Abstract
Objective
To characterize neuropsychological and academic status in children, age 8-18 years, with new/recent-onset idiopathic generalized epilepsy (IGE) and idiopathic localization-related epilepsy (ILRE) compared with healthy controls.
Study design
Participants underwent neuropsychological assessment and parents were interviewed regarding their child’s academic history. Cognitive scores for children with epilepsy were age- and sex-adjusted and compared with controls across both broad-band (IGE n = 41 and ILRE n = 53) and narrow-band (childhood/juvenile absence, juvenile myoclonic, benign epilepsy with centro-temproral spikes, and focal [temporal/frontal/NOS]) syndromes. Academic histories were examined including problems antecedent to epilepsy onset and diagnosis.
Results
Children with new-onset epilepsies exhibit considerable cognitive abnormality at baseline including patterns of shared abnormalities across syndromes (e.g., psychomotor slowing) as well as unique syndrome-specific cognitive effects (eg, executive function in IGE and language/verbal memory in ILRE) that are observed and sometimes exacerbated in specific IGE and ILRE syndromes. Academic difficulties are evident in approximately 50% of the children with epilepsy, affecting all syndrome groups to an equal degree.
Discussion
Patterns of shared and syndrome-specific cognitive abnormalities and academic problems are present early in the course of virtually all epilepsy syndromes examined here, including syndromes classically viewed as benign. This is the base upon which the effects of recurrent seizures, treatment and psychosocial effects will be added over time.
Keywords: Cognition, academic achievement, new onset-epilepsy, localization-related epilepsy, idiopathic generalized epilepsy, absence epilepsy, juvenile myoclonic epilepsy, BECTS
Community- and population-based investigations as well as reports from tertiary care centers demonstrate repeatedly that childhood epilepsy can be associated with abnormalities in cognition, academics, emotional status, and social function—complications referred to collectively as the neurobehavioral comorbidities of epilepsy [1-4]. A longstanding view of the etiology of these neurobehavioral complications is that they are due to the adverse effects of seizure frequency/severity, medications, social stigma, or stresses linked to recurrent seizures [5]. However, a recent literature has demonstrated that abnormalities in cognition, social and psychiatric status can be apparent at the time of epilepsy diagnosis and may even antedate the first recognized seizure. This effect has been reported in regard to general behavioral adjustment [6], cognition [7], academic achievement and school performance [8], attention deficit hyperactivity disorder (ADHD) [9], and depression [10].
These findings would not be unanticipated in children with complicated and symptomatic epilepsies, but they have been reported in children with so-called “epilepsy-only” [7], that is, children with average intelligence, normal neuroimaging and neurological exams, attending regular schools, with epilepsy subsyndromes perceived by many clinicians not to be worrisome and even benign (e.g., benign epilepsy with centro-temporal spikes [BECTS], childhood/juvenile absence epilepsy [Absence], Here we comprehensively examine baseline neuropsychological status in a large cohort of children with “epilepsy-only” compared with healthy controls. We have previously reported on cognitive abnormalities in a subset of this sample of children with epilepsy [11]; in the larger sample presented here, we examine the cognitive profiles of both broad syndrome groupings (idiopathic generalized epilepsy [IGE], idiopathic localization-related epilepsy [ILRE]) as well as narrow-band diagnostic subsyndromes (e.g., BECTS, Absence, JME, Focal). These comparisons demonstrate patterns of shared versus unique cognitive consequences of specific epilepsy syndromes close in time to diagnosis. Through structured interviews with parents, we examine rates of academic problems (AP) both at baseline and prior to the first recognized seizure and diagnosis, as well as the neuropsychological implications of this academic history.
Methods
Study participants were aged 8-18 including 72 healthy controls and 94 with new/recent-onset epilepsy including 53 with ILRE and 41 with IGE (Table I). All participants were followed for active epilepsy. Inclusion criteria were a diagnosis of epilepsy within the past 12 months, no other developmental disabilities or neurological disorders, normal neurological examinations and normal clinical imaging results. A board-certified pediatric neurologist (blinded to neuropsychological and interview data) confirmed that all patients had idiopathic epilepsies and provided independent confirmation of syndrome diagnosis. Four patients with IGE did not meet criteria for a specific syndrome diagnosis (eg, JME) and were excluded from analyses utilizing narrow-band syndrome diagnoses. Children with focal epilepsies (temporal lobe, frontal lobe, and focal NOS) were combined in one “focal” group as ictal confirmation of the localization/lateralization of seizure onset was not available for this sample. Healthy controls were age- and sex-matched first-degree cousins of epilepsy participants. Healthy controls presented with no history of seizures, early initial precipitating injuries (e.g., febrile convulsions), other developmental or neurologic disease, or loss of consciousness greater than 5 minutes.
Table 1.
Participant characteristics of controls and epilepsy participants by subsyndrome (means and standard deviations).
| Variable | Healthy controls (n = 72) |
BECTS (n=23) |
Focal (n=31) |
JME (n=26) |
Absence (n=11) |
|---|---|---|---|---|---|
| Age (years) | 12.86 (3.20) |
10.25 (1.40) |
11.82 (2.94) |
14.62 (3.06) |
12.24 (3.46) |
|
| |||||
| Sex (#/% female) | 37 (51%) | 10 (44%) | 14 (45%) | 14 (54%) | 4 (36%) |
|
| |||||
| FSIQ | 107.35 (12.00) |
103.00 (14.53) |
98.52 (10.90) |
101.62 (13.89) |
98.18 (11.16) |
|
| |||||
| Academic Problems(+/-) | 13/58 | 15/8 | 16/15 | 12/14 | 5/6 |
|
| |||||
|
Age of seizure onset (years) |
-- | 9.00 (2.41) |
10.51 (2.81) |
13.21 (4.09) |
11.20 (3.52) |
|
Seizure frequency (<1year, >1year) |
-- | 4/19 | 4/27 | 7/19 | 3/8 |
|
Epilepsy duration (months) |
-- | 7.22 (4.04) |
8.26 (3.56) |
8.46 (3.49) |
9.73 (3.17) |
|
Antiepileptic drugs (0/1/2+) |
-- | 9/14/0 | 6/24/1 | 0/25/1 | 0/9/2 |
BECTS: Benign epilepsy with centro-temporal spikes
JME: Juvenile myoclonic epilepsy
Research approval was obtained from the Health Sciences Institutional Review Board at the University of Wisconsin Medical School. Written informed consent was obtained from legal guardians of participating children and adolescents, written informed consent was obtained from participants age 18, and written informed assent was obtained from participants age 8-17. Children underwent comprehensive neuropsychological testing and MRI. Parents underwent a clinical interview and completed questionnaires to characterize gestation, delivery, neurodevelopment, and seizure history. All pertinent medical records were obtained after signed release of information was obtained from the parent. Parents were questioned through a structured interview about their child’s school progress and, in particular, any specific educational services provided to address academic problems (AP). These services included the traditional individualized educational plan (IEP) process, but also included early childhood interventions including speech therapy, physical therapy, occupational therapy, mandatory summer school, grade retention, special tutoring services (e.g., reading, math), and other specific educational services (inventory available from the authors). Also of interest was whether services were provided antecedent to the diagnosis of epilepsy as well as the first recognized seizure. This interview was conducted blinded to cognitive and behavioral results.
Neuropsychological assessment and analysis
All participants were administered a comprehensive test battery that included measures of intelligence, academic achievement, language, immediate and delayed verbal memory, executive function, and speeded fine motor dexterity (Table II). Regression techniques converted raw test scores to age- and gender-adjusted z-scores based on the control group.
Table 2.
Neuropsychological tests by domain.
| Domain | Ability | Test |
|---|---|---|
| Academic Achievement | Letter/word recognition | WRAT-3 (reading)[35] |
| Letter and word writing | WRAT-3 (spelling) | |
| Basic arithmetic | WRAT-3 (arithmetic) | |
| Intelligence | Verbal | WASI (verbal IQ)[36] |
| Nonverbal | WASI (performance IQ) | |
| Language | Confrontation naming | Boston Naming Test[37] |
| Expressive naming | Expressive Vocabulary Test[38] | |
| Receptive language | Peabody Picture Vocabulary Test-III[39] | |
| Generative naming | D-KEFS (letter fluency)[40] | |
| Memory | Verbal memory | CMS (word lists learning)[41] |
| CMS (word lists delayed) | ||
|
Executive
Function |
Problem solving | D-KEFS (confirmed correct sorts) |
| Response inhibition | D-KEFS (color-word interference test-Inhibition) | |
| Divided attention | D-KEFS (category switching accuracy) | |
| Inattentiveness | CCPT-II (omission and commission errors)[42] | |
| Motor Function | Speeded fine motor dexterity | Grooved Pegboard[43] |
| Psychomotor speed | WISC-III (digit symbol-coding)[44] |
WRAT-3: Wide Range Achievement Test - 3
WASI: Wechsler Abbreviated Scale of Intelligence
D-KEFS: Delis-Kaplan Executive Function System
CMS: Children's Memory Scale
CCPT-II: Conners' Continuous Performance Test -II
WISC-III: Wechsler Intelligence Scale for Children - III
Regression-based z-scores were submitted to a one-way MANOVA with group (IGE, ILRE, healthy controls) as the independent factor. A second MANOVA compared controls with specific epilepsy subsyndromes (BECTS, Focal, JME, Absence, healthy controls). For all analyses, post-hoc comparisons were made only following a significant omnibus F-value (< 0.05). Fisher’s LSD tests were used for multi-group post-hoc comparisons. To decrease the number of statistical comparisons (thus reducing the risk of family-wise error), post-hoc pairwise comparisons focused on the contrast of healthy controls to the epilepsy subsyndrome groups (BECTS, Focal, JME, Absence) of interest (i.e., all possible combinations of subsyndrome comparisons were not evaluated).
Results
IGE and ILRE
The overall MANOVA for Group (ILRE, IGE, healthy controls) across all cognitive tests was significant (F[38,256] = 2.49, p < 0.001). The univariate results were significant for 13 of 18 measures, with p-values ranging from < 0.001 (5/13 tests) to 0.05 (performance IQ). In all cases, significant differences reflected poorer performance in both epilepsy groups compared with healthy controls.
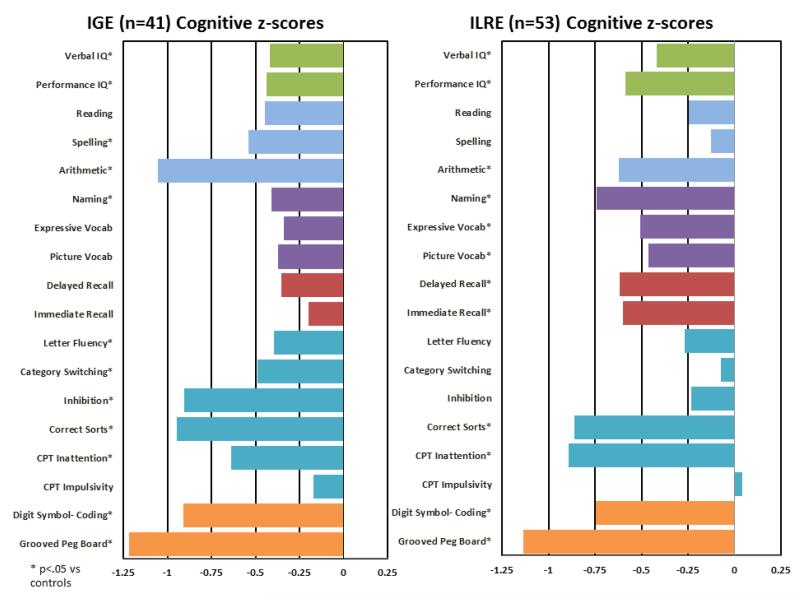
Post-hoc contrasts (Figure 1) revealed that participants with IGE performed significantly worse than healthy controls across measures of performance and verbal IQ, confrontation naming, executive functions (letter fluency, correct sorts, category switching, inhibition, CCPT inattention), speeded motor dexterity, and academic achievement (spelling and arithmetic). Figure 1 illustrates that motor and executive functions, as well as arithmetic, showed the greatest discrepancies from healthy controls. ILRE participants were significantly worse than healthy controls on measures of performance and verbal IQ, immediate and delayed memory, language (confrontation naming, picture and expressive vocabulary), executive functions (problem solving/correct sorts, inattention), speeded motor dexterity, and arithmetic. Motor and executive functions were the most affected domains (Figure 1). The IGE and ILRE groups were more similar than dissimilar. Participants with IGE scored significantly lower than ILRE participants only on tests of arithmetic (p = 0.037) and executive function including category switching (p = 0.02) and inhibition (p = 0.002).
Figure 1.
Cognitive performance by major epilepsy syndrome (IGE, ILRE). All scores have been age- and gender-corrected relative to controls, and are plotted here with z-scores relative to controls. Asterisks denote p-values of < .05. Test domain are identified by color:

LRE and IGE subsyndromes
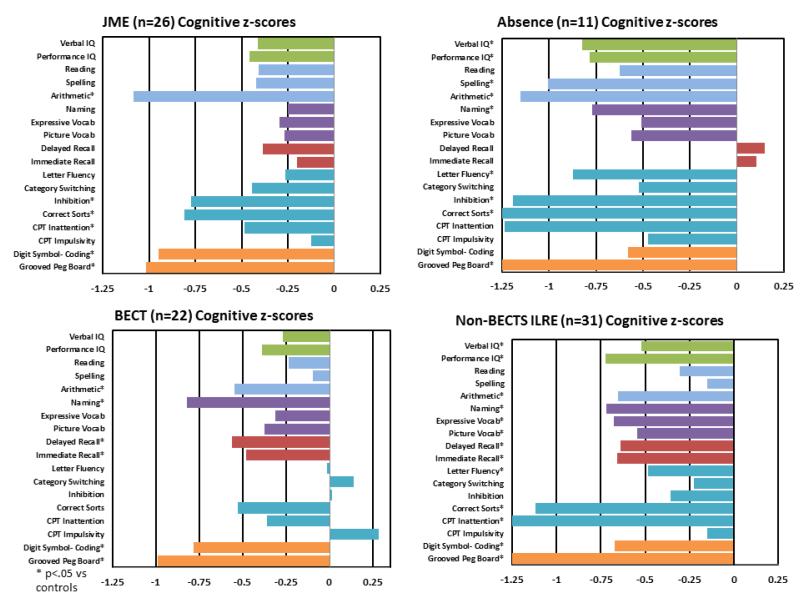
Table I characterizes the subsyndrome groups. Figure 2 shows age- and gender-corrected z-scores for each subsyndrome group compared with healthy controls. The overall MANOVA (BECTS, Focal, JME, Absence) across all cognitive tests was significant (F[72,486.025] = 1.652, p = 0.001). Univariate results were significant for 11 of 18 measures, ranging from a low of p < 0.001 (arithmetic, confrontation naming, inattention) to a high of p = 0.032 (immediate memory).
Figure 2.
Cognitive performance by epilepsy subsyndrome (BECTS, Absence, JME, Focal). All scores have been age- and gender-corrected relative to controls, and are plotted here with z-scores relative to controls. Asterisks denote p-values of < .05

Figure 2 shows each group’s performance relative to healthy controls. Participants with focal epilepsies showed significant deficits on 13 of 18 tests, with the largest deviations from healthy controls occurring on tasks of executive function (correct sorts, inattention) and psychomotor speed (grooved pegboard). Participants with Absence epilepsy differed significantly from healthy controls on 10 of 18 tests, particularly in the domains of motor (grooved pegboard) and executive functions (correct sorts, inhibition). JME and BECTS participants each performed poorly relative to healthy controls on 6 of 18 tests. While both groups showed performance deficits in motor function, JME participants showed widespread disruptions in executive function (number of correct sorts, inhibition errors, inattention), while BECTS participants performed significantly worse than healthy controls in confrontation naming, as well as immediate and delayed memory.
Academic Problems
45% of children with new/recent onset epilepsy exhibited a significantly higher rate of AP versus 18% for healthy controls (p < 0.001), a finding true for both children with ILRE (51%, p < 0.001 vs. healthy controls) and IGE (38%, p = 0.004 vs. healthy controls, with no ILRE vs. IGE difference (p = 0.252). AP antedated the diagnosis of epilepsy and first recognized seizures in 32% of the children with epilepsy, again true for both ILRE (31%) and IGE (32%). Table I shows that AP were elevated in all epilepsy syndromes including children with BECTS (p = 0.001), absence (p = 0.019), JME (p = 0.055), and focal (p = 0.003) epilepsies, with no significant difference across the syndrome groups (p = 0.663). Another indication of the clinical significance of AP is its relationship to cognitive status. Table III (available at www.jpeds.com) presents mean neuropsychological performance for epilepsy participants with versus without history of AP. The overall MANOVA (epilepsy + AP, epilepsy − AP, healthy controls) was significant (F[36,198] = 3.155, p < 0.001). Participants with ADHD were excluded from this analysis in order to provide a more stringent test of the effects of AP on cognitive performance. Among the children with epilepsy, scores on every cognitive measure but one (CCPT impulsivity errors) were significantly worse for AP children, all p’s < 0.05. Conversely, children with epilepsy without AP differed significantly from healthy controls only on two measures (Digit Symbol/Coding, p = 0.015) and CCPT inattention errors (p = 0.017).
Table 3.
Cognitive performance (means and standard deviations) in participants with epilepsy, with AP (AP+) and without AP (AP-), and healthy controls.
| Epilepsy | Control | ||
|---|---|---|---|
|
| |||
|
AP+
(n=29) |
AP-
(n=46) |
(n=65) |
|
| Verbal IQ | −0.812* | −0.508 | 0.031 |
| (0.707) | (1.146) | (0.969) | |
| Performance IQ | −1.166* | 0.161 | −0.025 |
| (1.039) | (1.311) | (1.01) | |
| Reading | −0.697* | 0.158 | 0.003 |
| (0.887) | (1.052) | (0.970) | |
| Spelling | −0.871* | 0.249 | −0.024 |
| (1.286) | (1.221) | (1.008) | |
| Arithmetic | −1.222* | −0.355 | 0.010 |
| (0.840) | (1.504) | (0.954) | |
| Naming | −1.007* | −0.199 | 0.046 |
| (1.346) | (1.132) | (0.982) | |
| Expressive Vocabulary | −0.857* | 0.022 | 0.045 |
| (0.670) | (0.996) | (0.982) | |
| Picture Vocabulary | −0.815* | −0.031 | −0.019 |
| (.961) | (1.285) | (0.963) | |
| Delayed Recall | −0.773* | −0.243 | 0.034 |
| (1.073) | (1.138) | (0.996) | |
| Immediate Recall | −0.760* | −0.236 | 0.010 |
| (1.407) | (1.037) | (0.994) | |
| Letter Fluency | −1.000* | 0.058 | 0.063 |
| (1.043) | (1.329) | (0.930) | |
| Category Switching | −0.533* | 0.084 | 0.033 |
| (0.989) | (1.095) | (0.971) | |
| Inhibition | 0.739* | 0.246 | −0.007 |
| (1.120) | (1.400) | (0.991) | |
| Correct Sorts | −1.455* | −0.455 | 0.075 |
| (1.504) | (1.624) | (1.006) | |
| CPT Inattention | 0.683* | 0.858* | −0.041 |
| (1.742) | (1.624) | (0.967) | |
| CPT Impulsivity | 0.254 | −0.023 | 0.012 |
| (1.207) | (0.954) | (0.999) | |
| Digit Symbol Coding | −1.039* | −0.671* | 0.033 |
| (1.276) | (1.072) | (0.999) | |
| Grooved Peg Board | 1.585* | 0.535 | 0.040 |
| (1.749) | (1.273) | (0.975) | |
All scores are age- and sex-corrected relative to controls, and are expressed as z-scores. Participants with ADHD have been excluded.
p < 0.05 compared with controls.
AP: Academic problems
ADHD: Attention deficit hyperactivity disorder
Discussion
Children with new- and recent-onset IGE and ILRE demonstrate a wide range of cognitive abnormalities early in the course of their disorder. Despite the unique pathophysiological characteristics of focal versus generalized epilepsies, the evident cognitive complications appear broad in spectrum, with many similarities across children with IGE and ILRE. That said, our ability to examine specific epilepsy subsyndromes reveals several unique patterns of cognitive vulnerability, even among childhood epilepsies viewed as among the most benign in nature (eg, Absence, BECTS). In addition to the cognitive abnormalities evident at baseline, there is a significantly increased rate of AP in the children with epilepsy, many of which were identified prior to the onset of epilepsy, diagnosis, and treatment. These academic difficulties did not respect epilepsy syndrome boundaries, affecting all groups, with clear cognitive correlates. As these cognitive and academic difficulties are present at or shortly after onset of epilepsy, they form the substrate upon which the effects of chronic epilepsy and treatment may manifest themselves.
Reports from population and community-based studies as well as tertiary care centers consistently point to an increased risk of neuropsychological and academic abnormalities in children with established epilepsy [2, 4, 12-14]. It is not surprising that the more severe and symptomatic epilepsies would be associated with cognitive complications, but perhaps less expected are abnormal cognitive abnormalities in children with less severe epilepsies including those with “benign” and idiopathic epilepsies. The nature, timing and evolution of these cognitive abnormalities have begun to be clarified.
Here we examined broad syndrome groups of children with IGE and ILRE, and found significant differences from healthy controls on measures of intelligence, language, memory, executive function and processing speed. Given that the underlying pathophysiological characteristics of these epilepsies are quite distinct, one might expect that the neuropsychological consequences would be very diverse. In contrast, we observed considerable similarity, with both IGE and ILRE groups significantly different from controls across 12 of 18 measures. Figure 1 depicts the very consistent overall trend of poorer cognitive performance compared with controls across all tests. Similarly affected in the IGE and ILRE groups are intelligence (verbal and performance IQ), academic achievement (arithmetic), aspects of executive function (attention and problem solving), and motor/psychomotor speed. In the context of a shared set of cognitive abnormalities across syndromes, there appear to be modest patterns of syndrome specific cognitive dysfunction. That is, executive functions appear to be additionally affected in IGE, while language and verbal memory are more impacted in ILRE compared with healthy controls. The cognitive impact shared by IGE and ILRE at this early stage could be related to the adverse effects of antecedent epileptogenesis on the developing brain and disruption of the supporting neurobiology for diverse cognitive processes. That this is not due to factors such as a lower intellectual environment in the families of children with epilepsy has been demonstrated in this cohort as the IQ of the parents of the children with epilepsy versus healthy controls do not differ (Walker et al, submitted). Finally, there is minimal effect of duration of epilepsy on cognition (accounting for only 5% of the variance on 2 of 18 test measures).
Growing experience indicates that so-called “benign” epilepsies may not be benign in terms of their neurobehavioral comorbidities including cognition [15-18]. While the outcomes in terms of seizures may be favorable, the effects on cognition and behavior can be detected both during the active period of seizures as well as after epilepsy remission [19, 20]. The current results reinforce this viewpoint and indicate evident cognitive consequences at or close to the time of diagnosis. Relative to healthy controls, children with BECTS showed significantly decreased cognitive performance on measures of academic achievement (arithmetic), language (confrontation naming), verbal learning and memory, and cognitive and psychomotor slowing. While affected, this was a relatively spared pattern compared with other epilepsy syndromes. Children with frontal and temporal lobe epilepsies exhibited broader cognitive disruption affecting intelligence, multiple aspects of expressive and receptive language, verbal learning and memory, multiple executive functions and cognitive/psychomotor speed. These findings are consistent with studies of children with chronic forms of these disorders [14, 21, 22]. Children with JME exhibited a profile of especially affected executive functions (response inhibition, attention, problem solving) and cognitive/psychomotor speed. Children with absence epilepsy were more broadly affected with the addition of intelligence and academic achievement, although it should be noted that our sample of children with absence epilepsy was relatively small (n = 11).
These baseline cognitive profiles raise concern regarding the early academic performance of the children with epilepsy [4, 7, 11, 12, 23]. A structured interview with parents was performed to inquire into indicators of academic performance problems including the traditional IEP, but also other indicators of difficulty including provision of tutors, grade retention, summer school, homework clubs, and other services. Indeed, rates of educational problems were significantly increased compared with controls not only in the broad IGE and ILRE syndromes, but in all the specific subsyndrome groupings including BECTS, Absence, and Focal epilepsies. Academic problems did not respect the boundaries of syndrome groupings and notions of the degree to which various epilepsies are “benign.” That presence of academic difficulties is a meaningful marker is further supported by the neuropsychological profile of children who did versus did not have AP, with poorer performance on nearly every measure of cognitive performance and academic achievement in the former. It is also important to note that for 32% of these children, academic difficulties were identified prior to the onset and diagnosis of epilepsy, again underscoring the need for early diagnosis and treatment of academic problems associated with epilepsy [4, 7, 11, 12, 23]. It has been shown that there is familial susceptibility in these epilepsies in regard to cognitive and specific academic skills [24].
These cognitive profiles (Figures 1 and 2) and academic difficulties become the baseline on which any effects of chronic epilepsy, its treatment, and the psychological and social complications will be based. Insights about these baseline cognitive and educational effects can be gained from recent neuroimaging research. Among the focal epilepsies, widespread cortical thinning has been reported in children with frontal lobe epilepsy [25], while in children with temporal lobe epilepsy widespread abnormalities have been reported in hippocampus, lateral temporal lobes, thalamus, posterior cingulum, and cerebellum [26]. BECTS is associated with abnormalities in the volumes and shape of subcortical structures [27]. Abnormalities in children with idiopathic generalized epilepsies have included abnormal thalamus and frontal lobe volumes and connectivity [28] and abnormalities in childhood absence epilepsy include thalamic [29] frontal and temporal regions [30], diffuse cortical morphometric features [31], and diffusion and volumes of subcortical nuclei [32] as well as prospective morphometric changes [33].
First, while the source of patients is not population-based, they came from two major medical centers covering broad community/rural portions of the state. Second, the sample sizes are modest in some subsyndrome groups. Third, with interest in many dependent measures across several diagnostic groups, the problem of over-comparison arises. For that reason, children with epilepsy were compared directly with healthy controls and not to each other to limit chance findings. Nevertheless, all test scores, with rare exception, reflected poorer performance in the children with epilepsy—making the general trend clear (Figures 1 and 2). Finally, while these children were seen early in the course of epilepsy, the impact of medications remains a concern. The majority of children were on monotherapy, were not seen and tested acutely after medication initiation, and examination of specific medication effects in a study like this is typically confounded with epilepsy syndrome. A clear assessment of adverse drug effects in children with epilepsy is dependent on controlled investigations [34].
Acknowledgments
Supported by National Institutes of Health (NIH; National Institute of Neurological Disorders and Stroke ROI 44351 to J.J., D.H., and M.S.). D.H. receives research support from Citizens United for Research on Epilepsy. C.S. has received compensation as a consultant for Questcor Pharmaceuticals (2011), serves on the Scientific Board of The Charlie Foundation, serves as an Associate Editor of Epilepsia and Co-Editor-in-Chief for Basic Science of Epilepsy Currents, and has received royalties from publication of Epilepsy and the Ketogenic Diet and Epilepsy: Mechanisms, Models and Translational Perspectives. B.H. serves as an Associate Editor for Epilepsia and receives research support from the NIH (RO1 AG027161 [coinvestigator], P50AG3314 [coinvestigator], 1RO1NS064034 [coinvestigator], and RO1AG031790 [coinvestigator]).
List of Abbreviations
- ADHD
Attention deficit hyperactivity disorder
- AP
Academic problems
- BECTS
Benign epilepsy with centro-temporal spikes
- CCPT-II
Conners’ Continuous Performance Test – II
- CMS
Children’s Memory Scale
- D-KEFS
Delis-Kaplan Executive Function System
- IGE
Idiopathic generalized epilepsy
- LRE
Localization-related epilepsy
- JME
Juvenile myoclonic epilepsy
- MRI
Magnetic resonance imaging
- NOS
Not otherwise specified
- WASI
Wechsler Abbreviated Scale of Intelligence
- WISC-III
Wechsler Intelligence Scale for Children – III
- WRAT-3
Wide Range Achievement Test - 3
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The other authors declare no conflicts of interest.
References
- [1].Camfield P, Camfield C. Prognosis in Epilepsies. John Libbey, Eurotext; Montrouge, France: 2003. [Google Scholar]
- [2].Hoie B, Mykletun A, Sommerfelt K, Bjornaes H, Skeidsvoll H, Waaler PE. Seizure-related factors and non-verbal intelligence in children with epilepsy. A population-based study from Western Norway. Seizure. 2005;14:223–31. doi: 10.1016/j.seizure.2004.10.006. [DOI] [PubMed] [Google Scholar]
- [3].Rutter M, Graham P, Yule W. A neuropsychiatric study in childhood. S.I.M.P./William Heineman Medical Books; London: 1970. [Google Scholar]
- [4].Berg AT, Langfitt JT, Testa FM, Levy SR, DiMario F, Westerveld M, et al. Global cognitive function in children with epilepsy: a community-based study. Epilepsia. 2008;49:608–14. doi: 10.1111/j.1528-1167.2007.01461.x. [DOI] [PubMed] [Google Scholar]
- [5].Dodrill CB. Neuropsychological effects of seizures. Epilepsy Behav. 2004;5(Suppl 1):S21–4. doi: 10.1016/j.yebeh.2003.11.004. [DOI] [PubMed] [Google Scholar]
- [6].Austin JK, Harezlak J, Dunn DW, Huster GA, Rose DF, Ambrosius WT. Behavior problems in children before first recognized seizures. Pediatrics. 2001;107:115–22. doi: 10.1542/peds.107.1.115. [DOI] [PubMed] [Google Scholar]
- [7].Oostrom KJ, Smeets-Schouten A, Kruitwagen CL, Peters AC, Jennekens-Schinkel A. Not only a matter of epilepsy: early problems of cognition and behavior in children with “epilepsy only”--a prospective, longitudinal, controlled study starting at diagnosis. Pediatrics. 2003;112:1338–44. doi: 10.1542/peds.112.6.1338. [DOI] [PubMed] [Google Scholar]
- [8].Berg AT, Smith SN, Frobish D, Levy SR, Testa FM, Beckerman B, et al. Special education needs of children with newly diagnosed epilepsy. Developmental Medicine & Child Neurology. 2005;47:749–53. doi: 10.1017/S001216220500157X. [DOI] [PubMed] [Google Scholar]
- [9].Hesdorffer DC, Ludvigsson P, Olafsson E, Gudmundsson G, Kjartansson O, Hauser WA. ADHD as a risk factor for incident unprovoked seizures and epilepsy in children. Archives of General Psychiatry. 2004;61:731–6. doi: 10.1001/archpsyc.61.7.731. [DOI] [PubMed] [Google Scholar]
- [10].Hesdorffer DC, Hauser WA, Olafsson E, Ludvigsson P, Kjartansson O. Depression and suicide attempt as risk factors for incident unprovoked seizures. Annals of Neurology. 2006;59:35–41. doi: 10.1002/ana.20685. [DOI] [PubMed] [Google Scholar]
- [11].Hermann B, Jones J, Sheth R, Dow C, Koehn M, Seidenberg M. Children with new-onset epilepsy: neuropsychological status and brain structure. Brain. 2006;129:2609–19. doi: 10.1093/brain/awl196. [DOI] [PubMed] [Google Scholar]
- [12].Farwell JR, Dodrill CB, Batzel LW. Neuropsychological abilities of children with epilepsy. Epilepsia. 1985;26:395–400. doi: 10.1111/j.1528-1157.1985.tb05670.x. [DOI] [PubMed] [Google Scholar]
- [13].Nolan MA, Redoblado MA, Lah S, Sabaz M, Lawson JA, Cunningham AM, et al. Memory function in childhood epilepsy syndromes. J Paediatr Child Health. 2004;40:20–7. doi: 10.1111/j.1440-1754.2004.00284.x. [DOI] [PubMed] [Google Scholar]
- [14].Cormack F, Cross JH, Isaacs E, Harkness W, Wright I, Vargha-Khadem F, et al. The development of intellectual abilities in pediatric temporal lobe epilepsy. Epilepsia. 2007;48:201–4. doi: 10.1111/j.1528-1167.2006.00904.x. [DOI] [PubMed] [Google Scholar]
- [15].Fastenau PS, Johnson CS, Perkins SM, Byars AW, deGrauw TJ, Austin JK, et al. Neuropsychological status at seizure onset in children: risk factors for early cognitive deficits. Neurology. 2009;73:526–34. doi: 10.1212/WNL.0b013e3181b23551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Caplan R, Siddarth P, Stahl L, Lanphier E, Vona P, Gurbani S, et al. Childhood absence epilepsy: behavioral, cognitive, and linguistic comorbidities. Epilepsia. 2008;49:1838–46. doi: 10.1111/j.1528-1167.2008.01680.x. [DOI] [PubMed] [Google Scholar]
- [17].Hommet C, Sauerwein HC, De Toffol B, Lassonde M. Idiopathic epileptic syndromes and cognition. Neurosci Biobehav Rev. 2006;30:85–96. doi: 10.1016/j.neubiorev.2005.06.004. [DOI] [PubMed] [Google Scholar]
- [18].MacAllister WS, Schaffer SG. Neuropsychological deficits in childhood epilepsy syndromes. Neuropsychol Rev. 2007;17:427–44. doi: 10.1007/s11065-007-9048-4. [DOI] [PubMed] [Google Scholar]
- [19].Berg AT, Langfitt JT, Testa FM, Levy SR, DiMario F, Westerveld M, et al. Residual cognitive effects of uncomplicated idiopathic and cryptogenic epilepsy. Epilepsy Behav. 2008;13:614–9. doi: 10.1016/j.yebeh.2008.07.007. [DOI] [PubMed] [Google Scholar]
- [20].Monjauze C, Broadbent H, Boyd SG, Neville BG, Baldeweg T. Language deficits and altered hemispheric lateralization in young people in remission from BECTS. Epilepsia. 2011;52:e79–83. doi: 10.1111/j.1528-1167.2011.03105.x. [DOI] [PubMed] [Google Scholar]
- [21].Braakman HM, Vaessen MJ, Hofman PA, Debeij-van Hall MH, Backes WH, Vles JS, et al. Cognitive and behavioral complications of frontal lobe epilepsy in children: a review of the literature. Epilepsia. 2011;52:849–56. doi: 10.1111/j.1528-1167.2011.03057.x. [DOI] [PubMed] [Google Scholar]
- [22].Guimaraes CA, Li LM, Rzezak P, Fuentes D, Franzon RC, Augusta Montenegro M, et al. Temporal lobe epilepsy in childhood: comprehensive neuropsychological assessment. J Child Neurol. 2007;22:836–40. doi: 10.1177/0883073807304701. [DOI] [PubMed] [Google Scholar]
- [23].McNelis AM, Dunn DW, Johnson CS, Austin JK, Perkins SM. Academic performance in children with new-onset seizures and asthma: a prospective study. Epilepsy Behav. 2007;10:311–8. doi: 10.1016/j.yebeh.2006.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Clarke T, Strug LJ, Murphy PL, Bali B, Carvalho J, Foster S, et al. High risk of reading disability and speech sound disorder in rolandic epilepsy families: case-control study. Epilepsia. 2007;48:2258–65. doi: 10.1111/j.1528-1167.2007.01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Widjaja E, Mahmoodabadi SZ, Snead OC, 3rd, Almehdar A, Smith ML. Widespread cortical thinning in children with frontal lobe epilepsy. Epilepsia. 2011;52:1685–91. doi: 10.1111/j.1528-1167.2011.03085.x. [DOI] [PubMed] [Google Scholar]
- [26].Cormack F, Gadian DG, Vargha-Khadem F, Cross JH, Connelly A, Baldeweg T. Extra-hippocampal grey matter density abnormalities in paediatric mesial temporal sclerosis. Neuroimage. 2005;27:635–43. doi: 10.1016/j.neuroimage.2005.05.023. [DOI] [PubMed] [Google Scholar]
- [27].Lin JJ, Riley JD, Hsu DA, Stafstrom CE, Dabbs K, Becker T, et al. Striatal hypertrophy and its cognitive effects in new-onset benign epilepsy with centrotemporal spikes. Epilepsia. 2012;53:677–85. doi: 10.1111/j.1528-1167.2012.03422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Anderson J, Hamandi K. Understanding juvenile myoclonic epilepsy: Contributions from neuroimaging. Epilepsy Res. 2011;94:127–37. doi: 10.1016/j.eplepsyres.2011.03.008. [DOI] [PubMed] [Google Scholar]
- [29].Pardoe H, Pell GS, Abbott DF, Berg AT, Jackson GD. Multi-site voxel-based morphometry: methods and a feasibility demonstration with childhood absence epilepsy. Neuroimage. 2008;42:611–6. doi: 10.1016/j.neuroimage.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Caplan R, Levitt J, Siddarth P, Wu KN, Gurbani S, Sankar R, et al. Frontal and temporal volumes in Childhood Absence Epilepsy. Epilepsia. 2009;50:2466–72. doi: 10.1111/j.1528-1167.2009.02198.x. [DOI] [PubMed] [Google Scholar]
- [31].Tosun D, Dabbs K, Caplan R, Siddarth P, Toga A, Seidenberg M, et al. Deformation-based morphometry of prospective neurodevelopmental changes in new onset paediatric epilepsy. Brain. 2011;134:1003–14. doi: 10.1093/brain/awr027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Luo C, Xia Y, Li Q, Xue K, Lai Y, Gong Q, et al. Diffusion and volumetry abnormalities in subcortical nuclei of patients with absence seizures. Epilepsia. 2011;52:1092–9. doi: 10.1111/j.1528-1167.2011.03045.x. [DOI] [PubMed] [Google Scholar]
- [33].Tosun D, Siddarth P, Toga AW, Hermann B, Caplan R. Effects of childhood absence epilepsy on associations between regional cortical morphometry and aging and cognitive abilities. Human Brain Mapping. 2011;32:580–91. doi: 10.1002/hbm.21045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Glauser TA, Cnaan A, Shinnar S, Hirtz DG, Dlugos D, Masur D, et al. Ethosuximide, valproic acid, and lamotrigine in childhood absence epilepsy. N Engl J Med. 2010;362:790–9. doi: 10.1056/NEJMoa0902014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Wilkinson GS. Wide Range Achievement Test: Manual. 3 ed Wide Range, Inc.; Wilmington, DE: 1993. [Google Scholar]
- [36].Wechsler D. Wechsler Abbreviated Scale of Intelligence. The Psychological Corporation; San Antonio, TX: 1999. [Google Scholar]
- [37].Kaplan EF, Goodglass H, Weintraub S. The Boston Naming Test. 2nd ed. Lippincott Williams & Wilkins; Philadelphia: 2001. [Google Scholar]
- [38].Williams KT. Expressive Vocabulary Test. American Guidance Service; Circle Pines, MN: 1997. [Google Scholar]
- [39].Dunn L, Dunn L, WIlliams KT. Peabody Picture Vocabulary Test. 3 rd ed. American Guidance Service; Circle Pines, MN: 1997. [Google Scholar]
- [40].Delis DC, Kaplan E, Kramer JH. Delis-Kaplan Executive Function System. The Psychological Corporation; San Antonion, TX: 2001. [Google Scholar]
- [41].Cohen MJ. Children’s Memory Scale. The Psychological Corporation; San Antonio, TX: 1997. [Google Scholar]
- [42].Conners CK. The Connors’ Continuous Performance Test. Multi-Heath Systems; Toronto, Canada: 1995. [Google Scholar]
- [43].Trites RL. Neuropsychological Test Manual. Royal Ottawa Hospital; Ottawa, Ontario, Canada: 1977. [Google Scholar]
- [44].Wechsler D. Wechsler Intelligence Scale for Children. 3 rd ed. The Psycholocal Corporation; San Antonio, TX: 1991. [Google Scholar]