to a pressure tubing (0.05 inch inner diameter (ID) tube, Mallinckrodt, Hazelwood, Missouri, USA) leading to a three-way stopcock. The stopcock was connected to a 5-mL syringe filled with PBS and to pressure tubing connected to a second three-way stopcock. A pressure transducer (Honeywell model 142PC05D, Honeywell Sensing and Control, MN, USA) and a vertical column of fluid (RenaPulse™ Tubing 0.08 mm ID, Braintree Scientific Inc. Braintree, MA, USA) were connected to the second stopcock. The transducer was connected to a PowerLab data acquisition system (ML870/P PowerLab 8/30, ADInstruments, Colorado Springs, Colorado, USA) linked to a computer running PowerLab software. One glass column (0.05 mm ID, Sulter Instrument Co., Novato, CA) was connected to the top of the vertical pressure tubing to ensure that the liquid was vertical and the inner diameter was uniform for outflow calculations. The height of the fluid in the column was adjusted using a fluid-filled syringe. Transducers were calibrated prior to each experiment to ensure accurate pressure measurements. The fluid height of the column set the pressure in the system. The fluid exiting the system was proportional to the decline in height of the column or slight decline in pressure over time, and thereby was used to determine the flow rate based on the dimensions of the fluid column. Outflow facility data were excluded if the mouse died or leaking occurred during outflow facility measurements.
To assess effects of intracameral infusion on outflow function, mice were infused with different volume of PBS (3, 5, 7 or 10 µl at rates of 1, 1.7, 2.34 or 3.33 µl/min, respectively) for 3 min or cannulated without infusion as control. Three to six days after infusion, outflow facility measurements were conducted in both mouse eyes at the same time. When the tip of a needle entered the tear film, the pressure was set to zero. The microneedle was then placed in the anterior chamber and IOP was recorded as initial intraocular pressure. IOPs were then set at pressures of 15, 25, or 35 mmHg by raising the fluid column to corresponding height. The pressure decline in each eye was monitored at 30-min intervals. At the end of the experiment, the needle again was placed in the tear-film to confirm a zero pressure. The slope of the pressure drop (mmHg/min) during each time interval was measured at three IOPs set by the fluid column. The data were recorded during the last 5 to 10 min of each interval.
Histological analysis of conventional outflow pathway
Under anesthesia, four different volumes of PBS (3, 5, 7 or 10 µl) were infused into mouse anterior chambers at four varies flow rates (1, 1.7, 2.34 or 3.33 µl/min) over 3 minutes using a glass microneedle connected to a syringe pump. After infusion, the needle was withdrawn and topical antibiotic was given to the infused eyes. Three days later mouse eyes were fixed in 2% paraformaldehyde + 2% glutaradehyde in PBS for 3h. Eyes were bisected at the equator and anterior segments were cut into four quadrants. Semi-thin sections of eye segments (0.5 µm) were stained with toluidine blue and visualized by light microscopy.
Statistical analysis
The data are presented as the mean ±SD. Statistical significance between groups was assessed by both the Mann-Whitney U Test and student t Tests with two-tails (assuming unequal variance and data is normally distributed). A value of p<0.05 was considered statistically significant.
Results
In preliminary experiments, our goal was to find the minimum titer needed to achieve high expression in the TM to avoid risk of inflammation or toxicity associated with higher virus titers. We tested between 1 × 105 and 1 × 107 infective viral particles. We observed that titers greater than 1 × 106 particles resulted in consistent transduction of corneal endothelia no matter the technique of delivery to intracameral space (not shown). With the infusion technique, we started to see GFP in some areas of outflow pathway with 1 × 105 viral particles, but much less than our optimal titer of 5 × 105 particles, which we used in the rest of the study. This dosage was much less than the 1 × 107-2 × 108 particles used in previous studies (Balaggan et al, 2006; Budenz et al, 1995; Millar et al, 2008; Shepard et al, 2007).
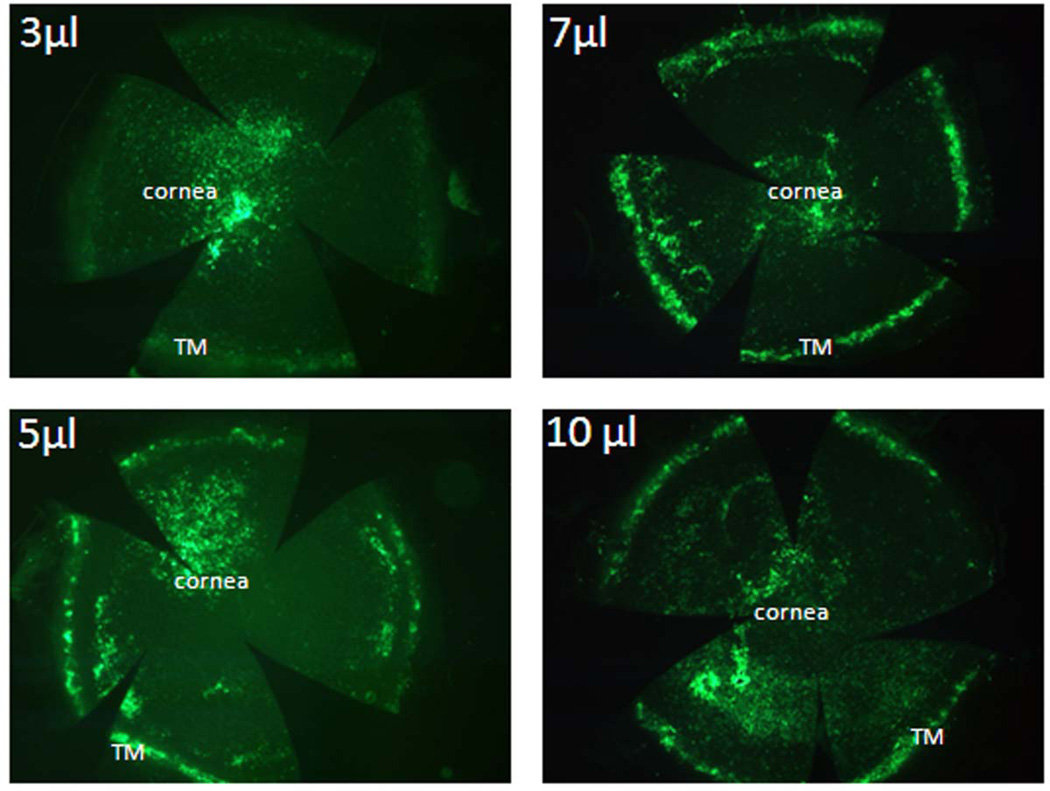
Once we found our optimal titer, we began analyzing the distribution and efficiency of gene delivery to mouse anterior segment tissues following single intracameral injection of different volumes of ad.cmv.gfp solution (3, 5, 7 or 10 µl), each containing 5 × 105 infective particles. Virus was injected intracamerally using a 33 gauge needle over 30 seconds, similar to reported by others (Balaggan et al, 2006; Budenz et al, 1995; Millar et al, 2008; Shepard et al, 2007). Figure 1A shows GFP reporter signal from flat mount of mouse anterior chamber, interior side up, that was injected with 3 µl of virus solution. Results display preferential targeting of virus and expression of GFP by corneal endothelial cells. In contrast, injection of the three greater volumes resulted in roughly equal transduction of corneal endothelia and conventional outflow cells (Figure 1B, C and D).
Figure 1. GFP expression in mouse anterior segments following intracameral injection of adenovirus encoding GFP.
Ad.cmv.gfp (5×105 particles in 3, 5, 7 or 10 µl volume diluted with PBS) was injected over 30 seconds into mouse anterior chamber using 33 gauge needle connected to a Hamilton syringe. Three to five days after injection, mouse eyes were collected and immersion fixed. Eyes were bisected, anterior segments were flat mounted and GFP expression was visualized using a fluorescence microscope. The images were captured with 2.5× amplification. The images are representative results of four individual experiments for each condition using both CD1 and C57BL/6 mice (8–30 weeks old).
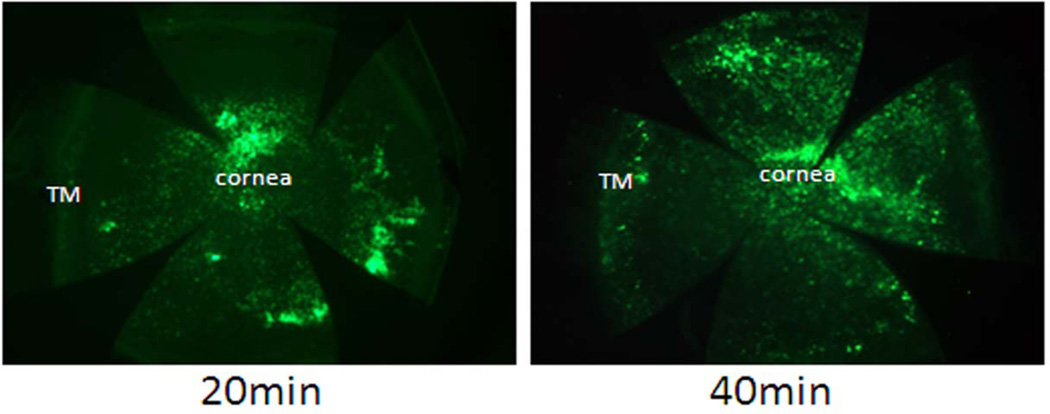
In an attempt to more specifically target conventional outflow cells in mice, we slowly infused virus solution (5 × 105 infective particles) over extended periods of time (20 or 40 minutes). While we found that this strategy was effective at targeting conventional outflow cells in rats (ARVO 2010 abstract #3106), it was extremely inefficient in mice. Figure 2 shows GFP fluorescence from flat mounts of mouse anterior chambers after prolonged infusion. Results illustrate that cornea endothelia were primarily targeted following slow infusion at either time point. When looked at more closely, we detected very few trabecular meshwork, Schlemm's canal and distal venous endothelia that expressed GFP at 20 minutes and no conventional outflow cells at 40 minutes (not shown).
Figure 2. GFP expression pattern after 20 and 40 min intracameral infusion of adenovirus encoding GFP.
Ad.cmv.gfp (5×105 particles) in 10 µl PBS were infused into mouse anterior chamber over 20 minutes (0.5 µl/min) or 40 minutes (0.25 µl/min). Three days after infusion, mouse eyes were collected and fixed. Anterior segments were flat mounted and the GFP expression was visualized and captured (2.5× amplification) using a fluorescence microscope. The images are representative results of four to five individual experiments for each condition using both CD1 and C57BL/6 mice (8–15 weeks old).
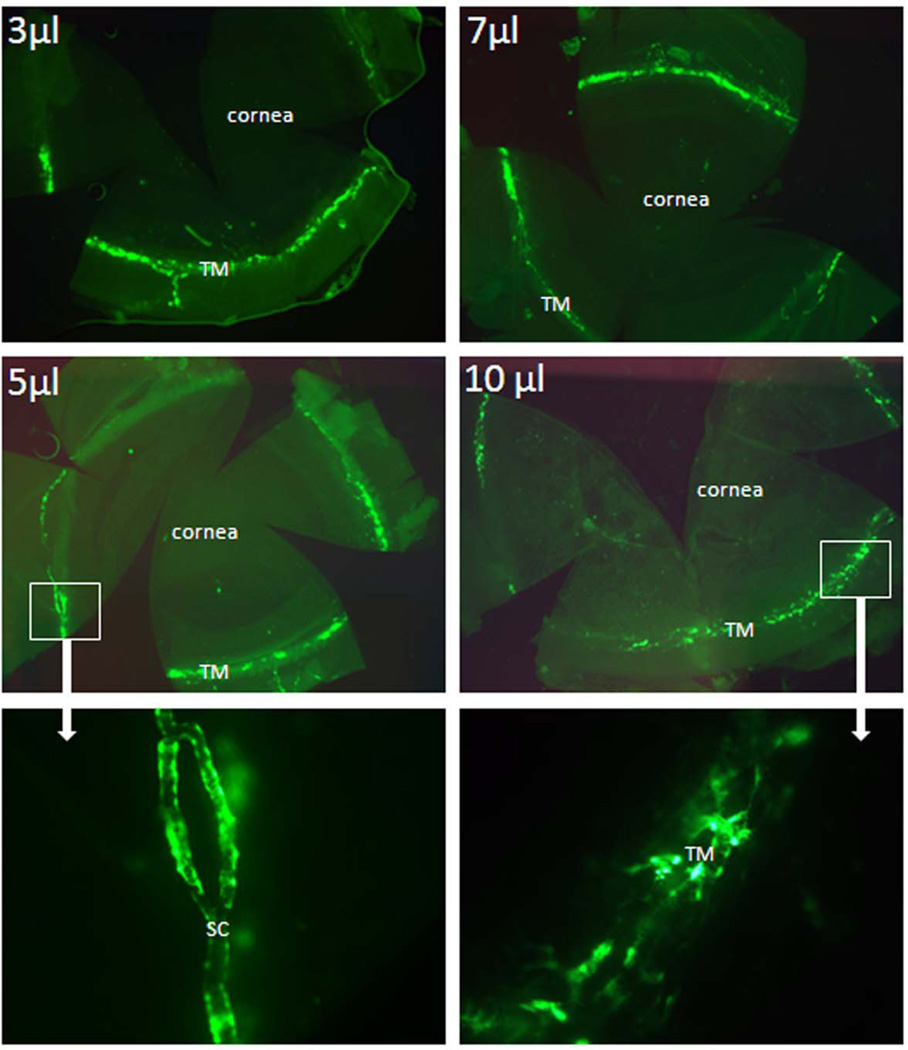
In the next set of experiments, we tried an alternative approach, infusing virus intracamerally over a very short period of time (3 min). We infused different volumes of virus solution (3, 5, 7 and 10 µl) containing the identical number of viral particles as before (5 × 105) into the anterior chamber over 3 minutes; giving corresponding flow rates of 1, 1.7, 2.34 or 3.33 µl/min. Figure 3 displays flat mounts of mouse anterior chambers from this set of experiments, at each of four conditions. Results show that fast infusion preferentially targeted outflow cells regardless of the infusion volume. Compared to infusions with 7 or 10 µl of virus solution, higher levels of GFP expression appear in conventional outflow tract of eyes infused with 3 or 5 µl of virus solution, with little to no collateral expression by cornea endothelia. In some experiments we did observe more efficient expression in conventional outflow tract using higher volumes (not shown). Regardless, a consistent finding was a segmental pattern of fluorescence, suggesting preferential flow pathways through the conventional outflow tract in mouse eyes.
Figure 3. GFP expression in mouse anterior segments following rapid intracameral infusion of adenovirus encoding GFP.
Ad.cmv.gfp (5×105 particles in 3, 5, 7, or 10 µl volume) was infused into mouse anterior chambers over 3 minutes (flow rate 1, 1.7, 2.34 or 3.33 µl/min). Three to five days after transduction, mouse eyes were fixed and dissected. The anterior segments were flat mounted and the images were captured with a fluorescence microscope. Note: top four panels (2.5 × amplification); bottom two panels (20 × amplification). The images are representative results of three to five individual experiments for each condition using both CD1 and C57BL/6 mice (8–30 weeks old).
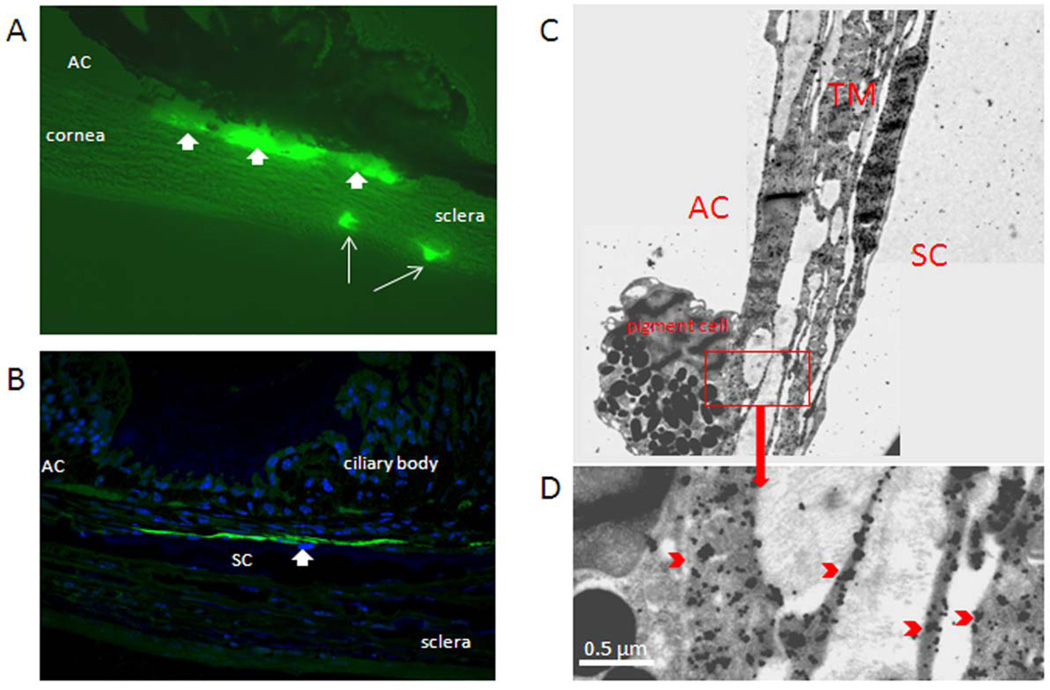
Anterior segment tissues from mouse eyes infused over 3 min were examined for GFP expression in sagittal section by three methods. Using fluorescence microscopy of frozen sections, we observed that only cells in the conventional outflow tract expressed GFP (figure 4). Specifically, data show that trabecular meshwork, Schlemm's canal and collector channel endothelia all express GFP (figure 4A), but not neighboring tissues. Semi-thin sections of mouse anterior chambers that were labeled with anti-GFP IgGs confirmed localization of signal to conventional pathway; in particular Schlemm's canal inner wall. We also performed immunogold labeling of GFP in mouse eyes rapidly infused with virus, and observed gold particles decorating trabecular meshwork and Schlemm's canal cells (figure 4C–D).
Figure 4. GFP expression in mouse aqueous humor outflow pathway following fast intracameral infusion of adenovirus encoding GFP.
Ad.cmv.gfp (5×105 particles in 10 µl volume) was infused into mouse anterior chamber over 3 minutes (flow rate 3.33 µl/min). Three to five days after transduction, mouse eyes were fixed and dissected. The anterior segments were then processed by cryosectioning (10 µm), semi-thin sectioning (0.5 µm) or thin sectioning (65 nm). A) Immunofluorescence image of sagittal plane section through mouse angle structures; B) Sagittal plane semi-thin section (60 × amplification) through angle structures of mouse eye. The section was immunostained with anti-GFP primary antibody and Alexa Fluor®488 goat anti-rabbit IgG (H+L) secondary antibody. Nuclei were stained with DAPI. The image was captured with a confocal microscope; C) Sagittal plane thin section through trabecular meshwork and Schlemm's canal. The section was immunostained with specific anti-GFP primary antibodies and 6 µm immunogold anti-rabbit secondary antibodies. Images were captured with a JEM-1400 electron microscope. Data are representative of 3 experiments using C57BL/6 mice (8–22 weeks old).
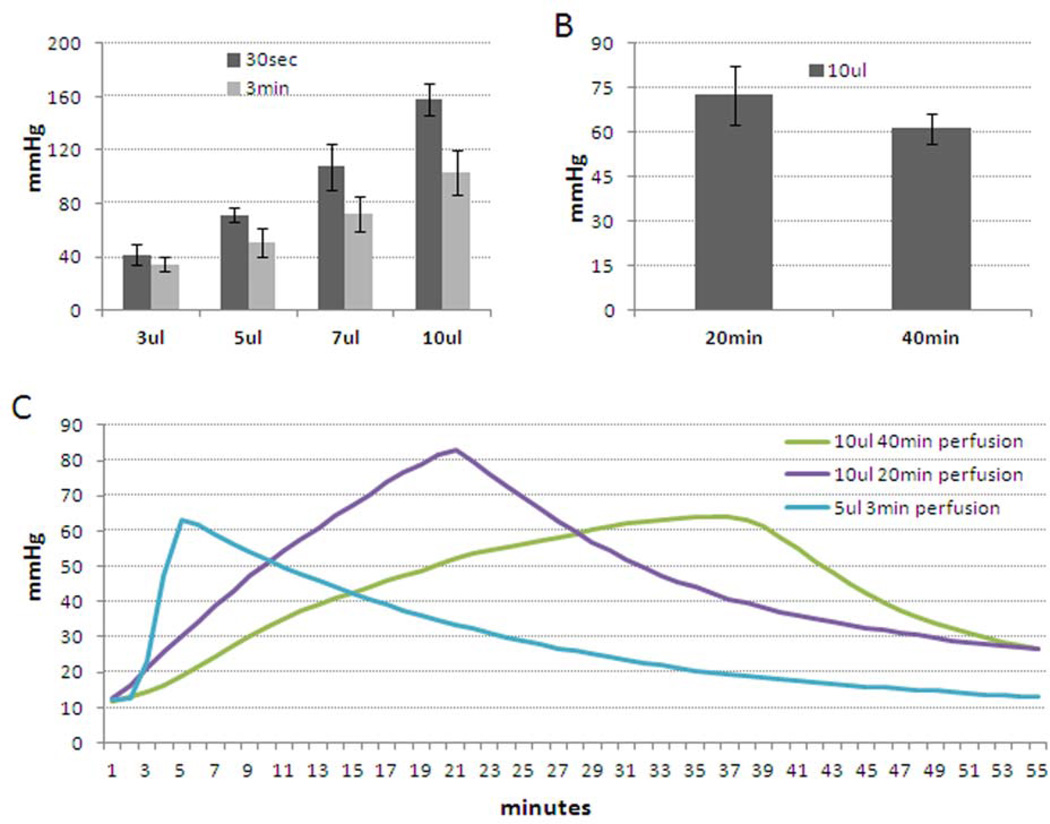
During infusion experiments, we monitored effects on intraocular pressure (IOP). Figure 5A shows a summary of results with infusions of PBS at two shorter time points, 30 seconds and 3 minutes. Not surprisingly, results show increasing IOP with increasing volume of solution. The highest IOPs were noted with 30 second injections, ranging from 40 mmHg with 3 µl volume to 160 mmHg with 10 µl volume. By comparison, 3 minute infusions caused IOP elevations of 40 mmHg for 3 µl volume to 100 mmHg for 10 µl volume. Following this trend, figure 5B shows that longer times to infuse 10 µl resulted in lesser effect on IOP. Thus, IOP spikes of 60 mmHg were noted for 40 minute infusions and 75 mmHg for 20 minute infusions. Representative IOP profiles for 3, 20 and 40 minute infusion times are shown in panel C.
Figure 5. Changes of intraocular pressure of mouse eyes during infusion.
Two needles were inserted into anterior chambers of living mouse eyes. One needle filled with PBS was connected to a one-mL syringe pump, and different volume of PBS at various flow rates were infused into anterior chambers. At the same time, a secondary needle which was connected to a pressure transducer recorded changes of IOP over time. A) Peak IOP recorded following infusion of different volumes of PBS over 30 seconds or 3 minutes (n=6–9). B) Peak IOP following infusion of 10 µl PBS over 20 or 40 minutes (n=3). C) Representative IOP profiles recorded during infusions over 3, 20 or 40 minutes using C57BL/6 mice (12–30 weeks old).
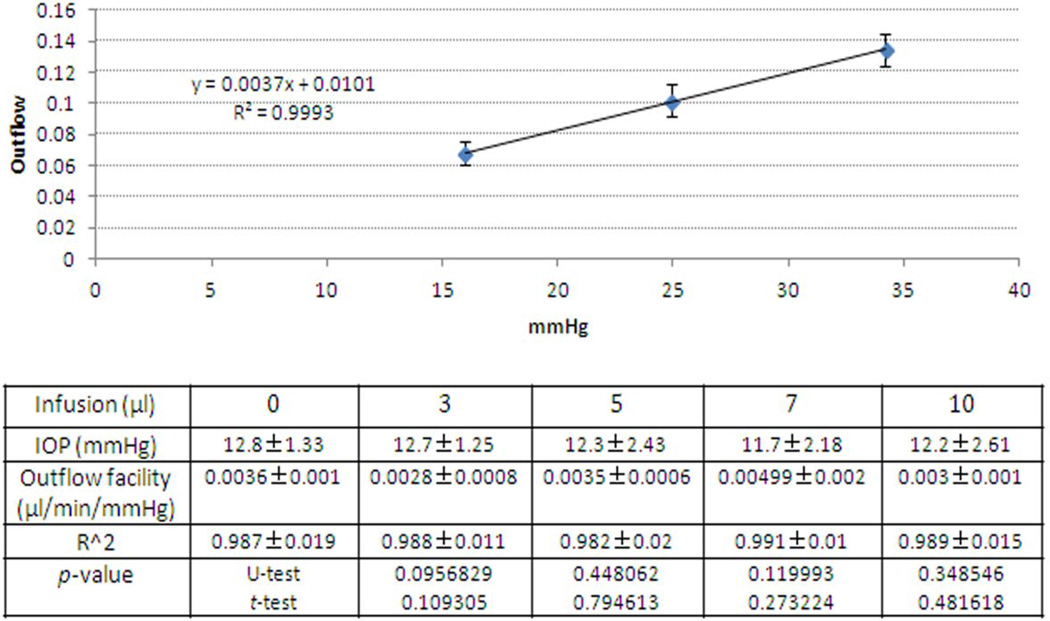
Due to IOP spikes during infusions, we were concerned about damage to conventional outflow structures and compromised function. We examined outflow function of 3 minute infused eyes, 3–6 days following infusion of PBS and compared findings to outflow measurements from eyes that had not been infused. Flow was measured at three different pressures (15, 25 and 35 mmHg) and outflow facility was calculated from the slope of best-fit regression lines to the data for each infusion volume. The upper panel in figure 6 shows a graph from control experiments (n=11 eyes) and the lower panel shows combined data in tabular form from all experiments. Notice that there does not appear to be any effect of infusion volume on outflow facility. Statistically, there was no difference between control eyes (0.0036 ± 0.001 µl/min/mmHg) and those infused with virus (0.0028–0.0050 µl/min/mmHg, p>0.05).
Figure 6. Outflow function of mouse eyes after fast intracameral infusions.
Mouse eyes were infused with different volume of PBS (0, 3, 5, 7 or 10 µl) over three minutes. Three to six days after infusions, outflow function was assessed in both mouse eyes simultaneously under anesthesia. Eyes were held at three different pressures (15, 25 and 35 mmHg) and flow measured. Top panel shows flow at each pressure in control eyes (n=11). The table below shows a summary of outflow facility (mean ± SD) for each volume of PBS infused that was derived from the best-fit slope of the flow/pressure relationship (n= 3–11) to obtained r2 values, and statistical comparisons (t-test and U-test). C57BL/6 mice (8–24 weeks) were used in this set of experiments.
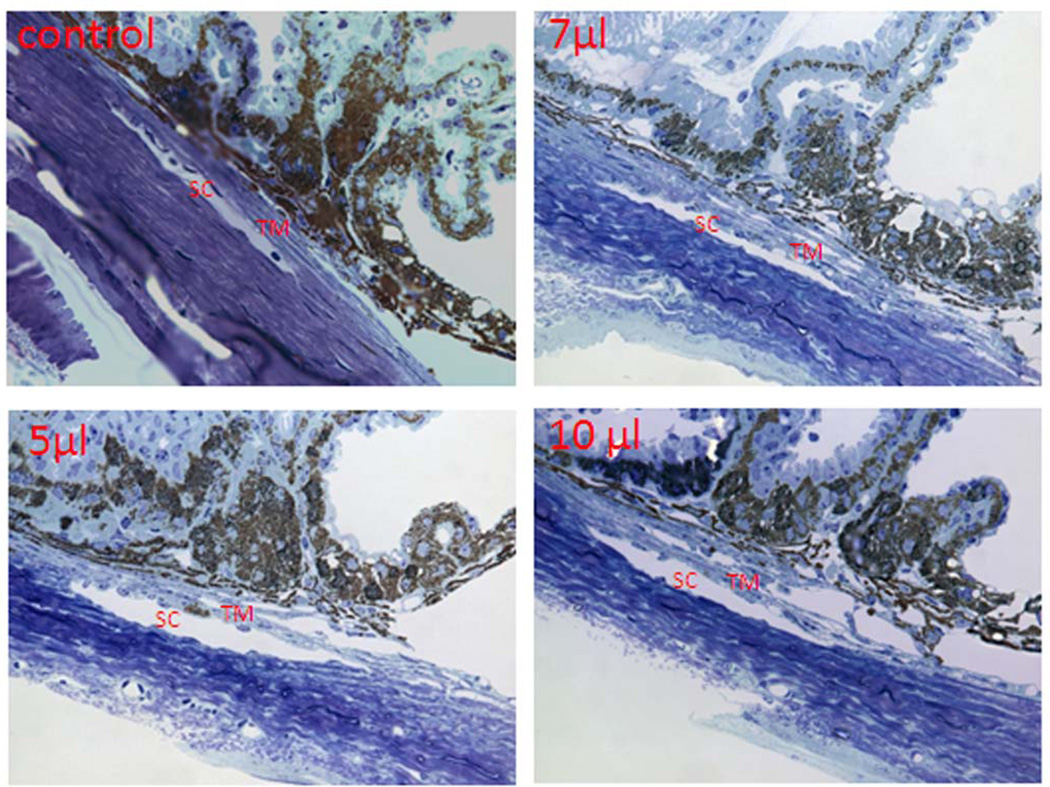
Mouse eyes that had been infused for 3 minutes with 3–10 µl volumes were examined histologically for potential structural damage due to IOP spikes during infusion. Results in figure 7 show toluidine blue staining of semi-thin sections taken from anterior segments of mouse eyes in sagittal section that had been infused with 5, 7 or 10 µl of PBS. Compared to control eyes, outflow structures from infused eyes look completely normal. The inner wall of Schlemm's canal was intact, trabecular tissue appears well populated with healthy cells and surrounding tissues appear undisturbed. Taken together, severe IOP spikes during infusions did not appear to alter conventional outflow structure or function.
Fig 7. Morphological analysis of outflow pathway after fast intracameral infusions.
Mouse eyes were infused with (0, 3, 5, 7 and 10 µl) of PBS over 3 minutes. Three days after infusions, mouse eyes were collected and fixed with 2% paraformaldehyde + 2% glutaraldehyde. Mouse anterior segments were dissected, processed and sectioned in sagittal plane (semi-thin, 0.5 µm) through angle structures. The images were visualized and captured with a light microscope. Images are representative results of three individual experiments for each condition using C57BL/6 mice (10–22 weeks old).
Discussion
The primary finding of the present study was that viruses can be introduced to mouse eyes intracamerally, in a manner that selectively transduces conventional outflow cells with minimal transduction of collateral tissues. Specifically, we discovered that infusion of 5–7 µl of virus solution over 3 minutes optimally transduced trabecular meshwork, Schlemm's canal and collector channel cells, with little to no transduction of corneal endothelia, lens epithelia or iris epithelia. These fast infusions resulted in transient IOP spikes that did not appear to adversely alter conventional outflow function or morphology.
Similar to what we observed following rapid infusions in mouse eyes, the human eye experiences dramatic, transient IOP fluctuations during daily activities. During routine activities such as saccades movements, blinking, accommodation and squinting, IOP in a living eye can fluctuate 10–80 mmHg (Coleman & Trokel, 1969; Downs et al, 2011). Thus, it appears that compliance in sclera and conventional outflow tissues normally accommodates dramatic IOP spikes. The magnitude of the spikes above baseline IOP we observed after intracameral infusions under optimal conditions (3–5 µl over 3 min.) in mouse eyes was <50 mmHg (figure 5) and without detectable damage to outflow function or structure (figure 6).
Prior to the present study, intravitreal injection was the preferred route for viral deliver of genes to conventional outflow cells in mice (Millar et al, 2008; Shepard et al, 2007; Shepard et al, 2010). While intravitreal delivery was successful in transducing trabecular meshwork and Schlemm's canal cells, many cell types in both the posterior and anterior mouse eye were also transduced including RPE, ciliary epithelia, lens epithelia and corneal endothelia (Budenz et al, 1995; Millar et al, 2008; Shepard et al, 2007; Spencer et al, 2000). To avoid this complication, intracameral injections were attempted, but abandoned due to preferential targeting of virus to corneal endothelia and little to conventional outflow cells; a finding that we reproduced in the present study (figure 1). The ability to avoid transduction of corneal endothelial cells during infusions of virus may be useful during aging experiments of mice where repeated IOP measurements may be necessary. Thus, adverse effects of virus on corneal endothelial health over time, and possible swelling, can be avoided.
Surprisingly, we observed significant differences in transduction efficiency between rats and mice using a slow, 20–40 minute virus infusion protocol. Infusions into both species were performed by the same three people (LC, PC and GL), suggesting differences were not technical in nature. We observed in a previous study that slow intracameral infusion into rat eyes resulted in preferential expression of transgene in conventional outflow tissues (ARVO 2010 abstract #3106). In contrast, results from the present study showed preferential expression of transgene by corneal endothelia (figure 2). The differences between species may be due to differences in anatomical dimensions of the anterior chamber and/or aqueous humor dynamics. Since outflow facility in rat is ~10 times higher than mouse, the clearance of virus through the conventional pathway is higher. Therefore, prolonged infusions in mice would result in higher number of viral particles that remain in anterior chamber over time, resulting in greater uptake by cells. In contrast, rapid infusions in mice caused rapid increase in IOP and possible compliance changes in TM (and corresponding change in flow) that may facilitate more efficient delivery of virus to the TM.
The mouse eye appears to be a good model to study conventional outflow function (Lei et al, 2011; Millar et al, 2011). For example, the conventional outflow tissues appear to have similar pharmacology to human; outflow facility increases with EP4 and FP receptor activation and decreases with S1P receptor activation (Boussommier-Calleja et al, 2012; Millar et al, 2011). The mouse eye is also similar to human, but unlike other non-primate models, in that it has a Schlemm's canal and does not appear to "wash out" (decrease outflow resistance with time of perfusion)(Lei et al, 2011). Thus, due to its anatomical and physiological similarities to human plus its genetic malleability, the mouse eye represents a useful model to better understand mechanistically conventional outflow function. In particular, results of the present study enable tissue-specific alterations in gene expression (due to new targeted delivery technique), that may otherwise be developmentally lethal to mice. For example, transduction of conventional outflow tissues of transgenic mice bearing loxP-flanked gene sequences with a viral vector expressing Cre recombinase provides a means of allowing flexible spatio-temporal control of target gene expression in conventional outflow cells, where tissue-specific promoters are not currently available.
Conclusion
Regulation of conventional outflow is complex, involving multiple levels of signaling, all working together to maintain IOP within a narrow range over a lifetime. In order to better understand these intricate processes, and what goes awry in ocular hypertension associated with POAG, models are needed that maintain the sophisticated architecture of outflow tissues and relationships between resident cells, but allows for targeted mechanistic manipulations. Results of the present study show optimized techniques to specifically deliver genetic material to conventional outflow cells of mouse eyes in vivo; enabling methodical testing of signaling components that contribute to conventional outflow and represent novel targets for future glaucoma therapies.
Highlights.
Fast intracameral infusion of virus selectively targets conventional outflow cells in mice
Fast infusions cause transient IOP spikes that may facilitate delivery of virus
IOP spikes do not cause morphological or functional damage to outflow tissues
Acknowledgements
We thank Ying Hao for help with electron microscopy and Dave Nyce for useful consultation with infusion system design. The work was supported in part by the following sources of funding: EY016228 (PG), R01EY022359 (WDS), P30EY005722 and Research to Prevent Blindness Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- AGIS-Investigators. The Advanced Glaucoma Intervention Study (AGIS): The relationship between control of intraocular pressure and visual field deterioration. Am J Ophthalmol. 2000;130:429–440. doi: 10.1016/s0002-9394(00)00538-9. [DOI] [PubMed] [Google Scholar]
- Aihara M, Lindsey JD, Weinreb RN. Aqueous humor dynamics in mice. Invest Ophthalmol Vis Sci. 2003;44:5168–5173. doi: 10.1167/iovs.03-0504. [DOI] [PubMed] [Google Scholar]
- Bahler C, Hann C, Fautsch M, Johnson D. Pharmacological disruption of Schlemm's canal cells and outflow facility in anterior segments of human eyes. Invest Ophthalmol Vis Sci. 2004;45:2246–2254. doi: 10.1167/iovs.03-0746. [DOI] [PubMed] [Google Scholar]
- Balaggan KS, Binley K, Esapa M, Iqball S, Askham Z, Kan O, Tschernutter M, Bainbridge JW, Naylor S, Ali RR. Stable and efficient intraocular gene transfer using pseudotyped EIAV lentiviral vectors. J Gene Med. 2006;8:275–285. doi: 10.1002/jgm.845. [DOI] [PubMed] [Google Scholar]
- Bill A, Phillips CI. Uveoscleral drainage of aqueous humour in human eyes. Experimental Eye Research. 1971;12:275–281. doi: 10.1016/0014-4835(71)90149-7. [DOI] [PubMed] [Google Scholar]
- Boland MV, Quigley HA. Risk factors and open-angle glaucoma: classification and application. J Glaucoma. 2007;16:406–418. doi: 10.1097/IJG.0b013e31806540a1. [DOI] [PubMed] [Google Scholar]
- Boussommier-Calleja A, Bertrand J, Woodward DF, Ethier CR, Stamer WD, Overby DR. Pharmacologic manipulation of conventional outflow facility in ex vivo mouse eyes. Invest Ophthalmol Vis Sci. 2012;53:5838–5845. doi: 10.1167/iovs.12-9923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley JM, Kelley MJ, Rose A, Acott TS. Signaling pathways used in trabecular matrix metalloproteinase response to mechanical stretch. Invest Ophthalmol Vis Sci. 2003;44:5174–5181. doi: 10.1167/iovs.03-0213. [DOI] [PubMed] [Google Scholar]
- Bradley JM, Kelley MJ, Zhu X, Anderssohn AM, Alexander JP, Acott TS. Effects of mechanical stretching on trabecular matrix metalloproteinases. Invest Ophthalmol Vis Sci. 2001;42:1505–1513. [PubMed] [Google Scholar]
- Budenz DL, Bennett J, Alonso L, Maguire A. In vivo gene transfer into murine corneal endothelial and trabecular meshwork cells. Invest Ophthalmol Vis Sci. 1995;36:2211–2215. [PubMed] [Google Scholar]
- Camras LJ, Sufficool KE, Camras CB, Fan S, Liu H, Toris CB. Duration of anesthesia affects intraocular pressure, but not outflow facility in mice. Curr Eye Res. 2010;35:819–827. doi: 10.3109/02713683.2010.494241. [DOI] [PubMed] [Google Scholar]
- Coleman DJ, Trokel S. Direct-recorded intraocular pressure variations in a human subject. Archives of Ophthalmology. 1969;82:637–640. doi: 10.1001/archopht.1969.00990020633011. [DOI] [PubMed] [Google Scholar]
- David R, Zangwill L, Stone D, Yassur Y. Epidemiology of intraocular pressure in a population screened for glaucoma. Br J Ophthalmol. 1987;71:766–771. doi: 10.1136/bjo.71.10.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs JC, Burgoyne CF, Seigfreid WP, Reynaud JF, Strouthidis NG, Sallee V. 24-hour IOP telemetry in the nonhuman primate: implant system performance and initial characterization of IOP at multiple timescales. Invest Ophthalmol Vis Sci. 2011;52:7365–7375. doi: 10.1167/iovs.11-7955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ethier C, Chan D. Cationic ferritin changes outflow facility whereas anionic ferritin does not. Invest Ophthalmol Vis Sci. 2001;42:1795–1802. [PubMed] [Google Scholar]
- Grant WM. Clinical tonography. Trans Am Acad Ophthalmol Otolaryngol. 1951;55:774–781. [PubMed] [Google Scholar]
- Grant WM. Experimental aqueous perfusion in enucleated human eyes. Arch Ophthalmol. 1963;69:783–801. doi: 10.1001/archopht.1963.00960040789022. [DOI] [PubMed] [Google Scholar]
- Johnson M, Shapiro A, Ethier CR, Kamm RD. Modulation of outflow resistance by the pores of the inner wall endothelium. Invest Ophthalmol Vis Sci. 1992;33:1670–1675. [PubMed] [Google Scholar]
- Klein BE, Klein R, Linton KL. Intraocular pressure in an American community. The Beaver Dam Eye Study. Invest Ophthalmol Vis Sci. 1992;33:2224–2228. [PubMed] [Google Scholar]
- Lei Y, Overby DR, Boussommier-Calleja A, Stamer WD, Ethier CR. Outflow physiology of the mouse eye: pressure dependence and washout. Invest Ophthalmol Vis Sci. 2011;52:1865–1871. doi: 10.1167/iovs.10-6019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, Leung CT, Peterson-Yantorno K, Stamer WD, Mitchell CH, Civan MM. Mechanisms of ATP release by human trabecular meshwork cells, the enabling step in purinergic regulation of aqueous humor outflow. J Cell Physiol. 2011 doi: 10.1002/jcp.22715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna C, Li G, Qiu J, Challa P, Epstein DL, Gonzalez P. Extracellular release of ATP mediated by cyclic mechanical stress leads to mobilization of AA in trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2009;50:5805–5810. doi: 10.1167/iovs.09-3796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäepa O, Bill A. Pressures in the juxtacanalicular tissue and Schlemm's canal in monkeys. Exp Eye Res. 1992;54:879–883. doi: 10.1016/0014-4835(92)90151-h. [DOI] [PubMed] [Google Scholar]
- Millar C, Clark AF, Pang IH. Assessment of aqueous humor dynamics in the mouse by a novel method of constant flow infusion. Invest Ophthalmol Vis Sci. 2011 doi: 10.1167/iovs.10-6069. [DOI] [PubMed] [Google Scholar]
- Millar JC, Pang IH, Wang WH, Wang Y, Clark AF. Effect of immunomodulation with anti-CD40L antibody on adenoviral-mediated transgene expression in mouse anterior segment. Mol Vis. 2008;14:10–19. [PMC free article] [PubMed] [Google Scholar]
- Ramos RF, Stamer WD. Effects of cyclic intraocular pressure on conventional outflow facility. Invest Ophthalmol Vis Sci. 2008;49:275–281. doi: 10.1167/iovs.07-0863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos RF, Sumida GM, Stamer WD. Cyclic mechanical stress and trabecular meshwork cell contractility. Invest Ophthalmol Vis Sci. 2009;50:3826–3832. doi: 10.1167/iovs.08-2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard AR, Jacobson N, Millar JC, Pang IH, Steely HT, Searby CC, Sheffield VC, Stone EM, Clark AF. Glaucoma-causing myocilin mutants require the Peroxisomal targeting signal-1 receptor (PTS1R) to elevate intraocular pressure. Hum Mol Genet. 2007;16:609–617. doi: 10.1093/hmg/ddm001. [DOI] [PubMed] [Google Scholar]
- Shepard AR, Millar JC, Pang IH, Jacobson N, Wang WH, Clark AF. Adenoviral gene transfer of active human transforming growth factor-{beta}2 elevates intraocular pressure and reduces outflow facility in rodent eyes. Invest Ophthalmol Vis Sci. 2010;51:2067–2076. doi: 10.1167/iovs.09-4567. [DOI] [PubMed] [Google Scholar]
- Spencer B, Agarwala S, Miskulin M, Smith M, Brandt CR. Herpes simplex virus-mediated gene delivery to the rodent visual system. Invest Ophthalmol Vis Sci. 2000;41:1392–1401. [PubMed] [Google Scholar]
- Stamer WD, Lei Y, Boussommier-Calleja A, Overby DR, Ethier CR. eNOS, a pressure-dependent regulator of intraocular pressure. Invest Ophthalmol Vis Sci. 2011;52:9438–9444. doi: 10.1167/iovs.11-7839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toris CB, Yablonski ME, Wang YL, Camras CB. Aqueous humor dynamics in the aging human eye. American Journal of Ophthalmology. 1999;127:407–412. doi: 10.1016/s0002-9394(98)00436-x. [DOI] [PubMed] [Google Scholar]
- Tumminia S, Mitton K, Arora J, Zelenka P, Epstein D, Russell P. Mechanical Stretch Alters The Actin Cytoskeletal Network And Signal Transduction In Human Trabecular Meshwork Cells. Invest Ophthalmol Vis Sci. 1998;39:1361–1371. [PubMed] [Google Scholar]