Abstract
Background and Objectives
Previous research suggests that attention bias toward threat contributes to the development and maintenance of anxiety. The current study extends this work by mapping the neural correlates of experimentally-induced changes in attention bias. The study examines both behavioral and psychophysiological changes associated with experimentally-induced changes in threat bias.
Methods
Thirty-four non-anxious female adults were randomly assigned to one of two conditions: training attention toward threat or placebo control. Attention bias was assessed and trained via a modified dot-probe task. Participants completed pre- and post-training assessments of attention bias and stress reactivity. As well, EEG was collected during pre- and post-test assessment of attention bias using the dot-probe task.
Results
Training induced significant changes in attention bias, though findings were complicated by group differences in baseline threat-bias scores. Compared to controls, those in the training group showed greater depression vulnerability to a post-training stressor and increased P2 amplitude, an ERP component associated with attention toward threat, during the dot-probe task.
Limitations
Although participants were randomly assigned to groups, there were still group differences in pre-training bias scores. Also, while the use of a stress task before the initial assessment of attention bias was used to control for initial differences in stress vulnerability, this may have altered pre-bias scores since participants completed this task immediately after being stressed.
Conclusions
These findings demonstrate training-induced changes in behavior and neural response patterns relevant to prior work on attention bias modification.
Keywords: Attention Bias, Anxiety, ERP, Attention Bias Modification
1. Introduction
Anxious individuals often preferentially allocate their attention toward threatening information in the environment over non-threatening stimuli, a pattern not detected in non-anxious individuals (Bar-Haim, Lamy, Pergamin, Bakermans-Kranenburg, & van IJzendoorn, 2007). This bias, referred to as an attention bias toward threat, is implicated in the development and maintenance of anxiety (Mathews & MacLeod, 2002; Mogg & Bradley, 1998). Prior experimental studies induced an attention bias toward threat in non-anxious individuals and found that this training to attend threats increased susceptibility to stress (Eldar, Ricon, & Bar-Haim, 2008; MacLeod, Rutherford, Campbell, Ebsworthy, & Holker, 2002). Both of these studies used a modified dot-probe task (MacLeod, Mathews, & Tata, 1986). This task involves the simultaneous presentation of two stimuli, one threatening and one neutral. After their offset, a target probe appears in the location previously occupied by one of the stimuli. Participants are instructed to identify the probe as quickly as possible. This task allows investigators to examine an individual’s pattern of attention allocation based on the response times to probes appearing at the location of the threatening stimuli relative to neutral stimuli (MacLeod, Mathews, & Tata, 1986). To induce attention bias, the probe always appears at the location of the emotionally valenced stimuli of interest and to induce a bias toward threat, the probe is always located behind the threat-related stimuli. Presenting the task in this manner implicitly focuses the participant's attention on the threatening stimulus. Repeated presentation of such trials "trains" an attention bias toward threat and is commonly referred to as Attention Bias Modification (ABM).
Whereas both the Macleod et al. and Eldar et al. studies demonstrated that training to attend toward threat increased stress vulnerability, the mechanisms underlying this effect remain unclear. First, in both studies, there were pre-to-post training differences between two active conditions, one where participants were actively trained to attend toward threat and a second where participants were actively trained to attend toward neutral stimuli. Group differences in reaction time and/or mood could reflect effects of either experimental condition. Comparing active attention training toward threat to an inactive placebo-training condition would address this issue. Second, there is only limited research that examines the neural correlates of ABM (e.g., Browning, Holmes, Murphy, Goodwin, & Harmer, 2010; Eldar & Bar-Haim, 2010). Demonstrating an effect of ABM on brain function could provide further insights into the cognitive mechanisms underlying training effects.
To study the neural correlates of ABM, the current study examined both behavioral and electrophysiological (event-related potentials: ERPs) data during bias assessments completed before and after an ABM procedure. The use of ERPs can provide information on the chronometry of neural activity associated with the acquisition of attention bias toward threat and allow the examination of where in the stream of neural information processing training exerts its effects. We examined components that represent early processing associated with attention orienting (i.e., P1 and N1), as well as later components associated with more complex processing (i.e., P2 and P3).
Previous research found that in addition to having an attention bias toward threat, anxious participants displayed enhanced amplitudes in ERP components when presented with threat-neutral facial stimuli relative to non-anxious participants. Specifically, several studies established relations among elevated P2, attention bias toward threat, and anxiety (e.g., Bar-Haim, Lamy, & Glickman, 2005; Carretie, Martin-Loeches, Hinojosa, & Mercado, 2001; Eldar, Yankelevitch, Lamy, & Bar-Haim, 2010). In an attention training study, anxious participants who were trained to avoid threat showed P2 amplitude reductions (Eldar & Bar-Haim, 2010). Thus, training the acquisition of attention bias toward threat might be expected to increase P2 amplitude.
Recently, O’Toole and Dennis (2012) used a modified dot-probe task with happy and angry faces to train non-anxious adults toward threat or away from threat and toward happy. In addition, ERPs during the pre- and post-training assessments were examined. For the threat training group, greater P2 amplitude and reduced N170 to threat versus happy cues were correlated with greater attention bias toward threat. While these findings highlight neural effects of attention training, the lack of a neutral control group make it difficult to distinguish between true training effects and physiological changes that may have occurred over time without attention manipulation.
Training effects might also be expected on earlier ERP components, such as the P1 and N1 components. These components reflect early attentional processing that vary as a function of visuospatial orienting (Hillyard et al., 1995). Increased occipital P1 amplitude has been related to the allocation of attention to visual stimuli, whereas greater N1 amplitude has been associated with the discrimination of attended stimuli (Mangun, 1995). Studies using the dot-probe paradigm have found an association between threat stimulus displays and increased P1 amplitude (Mueller et al., 2009; Pourtois, Dan, Grandjean, Sander, & Vuilleumier, 2005). Attention bias modification might therefore be expected to increase amplitudes of P1 and N1 components, reflecting enhanced attentional processing when threat is in the environment.
As well, studies have examined whether ABM leads to group differences in frontal recruitment following probe onset. Eldar and Bar-Haim (2010) found that participants who were trained away from threat displayed enhanced frontal P3 amplitdue to the trarget in angry-incongruent conditions. O’Toole and colleagues (2012) also examined P3 amplitdues to the target probe but did not finding significant between group differences. The current study also examined group differences in the P3 time-locked to the probe.
The current study aimed to extend a previous study by MacLeod et al (2002) by examining the acquisition of attention bias toward threat in a non-anxious population and exploring how ABM was associated with emotional vulnerability. Additionally, the current study explored changes in ERP correlates associated with the acquisition of an attention bias toward threat and examined how these electrophysiological changes were associated with stress vulnerability. A placebo training condition was used so the direct affects of training toward threat can be examined. We hypothesized that an attention bias toward threat can be induced in non-anxious participants and that such an induction would be associated with emotional vulnerability and ERP modulation.
2. Method
2.1 Participants
Thirty-four female young adult participants (M age=20.76, SD=1.41) scoring within one standard deviation of the normal range on the trait section of the Spielberger State-Trait Anxiety Inventory (STAI-Y: Speielberger, Gorsuch, & Lushene, 1983) were recruited for this study. We recruited only female subjects because of their greater stress-reactivity profile (De Rivera, De las Cuevas, Monterrey, Rodriguez-Pulido, & Gracia, 1993). Participants were randomly assigned to either the active training or placebo control condition. The mean STAI trait score did not differ between the placebo control group (M=38.59, SD=5.70) and the training group (M=40.82, SD=4.84), t(32)=−1.23, p=0.23. One participant from the training group was excluded from behavioral analyses due to extreme reaction times (greater than 2 SDs from group’s mean), during the pre-training attention bias assessment. Five outliers were removed from analyses due to poor behavioral performance on the dot-poor task (≥ 4.2 % incorrect trials; 1 from the control group and 4 from the ABM group). Thus, behavioral data analyses compared performance in 12 subjects in the active-training group and 16 participants in the control group. For ERP analyses, one additional participant from the control group was removed from analyses due to their extreme outlier status on usable epochs (greater than 2 SDs from group’s mean).
2.2 Measures
2.2.1 Attention bias measure: dot-probe experimental stimuli
The face stimuli consisted of 16 individuals (8 female) taken from the NimStim Face Stimulus Set (Tottenham et al., 2009). Each face pair display consisted of an individual’s angry and neutral facial expressions presented side-by-side. Each face was 11 cm tall by 8 cm wide, and the two facial expressions were presented at equal distance from the center of the screen. 11cm of white space separated the two face images. Probe arrows, oriented up or down, were 2 cm tall by 1 cm wide and presented in the center of the location previously occupied by one of the faces. A white fixation cross, 2.5 cm wide by 2.5 cm tall, was presented on the screen before the presentation of the faces. All stimuli were presented on a 17” monitor placed 0.5 meters away from the participant. Fixation, face, and probe images were all .tif files created on a black background.
2.2.2 Dot-probe task procedure: training and assessment
A modified dot-probe paradigm was used to examine and train attention bias toward threat. The task consisted of three separate blocks, two test phases and one training phase. Each trial began with a fixation cross presented in the center of the screen for 500 ms, followed by the horizontal faces display for 500 ms. After the face display presentation, an arrow, oriented up or down, appeared in the location of the previously viewed angry face (congruent trial) or neutral face (incongruent trial) for 200 ms. The arrow presentation was replaced by a blank screen in which the participants had up to 1400 ms to respond to the target orientation before the next trial began. Participants were asked to press one of two buttons on a button box as quickly and accurately as possible, to indicate the direction in which the arrow was pointing. Location of the angry face, location of the probe, and orientation of the probe were balanced across trials.
The pre-training and post-training blocks consisted of 100 trials each. Of the 100 trials, half the trials were congruent with the probe presented at the location of the angry face and the other half were incongruent with the probe presented at the location of the neutral face. The training session consisted of two blocks of 300 trials each (600 total training trials). Participants in the Training group only received threat congruent trials during training blocks. Participants in the Control group received an equal number of congruent and incongruent trials.
Dot-probe trials with incorrect response and reaction times (RTs) less than 200 ms after target presentation were excluded from further analyses. In addition, RTs above and below two standard deviations of the mean RT for each condition within each block for each subject were excluded from the mean reaction time calculations for each participant. To calculate bias scores, mean RTs on congruent trials were subtracted from mean RTs of incongruent trials, such that higher scores on the bias index reflect an attention bias toward threat and negative scores reflect an attention bias away from threat and toward neutral.
2.2.3 EEG collection
EEG was recorded from 16 scalp locations (F3, F4, F7, F8, T7, T8, C3, C4, P3, P4, O1, P7, P8, O2, Fz, and Pz) and the left and right mastoids sewn in a lycra Electro-Cap (Electro-Cap International Inc., Eaton, OH) according to the international 10–20 system. During data acquisition all electrodes were referenced to Cz and AFz served as ground. All impedances were kept below 10kΩ. Vertical electrooculogram (EOG) was recorded using tin electrodes placed above and below the left eye to record blinks and other eye movement. The EEG and EOG were amplified with a gain of 5000 and 2500, respectively, and band-pass filtered from 0.1 to 100 Hz using custom bioamplifiers from James Long Company (Caroga Lake, NY). Data was digitized at 512Hz onto a PC with a 12-bit A/D converter (±2.5 V input range) and Snap-Master acquisition software (HEM Data Corporation, Southfield, MI). Before each EEG recording session, a 50µV 10Hz signal was input into all recording channels and recorded for calibration purposes.
2.2.4 Data reduction
The EEG data were re-referenced through software to an average mastoid reference. Epochs containing blink artifact were automatically identified and EOG contamination was regressed from the EEG. Artifacts in the EEG signal that exceeded ±150µV were automatically scored and excluded from further analyses. The EEG data were then filtered with a 30 Hz low-pass filter. EEG data were then time-locked to the faces display onset and correct trials with reaction times greater than 200ms were used to create ERPs. The time window in which each component was scored was based on time windows used in previous studies as well as inspection of the grand mean ERPs. Peak amplitudes for face-evoked ERPs were computed for P1 (80–130ms) for sites O1 and O2 (e.g., Helfinstein, White, Bar-Haim, & Fox, 2008), N1 (115–165ms) for sites P3 and P4 (e.g., Helfinstein, et al., 2008), and P2 (125–225ms) for site Pz (e.g., Hanatani et al., 2005). Peak amplitudes for target-evoked ERPs were computed for P3 (285–400ms) for site Fz. All ERPs were computed relative to a 100 ms baseline immediately preceding stimuli onset. There were no group differences in the number of trials completed during the pre-test (t(25)= −.94, p=.36) or post test (t(25)= .90 p=.38).
2.2.5 Analogue mood scales
To assess changes in mood over the course of the experiment, anxiety and depression analogue moods scale were both administered throughout the task. Scales were those used in MacLeod et al. (2002), but adapted for paper administration. A 15 cm line was divided into 30 equal partitions. The anxiety scale had terminal labels “relaxed” and "anxious” and the depression scale had the terminal labels “happy” and “depressed”. Participants were asked to circle the mark on the scale that most accurately reflected their current mood state. Scores ranged from 1–30 where higher scores reflect a more anxious or depressed mood.
2.2.6 Stress induction tasks and stress vulnerability
Two types of stress induction tasks were used: The Anagram Stress Task and The Block-Design Stress Task. The order of the stress tasks was counter-balanced. Before each stress task, participants were told that the task was part of a department-wide initiative to assess the relation between academic performance and cognitive tasks and that a link had been established between intelligence and the ability to solve anagrams/puzzles. Participants were also told that their performance would be videotaped and although it was not likely, if their scores fell within the top or bottom 10% they would be asked for permission at the end of the experiment to use their video for teaching purposes in first-year psychology lab classes.
The anagram task was adapted from MacLeod et al. (2002) to create a stressful situation in which to elicit a negative mood state. Participants were told to unscramble a string of letters to make a word and write down the correct answer on a response sheet (provided by the experimenter). Once an anagram was completed participants were told to press a button on a response box to advance to the next anagram. Participants were told that if they could not solve an anagram they may press a button to skip ahead to the next anagram. Both the need for speed and accuracy in the task were emphasized. Before the experimenter started the timer and left the room, she walked over to the video camera to ensure the camera was pointing toward the participant and pretended to begin recording at that point.
Anagrams were impossible or extremely difficult to solve. Each anagram, a string of 5 cm white letters, was presented in the center of a black screen in white font. After 3 minutes, the experimenter walked back to the room and gathered the response sheet from each participant. After examining the responses, the experimenter informed each participant that her performance was unusually low and that she would like to use her video for later demonstration and that this would be discussed further at the end of the experiment.
The Block Design Task was adapted from a subset scale of the WAIS-III (Wechsler, 2002). All block designs were impossible to complete due to a missing block. Participants were given an assortment of red and white blocks and then shown a picture comprised of different orientations of these blocks. Participants were told to recreate as many picture designs as possible using the blocks provided. A similar timing and videotaping procedure to that used in the anagram task also was used for the block design task. Participants were told that the experimenter in an adjacent room would monitor their performance. Accuracy and speed were emphasized on the block design task. Participants were told once they successfully had completed one block design they were to move on to the next picture. Participants also were instructed that if they were unable to solve a specific block design, they may move on to the next design. After 3 minutes, the experimenter walked back to the room and informed each participant that her performance was unusually low and that she would like to use her video for later demonstration and that this would be discussed further at the end of the experiment.
Analogue moods scales for depression and anxiety were given to participants before and after each stress task. In order to examine differences in emotional vulnerability during a stressor, anxiety vulnerability and depression vulnerability scores were calculated for each stress task (i.e., pre-training, post-training). Scores were calculated by subtracting the pre-stress task mood rating from the post-stress task mood rating. For example, the pre-training anxiety vulnerability score for the first stress task was calculated by subtracting the anxiety scale rating before the first stressor (mood scale set 1) from the anxiety rating given after the first stressor (mood scale set 2). Similarly, depression vulnerability for time 1 was calculated by subtracting the depression scale rating given before the first stressor from the depression rating given after the first stressor. Vulnerability scores for time 2 used mood scale ratings from before and after the second stressor.
2.3 Procedure
Table 1 describes the sequence of events used in the procedure. The study took place during one session. After obtaining consent, participants were seated while the experimenter placed and prepared the EEG cap. Electrode impedances were also measured at this time. Participants then completed a set of anxiety and depression mood scales (mood scale set 1). Afterwards, they completed their first stress task (block design or anagram) and then another mood scale assessment (mood scale set 2). EEG was then collected while participants completed the dot-probe paradigm. Participants completed the pre-training attention bias assessment. The training group then completed 600 congruent training trials and the control group completed 600 trials of which half were congruent and the other half were incongruent trials. Participants were given a break every 100 trials. Both groups then completed another set of mood scales (mood scale set 3) followed by an attention bias post-test. Participants were then given the second stress task, filling out the anxiety and depression moods scales directly before (mood scale set 4) and after the stress task (mood scale set 5).
Table 1.
| Outline of General Procedure |
|---|
| Mood scales (set 1) |
| Stress task (anagram/block design) |
| Mood scale (set 2) |
| EEG collection begins |
| Attention bias assessment (pre-test) |
| Attention training |
| Mood scales (set 3) |
| Attention bias assessment (post-test) |
| Mood scales (set 4) |
| Stress task (anagram/block Design) |
| Mood scales (set 5) |
At the end of the session, participants were debriefed as to the purpose of the study and informed that the stress tasks were extremely difficult or unsolvable and that their performance was at no point videotaped during the experiment.
2.4 Data Analyses
To explore our hypotheses, an ANCOVA measures design was used to analyze group differences in behavior and psychophysiology after the training procedure (post-training assessment), controlling for differences in the measure before administration of the training procedure (pre-training assessment) (Davison & Sharma, 1994). Analyses of ANCOVA are best when studying treatment effects between randomly assigned groups because it is unbiased and has more power (Van Breukelen, 2006).
3. Results
3.1 Differences in Baseline Measures
To evaluate the presence of group differences prior to training, we compared the active and control groups on baseline measures of bias scores, ERP data, and emotional vulnerability. While groups did not differ statistically on any of the parameters, there was a non-significant trend for group difference in the pre-training attention bias, t(26)= 1.80, p=.08. The group randomized to train toward threat tended to manifest a greater bias away from threat than the control group. No differences in pre-training measures for anxiety or depression vulnerability or for pre-training ERP measures were detected (all ps >.05).
3.2 Effects of Attention Training on Response Time
Means and SDs of reaction times to each condition (congruent, incongruent trials), bias scores, and bias change scores are reported in Table 2. To examine changes in attention bias as a result of the attention training procedure, attention bias score data were subjected to an ANCOVA with post-training bias as the dependent measure and co-varying for pre-training bias. There were no group differences, F(1,25)=1.57, p=.22. Exploratory analyses were conducted by means of paired samples t-tests to examine within group changes in attention bias. When comparing bias scores pre- and post-training, the training group showed a significant change in attention bias scores after training (t(11) = −2.99, p =.01; 29.31 ms bias change), while there was no change in attention bias in the control group t(15) = −.24, p =.81). Despite the initial group difference in pre-training bias scores, the training group showed an increased attention bias toward threat from pre- to post-training, whereas the control group showed no change in bias scores (Table 2).
Table 2.
| Attention Bias by Group | |||
|---|---|---|---|
| Group | Mean (ms) | SD | |
| Control | |||
| Pre Congruent | 645.42 | 108.08 | |
| Pre Incongruent | 644.04 | 111.66 | |
| Post Congruent | 553.13 | 104.10 | |
| Post Incongruent | 553.62 | 98.79 | |
| Pre Bias | −1.37 | 26.78 | |
| Post Bias | 0.49 | 24.09 | |
| Bias Change | 1.86 | 30.69 | |
| Training | |||
| Pre Congruent | 653.93 | 97.72 | |
| Pre Incongruent | 630.86 | 78.55 | |
| Post Congruent | 590.97 | 128.61 | |
| Post Incongruent | 597.21 | 129.23 | |
| Pre Bias | −23.07 | 37.19 | |
| Post Bias | 6.25 | 22.64 | |
| Bias Change | 29.31 | 34.02 | |
3.3. Effects of Attention Training on Face-Evoked ERPs
To explore neural correlates associated with a change in attention bias toward threat, we examined whether there were group amplitude differences in the P1, N1, and P2 components post-attention training. For all components the ANCOVA design entered amplitudes at post-training as the dependent measure, with the pre-training assessment amplitude entered as a covariate. For the P1 component, we first examined hemispheric differences between O1 and O2 and because there were no differences, averages were computed between corresponding electrodes for both hemispheres. One outlier was removed from P1 analyses (greater than 2 SDs from group’s mean). No group differences were found, F(1,23)=.03, p=.87. There were no hemispheric differences for the N1 component in the P3 and P4 electrode sites. Thus, an average was created across these two sites. There were no group differences in peak amplitude, F(1,24)=.83, p=.37. The findings from the current study did not reveal group differences in early attentional components, as indexed by the P1 and N1 ERPs post-training.
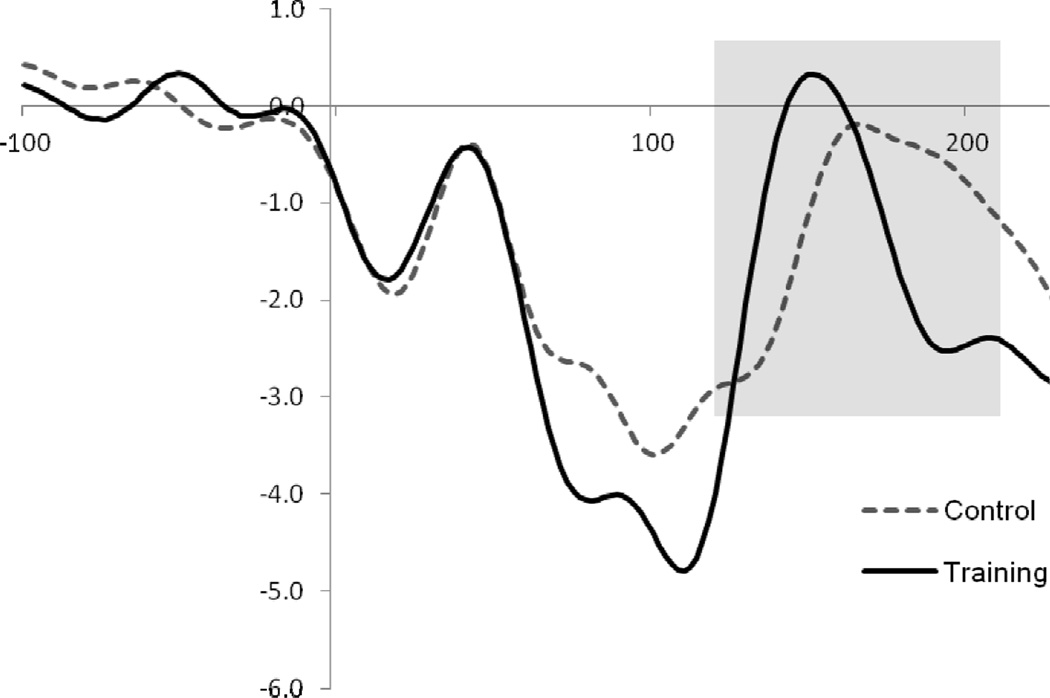
The P2 amplitude was examined at the Pz electrode. Three participants were removed from P2 analyses due to outlier status of amplitude values (greater than 2 SDs from group’s mean). When controlling for pre-training P2 amplitude, a significant group difference in post-training P2 amplitude was revealed, F(1,21)=4.31, p=.05 (Figure 1). Those trained toward threat (M=3.20, SE=.66) had greater P2 amplitude than those in the control group (M=1.35, SE=.60). These results suggest that undergoing training to attend toward threat was associated with modulation of P2 amplitude. There were no group differences in peak latency.
Figure 1.
P2 to face stimuli during post-test at Pz
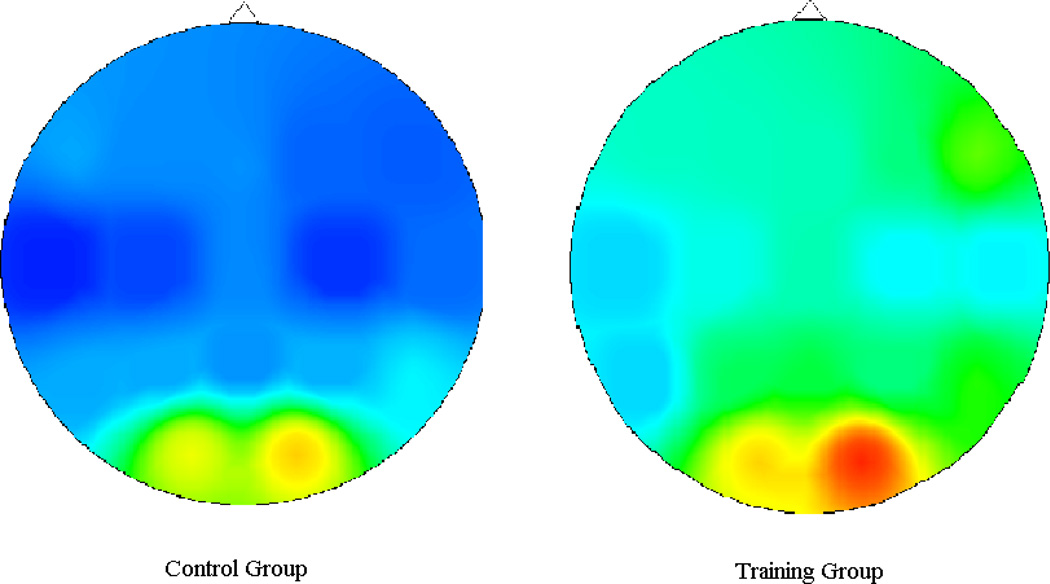
We conducted exploratory frontal P2 analyses to examine whether the findings were specific to parietal and not frontal regions. We examined P2 amplitude at the Fz electrode. When controlling for pre-training P2 amplitude, there were no group differences in post-training P2 amplitude at Fz, F(1,26)=.01, p=.91. See Figure 2 for scalp maps that represent the estimated marginal means of post-training scalp distributions during the P2 time window.
Figure 2.
Post-Training Scalp Distributions for P2 Time Window
3.4 Effects of Attention Training on Target-Evoked ERPs
We conducted exploratory analyses to examine whether there were group differences in target-evoked ERPs. We examined P3 amplitude at the Fz electrode separately for both angry-congruent trials and angry-incongruent trials. When controlling for pre-training P3 amplitude, there were no group differences in post-training P3 amplitude at Fz for angry-congruent trials, F(1,22)=.40, p=.54, nor were the group differences for angry-incongruent trials, F(1,22)=.2.49, p=.13.
3.5 Effects of Attention Training on Mood
We directly tested changes in mood as a function of the training procedure. Our dependent variables were the mood scales completed directly after the training procedure and our covariates were the mood scales completed at the start of the session. When controlling for pre-training mood scale scores (mood scale 1), no significant mood changes were detected immediately after attention training (mood scale 3) for either anxiety, F(1, 25)= .24, p=.63 or depression, F(1, 25)= .14, p=.71 (Table 3). This is consistent with previous findings (e.g., MacLeod, et al., 2002).
Table 3.
| Mood Scales | ||||
|---|---|---|---|---|
| Group | Mean | SD | ||
| Control | ||||
| Depression | ||||
| Time 1 | 8.69 | 4.08 | ||
| Time 2 | 15.25 | 6.87 | ||
| Time 3 | 11.81 | 5.37 | ||
| Time 4 | 10.80 | 3.76 | ||
| Time 5 | 13.69 | 6.12 | ||
| Vulnerability 1* | 6.56 | 6.78 | ||
| Vulnerability 2** | 2.87 | 4.58 | ||
| Anxiety | ||||
| Time 1 | 12.63 | 6.04 | ||
| Time 2 | 18.81 | 7.37 | ||
| Time 3 | 13.25 | 8.54 | ||
| Time 4 | 12.00 | 7.41 | ||
| Time 5 | 16.25 | 7.09 | ||
| Vulnerability 1* | 6.19 | 6.93 | ||
| Vulnerability 2** | 3.67 | 7.13 | ||
| Training | ||||
| Depression | ||||
| Time 1 | 6.58 | 4.52 | ||
| Time 2 | 14.46 | 6.90 | ||
| Time 3 | 11.71 | 7.75 | ||
| Time 4 | 7.29 | 5.38 | ||
| Time 5 | 14.29 | 8.19 | ||
| Vulnerability 1* | 7.88 | 6.94 | ||
| Vulnerability 2** | 7.00 | 5.22 | ||
| Anxiety | ||||
| Time 1 | 8.33 | 4.36 | ||
| Time 2 | 16.04 | 7.16 | ||
| Time 3 | 10.54 | 7.83 | ||
| Time 4 | 9.88 | 7.97 | ||
| Time 5 | 11.92 | 7.12 | ||
| Vulnerability 1* | 7.71 | 8.00 | ||
| Vulnerability 2** | 2.04 | 8.90 | ||
Note:
time 2 - time 1;
time 5 - time 4
3.6 Effects of Attention Training on Vulnerability to Stress
Controlling for pre-training stress vulnerability as well as for stress task order, there were group differences in depression vulnerability following the stress task, such that those in the training group showed greater depression vulnerability than did the controls, F(1,23)=4.98, p=0.04. This was not found for anxiety vulnerability, F(1,23)=.81, p= 0.38. In order to further examine this finding, we tested whether there were group differences in the individual depression mood scales before and after each stressor. Results suggest a trend group difference in the depression mood scale given before the second stressor (mood scale 4), t(25) = 1.99, p = .06.
3.7 Relations between Vulnerability to Stress and P2 and Bias Scores
Next, we explored how the changes in P2 amplitude, as a function of attention training, related to the group difference detected in depression vulnerability to stress. Within the training group we conducted a correlation analyses between change in P2 amplitude (post-training amplitude minus pre-training amplitude) and change in depression vulnerability change scores (post-training score minus pre-training score). Results revealed a moderate positive correlation significant at trend level in which greater increases of P2 amplitude as a result of training were related to greater increases in depression vulnerability, r(8)=.58, p=.08. We then examined the relations between change scores in depression vulnerability and change scores in attention bias. No such relation was found, r(8)= .001, p=.99.
4. Discussion
Findings from the current study link threat-related attention bias and emotional vulnerability while also delineating associated, underlying neural activation patterns. Specifically, participants trained to attend toward threat showed greater signs of stress-vulnerability than participants receiving the control-training regimen. These differences in vulnerability were paralleled by differences in the amplitude of P2.
Thus, the current study detected group differences in neural activation as a function of attention training toward threat. The active-training group displayed increased parietal P2 amplitude relative to the inactive-training group, suggesting that individuals trained toward threat may use greater attentional resources than individuals not receiving such training when presented with threatening stimuli. This is in line with research suggesting that the P2 component is a neural response that is sensitive to threat-related stimuli (e.g., O’Toole and Dennis, 2012). For example, Helfinstein and colleagues found that high socially anxious individuals tended to have greater P2 amplitude to faces in a dot-probe task than low socially anxious individuals (Helfinstein, et al., 2008). In the current study, the increased P2 found in the training group may reflect an enhanced neural sensitivity to threats induced by the attention training procedure. A number of theorists suggest that the difference between anxious and non-anxious individuals’ vigilant processing is due in part to a lower threshold for vigilant response to threatening stimuli amongst anxious participants (Mathews & MacLeod, 2002). In the current study, individuals in the training group learned to direct their attention toward threatening stimuli which may have activated or altered their threat-evaluation system, leading to a hypersensitivity in threat detection, as indexed by increased parietal P2 amplitude.
Findings also revealed a trend relation (correlation) between post-training P2 amplitude and post-training depression vulnerability in the training group. The modulated P2 found in the training group may be indicative of increased threat sensitivity and thus relate to emotion vulnerability. Non-anxious individuals in the training group appear to have increased their sensitivity toward threat. The current study adds to a growing literature that finds the amplitude and modulation of the P2 ERP component to be a potential biomarker of biased threat attendance (e.g., Eldar & Bar-Haim, 2010; Eldar, et al., 2010; O'Toole & Dennis, 2012). Future work should continue to examine the physiological processes underlying attention bias toward threat and how those relate to the development and maintenance of anxiety. No group differences in early attentional components, as indexed by P1 and N1 amplitudes were found. These null findings are similar to those reported by Eldar and Bar-Haim (2010) and may suggest that attention training modulates later processes as opposed to early attentional processing or may be the result of the small sample size in the current study.
Of note, findings for ERP data generally were clearer than for changes in attention bias. In particular, no significant group differences in attention bias were detected during the post-training assessment period. This suggests that ERP data may be more sensitive than behavioral data to the effects of attention training. Nevertheless, follow-up analyses did reveal some signs of an effect on behavioral indicators of attention, albeit at a relatively liberal statistical threshold. Namely, there was a significant change in attention bias in the training group, whereas no change was detected in the control group. However, these group differences in pre-to-post changes did not pass conventional levels of statistical significance.
Of note, unlike in the current study, both Macleod et al. (2002) and Eldar et al. (2008) found statistically significant post-training group differences in behavioral indicators of attention. Nevertheless, unlike the current study, both studies compared two active conditions, one trained to attend toward threat and another trained to avoid it. The current study employed a placebo control design, with an inactive control condition. The current procedures, relative to those in these two prior studies, may have minimized post-training group differences at the expense of more accurately quantifying the effects of active training; the current procedures thereby add to the literature by showing a direct effect of training toward threat on neural indicators of attention, as manifest in ERP data.
A recent meta-analysis reports that studies using a top-bottom presentation of the target stimuli have greater training effects than those that use a side-by-side presentation (Hakamata et al., 2010). As the current study replicated the design of MacLeod and colleagues (2002), it used the same side-by-side presentation. As well, presenting the target stimuli in a side-by-side formation is commonly used for attention modification studies that examine ERPs (e.g., Eldar & Bar-Haim, 2008; O’Toole & Dennis, 2012). The use of top-bottom stimuli presentation in future training studies may yield stronger training effects and should be further explored.
Results from the current study also revealed that attention bias training was related to elevated stress vulnerability during a stress task, indexed by increased depression ratings in individuals in the training group. Unlike previous attention training studies (Eldar, et al., 2008; MacLeod, et al., 2002), the current study showed significant vulnerability differences only in depression ratings: no group differences in anxiety vulnerability were detected. MacLeod and colleagues found that training was related to increased negative mood reactivity toward the stressor, but this was not modified by scale type (training effects on anxiety did not differ from training effects on depression), and Eldar and colleagues found increases in anxiety vulnerability. These discrepancies could be the result of several differences in procedural and population characteristics and warrants further investigation. First, compared to MacLeod and colleagues (2002), the current study used face stimuli in the dot-probe task as opposed to word stimuli. Second, the current study included a second type of stress task (the block design task). In addition, compared to the current study,Eldar et al. (2008) used a child population and a different stress induction procedure. Third, the use of a placebo control group as opposed to an active training control group helps tease apart the direct role that an induction of threat bias has on vulnerability and may explain the vulnerability differences found. The methodological differences may have specific influence on the type of emotional vulnerability induced.
There are several limitations to the current study that should be considered when interpreting the findings. One limitation, potentially related to a small sample size, was a possible between-group difference in the behavioral marker of attention bias. Although participants were randomly assigned to the active or control groups, the active and control groups tended to differ in pre-training bias scores, albeit only at a trend level statistical threshold. Since many group comparisons were conducted, this statistical trend could reflect a Type I error. While we did consider conducting additional analyses, stratifying participants based on pre-training attention scores, this would create additional problems, potentially leading to undetected group differences on the many other possible factors that might vary with attention bias scores. Future work should consider block randomizing on pre-training bias scores as way to ensure that there are no pre-training group differences in key variables of interest.
This first limitation also is confounded by the failure to detect between-group differences in attention following training. Namely, although analyses of ANCOVA were used to control for these pre-training differences, we did not find differences in post-bias scores. While further analyses revealed that those trained toward threat showed a significant change in bias scores compared to controls, this change did not differ on statistical grounds from the change in the control group. Accordingly, the change in the active group could reflect regression to the mean, in groups that tended to differ at baseline. Importantly, difficulties in measuring attention bias change in bias modification studies are not uncommon and may reflect particular aspects of the paradigm used (the dot probe task) rather than a true lack of change in attention-related patterns due to training.
Beyond these limitations related to attention bias data, the study possessed other limitations concerning pre-training characteristics. Specifically, groups also differed in their depression ratings prior to the second stressor. Although the data suggest that the training group showed a greater increase in depression vulnerability following the second stressor, this may in part be driven by the training group’s initial lower pre-stress depression ratings. While these findings should be interpreted with caution, our interest was in the change in depression ratings from pre to post stressor, as it is the change score that depicts the effects of training. Finally, while the use of a stress task before the initial assessment of attention bias was applied to control for initial differences in stress vulnerability, this may have altered pre-bias scores since participants completed this task immediately after being stressed. For instance, recent research has found that stress can suppress attention bias and induce threat avoidance (e.g., Bar-Haim et al., 2010; Shechner, Pelc, Pine, Fox, & Bar-Haim, 2012; Wald et al., 2011). To better differentiate between the effects of the stress task and the dot-probe task, future work should not include a pre-training stress task or consider the use of a between-group design in which half of the participants completed the initial stress task before training and the other half completed the task afterwards.
The present study extends work from MacLeod (MacLeod et al., 2002) and others (e.g., Eldar & Bar-Haim, 2010; O'Toole & Dennis, 2012) who have examined the chronometry of attention during training with the dot-probe. It highlights the particular importance of the P2 component as a possible bio-marker of ABM and suggests that the attentional mechanisms underlying attention bias toward threat may become a target for treatment. Indeed, there are now at least two meta-analyses of the success of ABM treatment for anxiety disorders that show a modest effect size for this approach (Beard, Sawyer, & Hofmann, 2012; Hakamata, et al., 2010). Future studies in this area may enhance the possibility of treatment development for anxiety disorders and depression.
We experimentally manipulated attention bias to threat in non-anxious individuals.
Behavioral and psychophysiological effects of attention training were assessed.
Those in the training group showed a significant change in attention bias.
The trained group showed greater depression vulnerability to a stressor.
Training increased the P2 amplitude response to faces.
Acknowledgments
Funding Source
The project described was supported by Grant Number P50 MH078105-01A2 from the National Institute Of Mental Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute Of Mental Health or the National Institutes of Health.”
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests
The authors do not have any interests that might be interpreted as influencing the research, and the study was conducted in accordance with APA ethical standards.
References
- Bar-Haim Y, Holoshitz Y, Eldar S, Frenkel TI, Muller D, Charney DS, Wald I. Life-threatening danger and suppression of attention bias to threat. Am J Psychiatry. 2010;167(6):694–698. doi: 10.1176/appi.ajp.2009.09070956. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y, Lamy D, Glickman S. Attentional bias in anxiety: A behavioral and ERP study. Brain and Cognition. 2005;59:11–22. doi: 10.1016/j.bandc.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg M, van IJzendoorn M. Threat-Related Attentional Bias in Anxious and Nonanxious Individuals: A Meta-Analytic Study. Psychological Bulletin. 2007;133(1):1–24. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- Beard C, Sawyer AT, Hofmann SG. Efficacy of attention bias modification using threat and appetitive stimuli: a meta-analytic review. Behavior Therapy. 2012;43(4):724–740. doi: 10.1016/j.beth.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning M, Holmes EA, Murphy SE, Goodwin GM, Harmer CJ. Lateral prefrontal cortex mediates the cognitive modification of attentional bias. [Randomized Controlled Trial Research Support, Non-U.S. Gov't] Biological Psychiatry. 2010;67(10):919–925. doi: 10.1016/j.biopsych.2009.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carretie L, Martin-Loeches M, Hinojosa J, Mercado F. Emotion and attention interaction studied through event-related potentials. Journal of Cognitive Neuroscience. 2001;13(8):1109–1128. doi: 10.1162/089892901753294400. [DOI] [PubMed] [Google Scholar]
- Davison M, Sharma A. ANOVA and ANCOVA of pre- and post-test, ordinal data. Psychometrika. 1994;59:593–600. [Google Scholar]
- De Rivera J, De las Cuevas C, Monterrey A, Rodriguez-Pulido F, Gracia R. Stress reactivity in the general population. European Journal of Psychiatry. 1993;7:5–5. [Google Scholar]
- Eldar S, Bar-Haim Y. Neural plasticity in response to attention training in anxiety. Psychol Med. 2010;40(4):667–677. doi: 10.1017/S0033291709990766. [DOI] [PubMed] [Google Scholar]
- Eldar S, Ricon T, Bar-Haim Y. Plasticity in attention: Implications for stress response in children. Behaviour Research and Therapy. 2008 doi: 10.1016/j.brat.2008.01.012. [DOI] [PubMed] [Google Scholar]
- Eldar S, Yankelevitch R, Lamy D, Bar-Haim Y. Enhanced neural reactivity and selective attention to threat in anxiety. Biol Psychol. 2010;85(2):252–257. doi: 10.1016/j.biopsycho.2010.07.010. [DOI] [PubMed] [Google Scholar]
- Hakamata Y, Lissek S, Bar-Haim Y, Britton JC, Fox NA, Leibenluft E, Pine DS. Attention bias modification treatment: A meta-analysis toward the establishment of novel treatment for anxiety. Biological Psychiatry. 2010;68:982–990. doi: 10.1016/j.biopsych.2010.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanatani T, Sumi N, Taguchi S, Fujimoto O, Nan-No H, Takeda M. Event-related potentials in panic disorder and generalized anxiety disorder. Psychiatry and clinical neurosciences. 2005;59(1):83–88. doi: 10.1111/j.1440-1819.2005.01336.x. [DOI] [PubMed] [Google Scholar]
- Helfinstein SM, White LK, Bar-Haim Y, Fox NA. Affective primes suppress attention bias to threat in socially anxious individuals. Behavior Research and Therapy. 2008;46:799–810. doi: 10.1016/j.brat.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillyard S, Anllo-Vento L, Clarck V, Heinze H, Luck S, Mangun G. Neuroimaging approaches to the study of visual attention. In: Kramer AF, Coles M, Logan G, editors. Converging operations in the study of visual selective attention. Washington: American Psychological Association; 1995. pp. 107–138. [Google Scholar]
- MacLeod C, Mathews A, Tata P. Attentional bias in emotional disorders. Journal of Abnormal Psychology. 1986;95:15–20. doi: 10.1037//0021-843x.95.1.15. [DOI] [PubMed] [Google Scholar]
- MacLeod C, Rutherford E, Campbell L, Ebsworthy G, Holker L. Selective attention and emotional vulnerability: Assessing causal bias of their association through the experimental manipulation of attentional bias. Journal of Abnormal Psychology. 2002;111:107–123. [PubMed] [Google Scholar]
- Mangun GR. Neural mechanisms of visual selective attention. [Review] Psychophysiology. 1995;32(1):4–18. doi: 10.1111/j.1469-8986.1995.tb03400.x. [DOI] [PubMed] [Google Scholar]
- Mathews A, MacLeod C. Induced processing biases have causal effects on anxiety. Cognition and Emotion. 2002;16(3):331–354. [Google Scholar]
- Mogg K, Bradley BP. A cognitive-motivational analysis of anxiety. Behaviour Research and Therapy. 1998;36:809–848. doi: 10.1016/s0005-7967(98)00063-1. [DOI] [PubMed] [Google Scholar]
- Mueller E, Hofmann S, Santesso D, Meuret A, Bitran S, Pizzagalli D. Electrophysiological evidence of attentional biases in social anxiety disorder. Psychological Medicine. 2009;39:1141–1152. doi: 10.1017/S0033291708004820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Toole L, Dennis TA. Attention training and the threat bias: an ERP study. [Research Support, N.I.H., Extramural] Brain and Cognition. 2012;78(1):63–73. doi: 10.1016/j.bandc.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourtois G, Dan ES, Grandjean D, Sander D, Vuilleumier P. Enhanced extrastriate visual response to bandpass spatial frequency filtered fearful faces: time course and topographic evoked-potentials mapping. [Comparative Study] Human brain mapping. 2005;26(1):65–79. doi: 10.1002/hbm.20130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechner T, Pelc T, Pine DS, Fox NA, Bar-Haim Y. Flexible Attention Deployment in Threatening Contexts: An Instructed Fear Conditioning Study. Emotion. 2012 doi: 10.1037/a0027072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speielberger CD, Gorsuch RL, Lushene R. Consulting Psychologists Press. Palo Alto: 1983. The State-Trait personality Inventory STAI-Y, Form Y. [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, Nelson C. The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Research. 2009;168(3):242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Breukelen G. ANCOVA versus change from baseline had more power in randomized studies and more bias in nonrandomized studies. Journal of Clinical Epidemiology. 2006;59:920–925. doi: 10.1016/j.jclinepi.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Wald I, Lubin G, Holoshitz Y, Muller D, Fruchter E, Pine DS, Bar-Haim Y. Battlefield-like stress following simulated combat and suppression of attention bias to threat. Psychol Med. 2011;41(4):699–707. doi: 10.1017/S0033291710002308. [DOI] [PubMed] [Google Scholar]
- Wechsler D. WPPSI-III administration and scoring manual. San Antonio, TX: 2002. [Google Scholar]