Abstract
This study examined mothers' physiological reactivity in response to infant distress during the Still-Face Paradigm. We aimed to explore normative regulatory profiles and associated physiological and behavioral processes in order to further our understanding of what constitutes regulation in this dyadic context. We examined physiological patterns—vagal tone, indexed by respiratory sinus arrhythmia (RSA)-- while mothers maintained a neutral expression over the course of the still face episode, as well as differential reactivity patterns in mothers with depression symptoms compared to non-depressed mothers. Behavioral and physiological data were collected from mothers of 5-month-old infants during the emotion suppression phase of the Still-Face Paradigm. We used Hierarchical Linear Modeling to examine changes in mothers' RSA during infant distress and explored maternal depression as a predictor of physiological profiles. Mothers were generally able to maintain a neutral expression and simultaneously demonstrated a mean-level increase in RSA during the still face episode compared to baseline, indicating an active regulatory response overall. A more detailed time-course examination of RSA trajectories revealed that an initial RSA increase was typically followed by a decrease in response to peak infant distress, suggesting a physiological mobilization response. However, this was not true of mothers with elevated depressive symptoms, who showed no change in RSA during infant distress. These distinct patterns of infant distress-related physiological activation may help to explain differences in maternal sensitivity and adaptive parenting.
Keywords: Emotion regulation, still face paradigm, respiratory sinus arrhythmia, infant, maternal depression
1. Introduction
While there is no definitive instruction book on parenting, most parents consider their main job to be supporting their child's development. This job involves coping with the often intense emotional needs of another, and doing so in a way that is both supportive and beneficial to that child. Particularly in infant caregiving, a primary role for parents is emotion regulation – of both the infant and themselves (Thompson, 1994). Before a mother can calm her crying baby, she needs to gain control over her own negative emotions (Ablow, Marks, Feldman & Huffman, in press; Diener, Mangelsdorf, McHale, & Frosch, 2002; Mills-Koonce, Gariepy, Propper, Sutton, Calkins, Moore, & Cox, 2007). For example, the sound of a distressed or hungry child crying in the middle of the night may be aversive for an exhausted mother and could trigger a negative emotional response. However, to comfort her distressed child, a parent often needs to tolerate and regulate her own distress while mobilizing to help the child soothe and regulate (Ablow et al., in press; Martini, Root, & Jenkins, 2004). This parental regulation may take the form of increases or decreases in physiological arousal, and this study aimed to examine these processes and their role in parenting.
Researchers alternately conceptualize emotion regulation as an intra-individual process and, within a co-regulation framework, as regulation of interpersonal interactions (Diamond & Aspinwall, 2003; Cole, Martin, & Dennis, 2004). Since young infants exist primarily within relationships with caregivers, researchers have proposed a dyadic basis for infant regulation at a behavioral and physiological level (Field, 1994; Dawson, Ashman, & Carver, 2000; Dawson, Hessel, & Frey, 1994). Infants rely on caregivers to regulate their emotions, until over time they internalize these abilities. While many developmental theorists propose dyadic co-regulation, most studies of emotion regulation in adult populations have examined individuals' regulatory strategies as internalized processes during relatively isolated situations, for example watching emotion-inducing film clips (e.g., Gross, 1998; 1999; 2001; 2002; Gross & Levenson, 1993; 1997). However, regulation of emotion in daily life, even as an adult, typically involves modulating the impact of one's emotions on others (Butler & Gross, 2009; Gross & Thompson, 2007; Rime, 2009), and this is particularly true in the context of parenting. Thus, as we move from primarily child-focused research toward understanding adult regulatory processes within this co-regulatory framework, it will be important to focus on the caregiver – in this study, the mother – to better understand how regulatory processes impact her role. Co-regulation (e.g., dyadic regulatory processes) has been posited as moving beyond achieving affective homeostasis and towards a capacity for dynamic flexibility in emotional experience and regulation, allowing for mobilizing emotions or actions in service of context-specific goals (Diamond & Aspinwall, 2003). However few researchers have investigated how dyadic features of a regulatory experience play a role in an individual's physiological regulation (Butler & Gross, 2009; Gross & Thompson, 2007; Rime, 2009).
1.1 Vagal Regulation and Respiratory Sinus Arrhythmia
Vagal tone is considered an important marker of physiological regulation. The vagus nerve, originating in the tenth cranial nerve in the brain stem, projects to numerous body organs including the heart; input from these vagal pathways regulates the heart, causing increases in heart rate during inhalation and decreases during exhalation (Moore, Hill-Soderlund, Propper, Calkins, Mills-Koonce, & Cox, 2009; Porges 1994). RSA is the rhythmic change in heart rate associated with respiration and is therefore used as an index of vagal tone, which in turn provides an index of parasympathetic nervous system activity (Beauchaine, 2001; Grossman & Taylor, 2007). According to Porges' Polyvagal Theory, these pathways are related to emotion and engagement with the environment (Porges 2007; 1994), and are central to the regulation of social interaction, behavior, and emotion (Moore et al., 2009; Wilhelm, Grossman, & Coyle, 2004).
During relaxed conditions, the vagal system serves a largely homeostatic purpose, and baseline vagal tone provides a measure of the body's ability to maintain homeostasis during periods with little external challenge. However, in situations of environmental challenge, the autonomic nervous system enables increased cardiac output that is often necessary to support coping behaviors. Modulation of vagal tone provides an index of emotional functioning distinct from baseline vagal tone, and both vagal tone withdrawal and the reciprocal excitation of sympathetic tone are associated with increased emotion regulation capabilities. RSA reflects these changes in vagal tone and is thought to indicate response to and recovery from stressful experiences (Grossman & Taylor, 2007). While decreases in RSA, or vagal withdrawal, are expected in fight-or-flight activation (e.g., response mobilization or active coping), increases in RSA are associated with early phases of a stress response and with efforts to return to resting states following stress exposure (e.g., emotion regulatory processes) (Beauchaine, 2001; Grossman & Taylor, 2007; Moore & Calkins, 2004). Despite advances in theoretical conceptualizations of RSA reactivity, understanding of individual differences in RSA reactivity and associated regulatory efforts is still developing.
1.2 Maternal vagal regulation during the Still-Face Paradigm
As interest in co-regulatory processes has increased, a growing body of research has begun to examine maternal physiological reactivity during mother-infant interactions such as the Still-Face Paradigm (SFP) (e.g., Conradt & Ablow, 2010; Ham & Tronick, 2006; Moore et al., 2009; Moore & Calkins, 2004), a dyadic interaction task used to understand early mother-infant relationships (Tronick, Als, Adamson, Wise, & Brazelton, 1978). The SFP involves a face-to-face interaction between the mother-infant dyad, followed by a task phase in which the mother maintains a neutral expression and refrains from playing with her infant. In a final “reunion” face-to-face episode, the mother returns to playing normally with her child. Past research has demonstrated associations between infant behavior during the SFP and maternal care-giving quality, future attachment quality, and subsequent child behavior problems (Mesman et al., 2009). Infants often become distressed during the neutral “still face” phase – confirmed across studies as the “still face effect”—and mothers generally report that maintaining neutrality toward their infant is quite stressful (Mesman et al., 2009). Past research using the SFP (Moore et al., 2009) has indicated that mothers show increased RSA during the neutral still face episode compared to baseline, consistent with theoretical expectations of an activated regulatory response (e.g., an attempted return to resting state or homeostasis), or an early arousal response during exposure to a stressor. Ham and Tronick (2006) similarly found that mothers showed increases in RSA level during the still face episode compared to RSA during a free play interaction before the still face. Additionally, mothers whose infants were distressed during the still face showed somewhat greater RSA increases over the task as a whole than did mothers of infants who did not show distress. Together, these studies suggest that mothers' reactivity in the SFP parallels the pattern of physiological reactivity expected during a regulatory response: greater vagal reactivity (i.e., increased RSA) during the task overall, and an even greater increase in vagal reactivity when regulatory demands (i.e., infant distress) increased beyond those of basic task engagement. However, both of these studies looked at overall levels of RSA reactivity across the neutral still face task. Thus, neither can address an important aspect of dyadic coregulation: associations between maternal RSA and infant distress patterns unfolding over time.
1.3 Vagal regulation and depression
Past studies have indicated that low resting levels of RSA may be a biomarker for poor coping and impaired self-regulation (Rottenberg, 2007). Meta-analytic findings have linked both depression diagnosis and severity of depression symptoms to lower baseline RSA (d=.3; see Rottenberg, 2007). Researchers have also begun to examine links between RSA reactivity and depression. Rottenberg and colleagues (Rottenberg, Wilhelm, Gross, & Gotlib, 2003) found elevations in RSA during crying in non-depressed individuals, but did not find these elevations in depressed individuals who cried. Thus, Rottenberg suggests that physiological self-regulatory mechanisms may be compromised, or muted, in depressed individuals.
Rottenberg and colleagues (Rottenberg, Salomon, Gross, & Gotlib, 2005) additionally examined RSA reactivity as a predictor of depression course, and found that depressed individuals who demonstrated greater RSA reactivity (i.e., vagal withdrawal) to a sad film were more likely to recover from depression, suggesting that a more sensitive and reactive physiological profile was indicative of, if not even supportive of, a recovery from depression (Gentzler, Santucci, Kovacs, & Fox, 2009; Rottenberg, 2007). Thus, either increases or decreases in RSA may indicate adaptive or functioning regulatory processes in individuals with depression, depending on the context. While research has been limited, these findings support a link between depression and physiological regulatory differences. The present study aimed to explore this link further in order to understand when a particular RSA response (i.e., increases or decreases) can truly be considered “regulatory.”
1.4 The current study
In the present study, we examined patterns of RSA reactivity in the mothers of 5-month-old infants asked to maintain a neutral facial expression as part of the Still-Face Paradigm (SFP). While past regulatory and developmental research has used predominantly low-risk middle-class populations, the participants in the current study were of lower socioeconomic status and at high risk for parenting problems and depression, theoretically increasing chances of a dysregulated physiological response from mothers during this task.
We asked the following questions about women's physiological response to their distressed infants during the SFP. First, how would mothers respond physiologically when maintaining a neutral expression across the still face episode, and would their response change as their infant became more distressed? While increases and decreases in RSA may indicate regulatory processes depending on the context, our investigation of mothers' reactivity aimed to understand when a particular RSA response could be considered “regulatory.” We predicted generally increased levels of RSA across the episode as women worked to maintain neutral expressions, replicating findings of increased vagal tone in previous research on maternal RSA reactivity during the still face period (Moore et al., 2009), and conceptualized this increase as indicating an active coping or regulatory response to the still-face task. However, because most prior research examining maternal physiological response has averaged a woman's autonomic response across the neutral still face episode, the dynamics or time course of women's RSA has not been studied. In line with polyvagal regulation theory, women might evidence a decrease in RSA levels (i.e., vagal withdrawal) over the course of the neutral still face episode as their infant becomes more distressed and as they prepare to mobilize a caregiving response. As such, we predicted that the use of linear growth models would reveal a more dynamic vagal response course--in particular, vagal withdrawal as infants became more distressed.
Next, we asked whether mothers' depression symptoms might alter typical patterns of RSA reactivity during the neutral still face episode. Prior research on patterns of RSA reactivity in depressed individuals suggests that depressed individuals show muted RSA reactivity to acute stressors, as well as restricted or inconsistent RSA patterns during periods of active regulation, compared to non-depressed controls (Gentzler et al., 2009; Rottenberg, 2003). We predicted that more depressed mothers of distressed infants would show less responsivity in their physiological regulation relative to baseline compared with non-depressed mothers, thus demonstrating less RSA reactivity across the neutral still face and a muted physiological response to infant distress.
2. Method
2.1 Participants
Participating mothers (N = 105) were recruited during their third trimester of pregnancy through local childbirth education classes, hospitals, and public assistance organizations as part of a longitudinal study of risk for insensitive or unresponsive parenting. Out of 299 interested participants, 130 were contacted for initial screening using the Screening Scale for Problems in Parenting (SSPP; Avison, Turner, & Noh, 1986) and 128 were screened using a 9-item version of the Center for Epidemiological Studies-Depression scale (CES-D; Radloff, 1977). During this prenatal stage, participants who scored in the clinical range on either measure (11 and above out of a possible 25 on the SSPP and 12 and above out of a possible 36 on the CES-D) were invited to participate in the prenatal laboratory visit. Our final sample demonstrated elevated levels on at least one of these measures (CES-D, M = 9.27, SD = 5.28; SSPP, M = 17.62, SD = 4.13). Ninety-five participants returned to the laboratory again when their infants were 5 months old (M = 20.99 weeks, SD = 2.55). Of the 10 participants who did not complete the 5-month visit, one mother's baby died, two moved, four could not be reached, two had infants who were too fussy to participate in the SFP, and one had volunteered for the prenatal assessment alone, so she was not contacted for the postnatal assessment. An additional 10 participants were excluded from analyses due to high frequency of movement interference during physiological acquisition leading to a high percentage of unusable data. Two dyads could not be coded for still-face behavior due to filming error, and two dyads did not complete the SFP. The differences in participant age, marital status, education, household income, or prenatal depression score between participants who completed the 5-month assessment and those with missing data were not statistically significant.
Our final analytic pool of dyads consisted of 81 mother-infant pairs. At the 5-month assessment, these infants (35 males, 46 females) ranged in age from 16 to 32 weeks (M = 21.12 weeks, SD = 2.71), and the mother's mean age was 23.94 years (SD = 4.27, range = 18–38). Approximately 95% of the sample had a personal income of less than $20,000 and 44% had household incomes below $20,000. Approximately 27% of mothers reported a high school diploma or GED as their highest level of education, while 48% attended some college or received a 2-year degree. Most of the mothers were either married (44.4%) or living with a partner (37%). Mothers were primarily European-Americans (85%), with 3.7% African American, 2.4% Hispanic, 3.7% American Indian, and 4.9% identifying themselves as “another group.”
2.2 Procedures
When infants were 5 months, they and their mothers came into the laboratory as part of a larger assessment of dyadic interactions. Experimenters attached heart rate and respiration monitoring equipment (described below) to both mothers and infants prior to the baseline episode. The dyads then watched a 2-minute Baby Einstein video (© 2002, The Baby Einstein, LLC) while the infant sat on the mother's lap. This baseline physiology assessment was used to examine mothers' RSA while in a resting state.
2.2.1 Mother-Infant Still Face Paradigm
The Still Face Paradigm (SFP) is a 6-minute laboratory procedure used to explore interaction patterns between infants and caregivers (Tronick, Als, Adamson, Wise, & Brazelton, 1978). Following the baseline, infants were placed in a high chair approximately 18 inches from their mother. The experimenter then left the room and communicated the specific procedures of the SFP over an intercom from a separate filming room. Mothers were first asked to play with their babies (with no toys) for 2 minutes. Following SFP procedures, mothers were then signaled to turn to their left for 15 seconds, and to turn around with a neutral face for 2 minutes. During the still face portion of the task, mothers were instructed to “show an expressionless or blank face to your child, and try not to touch or talk to your child.” Following this still-face episode, mothers were signaled to turn around to their left for 15 seconds, and then to play with their baby again for 1 minute. This slightly modified version of the SFP was adapted from Lewinsohn (1996) as reported in Forbes, Cohn, Allen, and Lewinsohn, (2004). If the infant was fussy for more than 15 seconds at the start of the procedure, the interaction was stopped and the SFP was attempted again after the baby was soothed.
The SFP was video-recorded with one camera on the mother and one on the infant. A split-screen generator combined the images so that the mother and infant behaviors could be observed simultaneously. A time code was added to the recording so that physiology and behavior could be examined simultaneously in a second-by-second manner. For the purpose of the current study, only the still face portion (third episode) of the SFP was coded and analyzed.
2.2.2 Coding of maternal behaviors
Maternal suppression of emotion behavior was assessed during the two-minute neutral episode of the SFP using an adaptation of the Emotional Expressive Behavior (EEB) Coding System (Gross, 1996). Two coders unaware of the study hypotheses independently examined two dimensions of maternal behavior during the SFP: emotion valence and emotion intensity. Emotion valence was coded on an ordinal scale from 1–3, where 1 represented negative expressions, 2 represented neutral expressions, and 3 represented positive expressions. Negative expressions included clear signs of negative affect, neutral indicated little or no emotionally expressive behavior, and positive expressions were defined as clear signs of positive affect (e.g., smiling). Emotion intensity was coded on an ordinal scale from 1–5, where higher scores represented greater intensity of emotion. Codes ranged from shows no emotion-expressive behavior (1) to is very emotionally expressive (5). All codes were recorded on a second-by-second, continuous basis using the James Long Company Behavioral Coding System (James Long Company, New York). The scores were then averaged into a grand mean over the 120 seconds of the still face episode in order to obtain a measure of general maternal neutrality. Intra-class correlations between these second-by-second codes were high (α =.95), suggesting some amount of autocorrelation and statistically supporting this method of collapsing data into a more manageable level of analysis.
All tapes were coded by two independent coders. After coding was complete, coders met with the first author and resolved differences in codes for a final revised version of ratings that were used in analyses. A subset of 32 tapes (39% of the sample) was randomly selected to evaluate inter-rater reliability prior to this resolution, which proved adequate (intra-class correlations of .92 and .87 and percent agreement of 97% and 96%, respectively, for emotion valence and intensity).
2.2.3 Coding of infant behaviors
An independent team of coders rated infants' distress during the neutral episode of the SFP. Infants were coded for emotion valence and intensity of distress, as well as frequency of crying over the 2-minute still face episode. Coding of infant facial expressions was based on parameters described by Oster and colleagues in their BabyFACS manual (Oster, Hegley, & Nagel, 1992; Oster, 2005). Similar to mothers, emotion valence was coded on an ordinal scale from 1–3, where 1 represented negative expressions (i.e. clear signs of negative affect or fussing), 2 represented neutral expressions, and 3 represented positive expressions. Distress intensity was coded on a 1–5 scale, where higher scores represented greater intensity of distress. Codes ranged from shows no distress (1) to big cry-face, defined as a facial expression with furrowed brows as well as eye movement/closed eyes and stretching of the mouth (5). Crying was coded as absent or present.
All codes were rated on a second-by second continuous basis using the James Long Company Behavioral Coding System. High intra-class correlations between second-by-second infant distress codes (α = .99), suggested substantial temporal autocorrelation and the advisability of aggregating scores. At the same time, our interest in differences in behavior across the episode made averaging across all data points undesirable. Therefore, we averaged distress scores over 10 second intervals, which paralleled the time-blocks for physiology data (see below) and facilitated comparisons of infant behavior and maternal physiology over the entire episode.
Tapes were coded by two independent coders using similar procedures as described above for coding of maternal behavior. Coder agreement was lower according to intra-class correlations, .66, .56, and .67 for emotion valence, distress intensity, and smile intensity respectively. However due to variations in, and limited range of, scales used for these codes, rates of coding disagreement may have been inflated by these Kappa calculations and percent agreement serves as a more accurate representation of coding reliability: 87.75% (emotion valence), 74.79% (distress intensity), and 91.66 (smile intensity).
2.3 Measures
2.3.1 Maternal depression
The Center for Epidemiologic Studies - Depression Scale (CES-D; Radloff, 1977) was used to assess maternal self-report of depression symptoms. Mothers rated 20 symptoms on a scale from 0 (rarely) to 3 (most or all of the time) for the past week, with a sum computed to index total symptoms. We also created a dichotomous scale for the CES-D based on the established cutoff of 16 for clinically significant symptoms (e.g., Wellisch & Lindberg, 2001). In our sample, 32% of women (n = 19) were classified as depressed according to this cutoff.
2.3.2 Mother RSA and movement measures
Mother's physiological responses were collected with a 21-channel Bioamplifier (model JCA-09) manufactured by the James Long Company (Caroga Lake, NY) and disposable self-adhesive vinyl electrodes (TD-142G) connected via alligator-clip electrode leads. The experimenter placed electrodes axially on the left-rib and right-rib at the same elevation as the heart. The ground electrode was placed on the left collarbone. A respiration bellows was placed at the height of the zyphoid process to measure inspiration and expiration (see Table 1 for sample-level respiration).
TABLE 1.
Demographic Data and Descriptive Statistics for Sample
| Percentage | M(SD) | Range | |
|---|---|---|---|
| Mother Age (years) | 23.94 (4.27) | 18–38 | |
| Education | |||
| Some HS or HS degree | 33.8 | ||
| Some college | 48.1 | ||
| 4-year college degree | 7.8 | ||
| Graduate or professional degree | 3.9 | ||
| European American | 81.0 | ||
| Married | 44.4 | ||
| Family incomea,b | 21,000–40,000 | 1–90,000 | |
| Employed full-time | 13.6 | ||
| Employed part-time | 25.9 | ||
| Child age (weeks) | 21.12 (2.71) | 16–32 | |
| Female children | 53 | ||
| Postnatal CES-D Score | 9.94(8.33) | 0–37 | |
| Depressed (CESD ≥16) | 32 (n=19) | ||
| Expressive Suppression Score | 2.00 (.11) | 1.78–2.63 | |
| Baseline RSA | .066 (.047) | .00–.26 | |
| Mean Still Face RSA | .104 (.066) | .014–.30 | |
| Baseline Heart Rate | 83.48 (10.13) | 53.64–104.49 | |
| Mean Still Face Heart Rate | 79.78 (9.50) | 54.69–102.84 | |
| Mean Baseline Respiration | 3.97 (2.46) | 1.98–15.47 | |
| Mean Still Face Respiration | 3.75 (1.90) | 2.63–19.13 | |
| Movement | .0172 (.008) | 0.0–.04 |
Median is reported;
Income reported in dollars
During the experimental session, electrocardiogram (ECG) and respiration physiological channels were sampled continuously with low-pass filtering at 1000 Hz. High-pass filtering was recorded at 0.03 Hz. All subsequent processing of interbeat intervals (IBIs) and editing of outliers in the R-R series were carried out using the IBI Analysis System and PHY General Analysis System from James Long (Caroga Lake, NY). Artifactual epochs were edited manually for each channel for incorrect detection of the R-wave or movement artifacts. For artifact editing, the sampled ECG signal was viewed graphically. When an R-wave was obscured or undetected by the software, a tick mark was manually inserted into the graphical ECG record based on the specific editing rules of Byrne and Porges (1993). Consistent with previous research (Moore & Calkins, 2004), editing the files included the identification of outlier points relative to adjacent data and replacing them by determining the time between successive interbeat intervals. Data files that required editing more than 2–3% of the data were not included in the analyses.
RSA was computed using respiration and IBI data as outlined by Grossman's peak-valley technique (Grossman, 1983; Grossman, van Beek, & Wientjes, 1990). The difference between the minimum IBI during inspiration and the maximum IBI during expiration, measured in seconds, was used to calculate RSA. The difference was computed twice for each respiration cycle, once for each inspiration and once for each expiration. The time for each arrhythmia sample was assigned as the midpoint between an inspiration time and an expiration time. Using this method, RSA was computed without being impacted by arrhythmia due to baroreceptor, thermoregulation, and tonic shifts in heart rate (Schuete, Eiden, & Coles, 2007). While a spectral method of estimating RSA is often used instead of the peak-to-valley method described above, studies suggest that the various methods of calculating RSA are highly correlated with each other (Beauchaine, 2001).
Continuous measures of whole-body activity were also monitored, as movement could affect heart rate and respiration. Movement was collected by placing a piezo-electric accelerometer (one axis) on the mother's chair. The gain was adjusted to take into account stiffness of chair and the weight of participant. The average movement score was .0172 (SD = .008, range = 0 – .04), with higher scores indicating greater movement (see Table 1). A score of 0 indicated that no movement was detected. In order to control for mothers' movement in analyses, we created a grand mean for movement during the still face episode, and found no differences based on maternal depression status (p = ns).
2.4 Analytic Strategy
Because the data under investigation – RSA levels within a mother-infant dyad measured over time – were dependent, multilevel modeling was selected as the analytic framework of choice. At Level 1, within-dyad variation in mother RSA across the still face episode was modeled with a set of growth parameters (i.e., intercept, slope, quadratic) that were allowed to vary across dyads. Other within-dyad characteristics varying over time that could help to explain RSA variability, such as infant distress, were added as covariates at Level 1. At Level 2, between-dyad variability in these Level 1 parameters was explained by characteristics that varied across mother-infant dyads – in this case, maternal CESD depressive symptoms.
3. Results
3.1 Preliminary Analyses
3.1.1 Data characteristics and assumptions
Variables of interest were checked for violations of normality and for patterns of missingness that could bias results. All variables of interest – i.e., mother RSA, infant distress, CES-D depression – showed acceptable levels of positive skew (skewness between 1 and 2, M of 1.48), with no influential outliers. HLM deals with missing data at Level 1 (in this case, mother RSA and infant distress values) through full information maximum likelihood estimation, which arrives at optimal parameter estimates based on available data. Out of 1,365 possible time points, 1,051 mother RSA values were available (77%), as were 1,101 infant distress values (81%), providing an adequate basis for model fitting. At Level 2, cases missing model-relevant data (in this case, maternal depression) were deleted; in this sample, missingness at Level 2 (affecting 29 out of a possible 105 cases) did not relate to any of the other variables tested, suggesting that missing data were unlikely to systematically bias the relationships found below.
3.1.2 Differences as a function of sample demographics
Based on a series of preliminary analyses (t-tests for continuous measures, Mann-Whitney for categorical measures), we found no significant differences in participant age, ethnicity, marital status, education, or household income related to depression status or RSA (see Table 1 for demographic data).
3.1.3 Maternal expressive suppression during the still face episode
Consistent with instructions for emotion suppression and maintenance of facial neutrality during the still face, women typically exhibited a neutral or non-emotional behavioral response across the episode as a whole (M = 2.00, SD = .11), and descriptive analyses of maternal expression codes showed minimal occasions of high emotion expression of either valence. These findings suggest that in general mothers were able to comply with the behavioral demands of the task by suppressing visible emotion behavior during the still face paradigm. Additional analyses confirmed that depressed (M = 1.98, SD = .07) and non-depressed (M = 2.02, SD = .12) mothers were comparable in their ability to maintain expressions of emotional neutrality, t(79) = .96, p = .34.
3.1.4 Baseline and Still Face RSA
Continuous RSA values were available across the still face episode and during the baseline preceding the SFP. We aggregated RSA levels (temporal autocorrelation α = .98) during the resting baseline to determine mean initial levels of RSA for our analytic models. We found no significant differences in resting baseline for depressed (M = .07, SD = .04) compared to non-depressed mothers (M = .07, SD = .07), t(79) = .06, p = .96.
We aggregated continuous RSA data during the still face episode as thirteen 10-second means to allow for analyses over time. Previous research examining infant and maternal RSA reactivity during the still-face has suggested calculation of short epochs in order to observe patterns over time (Huffman, Bryan, Del Carmen, Pedersen, Doussard-Roosevelt, et al., 1998; Moore et al., 2009). The grand mean of these thirteen, 10-second still face RSA averages showed no significant difference (t(79) = 1.22, p = .23) between depressed (M = .12, SD = .07) and non-depressed (M = .09, SD = .07) mothers during the still face episode. In addition, differences between depressed and non-depressed mothers' RSA failed to emerge across any of the individual 10-second epochs (range of t-scores = .15 – 1.38, range of p = .08 – .878).
3.2 Maternal RSA reactivity during the still face episode
Before beginning multilevel modeling, we examined the difference between mean-levels of RSA during the baseline segment and the still face episode to compare our sample to previous studies (Ham & Tronick, 2006; Moore et al., 2009). We found a significant differencebetween baseline RSA (M = .067, SD = .047) and mean RSA during the still face episode (M = .104, SD = .066, t(77) = 2.94, p < .01) that did not vary by maternal depression (t(76) =1.01, p = .32). This increase in average RSA levels was moderate in size (d = .67), suggesting a substantial increase in mothers' RSA in response to the still face.
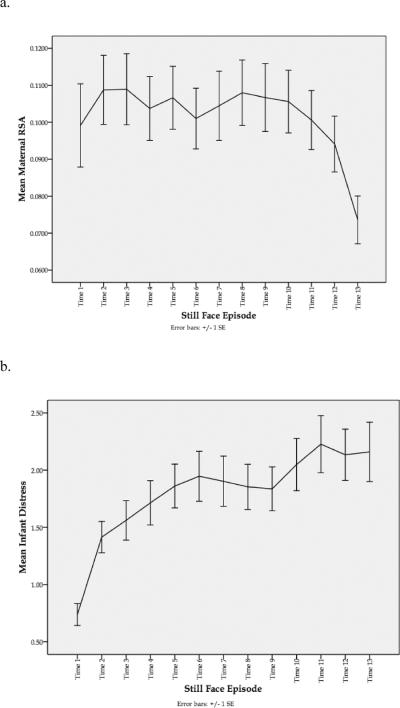
However, a graphical illustration of mothers' RSA levels across the entire still face episode (see Figure 1a) suggested that this type of data aggregation obscured dynamic aspects of mothers' physiological functioning during the task. As shown in Figure 1a, women tended to show a non-linear response pattern characterized by increased RSA during the earlier moments of the still face episode, followed by decreased levels of RSA during the latter moments of the episode. Additionally, the error bars presented in Figure 1a highlight the amount of individual variability in women's RSA levels during each time point of the still face. To understand better both the course and potential sources of variability in mothers' RSA across the episode, we next conducted a series of HLM analyses.
FIGURE 1.
Illustration of trajectories for maternal RSA and infant distress in 10-second increments across the neutral still face episode. (a) Mean level of mother RSA across the neutral still face episode. (b) Mean level of infant distress across the neutral still face episode.
3.3 HLM Analyses
Hierarchical Linear Modeling (HLM 6.0; Raudenbush & Bryk, 2002) was used to fit a series of growth models to describe mothers' RSA trajectories across the still face episode and to investigate the associations of individual growth curves with infant distress and maternal depression. First, a baseline model containing no predictors was run to derive average RSA trajectories and variability in trajectories between women. Then, a series of potential control variables were tested for inclusion, with those found significant retained for further model testing. The primary explanatory models tested a) covariation of mother RSA with infant distress across the episode (Level 1 prediction), and b) the effect of maternal depressive symptoms both on this within-dyad covariation and on between-dyad variability in mothers' RSA trajectories themselves (Level 2 prediction). Results of model testing are discussed below.
3.3.1 Baseline and control variables
All growth curve models were centered at time 6 so that intercept terms represented mothers' RSA level in the middle of the still face episode. This was also the point at which infant distress was observed to increase to a level indicating observable distress (coded greater than or equal to 2) in the sample. The change in maternal RSA and increase in infant distress are illustrated in Figure 1, with Figure 1a showing mothers' mean RSA trajectory across the still face episode and Figure 1b showing corresponding changes in infant distress across the same time episodes. Baseline model testing revealed that a quadratic growth curve explained the data better than a linear model alone, as shown by the deviance statistic, χ2(3) = 164.10, p < .001. A significant negative quadratic component demonstrated that, on average, mothers showed a rising and falling RSA curve across the still face. This means that even though mothers' RSA during the Still Face was higher, on average, than at baseline, within the Still Face episode their RSA followed a curvilinear pattern. The linear term – the slope of the curve at time 6 -- was nonsignificantly different from zero, indicating that the curve had reached its maximum, and that mothers tended to peak at this point roughly halfway through the episode. At the same time, all of the model terms exhibited significant variability, intercept χ2(75) = 1670.25, p < .001; linear slope χ2(75) = 113.42, p < .003; quadratic χ2(75) = 392.85, p < .001, meaning that individual mothers differed from this average trajectory in ways that might be explained by adding Level 2 (between-dyad) predictors. Furthermore, significant Level 1 variability (σ2 = .0022, SE = .0001) suggested that moment-to-moment variability in mother RSA was not fully explained by her fitted trajectory, thus within-dyad predictors might be added to characterize change in RSA.
Of the between-dyad control variables tested at Level 2 – mean mother movement across the still face and mean baseline mother RSA – only the latter significantly predicted mothers' trajectories; as would be expected, higher baseline RSA predicted a higher still face episode intercept. Variation in mothers' emotional expression across the still face episode was also tested as a within-dyad predictor of RSA at Level 1; this predictor was non-significant.
3.3.2 Explanatory models: Maternal RSA-infant distress associations (Table 2.)
TABLE 2.
Final Model Predicting Mothers' Still Face RSA
| Predictor | Coefficient | SE | P |
|---|---|---|---|
| Intercept (level at time 6) | .107 | .008 | <.001 |
| Mean Baseline RSA | .434 | .211 | .04 |
| Linear | 2.7 × 10−5 | 8.8 × 10−5 | .76 |
| Quadratic | −6 × 10−6 | 2 × 10−6 | .005 |
| Infant Distress | −.005 | .002 | .03 |
| Mother CESD | .0004 | .0002 | .02 |
Infant distress scores across the still face (centered around each infant's mean) were tested as Level 1 time-varying covariates of mothers' RSA. A significant inverse relationship was found such that as infant distress increased, maternal RSA tended to decrease. However, significant variability in this parameter, χ2(74) = 140.68, p < .001, also suggested that this was not characteristic of all dyads.
Mothers' CESD scores (centered around the grand mean for the sample) were entered as a Level 2 predictor of the infant distress covariate, and a significant positive effect revealed that maternal depression attenuated this negative within-dyad association (see Table 2). In other words, mothers with more depressive symptoms showed less RSA responsivity to their infant's distress across the episode.
Given interest not only in effects of continuous depressive symptoms, but also in clinically relevant differences between mothers below and above a critical depression threshold, we followed up on the above finding by testing a dichotomous variable indicating whether mothers were below (0) or above (1) the clinical cutoff of 16 points on the CESD. These results revealed that, whereas mothers below the clinical threshold showed the expected negative association between RSA and infant distress (coefficient = −.007, p = .01), mothers above the threshold did not show this association (coefficient = .0018, ns; see Figure 2). In other words, whereas non-depressed mothers' RSA decreased (becoming more physiologically activated) as their infants became more distressed, the depressed mothers showed little or no RSA change as a function of infant distress.
FIGURE 2.
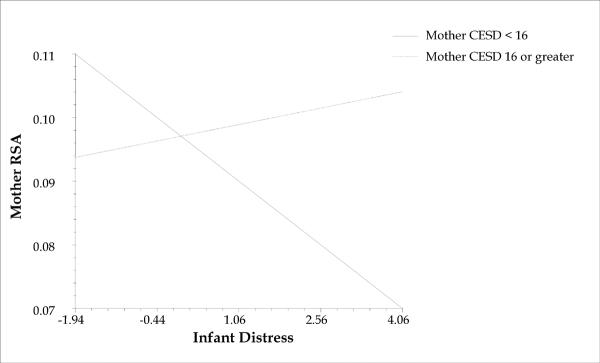
Relationship of mother RSA to infant distress for mothers above and below clinical cutoff for CESD depressive symptoms.
3.3.3 Explanatory models: Maternal RSA trajectories
Contrary to predictions, mothers' continuous CESD scores failed to predict variability in the RSA trajectories (intercept, linear, or quadratic terms) themselves, meaning that more depressed mothers did not differ from less depressed mothers in the level or shape of RSA response curves. Maternal depression also failed to predict RSA across the still face in a variable-intercept model that made no assumptions about a particular time course of RSA. Together, these models confirmed that mothers' depression did not predict the level or shape of their RSA profiles, though it did predict the way their physiology responded (or rather, failed to respond) to their infant's distress during the still face.
3.4 Summary
The model described above (and in Table 2) containing mothers' continuous depressive symptoms as the sole predictor of covariation with infant distress was retained as the final model. Further inferential analyses demonstrated no significant association between infant level of distress and maternal depressive symptoms, r = −.01, p = .92. Compared to baseline, our final model explained 12% of between-dyad variability in maternal RSA, 21% of within-dyad variability in maternal RSA, and 15% of overall variability in maternal RSA. Overall, then, mothers exhibited a pattern of rising and falling RSA across the still face episode, and this pattern was not associated with individual differences in women's depression scores. Instead, there was a significant association between depression and mothers' RSA as a function of infant distress such that non-depressed mothers showed a decrease in RSA (or increased arousal) in response to their infant's distress, and depressed mothers showed a slight albeit non-significant increase in RSA in response to their infant's distress.1
4. Discussion
The current study examined mothers' physiological regulation during infant distress in the Still-Face Paradigm. Overall, women in this sample demonstrated RSA reactivity across the still face period, evidenced by an increase in RSA over baseline levels that replicates prior research (Ham & Tronick, 2006; Moore et al., 2009). A more fine-grained temporal analysis revealed that mothers typically followed an initial RSA increase with a decrease during the period of peak infant distress, suggestive of an acute response to a stressor and an active regulatory response. This vagal withdrawal associated with infant distress may additionally reflect the physiological prerequisites for response mobilization, in this case preparation for soothing parenting behaviors. Finally, while maternal depression did not impact RSA trajectories themselves, depressed mothers failed to respond to their infants' distress with decreased RSA as did their non-depressed counterparts, tending instead to evidence either no change in RSA in response to their infant's distress or even increasing RSA levels in response to infant distress.
Our first group of analyses explored mothers' physiological responses during the still face episode while maintaining a neutral expression. It should be noted that mothers demonstrated uniform behavioral suppression across the task, regardless of infant distress or depression status, indicating that differences in expressive suppression behavior did not drive physiological differences. Studies by both Moore and colleagues (2009) and Ham and Tronick (2006) found RSA to increase in mothers during the still face episode of the SFP compared to a preceding baseline. These studies explained increased RSA as indicating an activation of regulatory processes in response to infant distress, a pattern echoed in the current sample when overall baseline versus still face episode means were compared. However, a slightly different picture emerged when we examined RSA patterns over the course of the entire still face episode. Specifically, we found that mothers' RSA levels demonstrated a pattern of initial increase across the first half of the episode, followed by a decrease. The initial increase may represent the expected regulated or calm state resulting from active regulatory processes in response to low-level infant distress signals or even a lack of distress (Butler, Wilhelm, & Gross, 2006). On the other side, the RSA decline coinciding with sample-level increases in infant distress may indicate a point at which mothers experienced their infant's distress as increasingly arousing and initiated an active coping or mobilizing regulatory response, activating physiologically and perhaps also experiencing a call to action for parenting behavior.
In the particular context of parenting, our findings are in line with prior research positing that mothers who more sensitively modulate their own internal emotions are better able to respond in a contingently responsive manner to their infants (Ablow et al., in press; Mills-Koonce et al., 2007; Moore et al., 2009). When an infant is distressed, a mother who responds with increased arousal and reactivity (i.e., decreased RSA, also indicating the initiation of an effective regulatory response) may be better able to respond actively, flexibly, and sensitively to the infant (Ablow et al., in press; Hill-Soderlund, Mills-Koonce, Propper, Calkins, Granger, Moore, et al., 2008; Moore et al., 2009), and this has been demonstrated in the animal literature as well (Mills-Koonce et al., 2007). Thus, the change from increasing to decreasing RSA shown by mothers as infants reached their peak levels of distress suggests an adaptive physiological reaction to this stressor (Ablow et al., in press; Hill-Soderlund et al., 2008; Mills-Koonce et al., 2007; Moore et al., 2009). In the context of the neutral still face episode, mothers are showing a potentially adaptive physiological response even in the absence of the freedom to express this mobilization behaviorally (Ablow et al., in press; Hill-Soderlund et al., 2008; Mills-Koonce et al., 2007; Moore et al., 2009).
In contrast, women who respond to their infants' distress with increased parasympathetic (RSA) regulation would appear not to be readying a comforting response aimed at the infant or responding with an active (e.g., dynamic) regulatory response, but rather are focused on maintaining homeostasis or retaining their own calm state. As such, the ability to maintain physiological arousal (decreased RSA) to infant distress observed in this sample overall may serve as a marker of maternal sensitivity to infant needs, whereas diminished arousal (increased RSA) may aid in maintaining intra-personal homeostasis but block a timely and sensitive parenting response. In line with this explanation, Ablow and colleagues (in press) found that expectant women who showed significant RSA withdrawal (decrease) in response to a recording of infant distress later demonstrated greater sensitivity with their own infants. It will be important in future studies of maternal RSA during the Still Face Paradigm to additionally measure regulatory responses during the post-still face reunion phase in order to determine whether this proposed differential pattern continues during a recovery phase and how reactivity/regulatory patterns relate to mothers' sensitivity in dyadic interactions.
In a final research question, to understand how depression status may impact patterns of RSA reactivity during parenting stress, we tested effects of both continuous depression symptoms and depression status (above/below clinical cutoff). We found that non-depressed mothers showed a clear pattern of decreased RSA as infant distress increased, paralleling the pattern observed in the overall sample. In contrast, depressed mothers showed a different – though statistically non-significant - response, specifically a pattern of stable or unchanged RSA with increased infant distress. This is consistent with past research showing less flexible or efficient RSA responses to regulation tasks among depressed individuals (e.g., Rottenberg, 2007). Further, the task demands of the still face - specifically, maintenance of a neutral face - may mimic a withdrawal strategy already employed by mothers suffering from depression, facilitating physiological disengagement in the face of infant distress (Conradt & Ablow, 2010). Such patterns can be typical of depressed mothers and often portend behavioral insensitivity (Field, 1994; Dawson, Ashman, & Carver, 2000; Dawson, Hessel, & Frey, 1994; Mesman et al., 2009). We speculate that these mothers may have learned to “regulate” physiologically by withdrawing from their infants in order to soothe themselves, though at a cost to the development of the mother-infant relationship.
4.1 Contributions and Limitations of the Present Study
The current study is the first that we know of to examine mothers' continuous parasympathetic activity across the still face episode. Most previous studies of mothers' physiological reactivity during the still face have examined mean levels of reactivity across the episode and, convergent with our findings, have found mean-level RSA increases during the still face compared to baseline consistent with what we would expect to see during regulatory processes according to the Polyvagal Theory (Ham and Tronick, 2006; Moore et al., 2009). However, our time-series examination of RSA patterns across the still face suggests that despite overall increases from baseline, maternal reactivity may fluctuate across the still face episode in response to infant cues, and during infant distress mothers actually show vagal withdrawal suggesting reactivity to stress or initiation of a regulatory response. These findings support the importance of multi-method statistical approaches to examining RSA reactivity.
In this study we examined regulatory behaviors and associated physiological reactivity in high-risk first-time mothers not as a purely intra-individual phenomenon, but rather through the lens of a well-established dyadic laboratory task, the Still-Face Paradigm. This novel approach to understanding emotion regulation provided an important extension to the field's current understanding of relations among RSA reactivity, emotion regulation, and depression. The atypical physiological pattern we observed of unchanged/increased RSA in depressed mothers during infant distress may play a role in development of early parenting problems and could serve as a mechanism linking maternal depression and associated child developmental outcomes, such as behavioral problems and increased prevalence of depression (Lovejoy, Graczyk, O'Hare, & Neuman, 2000).
However, this study is not without limitations. Our sample size was relatively small, particularly with regard to the number of mothers meeting our cut-off for depression, and generalizations to clinically diagnosed populations should be made with care. Despite this low number of depressed women, it should also be noted that our sample was selected for elevated depression symptoms during prenatal screening. Thus our overall sample represents a high-risk group even while our pool of mothers with current elevated depression was limited. Our reliance on depression symptomatology as a measure of maternal psychopathology may have limited our findings as well. Secondary models indicated a possible impact of anxiety on mothers' RSA reactivity, and past research has linked anxiety to atypical patterns of RSA reactivity (e.g., Watkins, Grossman, Krishnan, & Sherwood, 1998). Future research may tease apart the combined roles of anxiety, depression, and psychopathology in general in physiological and behavioral regulation.
Further, while changes in RSA are generally considered to indicate a regulatory response, there is often a lack of consensus among experts on the meaning of increases and decreases in RSA in terms of reactivity and regulatory processes. Interpretations of reactivity and arousal versus regulatory responses are often dependent on contextual factors, observations over time or in sequential episodes, and comparisons with other physiological measures. Since we examined solely maternal parasympathetic activity during a single episode of this dyadic paradigm, we are limited in drawing firm conclusions about the meaning of changes in RSA. However, our observation of normative and depression-related differences in RSA within a dyadic coregulation context helps to inform a concept of maternal regulation that includes both increasing and decreasing physiological arousal.
Finally, while past research has found the still face to be a stressful task for mothers and infants (Ham & Tronick, 2006), there are individual differences in the level of distress experienced, and we cannot be sure that this represented a regulatory task equally for all mothers. Future research measuring participants' self-reports on emotion experience during the task can further determine factors affecting behavioral and autonomic regulatory responses.
4.2 Conclusion
In summary, the current study was the first to examine patterns of maternal RSA reactivity across the duration of the still face episode. Our unique framework approached the still face episode as a dyadic regulatory task requiring mothers to modulate their individual emotion experience simultaneous with that of their infant, merging adult emotion-focused and child development research methodologies. These results begin to inform conceptualizations of regulation as a dyadic and physiological phenomenon across the lifespan. Findings additionally extend our understanding of the association between depression and reactivity to parenting stress, and indicate a pathway through which sensitive and responsive parenting may be impacted by current psychopathology.
Highlights
-
•
Mothers' behavior and physiology were measured during the Still Face Paradigm.
-
•
Patterns of physiological (RSA) reactivity during infant distress examined.
-
•
Mean-level increase in RSA during still face indicated overall regulatory response.
-
•
Time-course analysis of RSA patterns revealed initial increase then decrease.
-
•
In contrast to this mobilization response, depressed mothers had stable RSA pattern.
Acknowledgements
This research was supported by the National Institutes of Mental Health grant 1 R03 MH068692-01A1, the Associate Dean of Natural Sciences, University of Oregon, Discretionary Funds Award, an Oregon Community Credit Union Fellowship, and the National Science Foundation, BCS-Social Psychology Program. Additional funding was provided to the first author through the National Science Foundation Graduate Research Fellowship. Portions of this paper were presented as a poster at the 2008 Conference of the International Society for Infant Studies. We would also like to thank Elisabeth Conradt for editorial assistance and all the mothers and children who made this study possible.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Because anxiety symptoms are closely tied to depression symptoms and RSA reactivity (e.g., Tanaka-Matsumi & Kameoka, 1986; Watkins, Grossman, Krishnan, & Sherwood, 1998), in supplemental analyses we also ran our analytic model with anxiety alone (Beck Anxiety Inventory, BAI; Beck & Steer, 1990) and with both anxiety and depression. We found that, similar to depression, anxiety predicted a more positive though non-significant relationship between infant distress and mother RSA. In a model with continuous scores of anxiety and depression, we found that neither one contributed unique predictive power (both variables ns). We concluded that a distress component may be shared between depression and anxiety self-reports, and this component may play a role in predicting the RSA-infant distress covariance described in our primary analyses. This overlap is similar to past research finding high associations between anxiety and depression self-report (Tanaka-Matsumi & Kameoka, 1986) and to the negative affect factor described as an overlapping element of anxiety and depression in Clark and Watson's tripartite model (Mineka, Watson, & Clark, 1998). Thus, an important direction for future research will be examination and teasing apart of the joint role of anxiety and depression.
References
- Ablow JC, Marks AK, Feldman SS, Huffman LC. Associations between first-time expectant women's representations of attachment and their physiological reactivity to infant cry. Child Development. doi: 10.1111/cdev.12135. in press. [DOI] [PubMed] [Google Scholar]
- Avison WR, Turner RJ, Noh S. Screening for problem parenting: Preliminary evidence on a promising instrument. Child Abuse and Neglect. 1986;10(2):157–170. doi: 10.1016/0145-2134(86)90077-3. doi:10.1016/0145-2134(86)90077-3. [DOI] [PubMed] [Google Scholar]
- Beauchaine TP. Vagal tone, development, and Gray's motivational theory: Toward an integrated model of autonomic nervous system functioning in psychopathology. Development and Psychopathology. 2001;13:183–214. doi: 10.1017/s0954579401002012. doi:10.1017/S0954579401002012. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA. Manual for the Beck Anxiety Inventory. Psychological Corporation; San Antonio, TX: 1990. [Google Scholar]
- Butler EA, Wilhelm FH, Gross JJ. Respiratory sinus arrhythmia, emotion, and emotion regulation, during social interaction. Psychophysiology. 2006;43:612–622. doi: 10.1111/j.1469-8986.2006.00467.x. doi: 10.1111/j.1469-8986.2006.00467.x. [DOI] [PubMed] [Google Scholar]
- Butler EA, Gross JJ. Emotion and emotion regulation: Integrating individual and social levels of analysis. Emotion Review. 2009;1(1):86–87. doi: 10.1177/1754073908099131. [Google Scholar]
- Byrne EA, Porges SW. Data-dependent filter characteristics of peak-valley respiratory sinus arrhythmia estimation: A cautionary note. Psychophysiology. 1993;30:397–404. doi: 10.1111/j.1469-8986.1993.tb02061.x. doi: 10.1111/j.1469-8986.1993.tb02061.x. [DOI] [PubMed] [Google Scholar]
- Cole PM, Martin SE, Dennis TA. Emotion regulation as a scientific construct: Methodological challenges and directions for child development research. Child Development. 2004;75(2):317–333. doi: 10.1111/j.1467-8624.2004.00673.x. doi: 10.1111/j.1467-8624.2004.00673.x. [DOI] [PubMed] [Google Scholar]
- Conradt E, Ablow J. Infant physiological response to the still-face paradigm: Contributions of maternal sensitivity and infants' early regulatory behavior. Infant Behavior and Development. 2010;33:251–265. doi: 10.1016/j.infbeh.2010.01.001. doi: 10.1016/j.infbeh.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Dawson G, Ashman SB, Carver LJ. The role of early experience in shaping behavioral and brain development and its implications for social policy. Development and Psychopathology. 2000;12:695–712. doi: 10.1017/s0954579400004089. doi:10.1017/S0954579400004089. [DOI] [PubMed] [Google Scholar]
- Dawson G, Hessel D, Frey K. Social influences on early developing biological and behavioral systems related to risk for affective disorder. Development and Psychopathology. 1994;6:759–779. doi: 10.1017/S0954579400004776. [Google Scholar]
- Diamond LM, Aspinwall LG. Emotion regulation across the life span: An integrative perspective emphasizing self-regulation, positive affect, and dyadic processes. Motivation and Emotion. 2003;27(2):125–156. doi: 10.1023/A:1024521920068. [Google Scholar]
- Diener ML, Mangelsdorf SC, McHale JL, Frosch CA. Infants' behavioral strategies for emotion regulation with fathers and mothers: associations with emotional expressions and attachment quality. Infancy. 2002;3(2):153–174. doi: 10.1207/S15327078IN0302_3. doi: 10.1207/S15327078IN0302_3. [DOI] [PubMed] [Google Scholar]
- Field T. The effects of mothers' physical and emotional unavailability on emotion regulation. Monographs of the Society for Research in Child Development. 1994;59(2/3):208–227. doi:10.2307/1166147. [PubMed] [Google Scholar]
- Forbes EE, Cohn JF, Allen NB, Lewinsohn PM. Infant affect during parent-infant interaction at 3 and 6 months: Differences between mothers and fathers and influence of parent history of depression. Infancy. 2004;5(1):61–84. doi: 10.1207/s15327078in0501_3. doi: 10.1207/s15327078in0501_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentzler AL, Santucci AK, Kovacs M, Fox NA. Respiratory sinus arrhythmia reactivity predicts emotion regulation and depressive symptoms in at-risk and control children. Biological Psychology. 2009;82:156–163. doi: 10.1016/j.biopsycho.2009.07.002. doi: 10.1016/j.biopsycho.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JJ. Emotional Expressive Behavior (EEB) Coding System. 1996 [Google Scholar]
- Gross JJ. Antecedent- and response-focused emotion regulation: Divergent consequences for experience, expression, and physiology. Journal of Personality and Social Psychology. 1998;74(1):224–237. doi: 10.1037//0022-3514.74.1.224. doi: 10.1037/0022-3514.74.1.224. [DOI] [PubMed] [Google Scholar]
- Gross JJ. Emotion regulation: Past, present, future. Cognition and Emotion. 1999;13(5):551–573. doi: 10.1080/026999399379186. [Google Scholar]
- Gross JJ. Emotion Regulation in Adulthood: Timing is everything. Current Directions in Psychological Science. 2001;10(6):214–219. doi: 10.1111/1467-8721.00152. [Google Scholar]
- Gross JJ. Emotion regulation: Affective, cognitive, and social consequences. Psychophysiology. 2002;39:281–291. doi: 10.1017/s0048577201393198. doi: 10.1017/S0048577201393198. [DOI] [PubMed] [Google Scholar]
- Gross JJ, Levenson RW. Emotional Suppression: Physiology, self-report, and expressive behavior. Journal of Personality and Social Psychology. 1993;64(6):970–986. doi: 10.1037//0022-3514.64.6.970. doi: 10.1037/0022-3514.64.6.970. [DOI] [PubMed] [Google Scholar]
- Gross JJ, Levenson RW. Hiding feelings: The acute effects of inhibiting negative and positive emotion. Journal of Abnormal Psychology. 1997;106(1):95–103. doi: 10.1037//0021-843x.106.1.95. doi: 10.1037/0021-843X.106.1.95. [DOI] [PubMed] [Google Scholar]
- Gross JJ, Thompson RA. Emotion regulation: Conceptual foundations. In: Gross JJ, editor. Handbook of Emotion Regulation. Guilford Press; New York: 2007. pp. 3–24. [Google Scholar]
- Grossman P. Respiration, stress, and cardiovascular function. Psychophysiology. 20(3):284–300. doi: 10.1111/j.1469-8986.1983.tb02156.x. 1983 doi: 10.1111/j.1469-8986.1983.tb02156.x. [DOI] [PubMed] [Google Scholar]
- Grossman P, Taylor EW. Toward understanding respiratory sinus arrhythmia: Relations to cardiac vagal tone, evolution, and biobehavioral functions. Biological Psychology. 2007;74:263–285. doi: 10.1016/j.biopsycho.2005.11.014. doi: 10.1016/j.biopsycho.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Grossman P, van Beek J, Wientjes C. A comparison of three quantification methods for estimation of respiratory sinus arrhythmia. Psychophysiology. 1990;27:702–714. doi: 10.1111/j.1469-8986.1990.tb03198.x. doi: 10.1111/j.1469-8986.1990.tb03198.x. [DOI] [PubMed] [Google Scholar]
- Ham J, Tronick E. Infant resilience to the stress of the Still-Face: Infant and maternal psychophysiology are related. Annals of the New York Academy of Sciences. 2006;1094:297–302. doi: 10.1196/annals.1376.038. doi: 10.1196/annals.1376.038. [DOI] [PubMed] [Google Scholar]
- Hill-Soderlund AL, Mills-Koonce WR, Propper C, Calkins SD, Granger DA, Moore GA, Gariepy J, Cox MJ. Parasympathetic and sympathetic responses to the strange situation in infants and mothers from avoidant and securely attached dyads. Developmental Psychobiology. 2008;50:361–376. doi: 10.1002/dev.20302. doi: 10.1002/dev.20302. [DOI] [PubMed] [Google Scholar]
- Huffman LC, Bryan YE, Del Carmen R, Pedersen FA, Doussard-Roosevelt JA, Porges SW. Infant temperament and cardiac vagal tone: Assessments at twelve weeks of age. Child Development. 1998;69:624–635. doi: 10.1111/j.1467-8624.1998.tb06233.x. [PubMed] [Google Scholar]
- Lewinsohn . Oregon Research Institute infant development study wave 1–wave 4 assessment protocol and documentation. 1996. Unpublished manual. [Google Scholar]
- Lovejoy MC, Craczyk PA, O'Hare E, Neuman G. Maternal depression and parenting behavior: A meta-analytic review. Clinical Psychology Review. 2000;20(5):561–592. doi: 10.1016/s0272-7358(98)00100-7. doi: 10.1016/S0272-7358(98)00100-7. [DOI] [PubMed] [Google Scholar]
- Martini TS, Root CA, Jenkins JM. Low and middle income mothers' regulation of negative emotion: Effects of children's temperament and situational emotional responses. Social Development. 2004;13(4):515–530. doi: 10.1111/j.1467-9507.2004.00281.x. [Google Scholar]
- Mesman J, van Ijzendoorn MH, Bakermans-Kranenburg MJ. The many faces of the Still-Face Paradigm: A review and meta-analysis. Developmental Review. 2009;29(2):120–162. doi: 10.1016/j.dr.2009.02.001. [Google Scholar]
- Mills-Koonce WR, Gariepy J, Propper C, Sutton K, Calkins S, Moore G, Cox M. Infant and parent factors associated with early maternal sensitivity: A caregiver attachment systems approach. Infant Behavior and Development. 2007;30:114–126. doi: 10.1016/j.infbeh.2006.11.010. doi: 10.1016/j.infbeh.2006.11.010. [DOI] [PubMed] [Google Scholar]
- Mineka S, Watson D, Clark LA. Comorbidity of anxiety and unipolar mood disorders. Annual Review of Psychology. 1998;49:377–412. doi: 10.1146/annurev.psych.49.1.377. doi: 10.1146/annurev.psych.49.1.377. [DOI] [PubMed] [Google Scholar]
- Moore G, Calkins SD. Maternal behavior mediates the relation between infant physiological and behavioral distress in the Still-Face paradigm. Developmental Psychology. 2004;40:1068–1080. doi: 10.1037/0012-1649.40.6.1068. doi: 10.1037/0012-1649.40.6.1068. [DOI] [PubMed] [Google Scholar]
- Moore GA, Hill-Soderlund AL, Propper CB, Calkins SD, Mills-Koonce WR, Cox MJ. Mother-infant vagal regulation in the face-to-face still-face paradigm is moderated by maternal sensitivity. Child Development. 2009;80(1):209–223. doi: 10.1111/j.1467-8624.2008.01255.x. doi: 10.1111/j.1467-8624.2008.01255.x. [DOI] [PubMed] [Google Scholar]
- Oster H. BabyFACS: Facial Action Coding System for Infants and Young Children. 2005 [Google Scholar]
- Oster H, Hegley D, Nagel L. Adult judgments and fine-grained analysis of infant facial expressions: Testing the validity of a priori coding formulas. Developmental Psychology. 1992;28:1115–1131. doi: 10.1037/0012-1649.28.6.1115. [Google Scholar]
- Porges SW, Doussard-Roosevelt JA, Maiti AK. Vagal tone and the physiological regulation of emotion. Monographs of the Society for Research in Child Development. 1994;59(2/3):167–186. doi:10.2307/1166144. [PubMed] [Google Scholar]
- Porges SW. The polyvagal perspective. Biological Psychology. 2007;74:116–143. doi: 10.1016/j.biopsycho.2006.06.009. doi:10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. doi: 10.1177/014662167700100306. [Google Scholar]
- Raudenbush SW, Bryk AS. Hierarchical linear models: Applications and data analysis methods. 2nd edition Sage; Newbury Park, CA: 2002. [Google Scholar]
- Rime B. Emotion elicits the social sharing of emotion: Theory and empirical review. Emotion Review. 2009;1(1):60–85. doi: 10.1177/1754073908097189. [Google Scholar]
- Rottenberg J. Cardiac vagal control in depression: A critical analysis. Biological Psychology. 2007;74:200–211. doi: 10.1016/j.biopsycho.2005.08.010. doi: 10.1016/j.biopsycho.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Rottenberg J, Salomon K, Gross JJ, Gotlib IH. Vagal withdrawal to a sad film predicts subsequent recovery from depression. Psychophysiology. 2005;42:277–281. doi: 10.1111/j.1469-8986.2005.00289.x. doi:10.1111/j.1469-8986.2005.00289.x. [DOI] [PubMed] [Google Scholar]
- Rottenberg J, Wilhelm FH, Gross JJ, Gotlib IH. Vagal rebound during resolution of tearful crying among depressed and nondepressed individuals. Psychophysiology. 2003;40:1–6. doi: 10.1111/1469-8986.00001. doi: 10.1111/1469-8986.00001. [DOI] [PubMed] [Google Scholar]
- Schuetze P, Eiden RD, Coles CD. Prenatal cocaine and other substance exposure: Effects on infant autonomic regulation at 7 months of age. Developmental Psychobiology. 2007;49:276–289. doi: 10.1002/dev.20215. doi: 10.1002/dev.20215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka-Matsumi J, Kameoka VA. Reliabilities and concurrent validities of popular self-report measures of depression, anxiety, and social desirability. Journal of Consulting and Clinical Psychology. 1986;54(3):328–333. doi: 10.1037//0022-006x.54.3.328. doi: 10.1037//0022-006X.54.3.328. [DOI] [PubMed] [Google Scholar]
- Thompson R. Emotion regulation: A theme in search of definition. In: Fox N, editor. The development of emotion regulation: Biological and behavioral considerations. Monographs of the Society for Research in Child Development. 240. Vol. 59. 1994. pp. 25–52. doi: 10.2307/1166137. [PubMed] [Google Scholar]
- Tronick E, Als H, Adamson L, Wise S, Brazelton TB. The infant's response to entrapment between contradictory messages in face-to-face interaction. Journal of the American Academy of Child Psychiatry. 1978;17(1):1–13. doi: 10.1016/s0002-7138(09)62273-1. doi: 10.1016/S0002-7138(09)62273-1. [DOI] [PubMed] [Google Scholar]
- Watkins LL, Grossman P, Krishnan R, Sherwood A. Anxiety and vagal control of heart rate. Psychosomatic Medicine. 1998;60(4):498–502. doi: 10.1097/00006842-199807000-00018. Retrieved from http://www.psychosomaticmedicine.org/ [DOI] [PubMed] [Google Scholar]
- Wellisch DK, Lindberg NM. A psychological profile of depressed and non- depressed women at high risk for breast cancer. Psychosomatics. 2001;42:330–336. doi: 10.1176/appi.psy.42.4.330. doi: 10.1176/appi.psy.42.4.330. [DOI] [PubMed] [Google Scholar]
- Wilhelm FH, Grossman P, Coyle MA. Improving estimation of cardiac vagal tone during spontaneous breathing using a paced breathing calibration. Biomedical Science and Instruments. 2004:317–323. [PubMed] [Google Scholar]