Abstract
Background
Recent evidence implicates toll-like receptor 4 (TLR4) in opioid analgesia, tolerance, conditioned place preference, and self-administration. Here we determined the effect of the TLR4 antagonist (+)-naltrexone (a μ-opioid receptor inactive isomer) on the time-dependent increases in cue-induced heroin seeking after withdrawal (incubation of heroin craving).
Methods
In an initial experiment, we trained rats for 9 h/day to self-administer heroin (0.1 mg/kg/infusion) for 9 days; lever presses were paired with a 5-sec tone-light cue. We then assessed cue-induced heroin seeking in 30-min extinction sessions on withdrawal day 1; immediately after testing, we surgically implanted rats with Alzet minipumps delivering (+)-naltrexone (0, 7.5, 15, 30 mg/kg/day, s.c.) for 14 days. We then tested the rats for incubated cue-induced heroin seeking in 3-h extinction tests on withdrawal day 13.
Results
We found that chronic delivery of (+)-naltrexone via minipumps during the withdrawal phase decreased incubated cue-induced heroin seeking. In follow-up experiments, we found that acute injections of (+)-naltrexone immediately before withdrawal day 13 extinction test had no effect on incubated cue-induced heroin seeking. Furthermore, chronic delivery of (+)-naltrexone (15 or 30 mg/kg/day) or acute systemic injections (15 or 30 mg/kg) had no effect on ongoing extended access heroin self-administration. Finally, in rats trained to self-administer methamphetamine (0.1 mg/kg/infusion, 9 h/d, 9 days), chronic delivery of (+)-naltrexone (30 mg/kg/day) during the withdrawal phase had no effect on incubated cue-induced methamphetamine seeking.
Conclusions
The present results suggest a critical role of TLR4 in the development of incubation of heroin, but not methamphetamine, craving.
Keywords: TLR4, glia, relapse, opioid drugs, heroin self-administration, craving, reinstatement, extinction
Introduction
A high rate of relapse to drug use is a main feature of heroin addiction (1, 2). One factor thought to contribute to heroin relapse and craving in humans, even after prolonged abstinence, is exposure to environmental cues previously associated with drug use (3). In rat models of drug relapse and craving (4), r to cues previously associated with self-administration of heroin (5, 6) and other abused drugs (7-11), progressively increases after withdrawal. We have termed this phenomenon ‘incubation of drug craving’ (7, 12). Over the last decade, we and others have identified several critical mechanisms of incubation of cocaine craving (13, 14). In contrast, mechanisms underlying incubation of craving for heroin and other drugs are largely unknown (13). Here, we assessed the role of toll-like receptor 4 (TLR4) in incubation of heroin craving.
Emerging evidence indicates that exposure to opioids and other abused drugs activates non-neuronal (glia, microglia, astrocytes) cells of the central immune system, and that this activation plays a role in the behavioral effects of opioids and possibly other drugs (15-18). TLR4 is an innate immune system pattern recognition receptor and a member of the TLR family; this family includes 13 innate immune system receptors traditionally thought to primarily respond to pathogen-derived (pathogen associated molecular patterns; PAMPs) and tissue damage-related (damage associated molecular patterns; DAMPs) ligands (19, 20). TLR4, the first discovered mammalian TLR, was initially found to recognize and to be activated by bacterial lipopolysaccharide (LPS) (21). Subsequent studies have demonstrated that TLR4 is also activated by other foreign substances such as small molecule xenobiotics (xenobiotic associated molecular patterns; XAMPs) (22) and several abused drugs (15, 16).
TLR4 activation within the central nervous system causes the release of pro-inflammatory and neuroexcitatory cytokines, such as tumor necrosis factor- and interleukin-1 (20, 23). TLR4 and other TLRs are widely distributed in the brain, where they form an essential link between the innate immune system and the central nervous system (20, 24). These innate immune receptors are expressed in different immunocompetent cells (20, 24), including microglia (25), astrocytes (26), and oligodendrocytes (27). There is also evidence that TLR4 is expressed in cortical CNS neurons (28).
Recent studies indicate that morphine and other μ-opioid receptor (MOR) agonists, which stereoselectively activate MOR (29), induce non-stereoselective activation of TLR4 by binding to an accessory protein of TLR4, myeloid differentiation protein 2 (MD-2). Activation of TLR4 triggers oligomerization and subsequent glia-mediated pro-inflammatory responses (30, 31). Conversely, the preferential MOR antagonists (−)-naloxone and (−)-naltrexone non-stereoselectively inhibit TLR4 activation by opioid agonists and other stimuli (e.g., stressors, pain manipulations) (16, 24). Results from in vivo, in vitro, and in silico studies demonstrate that (+)-naloxone and (+)-naltrexone, the MOR inactive isomers of (−)-naloxone and (−)-naltrexone, are selective TLR4 antagonists (31-33). Importantly, blockade of TLR4 with (+)-naloxone or (+)-naltrexone attenuates neuropathic pain, morphine analgesic tolerance, and opioid withdrawal symptoms (16, 33). Most recently, Hutchinson et al. (31) reported that blockade of TLR4 with (+)-naloxone decreased morphine conditioned place preference (CPP), and remifentanil (a short-acting MOR agonist) self-administration in rats.
The studies described above implicate TLR4 in the acute rewarding effects of opioid drugs, as assessed in CPP (34) and drug self-administration (35) procedures. The role of TLR4 in relapse to opioid seeking is unknown; additionally, mechanisms of drug reward, as assessed in these procedures, are often dissociable from those mediating relapse to drug seeking in rat models (36, 37). Therefore, in the present study we explored the role of TLR4 in relapse to heroin seeking using an incubation of heroin craving procedure in which the response to heroin cues in extinction tests progressively increases after withdrawal from the drug (5, 6). In the experiments described below, we used (+)-naltrexone as a long-acting TLR4 antagonist. After assessing its receptor selectivity, we determined the effect of acute and chronic (+)-naltrexone exposure on incubation of heroin craving. We also studied the effect of chronic delivery and acute injections of (+)-naltrexone on ongoing heroin self-administration, and incubation of methamphetamine craving. To the degree that (+)-naltrexone is a selective TLR4 antagonist, our results demonstrate a novel role of TLR4 in the development of incubation of heroin but not methamphetamine craving.
Methods
Overview of the behavioral experiments
Using procedures similar to the ones described in the SOM Section, we found that acute injections of the short-acting TLR4 antagonist (+)-naloxone (10 or 30 mg/kg, s.c.) had an inconsistent effect on cue-induced heroin-seeking in extinction tests (3 h) on withdrawal days 1 and 15 (unpublished data). We also found in these pilot studies that twice daily repeated injections of (+)-naloxone (30 mg/kg) during the withdrawal period had no effect on incubated cue-induced heroin-seeking on day 15.
Thus, in Exp. 1 reported here, we employed an extended access heroin self-administration training procedure (9 h of heroin access per day over 9 days) and used Alzet minipumps (14-day delivery) to chronically deliver the long-acting TLR4 antagonist (+)-naltrexone during the two weeks of withdrawal from heroin. We tested the rats for ‘incubated’ cue-induced heroin-seeking in 3-h extinction tests on withdrawal day 13. Prior to minipump implantation, we gave rats a 30-min extinction session on day 1. This was done in order to verify that incubation of craving is reliably observed in each experiment in the minipump-vehicle condition and to allow us to match the different groups for baseline early withdrawal extinction responding.
In Exp. 2, we determined whether the effect of chronic delivery of (+)-naltrexone on “incubated” cue-induced heroin-seeking is mimicked by acute pre-test injections of the TLR4 antagonist. We also used 12 rats that previously participated in Exp. 2 to assess the effect of chronic delivery of (+)-naltrexone on operant responding maintained by palatable food pellets (38). In Exp. 3, we surgically implanted rats with the minipumps containing (+)-naltrexone two days prior to the training phase to determine whether chronic delivery of the TLR4 antagonist would decrease ongoing extended-access heroin self-administration. We also assessed the effect of acute systemic injections of both (+)-naltrexone (both s.c. and i.p.) and for comparison purposes (+)-naloxone (used in Hutchinson et al. (31) study), on ongoing extended access heroin self-administration. Finally, in Exp. 4 we used the same experimental conditions used in Exp. 1, with the exception that lever-presses during the training phase led to methamphetamine infusions, to determine whether chronic delivery of (+)-naltrexone would also decrease “incubated” cue-induced methamphetamine seeking. The details of the experimental procedures for these experiments are provided in the Supplemental Online Methods Section, which also provides a description of the initial in vitro experiments to assess potential non-TLR4 receptor binding sites or enzymatic activity of (+)-naltrexone.
Results
In vitro assays
Results from the target screening performed by Caliper Life Sciences showed that (+)-naltrexone displays no significant activity at the 64 biological targets examined. The summary data in Table S1 show that (+)-naltrexone has greater than 10 μM affinity for the receptors and ion channels tested, and failed to exhibit significant inhibition of the enzymes tested. Fig. 1 depicts the dose-response curves for (+) and (−) isomers of naltrexone in the binding assay for human MOR. Ki for (+)- and (−)-naltrexone were 1634±146 nM and 0.68±0.04 nM, respectively; the Ki for DAMGO was 11.1±0.08 nM. The binding data indicate that (+)-naltrexone is at least 2400-fold less potent than (−)-naltrexone in its binding affinity at MOR.
Figure 1. Dose-response curves for inhibition of [3H]DAMGO binding for isomers of naltrexone: (−)-naltrexone and (+)-naltrexone.
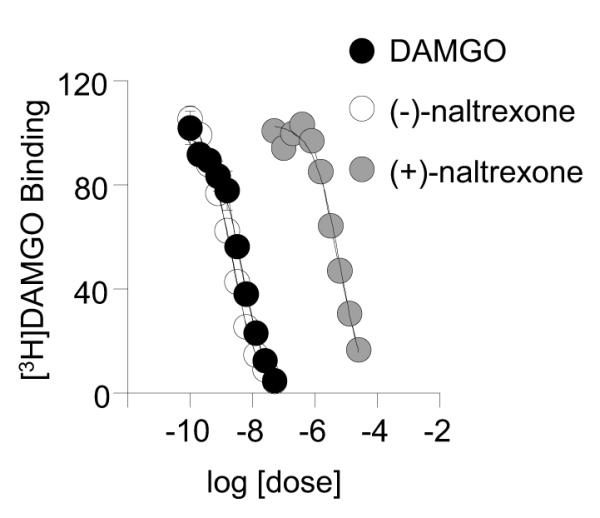
Membranes from CHO cells expressing human mu opioid receptors were prepared as described in Methods. Ten concentrations of each test drug were incubated in the presence of 3 nM [3H]DAMGO to generate curves. Data are expressed as mean±SD for 3 separate runs performed in triplicates.
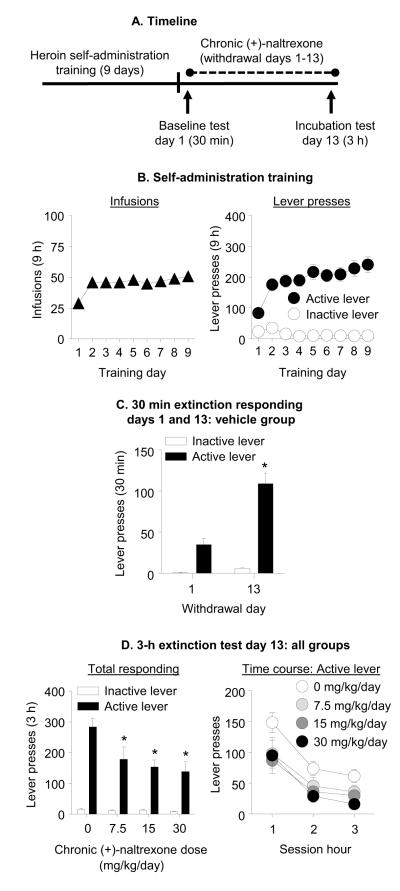
Exp. 1: Effect of chronic delivery of (+)-naltrexone during the withdrawal phase on incubated cue-induced heroin-seeking (Fig. 2A)
Figure 2. Chronic delivery of (+)-naltrexone during the withdrawal phase decreased incubated cue-induced heroin seeking.
(A) Timeline of the experiment. (B) Heroin self-administration training. Data are mean±SEM number of heroin infusions (0.1 mg/kg/infusion), and active and inactive lever-presses during the nine 9-h daily self-administration sessions (total n=57). During training, active lever presses were reinforced on an FR1 20-sec timeout reinforcement schedule and heroin infusions were paired with a 5-sec tone-light cue. (C) Extinction test withdrawal day 1 and 13 (vehicle group): Data are mean±SEM of responses on the previously active lever and on the inactive lever in the vehicle-treated rats (n=28) during the 30 min extinction test on withdrawal day 1 and the first 30 min of the 3 h extinction test on withdrawal day 13. * Different from withdrawal day 1, p<0.05. (D) Extinction test withdrawal day 13: Data are mean±SEM of responses on the active and inactive levers during the 3-h extinction test. During testing, lever-presses led to contingent presentations of the tone-light cue previously paired with heroin infusions during training, but not heroin. The rats were tested on withdrawal day 13 with Alzet osmotic minimpumps that were implanted s.c. on withdrawal day 1 with either vehicle (sterile water, n=28) or (+)-naltrexone: 7.5, 15, or 30 mg/kg/day (n=9-10 per dose). * Different from vehicle, p<0.05.
Self-administration training
The rats increased their number of heroin infusions over days (F(8,424)=12.0, p<0.001; Fig. 2B). Additionally, active but not inactive lever-presses increased over days (Lever × Day interaction (F(8,424)=10.9, p<0.001; Fig. 2B)).
Extinction tests
The rats in the chronic vehicle group demonstrated time-dependent increases in cue-induced heroin-seeking in the extinction tests (incubation of heroin craving, Fig. 2C). The statistical analysis, which included the within-subjects factors of Withdrawal Day and Lever, demonstrated a significant interaction of Withdrawal Day × Lever (F(1,27)=27.6, p<0.001). Chronic delivery of (+)-naltrexone decreased incubated cue-induced heroin-seeking on withdrawal day 13 (Fig. 2D). The analysis of total active and inactive lever presses, which included the between-subjects factor of (+)-Naltrexone Dose and the within-subjects factor of Lever, demonstrated a significant interaction of (+)-Naltrexone Dose × Lever (F(3,53)=4.9, p=0.005). Subsequent one-way ANCOVA of active lever-presses on withdrawal day 13, using day 1 active lever-presses (30 min) as a covariate, demonstrated a main effect of (+)-Naltrexone Dose (F(3,52)=5.02, p=0.004); post-hoc tests (FDR corrected) demonstrated that the 7.5, 15, and 30 mg/kg/day (+)-naltrexone groups were significantly different from the vehicle group (p<0.05). Analysis of time course of active lever-presses, which included the between-subjects factor of (+)-Naltrexone Dose and Session Time (hour 1, 2, 3), demonstrated significant effects of (+)-Naltrexone Dose (F(3,53)=5.1, p=0.004) and Session Time (F(2,106)=27.9, p<0.001) but no interaction between the two factors (Fig. 2D).
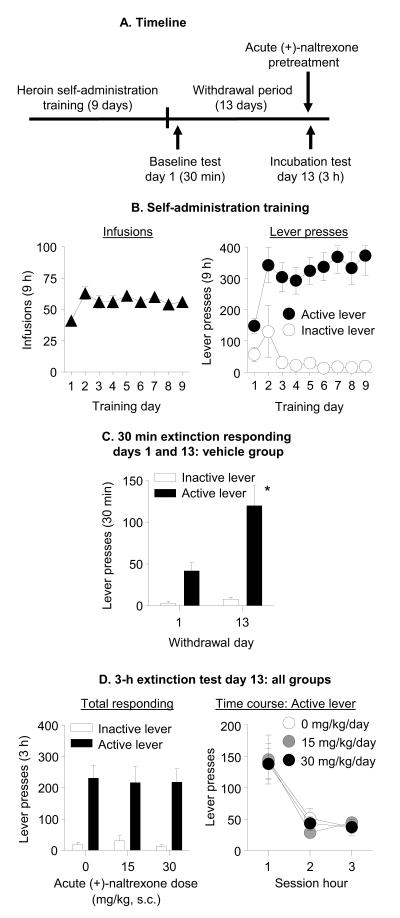
Exp. 2: Effect of acute injection of (+)-naltrexone on incubated cue-induced heroin-seeking (Fig. 3A)
Figure 3. Acute injection of (+)-naltrexone had no effect on incubated cue-induced heroin seeking on withdrawal day 13.
(A) Timeline of the experiment. (B) Heroin self-administration training. Data are mean±SEM number of heroin infusions (0.1 mg/kg/infusion), and active and inactive lever-presses during the nine 9-h daily self-administration sessions (total n=30). (C) Extinction test withdrawal day 1 and 13 (vehicle group): Data are mean±SEM of responses on the active and inactive levers in the vehicle-treated group (n=10) during the 30 min extinction test on withdrawal day 1 and the first 30 min of the 3 h extinction test on withdrawal day 13. * Different from withdrawal day 1, p<0.05. (D) Extinction test withdrawal day 13: Data are mean±SEM responses on the active and inactive levers during the 3-h extinction test. On withdrawal day 13, rats were injected acutely with either vehicle (sterile water, n=10) or (+)-naltrexone (15 or 30 mg/kg, s.c., n=10 per dose) 10-15 min prior to the extinction test.
Self-administration training
The rats increased their number of heroin infusions over days (F(8,216)=6.1, p=0.001; Fig 3B). Additionally, active but not inactive lever-presses increased over days (Lever × Day (F(8,216) = 3.9, p<0.001; Fig 3B).
Extinction tests
The rats in the acute vehicle group demonstrated time-dependent increases in cue-induced heroin-seeking in these tests (Withdrawal Day × Lever (F(1,9)=13.1, p=0.006); Fig. 3C). Acute subcutaneous injections of (+)-naltrexone prior to the extinction test on withdrawal day 13 had no effect on cue-induced heroin-seeking on that day (a significant effect of Lever (F(1,27)=72.8, p<0.001) but no effects of (+)-Naltrexone Dose or (+)-Naltrexone Dose × Lever (p values > 0.05), Fig. 3D). Analysis of the time course of active lever-presses demonstrated a significant effect of Session Time (F(2,54)=37.2, p<0.001) but no effects of (+)-Naltrexone Dose or interaction between the two factors (p values > 0.05; Fig. 3D).
Food-reinforced responding
Seven days after withdrawal day 13 testing, 12 rats received surgically implanted minipumps containing sterile water (vehicle, n=6) or (+)-naltrexone (30 mg/kg/day, n=6) to determine the effect of (+)-naltrexone on operant responding for food pellets (FR-1, 20-sec timeout reinforcement schedule). (+)-Naltrexone had no effect on food-reinforced responding (Fig. S1 in Supplement 1). The analysis of total pellets earned, which included (+)-Naltrexone Dose as the between-subjects factor and Training Day as the within-subjects factor, did not show significant effects of (+)-Naltrexone Dose or interaction between the two factors (p values > 0.05). The analysis of total active and inactive lever-presses demonstrated a significant effect of Lever (F(1,10)=37.5, p<0.001) but no effects of (+)-Naltrexone Dose or interaction between the two factors (p values > 0.05) (Fig. S1 in Supplement 1).
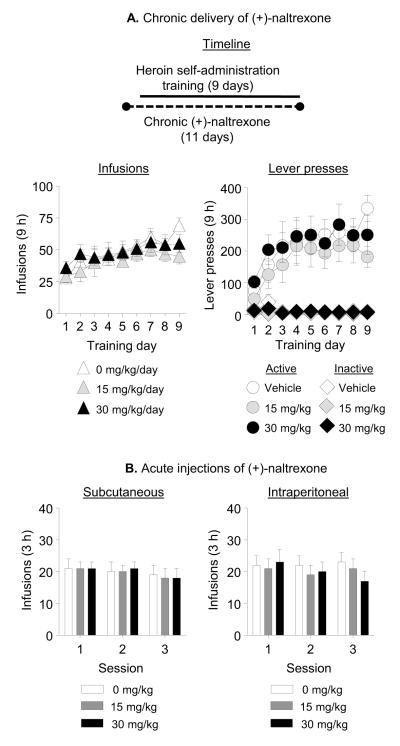
Exp. 3: Effect of chronic delivery or acute injections of (+)-naltrexone on heroin self-administration
Exp. 3a: Chronic minipump delivery
Chronic delivery of (+)-naltrexone during the training phase had no effect on acquisition and maintenance of heroin self-administration (Fig. 4A). The analysis of the number of heroin infusions, which included the between-subjects factor of (+)-Naltrexone Dose and the within-subjects factor of Training Day, demonstrated a significant effect of Day (F(8,200)=9.1, p<0.001), but no significant effects of (+)-Naltrexone Dose or interaction between the two factors (p values >0.05). The analysis of the number of active and inactive lever-presses demonstrated significant effects of Lever (F(1,25)=68.1, p<0.001) and Lever × Training Day (F(8,200)=10.5, p<0.001), but no significant effects of (+)-Naltrexone Dose or interactions between this factor and Lever or Training Day (p values > 0.05).
Figure 4. Chronic (minipump) delivery or acute systemic injections of (+)-naltrexone had no effect on ongoing heroin self-administration.
A) Chronic delivery: Data are total mean±SEM of heroin infusions (0.1 mg/kg/infusion), and active and inactive lever-presses during heroin self-administration training (three 3-h sessions separated by 1 h). Two days prior to training, rats were implanted with s.c. with Alzet osmotic minipumps that delivered either vehicle (sterile water, n=10) or (+)-naltrexone (15 or 30 mg/kg/day, n=8-10 per dose) during the training period. (B) Acute injections: Data are mean±SEM of heroin infusions (0.1 mg/kg/infusion) during the first, second, and third daily sessions. Systemic injections (s.c. or i.p.) of (+)-naltrexone (0, 15, and 30 mg/kg; n=10-11) were given 10-15 min prior to the start of the first 3 h daily session.
Exp. 3b: Acute injections
We trained rats (n=11) to self-administer heroin for 8 days (3 × 3-h sessions days separated by 1 h) and then tested them repeatedly (counterbalanced order) for the effects of acute systemic injections of (+)-naltrexone and (+)-naloxone prior to the first 3-h session of the 9-h daily sessions on heroin self-administration. Acute i.p. or s.c systemic injections of (+)-naltrexone had no effect on heroin self-administration (Fig. 4B). Data (infusions/3 h) were analyzed using the within-subjects factors of (+)-Naltrexone Dose and Session Time (first, second, and third 3-h session). For s.c. injections, there were no effects of (+)-Naltrexone Dose, Session Time, or interaction between the two factors (p values >0.05). For i.p. injections of (+)-naltrexone, there was a significant (+)-Naltrexone Dose × Session Time interaction (F(4,40)=2.9, p=0.032) but no effects of (+)-Naltrexone Dose or Session Time (p values >0.05); this interaction is due to the somewhat higher and lower heroin intake in the 30 mg/kg dose condition in the first and third session, respectively. There were no statistically significant effects of acute i.p or s.c (+)-naloxone injections on heroin self-administration (p values >0.05; Fig. S2 in Supplement 1).
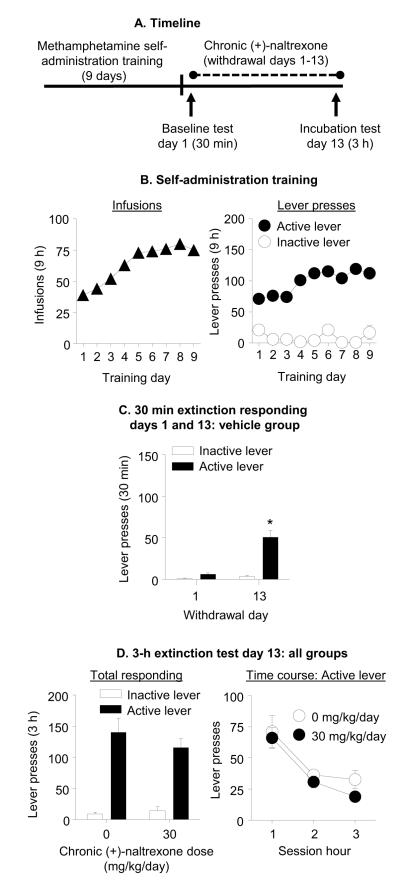
Exp. 4: Effect of chronic delivery of (+)-naltrexone during the withdrawal phase on incubated cue-induced methamphetamine seeking (Fig. 5A)
Figure 5. Chronic delivery of (+)-naltrexone during the withdrawal phase had no effect on incubated cue-induced methamphetamine seeking.
(A) Timeline of the experiment. (B) Methamphetamine self-administration training. Data are mean±SEM number of methamphetamine infusions (0.1 mg/kg/infusion), and active and inactive lever-presses during the nine 9-h daily self-administration sessions (total n=26). (C) Extinction test withdrawal day 1 and 13 (vehicle group): Data are mean±SEM of responses on the active and inactive levers in the vehicle-treated group (n=13) during the 30 min extinction test on withdrawal day 1 and the first 30 min of the 3-h extinction test on withdrawal day 13. * Different from withdrawal day 1, p<0.05. (D) Extinction test withdrawal day 13: Data are mean±SEM responses on the active and inactive levers during the 3-h extinction test. During testing, lever presses led to contingent presentations of the tone-light cue previously paired with methamphetamine infusions during training, but not methamphetamine. The rats were tested on withdrawal day 13 with Alzet osmotic minimpumps that were implanted s.c. on withdrawal day 1 with either vehicle (sterile water, n=13) or (+)-naltrexone, 30 mg/kg/day (n=13).
Self-administration training
The rats increased their number of methamphetamine infusions over days (F(8,192)=48.3, p<0.001; Fig. 5B). Analysis of active and inactive lever-presses demonstrated significant effects of Lever (F(1,192)=4.4, p=0.047) and Training Day (F(8,192)=2.15, p=0.033, Fig. 5B) but not Lever × Training Day (p>0.1). The lack of significant interaction is likely due to the fact that 5 of the 26 rats developed stereotypic responding on the inactive lever on some of the training sessions, resulting in high rate of responding on this lever (over 300 per day). This stereotyped responding occurred on 13 daily sessions across these 5 rats; these outlier values (>3 standard deviation from the sample mean) were included in the statistical analysis but were excluded from the data present in Fig. 5B, which includes 221 individual data points out of the 234 possible data points from the 26 rats across the 9 training days.
Extinction tests
The rats in the vehicle group demonstrated time-dependent increases in cue-induced methamphetamine seeking in the extinction tests (incubation of methamphetamine craving, Fig. 5C). The analysis demonstrated a significant interaction of Withdrawal Day × Lever (F(1,12)=39.8, p<0.001).
Chronic delivery of (+)-naltrexone had no effect on incubated cue-induced methamphetamine seeking on withdrawal day 13 (Fig. 5D). The analysis demonstrated a significant effect of Lever (F(1,24)=101.2, p<0.001), but no effect of (+)-Naltrexone Dose or (+)-Naltrexone Dose × Lever (p values > 0.1). Analysis of the time course of active lever-presses demonstrated a significant effect of Session Time (F(2,48)=40.6, p<0.001), but no significant effect of (+)-Naltrexone Dose or interaction between the two factors (p values > 0.05; Fig. 5D).
Discussion
We used (+)-naltrexone to study the role of TLR4 in incubation of heroin craving, operationally defined as time-dependent increases in cue-induced heroin-seeking in extinction tests after withdrawal from self-administered heroin. We first performed in vitro binding experiments to determine the possibility of non-TLR4 effects of (+)-naltrexone and found that (+)-naltrexone had minimal activity at a number of biologically relevant targets, as well as low binding affinity to MOR. In the in vivo experiments, we found that chronic delivery of (+)-naltrexone during the withdrawal phase attenuated ‘incubated’ cue-induced heroin-seeking in extinction tests performed on withdrawal day 13. This effect was not statistically dose-dependent due to large individual variability in non-reinforced lever presses during testing, a common observation in extinction-reinstatement (39, 40) and incubation (41) studies. In contrast, acute (+)-naltrexone injections immediately before withdrawal day 13 extinction tests were ineffective. Chronic delivery of (+)-naltrexone or acute pre-session injections of (+)-naltrexone (or (+)-naloxone) had no effect on ongoing extended access heroin self-administration; additionally, chronic delivery of (+)-naltrexone had no effect on high rate food-reinforced responding. Finally, we assessed the generality of our findings to incubation of psychostimulant craving and found that chronic delivery of (+)-naltrexone during the withdrawal phase had no effect on incubated cue-induced methamphetamine seeking. Our data indicate a role of TLR4 in the development of incubation of heroin, but not methamphetamine, craving. The present findings provide additional evidence for the important role of non-neuronal glia-related mechanisms in the behavioral effects of opioid drugs (17, 18, 31).
Methodological considerations
Several methodological issues should be considered in the interpretation of our data. One issue is the behavioral specificity of chronic (+)-naltrexone’s effect for incubated cue-induced heroin-seeking. Decreased active-lever responding after chronic delivery of (+)-naltrexone may be caused by motor deficits or other non-specific performance deficits. However, this is unlikely because chronic delivery of (+)-naltrexone had minimal effects on heroin self-administration, lever responding for palatable food, or cue-induced methamphetamine seeking. It is also unlikely that a short extinction session on withdrawal day 1 confounds data interpretation. In the present and previous studies, we observed reliable incubation of craving for both heroin (5, 42) and cocaine (43, 44) in rats repeatedly tested during early and late withdrawal. Additionally, it is unlikely that a short extinction session on withdrawal day 1 promotes long-term extinction learning and consequently decreased cue responding on day 13, because it takes several weeks to extinguish heroin self-administration behavior in rats (45, 46).
Another issue is the pharmacological specificity of (+)-naltrexone to TLR4. We found that in vitro (+)-naltrexone had no significant activity at a number of potential non-TLR4 sites, including MOR. A MOR-mediated effect is also unlikely, because we recently found that acute injections of the preferential MOR antagonist, (−)-naloxone (1 mg/kg), decreased ‘incubated’ cue-induced heroin-seeking on withdrawal day 15 (42). In contrast, acute injections of higher (+)-naltrexone doses (15-30 mg/kg) prior to withdrawal day 13 testing were ineffective. A MOR-mediated effect of (+)-naltrexone or potentially its metabolites is also unlikely, because with this scenario, (+)-naltrexone would have also decreased heroin self-administration, a MOR-dependent behavior (47, 48). Finally, other non-TLR4 targets of (+)-naloxone (and by extension (+)-naltrexone) were recently reported, including Filamin A (49) and NADPH oxidase (50). However, it is unlikely that these targets mediated (+)-naltrexone’s effect on incubation of heroin craving, because the effects of (+)-naloxone or (+)-naltrexone on behavioral effects of opioid drugs (e.g., tolerance, dependence, CPP) are not observed in the TLR4 knockout mice (16).
Role of TLR4 in opioid reward
Hutchinson et al. (31) recently reported that acute injections of the TLR4 antagonist (+)-naloxone decreased morphine-induced CPP and remifentanil self-administration in rats. They also reported that TLR4 or MyD88 (a TLR4 accessory signaling protein) knockout mice do not develop CPP for the opioid agonist oxycodone. In contrast, we found that chronic delivery of (+)-naltrexone or acute injections of (+)-naltrexone or (+)-naloxone had no effect on heroin self-administration. What might account for these different results beyond differences in the opioid agonist (remifentanil or oxycodone versus heroin)?
It is perhaps not surprising that TLR4 antagonism prevented CPP for response-independent morphine injections but had no effect on response-contingent operant heroin self-administration. While both CPP and drug self-administration procedures have been used to measure opioid reward (51-53), previous studies demonstrated dissociable neurobiological mechanisms for opioid CPP versus self-administration. For example, mesoaccumbens dopamine plays a critical role in morphine and heroin CPP (54, 55) but not heroin self-administration (47, 56, 57).
It is somewhat more difficult to reconcile our negative findings for chronic (+)-naltrexone or acute injections of (+)-naltrexone or (+)-naloxone effects on heroin self-administration with those reported by Hutchinson et al. (31) who found that acute (+)-naloxone decreased remifentanil self-administration. These differences might be due to two main factors. The first is that Hutchinson et al. (31) trained rats for cocaine self-administration and then assessed the effect of (+)-naloxone on remifentanil self-administration during ‘substitution’ sessions in which cocaine was intermittently replaced with remifentanil, an opioid agonist with a half-life that is significantly shorter than heroin (1). Another potential factor is that the rats in Hutchinson et al. (31) were trained under a limited-access drug self-administration condition (2 h/d) for cocaine while our rats were trained under an extended-access condition (9 h/d) for heroin. Even within a given drug class, these different access conditions lead to different patterns of drug self-administration (58, 59), brain neuroadaptations (60-63), and differential responses to pharmacological manipulations (64-67).
Mechanisms of TLR4 role in incubation of heroin craving
Our pharmacological finding with (+)-naltrexone suggests a role of TLR4 in incubation of heroin craving. As in other systemic pharmacology studies, our positive findings inspire follow-up questions on downstream molecular mechanisms. Below we briefly speculate on potential mechanisms within a conceptual framework of two distinct molecular mechanisms of incubation of drug craving (13). The first involves the acute expression of incubation of drug craving or the acute “incubated” response to drug cues after prolonged withdrawal that occurs on a time scale of minutes. The second involves the development of incubation of drug craving or the time-dependent drug-induced neuroadaptations that take weeks to develop after withdrawal but are not directly involved in the acute incubated response to drug cues during testing.
Regarding the first mechanism, one possibility is that acute conditioned TLR4 activation by heroin cues in brain areas critical for cue-induced heroin-seeking (e.g., nucleus accumbens (46)) directly mediates the ‘incubated’ response on withdrawal day 13. Since the seminal work of Ader (68) many studies have shown that conditioned cues can activate (or inhibit) the immune system (69), including cues associated with opioid-induced immune activation/suppression (70). There is also evidence for modulation of conditioned responses to opioids by central glia immune-related mechanisms (15, 17), including TLR4-related mechanisms (31). However, it is unlikely that direct heroin-cue-induced TLR4 activation contributes to the acute expression of incubation of heroin craving. This is because acute injections of high (+)-naltrexone doses prior to the extinction tests on withdrawal day 13 had no effect on ‘incubated’ cue-induced heroin-seeking.
The finding that chronic but not acute (+)-naltrexone delivery decreased incubated cue-induced heroin-seeking suggests that TLR4 plays a unique role in the development of incubation of heroin craving. The causes of these TLR4-related neuroadaptations, induced by heroin self-administration and subsequent withdrawal, are unknown. One potential downstream mechanism is TLR4-mediated activation of nuclear factor kappa-B (NF B) (19), which is activated by opioid agonists (71), and recently implicated in the maintenance of memories for morphine-associated cues (72), opioid withdrawal symptoms (73), as well as other behavioral effects of drugs (74). We assessed the potential role of this downstream mechanism by determining the effect of chronic (minipump) delivery of the NF B antagonist sc-514 (75) into the lateral ventricles during the withdrawal period using experimental conditions similar to those used in Exp. 1 (see legend of Fig. S3 in Supplement 1). We found that this manipulation had no effect on incubated cue-induced heroin-seeking on withdrawal day 13 (Fig. S3 in Supplement 1). These data may suggest that NF B is not a downstream mechanism for the TLR4-mediated effect of chronic delivery of (+)-naltrexone on development of incubation of craving. However, an alternative interpretation of these negative data with the NF B antagonist is that ventricular delivery of sc-514 (a compound that is very difficult to dissolve, even in 50% DMSO) either did not reach critical brain areas involved in incubation of heroin craving or that the drug did not remain in solution in the minipump for the duration of the experiment. Thus, whether or not NF B is a potential downstream for the putative TLR4-mediated effect of chronic delivery of (+)-naltrexone on incubation of heroin craving is a subject for future research.
Another downstream mechanism of TLR4 activation that may contribute to the development of incubation of heroin craving is activation of TNF- and subsequent regulation of synaptic strength at glutamate synapses (76). In hippocampal cultured neurons and slices, TNF-promotes the insertion of AMPA receptors into plasma membranes (76) and the formation of GluA2-lacking AMPA receptors (77). Time-dependent accumulation of GluA2-lacking AMPA receptors in nucleus accumbens after withdrawal is critical for incubation of cocaine craving (14, 78). However, whether this speculative mechanism contributes to incubation of heroin craving is a subject for future research, because it has not been established that withdrawal from heroin self-administration induces the accumulation of GluA2-lacking AMPA receptors in nucleus accumbens or that TNF-modulates glutamatergic synapse strength in this brain area.
Concluding remarks
Our results suggest a novel role of TLR4 in incubation of heroin, but not methamphetamine, craving. This selective role of TLR4 in incubation of heroin craving is in agreement with results from our recent studies suggesting different mechanisms for incubation of opioid versus psychostimulant craving (5, 13, 42). These previous and present results also extend previous reports demonstrating that mechanisms of opioid- and psychostimulant-taking behaviors are often dissociable (48, 79, 80). Finally, a question for future research will be to identify brain sites and downstream cellular mechanisms that contribute to incubation of heroin craving whose function is altered by chronic delivery of (+)-naltrexone during the withdrawal phase.
Supplementary Material
Acknowledgments
The work was supported by the Intramural Research Programs of NIDA and NIAAA. We thank Dr. David Epstein for calculating the FDR post-hoc test results and Hila Eichenbaum and Dr. Kimberlei Richardson for helping with the intravenous catheter surgery. MRH is supported by an Australian Research Council Research Fellowship (DP110100297). LRW is supported by NIDA K05-DA024044. KCR is also affiliated with the Intramural Research Program of NIAAA, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Jaffe JH. Drug addiction and drug abuse. In: Gilman AG, Rall TW, Nies AS, Taylor P, editors. Goodman & Gilman’s the pharmacological basis of therapeutics. 8th ed Pergamon Press; New York: 1990. pp. 522–573. [Google Scholar]
- 2.Wikler A. Dynamics of drug dependence. Implications of a conditioning theory for research and treatment. Arch Gen Psychiatry. 1973;28:611–616. doi: 10.1001/archpsyc.1973.01750350005001. [DOI] [PubMed] [Google Scholar]
- 3.O’Brien CP, Ehrman RN, Ternes JW. Classical conditioning in human opioid dependence. In: Goldberg S, Stolerman I, editors. Behavioral analysis of drug dependence. Academic Press; Orlando: 1986. pp. 329–356. [Google Scholar]
- 4.Epstein DH, Preston KL, Stewart J, Shaham Y. Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology. 2006;189:1–16. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Airavaara M, Pickens CL, Stern AL, Wihbey KA, Harvey BK, Bossert JM, et al. Endogenous GDNF in ventral tegmental area and nucleus accumbens does not play a role in the incubation of heroin craving. Addict Biol. 2011;16:261–272. doi: 10.1111/j.1369-1600.2010.00281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shalev U, Morales M, Hope B, Yap J, Shaham Y. Time-dependent changes in extinction behavior and stress-induced reinstatement of drug seeking following withdrawal from heroin in rats. Psychopharmacology. 2001;156:98–107. doi: 10.1007/s002130100748. [DOI] [PubMed] [Google Scholar]
- 7.Grimm JW, Hope BT, Wise RA, Shaham Y. Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neisewander JL, Baker DA, Fuchs RA, Tran-Nguyen LT, Palmer A, Marshall JF. Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment. J Neurosci. 2000;20:798–805. doi: 10.1523/JNEUROSCI.20-02-00798.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shepard JD, Bossert JM, Liu SY, Shaham Y. The anxiogenic drug yohimbine reinstates methamphetamine seeking in a rat model of drug relapse. Biol Psychiatry. 2004;55:1082–1089. doi: 10.1016/j.biopsych.2004.02.032. [DOI] [PubMed] [Google Scholar]
- 10.Abdolahi A, Acosta G, Breslin FJ, Hemby SE, Lynch WJ. Incubation of nicotine seeking is associated with enhanced protein kinase A-regulated signaling of dopamine- and cAMP-regulated phosphoprotein of 32 kDa in the insular cortex. Eur J Neurosci. 2010;31:733–741. doi: 10.1111/j.1460-9568.2010.07114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bienkowski P, Koros E, Kostowski W, Bogucka-Bonikowska A. Reinstatement of ethanol seeking in rats: behavioral analysis. Pharmacol Biochem Behav. 2000;66:123–128. doi: 10.1016/s0091-3057(00)00194-5. [DOI] [PubMed] [Google Scholar]
- 12.Lu L, Grimm JW, Hope BT, Shaham Y. Incubation of cocaine craving after withdrawal: a review of preclinical data. Neuropharmacology. 2004;47(Suppl 1):214–226. doi: 10.1016/j.neuropharm.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 13.Pickens CL, Airavaara M, Theberge F, Fanous S, Hope BT, Shaham Y. Neurobiology of incubation of drug craving. Trends Neurosci. 2011;34:411–420. doi: 10.1016/j.tins.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolf ME, Ferrario CR. AMPA receptor plasticity in the nucleus accumbens after repeated exposure to cocaine. Neurosci Biobehav Rev. 2010;35:185–211. doi: 10.1016/j.neubiorev.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coller JK, Hutchinson MR. Implications of central immune signaling caused by drugs of abuse: mechanisms, mediators and new therapeutic approaches for prediction and treatment of drug dependence. Pharmacol Ther. 2012;134:219–245. doi: 10.1016/j.pharmthera.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 16.Hutchinson MR, Shavit Y, Grace PM, Rice KC, Maier SF, Watkins LR. Exploring the neuroimmunopharmacology of opioids: an integrative review of mechanisms of central immune signaling and their implications for opioid analgesia. Pharmacol Rev. 2011;63:772–810. doi: 10.1124/pr.110.004135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwarz JM, Hutchinson MR, Bilbo SD. Early-life experience decreases drug-induced reinstatement of morphine CPP in adulthood via microglial-specific epigenetic programming of anti-inflammatory IL-10 expression. J Neurosci. 2011;31:17835–17847. doi: 10.1523/JNEUROSCI.3297-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Narita M, Suzuki M, Kuzumaki N, Miyatake M, Suzuki T. Implication of activated astrocytes in the development of drug dependence: differences between methamphetamine and morphine. Ann N Y Acad Sci. 2008;1141:96–104. doi: 10.1196/annals.1441.032. [DOI] [PubMed] [Google Scholar]
- 19.Okun E, Griffioen KJ, Mattson MP. Toll-like receptor signaling in neural plasticity and disease. Trends in neurosciences. 2011;34:269–281. doi: 10.1016/j.tins.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miyake K. Innate immune sensing of pathogens and danger signals by cell surface Toll-like receptors. Seminars in immunology. 2007;19:3–10. doi: 10.1016/j.smim.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 21.Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 22.Wang X, Loram LC, Ramos K, de Jesus AJ, Thomas J, Cheng K, et al. Morphine activates neuroinflammation in a manner parallel to endotoxin. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:6325–6330. doi: 10.1073/pnas.1200130109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yirmiya R, Goshen I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav Immun. 2011;25:181–213. doi: 10.1016/j.bbi.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 24.Nicotra L, Loram LC, Watkins LR, Hutchinson MR. Toll-like receptors in chronic pain. Exp Neurology. 2012;234:316–329. doi: 10.1016/j.expneurol.2011.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olson JK, Miller SD. Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J Immun. 2004;173:3916–3924. doi: 10.4049/jimmunol.173.6.3916. [DOI] [PubMed] [Google Scholar]
- 26.Bowman CC, Rasley A, Tranguch SL, Marriott I. Cultured astrocytes express toll-like receptors for bacterial products. Glia. 2003;43:281–291. doi: 10.1002/glia.10256. [DOI] [PubMed] [Google Scholar]
- 27.Aravalli RN, Peterson PK, Lokensgard JR. Toll-like receptors in defense and damage of the central nervous system. J Neuroimmune Pharmacol. 2007;2:297–312. doi: 10.1007/s11481-007-9071-5. [DOI] [PubMed] [Google Scholar]
- 28.Tang SC, Arumugam TV, Xu X, Cheng A, Mughal MR, Jo DG, et al. Pivotal role for neuronal Toll-like receptors in ischemic brain injury and functional deficits. Proc Nat Acad Sci (USA) 2007;104:13798–13803. doi: 10.1073/pnas.0702553104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldstein A, Naidu A. Multiple opioid receptors: ligand selectivity profiles and binding sties signatures. Mol Pharmacol. 1989;36:265–272. [PubMed] [Google Scholar]
- 30.Wang X, Loram LC, Ramos K, de Jesus AJ, Thomas J, Cheng K, et al. Morphine activates neuroinflammation in a manner parallel to endotoxin. Proc Nat Acad Sci (USA) 2012;109:6325–6330. doi: 10.1073/pnas.1200130109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hutchinson MR, Northcutt AL, Hiranita T, Wang X, Lewis SS, Thomas J, et al. Opioid activation of toll-like receptor 4 contributes to drug reinforcement. J Neurosci. 2012;32:11187–11200. doi: 10.1523/JNEUROSCI.0684-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hutchinson MR, Zhang Y, Brown K, Coats BD, Shridhar M, Sholar PW, et al. Non-stereoselective reversal of neuropathic pain by naloxone and naltrexone: involvement of toll-like receptor 4 (TLR4) Eur J Neurosci. 2008;28:20–29. doi: 10.1111/j.1460-9568.2008.06321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hutchinson MR, Zhang Y, Shridhar M, Evans JH, Buchanan MM, Zhao TX, et al. Evidence that opioids may have toll-like receptor 4 and MD-2 effects. Brain Behav Immun. 2010;24:83–95. doi: 10.1016/j.bbi.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mucha RF, van der Kooy D, O’Shaughnessy M, Bucenieks P. Drug reinforcement studied by the use of place conditioning in rat. Brain Res. 1982;243:91–105. doi: 10.1016/0006-8993(82)91123-4. [DOI] [PubMed] [Google Scholar]
- 35.Schuster CR, Thompson T. Self administration of and behavioral dependence on drugs. Annu Rev Pharmacol. 1969;9:483–502. doi: 10.1146/annurev.pa.09.040169.002411. [DOI] [PubMed] [Google Scholar]
- 36.Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nature reviews Neuroscience. 2009;10:561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- 37.Shalev U, Grimm JW, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev. 2002;54:1–42. doi: 10.1124/pr.54.1.1. [DOI] [PubMed] [Google Scholar]
- 38.Pickens CL, Cifani C, Navarre BM, Eichenbaum H, Theberge FR, Baumann MH, et al. Effect of fenfluramine on reinstatement of food seeking in female and male rats: implications for the predictive validity of the reinstatement model. Psychopharmacology. 2012;221:341–353. doi: 10.1007/s00213-011-2585-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Erb S, Hitchcott PK, Rajabi H, Mueller D, Shaham Y, Stewart J. Alpha-2 adrenergic receptor agonists block stress-induced reinstatement of cocaine seeking. Neuropsychopharmacology. 2000;23:138–150. doi: 10.1016/S0893-133X(99)00158-X. [DOI] [PubMed] [Google Scholar]
- 40.Shaham Y, Highfield D, Delfs JM, Leung S, Stewart J. Clonidine blocks stress-induced reinstatement of heroin seeking in rats: an effect independent of the locus coeruleus noradrenergic neurons. Eur J Neurosci. 2000;12:292–302. doi: 10.1046/j.1460-9568.2000.00899.x. [DOI] [PubMed] [Google Scholar]
- 41.Lu L, Uejima JL, Gray SM, Bossert JM, Shaham Y. Systemic and central amygdala injections of the mGluR(2/3) agonist LY379268 attenuate the expression of incubation of cocaine craving. Biol Psychiatry. 2007;61:591–598. doi: 10.1016/j.biopsych.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 42.Theberge FR, Pickens CL, Goldart E, Fanous S, Hope BT, Liu QR, et al. Association of time-dependent changes in mu opioid receptor mRNA, but not BDNF, TrkB, or MeCP2 mRNA and protein expression in the rat nucleus accumbens with incubation of heroin craving. Psychopharmacology. 2012;224:559–571. doi: 10.1007/s00213-012-2784-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lu L, Dempsey J, Liu SY, Bossert JM, Shaham Y. A single infusion of brain-derived neurotrophic factor into the ventral tegmental area induces long-lasting potentiation of cocaine seeking after withdrawal. J Neurosci. 2004;24:1604–1611. doi: 10.1523/JNEUROSCI.5124-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lu L, Wang X, Wu P, Xu C, Zhao M, Morales M, et al. Role of ventral tegmental area glial cell line-derived neurotrophic factor in incubation of cocaine craving. Biol Psychiatry. 2009;66:137–145. doi: 10.1016/j.biopsych.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bossert JM, Wihbey KA, Pickens CL, Nair SG, Shaham Y. Role of dopamine D(1)-family receptors in dorsolateral striatum in context-induced reinstatement of heroin seeking in rats. Psychopharmacology. 2009;206:51–60. doi: 10.1007/s00213-009-1580-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bossert JM, Poles GC, Wihbey KA, Koya E, Shaham Y. Differential effects of blockade of dopamine D1-family receptors in nucleus accumbens core or shell on reinstatement of heroin seeking induced by contextual and discrete cues. J Neurosci. 2007;27:12655–12663. doi: 10.1523/JNEUROSCI.3926-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ettenberg A, Pettit HO, Bloom FE, Koob GF. Heroin and cocaine intravenous self-administration in rats: mediation by separate neural systems. Psychopharmacology. 1982;78:204–209. doi: 10.1007/BF00428151. [DOI] [PubMed] [Google Scholar]
- 48.Mello NK, Negus SS. Preclinical evaluation of pharmacotherapies for treatment of cocaine and opioid abuse using drug self-administration procedures. Neuropsychopharmacology. 1996;14:375–424. doi: 10.1016/0893-133X(95)00274-H. [DOI] [PubMed] [Google Scholar]
- 49.Burns LH, Wang HY. PTI-609: a novel analgesic that binds filamin A to control opioid signaling. Recent Pat CNS Drug Discov. 2010;5:210–220. doi: 10.2174/157488910793362386. [DOI] [PubMed] [Google Scholar]
- 50.Wang Q, Zhou H, Gao H, Chen SH, Chu CH, Wilson B, et al. Naloxone inhibits immune cell function by suppressing superoxide production through a direct interaction with gp91phox subunit of NADPH oxidase. Journal of neuroinflammation. 2012;9:32. doi: 10.1186/1742-2094-9-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tzschentke TM. Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict Biol. 2007;12:227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- 52.De Vries TJ, Shippenberg TS. Neural systems underlying opiate addiction. J Neurosci. 2002;22:3321–3325. doi: 10.1523/JNEUROSCI.22-09-03321.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Van Ree JM, Gerrits MA, Vanderschuren LJ. Opioids, reward and addiction: An encounter of biology, psychology, and medicine. Pharmacol Rev. 1999;51:341–396. [PubMed] [Google Scholar]
- 54.Spyraki C, Fibiger HC, Phillips AG. Attenuation of heroin reward in rats by disruption of the mesolimbic dopamine system. Psychopharmacology. 1983;79:278–283. doi: 10.1007/BF00427827. [DOI] [PubMed] [Google Scholar]
- 55.Fenu S, Spina L, Rivas E, Longoni R, Di Chiara G. Morphine-conditioned single-trial place preference: role of nucleus accumbens shell dopamine receptors in acquisition, but not expression. Psychopharmacology. 2006;187:143–153. doi: 10.1007/s00213-006-0415-2. [DOI] [PubMed] [Google Scholar]
- 56.Gerrits MAFM, Ramsey NF, Wolterink G, Van Ree JM. Lack of evidence for an involvement of nucleus accumbens dopamine D1 receptors in the initiation of heroin self-administration. Psychopharmacology. 1994;114:486–494. doi: 10.1007/BF02249340. [DOI] [PubMed] [Google Scholar]
- 57.Pettit HO, Ettenberg A, Bloom FE, Koob GF. Destruction of dopamine in the nucleus accumbens selectively attenuates cocaine but not heroin self-administration. Psychopharmacology. 1984;84:167–173. doi: 10.1007/BF00427441. [DOI] [PubMed] [Google Scholar]
- 58.Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- 59.Ahmed SH, Walker JR, Koob GF. Persistent increase in the motivation to take heroin in rats with history of drug escalation. Neuropsychopharmacology. 2000;22:413–421. doi: 10.1016/S0893-133X(99)00133-5. [DOI] [PubMed] [Google Scholar]
- 60.Ferrario CR, Gorny G, Crombag HS, Li Y, Kolb B, Robinson TE. Neural and behavioral plasticity associated with the transition from controlled to escalated cocaine use. Biol Psychiatry. 2005;58:751–759. doi: 10.1016/j.biopsych.2005.04.046. [DOI] [PubMed] [Google Scholar]
- 61.Ben-Shahar O, Obara I, Ary AW, Ma N, Mangiardi MA, Medina RL, et al. Extended daily access to cocaine results in distinct alterations in Homer 1b/c and NMDA receptor subunit expression within the medial prefrontal cortex. Synapse. 2009;63:598–609. doi: 10.1002/syn.20640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ben-Shahar O, Ahmed SH, Koob GF, Ettenberg A. The transition from controlled to compulsive drug use is associated with a loss of sensitization. Brain Res. 2004;995:46–54. doi: 10.1016/j.brainres.2003.09.053. [DOI] [PubMed] [Google Scholar]
- 63.Im HI, Hollander JA, Bali P, Kenny PJ. MeCP2 controls BDNF expression and cocaine intake through homeostatic interactions with microRNA-212. Nat Neurosci. 2010;13:1120–1127. doi: 10.1038/nn.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Greenwell TN, Walker BM, Cottone P, Zorrilla EP, Koob GF. The alpha1 adrenergic receptor antagonist prazosin reduces heroin self-administration in rats with extended access to heroin administration. Pharmacol Biochem Behav. 2009;91:295–302. doi: 10.1016/j.pbb.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Greenwell TN, Funk CK, Cottone P, Richardson HN, Chen SA, Rice KC, et al. Corticotropin-releasing factor-1 receptor antagonists decrease heroin self-administration in long- but not short-access rats. Addict Biol. 2009;14:130–143. doi: 10.1111/j.1369-1600.2008.00142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wee S, Orio L, Ghirmai S, Cashman JR, Koob GF. Inhibition of kappa opioid receptors attenuated increased cocaine intake in rats with extended access to cocaine. Psychopharmacology (Berl) 2009;205:565–575. doi: 10.1007/s00213-009-1563-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Orio L, Edwards S, George O, Parsons LH, Koob GF. A role for the endocannabinoid system in the increased motivation for cocaine in extended-access conditions. J Neurosci. 2009;29:4846–4857. doi: 10.1523/JNEUROSCI.0563-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ader R, Cohen N. Behaviorally conditioned immunosuppression. Psychosomatic Med. 1975;37:333–340. doi: 10.1097/00006842-197507000-00007. [DOI] [PubMed] [Google Scholar]
- 69.Lysle DT, Coussons-Read ME. Mechanisms of conditioned immunomodulation. Int J Immunopharmacol. 1995;17:641–647. doi: 10.1016/0192-0561(95)00050-c. [DOI] [PubMed] [Google Scholar]
- 70.Saurer TB, Ijames SG, Carrigan KA, Lysle DT. Neuroimmune mechanisms of opioid-mediated conditioned immunomodulation. Brain Behav Immun. 2008;22:89–97. doi: 10.1016/j.bbi.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen YL, Law PY, Loh HH. Nuclear factor kappaB signaling in opioid functions and receptor gene expression. J Neuroimmune Pharmacol. 2006;1:270–279. doi: 10.1007/s11481-006-9028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang J, Yu J, Jia X, Zhu W, Zhao L, Li S, et al. Inhibition of nuclear factor-kappaB impairs reconsolidation of morphine reward memory in rats. Behav Brain Res. 2011;216:592–596. doi: 10.1016/j.bbr.2010.08.047. [DOI] [PubMed] [Google Scholar]
- 73.Rehni AK, Bhateja P, Singh TG, Singh N. Nuclear factor-kappa-B inhibitor modulates the development of opioid dependence in a mouse model of naloxone-induced opioid withdrawal syndrome. Behav Pharmacol. 2008;19:265–269. doi: 10.1097/FBP.0b013e3282febcd9. [DOI] [PubMed] [Google Scholar]
- 74.Russo SJ, Wilkinson MB, Mazei-Robison MS, Dietz DM, Maze I, Krishnan V, et al. Nuclear factor kappa B signaling regulates neuronal morphology and cocaine reward. J Neurosci. 2009;29:3529–3537. doi: 10.1523/JNEUROSCI.6173-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Koo JW, Russo SJ, Ferguson D, Nestler EJ, Duman RS. Nuclear factor-kappaB is a critical mediator of stress-impaired neurogenesis and depressive behavior. Proc Natl Acad Sci U S A. 2010;107:2669–2674. doi: 10.1073/pnas.0910658107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Beattie EC, Stellwagen D, Morishita W, Bresnahan JC, Ha BK, Von Zastrow M, et al. Control of synaptic strength by glial TNFalpha. Science. 2002;295:2282–2285. doi: 10.1126/science.1067859. [DOI] [PubMed] [Google Scholar]
- 77.Stellwagen D, Beattie EC, Seo JY, Malenka RC. Differential regulation of AMPA receptor and GABA receptor trafficking by tumor necrosis factor-alpha. J Neurosci. 2005;25:3219–3228. doi: 10.1523/JNEUROSCI.4486-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, et al. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Badiani A, Belin D, Epstein D, Calu D, Shaham Y. Opiate versus psychostimulant addiction: the differences do matter. Nat Rev Neurosci. 2011;12:685–700. doi: 10.1038/nrn3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Caprioli D, Celentano M, Paolone G, Badiani A. Modeling the role of environment in addiction. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1639–1653. doi: 10.1016/j.pnpbp.2007.08.029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.