Abstract
Recent discoveries suggest that aging is neither driven by accumulation of molecular damage of any cause, nor by random damage of any kind. Some predictions of a new theory, quasi-programmed hyperfunction, have already been confirmed and a clinically-available drug slows aging and delays diseases in animals. The relationship between diseases and aging becomes easily apparent. Yet, the essence of aging turns out to be so startling that the theory cannot be instantly accepted and any possible arguments are raised for its disposal. I discuss that these arguments actually support a new theory. Are any questions remaining? And might accumulation of molecular damage still play a peculiar role in aging?
Keywords: MTOR, TOR, rapamycin, senescence, cancer, stroke, development, growth
It is commonly believed that aging is caused by random accumulation of molecular damage due to failure of maintenance, because repair is costly [1, 2]. As emphasized by Kirkwood, “the aging process is caused by the gradual buildup of a huge number of individually tiny faults - some damage to a DNA strand here, a deranged protein molecule there, and so on” [2]. The view is very logical, intuitive and simple. As argued recently [3], the scholastic philosopher William of Ockham would surely have liked it. Yet, the damage/repair theory leads to incorrect predictions and to bizarre paradoxes [4, 5]. Also, the free radical version of this theory has not been confirmed [6-15]. After all, William of Ockham lived before Galileo. Now we know that a theory must make correct predictions and be useful, rather than just be elegant.
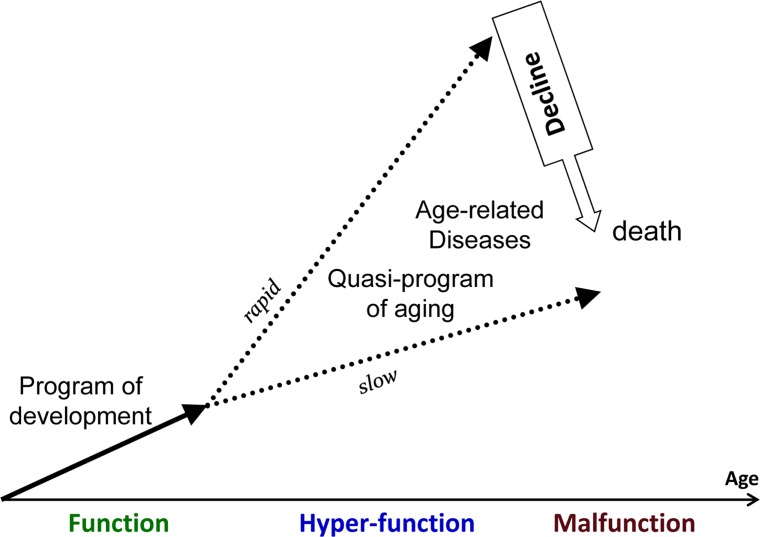
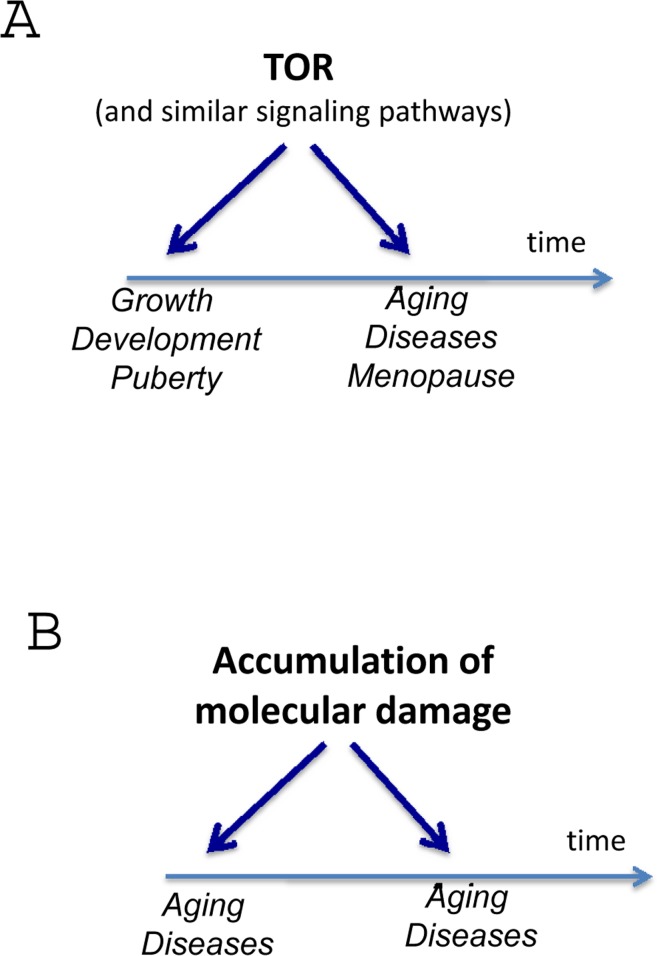
What if aging is not caused by accumulation of molecular damage? What if random accumulation of molecular damage is irrelevant to aging? Then the cause of molecular damage is not really important. What if aging does not start from day one. In fact, the mortality rate is lower in 10-years old children than in infants. So the period of growth is hardly aging. But when developmental growth is finished, growth-signaling pathways may continue to run on inertia (Fig. 1). Where would that lead the soma?
Figure 1. Aging as a quasi-program of development.
When development is finished cellular normal functions become excessive (hyperfunctions). They lead to diseases. Development is strictly programmed and therefore is precise (one line), whereas aging is not (a continuation of developmental program or quasi-program), and so age-related diseases (ADR) occur at different age.
The hyperfunction theory
In cell culture, when actual proliferation is blocked, then still active growth-signaling and nutrient-sensing pathways such as the TOR (Target of Rapamycin) pathway cause senescence [16-27]. TOR can convert quiescent cells into senescent cells without any involvement of molecular damage [28-36]. The TOR pathway is involved in yeast and organismal aging from worm to mammals [37-53] as well as in age-related disease in mammals [54-65]. The same pathway, which drives developmental growth, later drives aging and its associated diseases. As discussed in detail previously [54, 66], aging is of course not a program, but it is a quasi-program, a useless and unintentional continuation (or run on) of developmental programs. Similarly, cellular senescence is a continuation of cellular growth [36, 67, 68]. In brief, over-stimulation leads to increased functions. Such functions include secretion by fibroblasts, contraction by arterial smooth muscle cells (SMC), aggregation by platelets, bone resorption by osteoclasts, lipogeneisis by fat cells, glycogenesis by liver cells, inflammation by neutrophils, phagocytosis by macrophages and so on and so one. Also, overstimulation may render cell signal resistance due to feedback block of signaling pathways. In turn, hyperfunction coupled with signal-resistance causes loss of homeostasis, diseases, organ damage and eventually death of the organism [54]. For example, taken together, hyperfunctions of arterial smooth muscle cells, macrophages, hepatocytes, fat cells, blood platelets, neurons and glial cells, fibroblasts, beta-cells cause organ hypertrophy and fibrosis, atherosclerosis and hypertension (and their complications such as stroke and infarction), osteoporosis and (as complication, born rupture), age-related blindness, gangrenes, renal and heart failure and even cancer. There is no ARD that cannot be linked to initial cellular hyperfunction, in part, driven by mTOR [54]. The senescence-associated secretory phenotype (SASP) [69- 73] is a characteristic hyperfunction of fibroblasts (and some other cells) caused by hyper-mitogenic stimulation of arrested cells [74, 35]. Chronic inflammation, a classic example of hyperfunction, is associated with aging and age-related diseases [75- 82]. Even telomere shortening [83-93] can also be viewed as a consequence of hyperfunction insofar as it is promoted by hyper-proliferation, and perhaps, therefore, is associated with accelerated age-related diseases (ARD). Although loss of functions is often in terminal aging, loss of function always results from initial hyperfunction (and no another example could be found [54]. This is also applicable to simple multicellular organisms such as Drosophila and C. Elegans [94, 95].
Given that cellular hyperfunction is one of the main characteristics of aging, David Gems, Yila de la Guardia and Linda Partridge suggested a short name “hyperfunction theory” [94, 95], which I will use here.
Many predictions of the hyperfunction theory have already been confirmed [96]. Pro-aging signal-transduction pathways and potential anti-aging agents that target them have been revealed, including several existing drugs such as rapamycin and metformin [97]. Moreover, inhibition of hyperfunction in downstream processes regulated by aging pathways, e.g. attenuation of protein synthesis, extends lifespan [98-100]. Intriguingly, some anti-hypertensive drugs “calm down” hyper-functional signaling-pathways, simultaneously preventing other age-related diseases such as cancer (see for references [101]). Examples include inhibitors of beta-adrenergic [101-103] as well as of angiotensin II signaling [104, 105], which are both linked to mTOR signaling [106]. Metformin, an anti-diabetic drug, which indirectly inhibits the mTOR pathway, decreases cancer incidence, prevents premature menopause and increases lifespan in rodents [107-112]. Rapamycin not only delays typical age-related diseases in animal models but also extends life span in mice [113-119]. Thus, there exists the opportunity to extend both health span and lifespan in our life time [120, 121].
The molecular damage theory dies hard
But what about the molecular damage? It was assumed that molecular damage contributes to aging because it accumulates with time. Well, over time you may accumulate money in your bank account. However, neither accumulation of molecular damage nor accumulation of money is a cause of your aging. Yes, molecular damage must accumulate. But although molecular damage accumulates, it does not necessarily limit lifespan, particularly if other causes limit life span. By analogy, if everyone died from accidents, starvation and infection early in life, then aging and age-related diseases (such as obesity and atherosclerosis) would not even be known. By the same token, “aging” due to molecular damage will not manifest itself, if aging due to hyperfunction invariably limits life span [122]. Notably, as a marker of hyperfunction in senescent cells, DNA damage response-signaling pathways can be hyper-activated even without DNA damage [123-126].
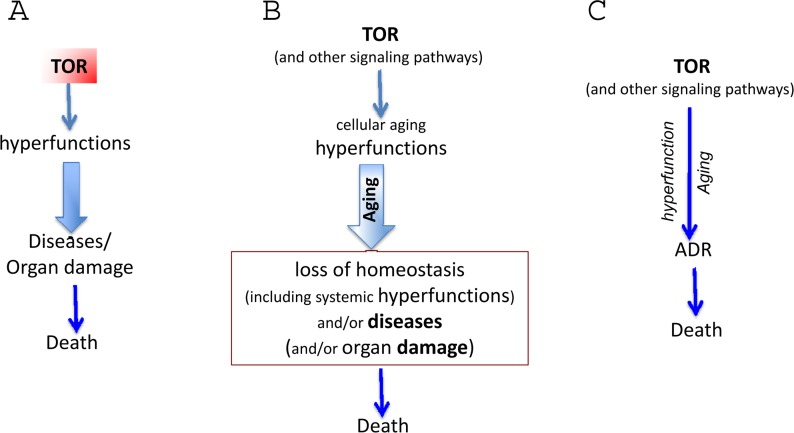
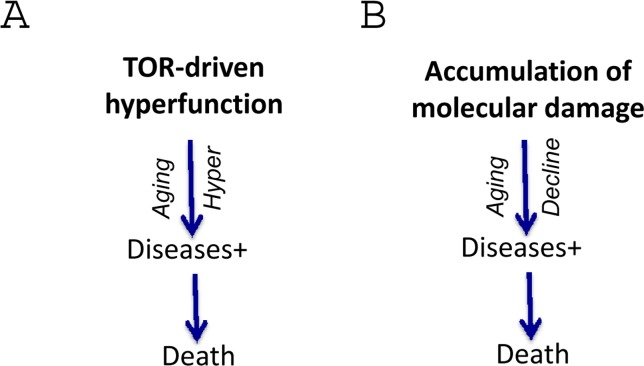
The hyperfunction theory suggests that repair of molecular damage is important for long life, exactly because it is harmful from day one. But the importance of any process for viability does not imply its role in aging. For example, although DNA replication is important, it (or its abnormalities) does not drive aging. Nonetheless, with a few exceptions, most gerontologists cannot let go of the damage accumulation theory, historically the dominant paradigm in the field. This outlook is superbly expressed by Piotr Zimniak, who argues that the molecular damage theory cannot be replaced by the hyperfunction theory [3] He has briefly summarized the hyperfunction theory in figures 1 A (here figure 1A). First, I will extend figure 2 from A to B (Fig. 2 A,B) in part because the terms “loss of homeostasis” and “age-related diseases” are manifestations of aging either according to molecular-damage or to hyperfunction theories, retrospectively. “Loss of homeostasis” and “age-related diseases” are not alternatives, but instead overlapping terms, almost synonyms, closely related phenomena.
Figure 2. The hyperfunction theory: three representations.
(A) The simplest model. Cause-effect relationship between TOR-driven hyperfunction and death via age-related diseases (diseases). For diseases, we mean age-related diseases (ADR). (B) Extended model. Diseases include initial loss of homeostasis and systemic hyperfunction (an increase in blood pressure and glucose) leading to organ damage like stroke, menopause and diabetes. Cellular hyperfunctions (e.g. hyper-secretion) can be viewed as cellular aging. (C) Unification of the hyper-function theory. Since cellular aging is cellular hyperfunction, it can be unified with systemic hyperfinction. In brief, aging = hyperfunction. Loss of homeostasis, decline and organ damage, which can be unified as ADR (age-related DISEASES).
Loss of homeostasis and age-related disease and death
Cellular hyper-function (and secondary signal resistance) must cause loss of homeostasis and, eventually, organ damage and death. Loss of homeostasis encompasses pre-diseases (e.g., glucose intolerance or insulin-resistance), syndromes such as metabolic and diseases such as hypertension, obesity, diabetes, atherosclerosis, renal and cardiac failure and so on. Obesity, hypertension, hyperlipidemia, hyperglycemia, hyperinsulinemia, hyperprolactinemia (and other hypers) are all systemic hyperfunctions, measurable by standard medical methods. They are systemic manifestations of cellular hyperfunction. Such systemic hyperfunctions also constitute loss of homeostasis.
Yet, given my medical background, I prefer the term “disease” to “loss of homeostasis”. Otherwise patients with blindness due to diabetes and patients with stroke due to hypertension and atherosclerosis would both be just suffering from loss of homeostasis. Second, dividing “loss of homeostasis” into specific diseases allows us to better apply the wealth of biomedical knowledge. It is known in detail how hyperfunctions such as an increased lipogenesis by fat and liver cells, hyper-aggregation of platelets, increased contractility and hypertrophy of arterial smooth muscle cells, migration and phagocytosis by macrophages - after many steps that are very well-known in pathology and medicine – cause, for example, damage to the brain via stroke. The sequence of events from TOR-driven hyperfunction to atherosclerosis, type II diabetes and menopause were recently discussed [58, 59, 63, 127-130] or will be discussed soon.
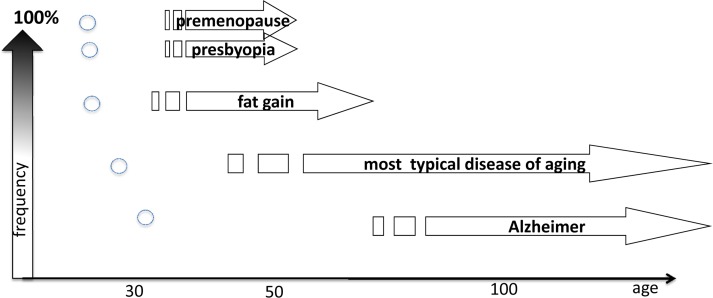
Age-related diseases (ARD), or if you prefer “loss of homeostasis (LOH)” limit lifespan in protected environments. ARD can be delayed by slowing down aging due to deactivation of the mTOR pathway by calorie restriction, genetic manipulation and drugs. The incidence of age-related diseases increases exponentially with aging. ARD include cancer, hypertension, atherosclerosis, diabetes type II, osteoporosis, sarcopenia, Alzheimer and Parkinson diseases, macular degeneration, organ fibrosis and hypertrophy and many more. Their complications (and often an immediate cause of death) include stroke, infarction, ventricular fibrillation, complications of diabetes such as renal failure, and so on. Also, menopause (in females) and presbyopia are normal age-related diseases, whose incidence does not increase indefinitely since almost all are affected by age 55 (Fig. 3). And of course “cosmetic diseases” such as baldness can be linked to hyper-function too, but this is a topic for another article.
Figure 3. The relationship between the onset of age-related disease (ADR) and it frequency.
The onset of the ADR determines its frequency and the precision of the onset. Arrows: Age of age-related diseases and its frequency in general population. Small circles: Genertic variants of the similar disease, which is a rare case in general population. High frequency is only (!) in very small groups of genetically-abnormal patients.
I combine the terms “loss of homeostasis and/or disease” (and call them ADR in this article; may be another term could be malignant hyperfunction or even malfunction). Mild and brief loss of homeostasis is not necessarily called disease but they are not lethal anyway. Some forms of damages such as hip fracture due to osteoporosis are not viewed as loss of homeostasis. Therefore, “and/or” (Fig. 2B). Organ damage or organ malfunctions could occur either early or late in the pathogenesis of ADR. There are positive-feedback loops between cellular hyperfunction and loss of homeostasis as well as vicious cycles between “loss of homeostasis” and “organ damage”. Organ damage is the end point: cardiac arrest and stem brain damage always happen as an immediate cause of death. But all that is well described in the medical literature. All these late and terminal events MUST be the same regardless of either the “molecular damage” or the “hyperfunction theories. In contrast, gerontology is concerned whether it is “molecular damage” or “hyperfunction” cause malfunctions and diseases (causes of death). And the molecular damage theory cannot explain hyperfunctions.
For brevity, I will use the term disease, which include loss of homeostasis (biomedical term) and non-random organ damage (Fig. 2C). Also, TOR causes cellular and system hyperfunctions (e.g., hyperlipidemia and hypertension) are continuation of cellular hyper-functions. Cellular hyperfunction is the essence of cellular aging. The consequence of hyperfunction is the organismal aging, defined as a progressive increase in death rate. Therefore, in figure 1 C, cellular and systemic hyperfunction are combined, as an essence and equivalent of aging, which drives loss of homeostasis, diseases, macro-damage and secondary decline (all combined as “disease” or malfunction in figure 3C).
Finally, TOR-driven hyperfunctions are not only involved in diseases but also in “cosmetic” age-related alterations/diseases. Male pattern of baldness is often associated with hyper-stimulation with testosterone. I will not discuss cosmetic alterations in detail here. Many of them are deadly in some environmental conditions. For example, loss of teeth or vision is deadly in the wild. These are conditional diseases, so I will include them in the term “disease” or ARD.
For complex organisms like mammals the relationships between hyperfunctions (aging), diseases and damage (decline) are:
Hyperfunctions (increased cellular functions) including hypertrophy are primary. This is the essence of aging, which silently causes malfunctions and age-related diseases (ADR).
Decline of functions, malfunctions and atrophy are secondary. For example, hyper-stimulation of beta-cells by nutrients and mitogens can cause its apoptosis. Here is important to emphasize however that apoptosis can be also a form of hyperfunction, unneeded continuation primary function such as apoptosis during development of the immune system.
Damage is caused by aging, not the reverse.
Damage is not molecular. It is macro-damage (tissue, system and organ damage), like stroke, infarction, metastases, broken hip fracture and renal failure. Damage may take a form of sudden “catastrophe”, even though hyperfunctional aging slowly generates diseases for decades. If a patient survives infarction (due to medical intervention), she can live for many years, reflecting the fact that catastrophe was not due to the burden of molecular damage. In small organisms (e.g., Drosophila) organ can consist of one cell or even cell's part, but still an organ.
Diseases and organ damage are not random. There is a limited number of common causes of death. This is because hyperfunction is a continuation of develop-mental function, not a random process. Stroke, infarction and cancer are common. Cessation of thrombocytopoiesis (for example) is not. In C elegance and humans, “age-related diseases” are very different of course, because physiology and anatomy is so different [131]. For example, nutrient-sensitive organ (“hypothalamus”) contains 2 neurons only. These two neurons mediate diet-restriction-induced longevity in C. elegans [132]. In all multi-cellular organisms, the rule is: function, then hyperfunction (aging), then mal-function.
Argument 1: As argued by Piotr Zimniak, “I would hesitate to accept that catastrophic events, such as a stroke in a middle-aged person or sepsis in an otherwise healthy individual, are aging” [3].
Me too. To say that “aging causes diseases” is not to mean that “all diseases are caused by aging”. Clearly, not all diseases are caused by aging. For example, sepsis is caused by microorganisms. Even “age-related” diseases and their complications such as stroke could result from genetic defects, developmental abnormalities, environmental factors and so on. Or stroke could be due to accelerated atherosclerosis and hypertension, which in turn could be due to either accelerated aging, genetic/environmental factors or both. It could be a combination of genetic defects, environmental factors (smoking) and aging, which drives atherosclerosis, platelet hyperfunction and hypertension. For details here, I refer the readers to medical textbooks.
The point is not that age-related diseases are always caused by aging but that aging is sufficient to cause age-related diseases (either organ hyperfunction or its malfunction/failure). Even without genetic predisposition and environmental causes, aging causes stroke and other diseases, which together kill every human being (so far) by the age of 122 (the age at death of the oldest woman, Jeanne Calment). True age-related diseases (ARD) are manifestations and exacerbations of the normal aging process. Any particular ARD (age-related diseases) may not happen in any given individual simply because other ARD can terminate life first (Fig. 4). As we will discuss, time of onset, the frequency and the inevitability of ARD vary because aging is not programmed but an imprecise continuation of development. But here is a second argument against the hyperfunction theory.
Figure 4. The molecular damage theory.
Elimination of the repetitive terms. (A) Repetitive links between aging and death. (B) The only link between aging and death.
Argument 2: Aging kills not by causing catastrophic damage but “-rather loss of homeostasis, i.e., aging, can lower cell/tissue robustness and precipitate catastrophic events” [3].
In other words, by lowering robustness “accumulation of molecular damage” in the brain would precipitate stroke. This scenario is imaginary. This is not how it happens. Rather hyperfunctions such as hypertension, propensity to thrombosis, rapture of or stenosis by atherosclerotic plaque cause stroke. By analogy, hurricanes cause damage due to their “hyperfunction and robustness”. In contrast, weakness of construction does not precipitate catastrophe in good weather. Let us extend the analogy. An increased use of oil and coal (purposeful program) leads to carbon emissions, which lead to global warming (aimless quasi-program, analogous to hypertension, atherosclerosis and hyper-coagulation), which contribute to other factors that generate a particularly powerful storm, which can be damaging, if it strikes a vital location such as New Orleans (or the brain, in the case of stroke).
Should we re-invent medical science to fit the theory of molecular damage? More plausibly, it is any theory of aging that should reconcile itself with physiology, pathology and medicine, rather than vice versa. Hyperfunction (hypertension, propensity to thrombosis, etc) causes catastrophic events. Even cancer cells, which actually accumulate molecular damage, are robust and in turn damage and kill the organism due to their robustness. Only after non-random system/organ damage, is there decline and weakness. The decline phase is not driven by TOR and is only marginally quasi-programmed, a process run loose. This is a subject for emergency medicine, not gerontology.
Elderly patients who are immobilized by stroke, for example, are vulnerable to infections and sepsis. However, it is not aging per se that provoked sepsis but is rather immobolization caused by stroke (or in other words complications of age-related diseases, which then take their own TOR-independent course). But even then hyperfunctions contribute to deadly outcome such as fatal septic shock, which can be prevented with rapamycin [133].
Normal and hyper-functions
Hyper-functions result from the continuation (or running on) of normal functions. For example, blood pressure rises from birth to adulthood. This developmental program increases robustness by assuring optimal blood pressure. But its continuation (hyperfunction) leads to hypertension. As another example, at puberty in girls, a carefully-regulated increase of estrogen and gonadotropin levels switch on the reproduction (program, function). A continuation of the same process (quasi-program, hyperfunction) progressively impairs fertility after 30 (Fig. 2) and eventually culminates in ovarian failure and menopause [128, 134, 135]. Then, levels of estrogens drop (decline), accelerating osteoporosis. Menopause is a typical age-related disease [136]. It is not called a disease simply because it happens in all women (Fig. 3). Actually, it does not: some women die before menopause. Just 300 years ago most women died before menopause. Menopause is a quasi-programmed disease [128]. Menopause is particularly program-like, because it happens relatively early in life, when quasi-program (hyperfunction) is still very directional, a precise continuation of the developmental program for reproduction.
I need to emphasize that hyper-function is not always an increased function. It may be unneeded normal function like growth and apoptosis. In analogy, a car that is driving at 65 pmh at small parking lot is “hyperfunctional”, even if at the highway, this speed isr normal. Similarly, the TOR activity that is constantly and chronically at the level of rapidly dividing cells, (“proliferating cell” level) is gerogeni in resting postmitotic cells.
Some facts on age-related diseases (ARD)
Any theory of aging (regardless of the cause of aging) must be in agreement with the following medical facts:
The incidence of age-related diseases (ARD) increases exponentially with age in parallel with death rate. This is not co-incidental but rather reflects the fact that ARD cause death. If an individual does not die from one disease (e.g., cancer), he/she will eventually die from another (e.g., stroke and myocardial infarction). Of course, small organisms, which even lack the heart have different ARD, which would be better studied, if these small animals “complain to their “doctors”.
Aging kills via diseases. In fact, no one has ever died without a cause. And “natural causes” always denotes disease. In very elderly people, the diagnosis of “death from natural causes” means many competing causes (diseases) simultaneously. Death from natural causes simply excludes death from suicide and homicide. But there is no such medical diagnosis as “death from accumulation of molecular damage”. This “nonexistent diagnosis” is simply not needed because one or several deadly diseases can be always (always) found (unless homicide). Even sudden cardiac death usually results from myocardial fibrillation (archetypical hyper-function of electrical myocytes andventricular hypertrophy).
The later the onset of a disease, the greater the variability in the time of its onset (Fig. 2). This is because aging is not programmed, but quasi-programmed. It is a continuation of development. Development is strictly regulated. But aging and its diseases are not because they are an unintended, non-guided continuation of developmental growth. By analogy, the longer you walk with your eyes closed, the less precisely you continue in the same direction. The further from the end of development, the bigger deviation and the higher imprecision. A particular age-related disease (ARD) could strike at 40 and at 110 and at 300 in some people, if they lived so long). It would strike everyone, if other diseases would not terminate their lives first (Fig. 2). It is perhaps the case that any age-related disease would develop sooner or later in anyone, and only death from other diseases precludes death from any given one.
The time of onset of ARD determines the frequency and inevitability of the disease (Fig. 2). Importantly, this also correlates with the invariability of age of disease in everyone and its precision. Early ARD is a direct continuation of developmental programs. I already mentioned progressive loss of fertility and menopause in women, which is a direct continuation of reproductive function. Another example is presbyopia, a progressive hardening of lens that prevents focusing at close small objects [137, 138]. Symptoms such as problems focusing on fine print, requiring glasses, are noticed between the ages of 40 and 50, very often almost suddenly. This ARD is a quasi-program, a continuation of developmental program. The near point of vision is very close in infants and then progressively moves further away as an organism grows larger. The same process continues later in life: from 7 cm at age 10, to16 cm at age 40, to 100 cm at age 60. Mechanistically, this is hyperfunction due to progressive increase of thickness and stiffness of the crystalline lens as well as continual growth of the lens [139, 140]. Most importantly, a continuation of this quasi-program (quasi-quasi-program) is age-related nuclear cataract, which is a cause of blindness much later in life, involving genetic and environmental factors [141]. Therefore, cataract does not happen to everyone and is variable in its timing of onset.
Argument 3: “it would be difficult to identify catastrophic death events in, for example, bacteria, organisms that also age”.
Yes, I agree of course. If bacteria indeed age, they may age from accumulation of molecular damage, precisely because they do not undergo hyperfunctional aging and have a chance to experience (or not) aging from accumulation of damage. But multicellular organisms do not die from the same “aging” as bacteria. In multicellular organisms, hyperfunctions lead to eventual disintegration of the soma. In contrast bacteria may age from accumulation of moleculer damage (most probable) but they have no multicellular soma and never dir from stroke anyway. Bacteria is irrelevant example of commom aging.
Age-related diseases in worm and flies
Due to extensive biomedical research, humans are the most studied animal. No one dies from “healthy aging”, without a cause: either natural causes such as disease or homicide/suicide. Similar, all mammals die from age-related diseases (ARD), albeit their frequencies vary dramatically. This could be expected given a quasi-programmed nature of aging and ARD. If one disease occurs earlier than other diseases, it will mask all other diseases. Likewise, Pacific Salmon die from quasi-programmed ADR, namely massive organ damage, resulting from continuation of the reproductive program. This is a particular clear example of quasi-programmed hyperfunction [142]. It is often stated that all Pacific Salmon die from aging/ADR, and therefore that this is a program. Not true. Only 1-2% Pacific Salmon die from ADR, all others die earlier from accidental causes, which must be expected in the wild [142], (see figure 3 in ref.[4]).
Fruit flies and worms die from manifestations of aging or ARD. ADR as causes of death are more specific than vague death from aging. In fruit flies, diseases include neurodegeneration [143-146], cardiac dysfunction [147, 148] and even “diabetes” [149]. TOR is involved in age-related pathologies in flies [150, 46, 47, 150]. Insulin, which activates TOR, is implicated in pathologies resembling mammalian metabolic syndrome [53, 151], diet-induced obesity, diabetes and cardiac dysfunction [152-156]. As in mammals, high-fat-diet-induced obesity and heart dysfunction are regulated by the TOR pathway [147]. Thus main pro-aging pathways in Drosophila include insulin/FOXO/TOR, TOR, JNK, NF-kB [46, 47, 145, 147, 157-162].
In the nematode worm Caenorhabditis elegans, several “diseases” can be identified [163-173], including infections [174]. Many of the diseases of aging seen in worms are consistent with quasi-programmed hyperfunction, and within its short 2-3 week lifespan C. elegans develops a number of pathologies involving extreme hypertrophy [94, 95]. One example, discussed by David Gems and Linda Partridge [94], involves yolk, which is synthesized in the intestine in large quantities to provision the developing oocytes. After several days of reproduction, reproduction ceases. However, production of yolk continues, and consequently it accumulates in large pools within the body cavity. This accumulation is suppressed in long-lived daf-2 insulin/IGF-1 receptor mutants. Moreover, inhibition of yolk protein gene expression extends worm lifespan, suggesting that quasi-programmed yolk accumulation increases age-related mortality [94]. So worm and fly die from ADR too, just different ADR. Needless to say that even different mammals have different frequency of common ADR. Needless to say that even different nations have different prevalence of ADR.
Argument 4: If aging is quasi-programmed hyper-function, why then is aging associated with decline and atrophy? The critic agrees that “atrophy, a classical sign of aging-related decline, can be in fact secondary to an initial hyperfunction and hypertrophy (Blagosklonny, 2012).” This is correct: “signs of age-related decline” are secondary.
From hyperfunction/hypertrophy to decline/atrophy
There are diverse mechanisms of secondary atrophy during aging.
Quasi-programmed (hyperfunction-driven) apoptosis. In this case, atrophy is secondary to signal resistance due to mTOR overactivation. For example, in insulin-secreting beta-cells overactivated by nutrients and insulin, mTOR causes cellular hypertrophy/hyperplasia/hyperfunction and secondary insensitivity to IGF-1 and deactivation of Akt, leading to beta-cell death [130, 175, 176]. mTOR-dependent hyperfunction and hypertrophy of beta-cells, may eventually culminate in cell loss and decline of function [177, 178]. Similar quasi-programmed apoptosis could be observed in the muscle, the immune system and subcutaneous adipocytes. Apoptosis is programmed in development but quasi-programmed in aging. Also, strong hyper-mitogenic drive can force post-mitotic neurons into the cell cycle leading to apoptosis in Alzheimer disease [179-184]. Importantly, cellular senescence is associated with both hyper-mitogenic drive and death in mitosis, explaining this phenomenon [124].
Hyper-stimulation-driven cell exhaustion. For example, mTOR overactivation [68, 185, 186] or growth factor stimulation [187] drives exhaustion of stem cells and ovarian oocytes [188-191]
Poor wound-healing could be due to signal resistance secondary to cellular aging and hyperglycemia [192].
Metabolic-self destruction due to hyper-active TOR. [108, 193, 194]
Common atrophy is secondary not to aging itself but to age-related diseases. This is disease-driven atrophy, the end point of some diseases. This is so far away from initial cause that it is completely unrelated to aging and is mTOR-independent. Let me provide two examples. Atherosclerosis of the femoral artery can cause not only atrophy but even gangrene of the feet. Atrophy is very common in ischemia due to atherosclerosis. As another example, hip fracture in an elderly person often leads to prolonged immobilization. Muscle atrophy is secondary to immobilization, which is secondary to the broken hip, which is secondary to osteoporosis, which is secondary to hyperfunction of osteoclasts… and so on. This is disease-driven atrophy. Atrophy is common because it is secondary to diseases. This further supports the thesis that there is no healthy aging (healthy aging is no aging or very slow aging).
Argument 5: When cellular hyperfunction causes atrophy, there must be molecular mechanisms such as interaction of ligand with receptor, protein aggregation and so on. More specifically, the critic claims that “an overproduced ligand may over stimulate or desensitize a receptor” [3] For example, an overproduced ligand may over stimulate or desensitize a receptor, and an overabundant protein may aggregate and interfere with intracellular trafficking, or co-precipitate with and thus withdraw essential cell constituents. First, this is still an example of hyper-function. Importantly, rapamycin alleviates toxicity of different aggregate-prone proteins [195] and decreases aggregate-prone proteins [196-200]Second, this is not molecular damage but signal transduction. In contrast, the molecular damage theory is about life long accumulation (!) of random (!) molecular damage due to failure of repair/maintenance (!). As emphasized by Kirkwood, “the aging process is caused by the gradual buildup of a huge number of individually tiny faults - some damage to a DNA strand here, a deranged protein molecule there, and so on” [2].
If we redefine “signal transduction” as “accumulation of random molecular damage due to failure of maintenance”, then yes, this is a cause of aging and age-related diseases. Then driven by mTOR, “molecular damage” (formerly, signal transduction) includes protein phosphorylation as well as protein synthesis, inhibition of autophagy and caspase activation. But this is hyperfunction, not failure of maintenance. Phosphorylation of S6K by mTOR, modification of NF-kB or dephosphorylation of Akt, for instance, are not molecular damage. This is signal transduction. Exactly the same signal transduction is involved in development, cell growth, differentiation and apoptosis. These are normal functions. Since the same molecular events are involved in development and developmental growth, this would lead to the reductio ad absurdum that development is caused by damage. More plausibly, their (developmental functions) continuation gives rise to quasi-programmed hyperfunction. These normal functions and hyperfunctions can be inhibited by signal transduction inhibitors including rapamycin.
Life long accumulation of molecular damage is irrelevant to aging. Aging (hyper-function of signaling pathway) causes damage and this damage is organ/system/organismal damage (not molecular damage). Hyperfunction of liver cells, for instance, after several decades, contributes to brain damage via stroke.
Healthy death in molecular damage theory
As commonly depicted [3], aging (loss of homeostasis), caused by molecular damage, in turn causes death via two independent ways (Fig. 4A). The first way is via an increased susceptibility to diseases. The second way is directly without any diseases, and is the true aging mechanism, according the molecular damage theory. This is incorrect. Consider young healthy constructor worker fall to death from the storm (Yes, this is “healthy” death but not from aging). Another example. The fall from the height of 100 year old person is due to either age-related Parkinson's disease or due to infarction. This is not death from healthy aging. This is death due to an age-related disease.
As we discussed, death from aging is death from diseases (natural causes) (Figs. 5,6). Even the oldest people do not die from healthy aging. There is no such medical diagnosis as healthy death or death from asymptomatic accumulation of molecular damage. Of course, we can consider “loss of homeostasis” broadly, including severe deviations of homeostasis or diseases. But then there is no other “disease pathway” anyway. Regardless of the causes of aging, the causes of death and the path from “loss of homeostasis” to death are well known. In all theories of aging, this must be identical because this is a medical fact (Fig. 5). There is no death directly from healthy aging (of healthy loss of homeostasis). This is a part of the same path (Fig. 5). So, shift from A to B (Fig. 4), exactly as in figure 2 B.
Figure 5. Harmonizing two theories for direct comparison.
The causes of molecular damage are mostly unknown and also irrelevant.
Figure 6. Harmonizing two theories for direct comparison.
The differences are obvious.
Now the question is how accumulation of molecular damage drives each age-related diseases (Figs. 5, 6). For example, how accumulation of molecular damage causes insulin resistance, hypertension and obesity. There is no obvious answer.
Is TOR-driven hyperfunction true aging?
Is it true aging? Or is it just a process related to disease and mortality [3]? No, it is genuine aging. Not only because it determines mortality (a hallmark of aging is an exponential increase in mortality rate) but also because mTOR-dependent hyperfunctions, signal-resistance, hypertrophy, hypermitogenic drive coupled with loss of regenerative potential are markers of cellular senescence. Cellular senescence can be caused by mTOR activation in cell culture. Although by arresting cell cycle, DNA damage response (DDR) creates conditions for senescence (if mTOR is active), the accumulation of molecular damage itself does not cause senescence in cell culture [35, 36]. In contrast, accumulation of molecular damage contributes to cancer cell immortality [122]. Thus TOR-driven hyperfunction links cellular aging to age-related diseases and organismal aging, defined as an increase of the probability of death.
Do any questions remain unanswered?
Although we have answered most of the issues relating to the damage/maintenance vs hyperfunction debate raised by P. Zimniak (one is left for the conclusion), it may seem that many more unanswered questions remain. Yet, some of them have been answered previously (see PubMed “Blagosklonny” and related references within) and others will be answered in forthcoming articles. The evolutionary aspects, the links between development and aging, pro-aging and anti-aging genes, common signaling pathways that drive aging, cellular senescence and diseases such as cancer have been extensively discussed. According to the hyperfunction theory, aging and its manifestations are never programmed: even menopause and the death of Pacific Salmon are not programmed. Both are excellent examples of quasi-program, a non-adoptive, aimless, harmful continuation of a reproductive program.
Also, as already discussed, lifespan is determined not only by the aging process but also by aging-tolerance, an ability to tolerate disease of aging and their complications. As a matter of fact, almost all medical interventions (including by-pass surgery and teeth proteases) increase aging tolerance rather than slow down aging. When needed, natural selection may favor anatomical and molecular adaptations such as collateral blood vessels and heat shock proteins, for example. Thus extra coronary arteries would increase lifespan despite age-related atherosclerosis, hypertension and thrombosis. Aging-tolerance is a concept that can solve some mysteries of aging. Many potential questions that might be asked are purely medical. Their answers can be found in medical textbooks.
There are a few questions that are difficult to answer now:
What TOR-independent pathways contribute to hyperfunctional aging? For example, sirtuins, FOXO, AMPK and IGF-1 can all be linked to the mTOR networks [201-206]. What about JNK [158, 207-209] and NF-kB [210], [162, 211, 212]? Are these pathways TOR-independent? And what are crucial downstream effectors of TOR that control aging?
It seems that rapamycin should be used in intermittent fashion, perhaps in combinations with e.g. metformin, lipid-lowering drugs, and beta-blockers and angiotens together with dietary restriction and physical exercise. But what are the exact doses and schedules maximize positive and minimize negative effects?
What would be the causes of death if TOR-driven aging were suppressed? Hyperfunction driven by run on of other pathways? Accumulation of molecular damage? Mitochondrial expansion? Other types of unknown aging? Would anti-oxidants become useful for that types of aging? And what are the pathological manifestations of accumulation of random molecular damage?
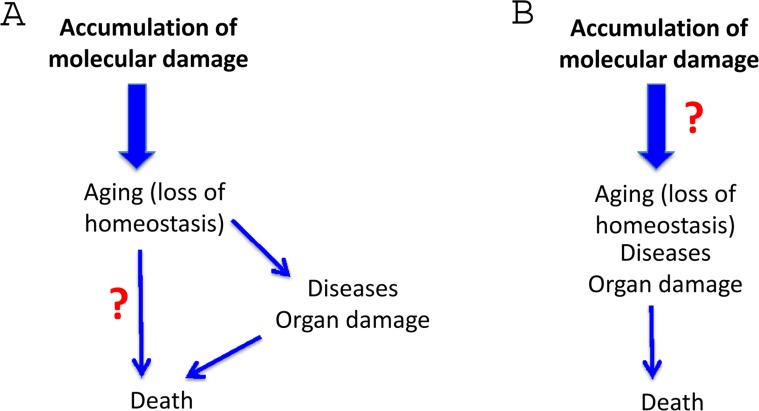
The peculiar role of molecular damage
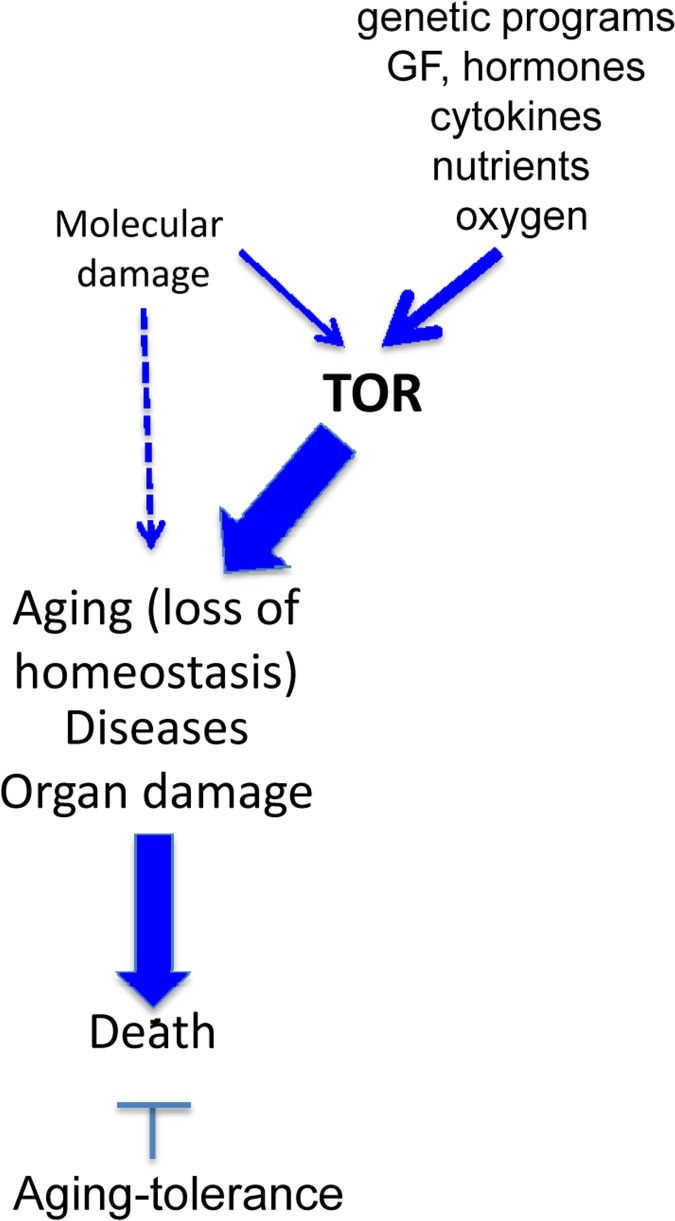
Repair of random molecular damage is so important that cumulative damage does not reach a deadly threshold during the lifetime. In progeria [213], fitness is low from day one. There is a very strong natural selection for repair and maintenance. In contrast, mTOR-driven functions are essential early in life and there is a very strong selection for robust mTOR-dependent functions, even if their continuation (hyperfunctions) are harmful in old age. Still, we cannot exclude contribution of molecular damage to some symptoms of aging (Fig.7). This is simply unknown. May it decrease aging-tolerance [127, 214]? This is a fascinating question to answer.
Figure 7. Incorporation of a hypothethetical role of molecular damage in the hyperfunction theory.
A peculiar case is cancer. Accumulation of damage does not make a cancer cell fragile, arguing against the molecular damage theory, but instead via rounds of selection and proliferation which create robust cells that damage the organism. Notably, such selection of random mutations culminates in non-random activation of the mTOR pathway [122]. Activation of the PI3K/mTOR pathway is the most common alteration (and therapeutic target) in cancer [215-227]. Also, hyper-activation of the DNA damage response, involving TOR-like kinases, may contribute to hyperfunction. Therefore, molecular damage and autonomous activation of damage-sensing signal-transduction pathways may contribute to hyperfunction, not vice versa.
The last argument for molecular damage theory
The last argument by Zimniak is: “Hyperfunction is one of several sources of molecular damage, on equal footing with reactive metabolites, toxicants, ROS, electrophiles, stochastic events, and many others” [3]. This argument will not be discussed all over again. Not only because hyper-functions are not a source of accumulation of molecular damage. But mostly because the starting point of this article is that the theory of molecular damage did not fit numerous observation, made incorrect predictions, did not contribute to medical advances, and did not lead to any practical application. As philosophers teach us, the theory cannot be wrong (or right), but it can be useless.
REFERENCES
- Kirkwood TB. Understanding the odd science of aging. Cell. 2005;120:437–447. doi: 10.1016/j.cell.2005.01.027. [DOI] [PubMed] [Google Scholar]
- Kirkwood T. Why women live longer. Stress alone does not explain the longevity gap. Sci Am. 2010;303:34–35. [PubMed] [Google Scholar]
- Zimniak P. What is the proximal cause of aging? Front Genet. 2012;3:189. doi: 10.3389/fgene.2012.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagosklonny MV. Paradoxes of aging. Cell Cycle. 2007;6:2997–3003. doi: 10.4161/cc.6.24.5124. [DOI] [PubMed] [Google Scholar]
- Blagosklonny MV. Why the disposable soma theory cannot explain why women live longer and why we age. Aging (Albany NY) 2010;2:884–887. doi: 10.18632/aging.100253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagosklonny MV. Aging: ROS or TOR. Cell Cycle. 2008;7:3344–3354. doi: 10.4161/cc.7.21.6965. [DOI] [PubMed] [Google Scholar]
- Doonan R, McElwee JJ, Matthijssens F, Walker GA, Houthoofd K, Back P, Matscheski A, Vanfleteren JR, Gems D. Against the oxidative damage theory of aging: superoxide dismutases protect against oxidative stress but have little or no effect on life span in Caenorhabditis elegans. Genes Dev. 2008;22:3236–3241. doi: 10.1101/gad.504808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gems D, Doonan R. Antioxidant defense and aging in C. elegans: Is the oxidative damage theory of aging wrong? Cell Cycle. 2009;8:1681–1687. doi: 10.4161/cc.8.11.8595. [DOI] [PubMed] [Google Scholar]
- Perez VI, Van Remmen H, Bokov A, Epstein CJ, Vijg J, Richardson A. The overexpression of major antioxidant enzymes does not extend the lifespan of mice. Aging Cell. 2009;8:73–75. doi: 10.1111/j.1474-9726.2008.00449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pani G. P66SHC and ageing: ROS and TOR? Aging (Albany NY) 2010;2:514–518. doi: 10.18632/aging.100182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabreiro F, Ackerman D, Doonan R, Araiz C, Back P, Papp D, Braeckman BP, Gems D. Increased life span from overexpression of superoxide dismutase in Caenorhabditis elegans is not caused by decreased oxidative damage. Free Radic Biol Med. 2011;51:1575–1582. doi: 10.1016/j.freeradbiomed.2011.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapointe J, Hekimi S. When a theory of aging ages badly. Cell Mol Life Sci. 2009;67:1–8. doi: 10.1007/s00018-009-0138-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Raamsdonk JM, Hekimi S. Deletion of the mitochondrial superoxide dismutase sod-2 extends lifespan in Caenorhabditis elegans. PLoS Genet. 2009;5:e1000361. doi: 10.1371/journal.pgen.1000361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speakman JR, Selman C. The free-radical damage theory: Accumulating evidence against a simple link of oxidative stress to ageing and lifespan. Bioessays. 2011;33:255–259. doi: 10.1002/bies.201000132. [DOI] [PubMed] [Google Scholar]
- Ristow M, Schmeisser S. Extending life span by increasing oxidative stress. Free Radic Biol Med. 2011;51:327–336. doi: 10.1016/j.freeradbiomed.2011.05.010. [DOI] [PubMed] [Google Scholar]
- Demidenko ZN, Blagosklonny MV. Growth stimulation leads to cellular senescence when the cell cycle is blocked. Cell Cycle. 2008;7:3355–3361. doi: 10.4161/cc.7.21.6919. [DOI] [PubMed] [Google Scholar]
- Demidenko ZN, Zubova SG, Bukreeva EI, Pospelov VA, Pospelova TV, Blagosklonny MV. Rapamycin decelerates cellular senescence. Cell Cycle. 2009;8:1888–1895. doi: 10.4161/cc.8.12.8606. [DOI] [PubMed] [Google Scholar]
- Demidenko ZN, Shtutman M, Blagosklonny MV. Pharmacologic inhibition of MEK and PI-3K converges on the mTOR/S6 pathway to decelerate cellular senescence. Cell Cycle. 2009;8:1896–1900. doi: 10.4161/cc.8.12.8809. [DOI] [PubMed] [Google Scholar]
- Demidenko ZN, Blagosklonny MV. Quantifying pharmacologic suppression of cellular senescence: prevention of cellular hypertrophy versus preservation of proliferative potential. Aging (Albany NY) 2009;1:1008–1016. doi: 10.18632/aging.100115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demidenko ZN, Korotchkina LG, Gudkov AV, Blagosklonny MV. Paradoxical suppression of cellular senescence by p53. Proc Natl Acad Sci U S A. 2010;107:9660–9664. doi: 10.1073/pnas.1002298107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leontieva OV, Natarajan V, Demidenko ZN, Burdelya LG, Gudkov AV, Blagosklonny MV. Hypoxia suppresses conversion from proliferative arrest to cellular senescence. Proc Natl Acad Sci U S A. 2012;109:13314–13318. doi: 10.1073/pnas.1205690109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leontieva OV, Demidenko ZN, Gudkov AV, Blagosklonny MV. Elimination of proliferating cells unmasks the shift from senescence to quiescence caused by rapamycin. PLoS One. 2011;6:e26126. doi: 10.1371/journal.pone.0026126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schug TT. mTOR favors senescence over quiescence in p53-arrested cells. Aging (Albany NY) 2010;2:327–328. doi: 10.18632/aging.100164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi L, Kepp O, Kroemer G. TP53 and MTOR crosstalk to regulate cellular senescence. Aging (Albany NY) 2010;2:535–537. doi: 10.18632/aging.100202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanov VS, Abramova MV, Svetlikova SB, Bykova TV, Zubova SG, Aksenov ND, Fornace AJ, Jr., Pospelova TV, Pospelov VA. p21(Waf1) is required for cellular senescence but not for cell cycle arrest induced by the HDAC inhibitor sodium butyrate. Cell Cycle. 2010;9:3945–3955. doi: 10.4161/cc.9.19.13160. [DOI] [PubMed] [Google Scholar]
- Kolesnichenko M, Hong L, Liao R, Vogt PK, Sun P. Attenuation of TORC1 signaling delays replicative and oncogenic RAS-induced senescence. Cell Cycle. 2012;11:2391–2401. doi: 10.4161/cc.20683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pospelova TV, Leontieva OV, Bykova TV, Zubova SG, Pospelov VA, Blagosklonny MV. Suppression of replicative senescence by rapamycin in rodent embryonic cells. Cell Cycle. 2012;11:2402–2407. doi: 10.4161/cc.20882. [DOI] [PubMed] [Google Scholar]
- Leontieva OV, Blagosklonny MV. DNA damaging agents and p53 do not cause senescence in quiescent cells, while consecutive re-activation of mTOR is associated with conversion to senescence. Aging (Albany NY) 2010;2:924–935. doi: 10.18632/aging.100265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leontieva O, Gudkov A, Blagosklonny M. Weak p53 permits senescence during cell cycle arrest. Cell Cycle. 2010;9:4323–4327. doi: 10.4161/cc.9.21.13584. [DOI] [PubMed] [Google Scholar]
- Dulic V. Be quiet and you'll keep young: does mTOR underlie p53 action in protecting against senescence by favoring quiescence? Aging (Albany NY) 2011;3:3–4. doi: 10.18632/aging.100257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki CG. Decision-making by p53 and mTOR. Aging (Albany NY) 2010;2:324–326. doi: 10.18632/aging.100166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesierska-Gadek J. mTOR and its link to the picture of Dorian Gray - re-activation of mTOR promotes aging. Aging (Albany NY) 2010;2:892–893. doi: 10.18632/aging.100240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano M. Shifting senescence into quiescence by turning up p53. Cell Cycle. 2010;9:4256–4257. doi: 10.4161/cc.9.21.13785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane DP, Verma C, Fang CC. The p53 inducing drug dosage may determine quiescence or senescence. Aging (Albany NY) 2010;2:748. doi: 10.18632/aging.100229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagosklonny MV. Cell cycle arrest is not senescence. Aging (Albany NY) 2011;3:94–101. doi: 10.18632/aging.100281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagosklonny MV. Cell cycle arrest is not yet senescence, which is not just cell cycle arrest: terminology for TOR-driven aging. Aging (Albany NY) 2012;4:159–165. doi: 10.18632/aging.100443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stipp D. A new path to longevity. Sci Am. 2012;306:32–39. doi: 10.1038/scientificamerican0112-32. [DOI] [PubMed] [Google Scholar]
- Kapahi P, Zid BM, Harper T, Koslover D, Sapin V, Benzer S. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr Biol. 2004;14:885–890. doi: 10.1016/j.cub.2004.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia K, Chen D, Riddle DL. The TOR pathway interacts with the insulin signaling pathway to regulate C. elegans larval development, metabolism and life span. Development. 2004;131:3897–3906. doi: 10.1242/dev.01255. [DOI] [PubMed] [Google Scholar]
- Kaeberlein M, Powers RWr, Steffen KK, Westman EA, Hu D, Dang N, Kerr EO, Kirkland KT, Fields S, Kennedy BK. Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Science. 2005;310:1193–1196. doi: 10.1126/science.1115535. [DOI] [PubMed] [Google Scholar]
- Ayyadevara S, Alla R, Thaden JJ, Shmookler Reis RJ. Remarkable longevity and stress resistance of nematode PI3K-null mutants. Aging Cell. 2008;7:13–22. doi: 10.1111/j.1474-9726.2007.00348.x. [DOI] [PubMed] [Google Scholar]
- Stanfel MN, Shamieh LS, Kaeberlein M, Kennedy BK. The TOR pathway comes of age. Biochim Biophys Acta. 2009;1790:1067–1074. doi: 10.1016/j.bbagen.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selman C, Lingard S, Choudhury AI, Batterham RL, Claret M, Clements M, Ramadani F, Okkenhaug K, Schuster E, Blanc E, Piper MD, Al-Qassab H, Speakman JR, Carmignac D, Robinson IC, Thornton JM, Gems D, Partridge L, Withers DJ. Evidence for lifespan extension and delayed age-related biomarkers in insulin receptor substrate 1 null mice. FASEB J. 2008;22:807–818. doi: 10.1096/fj.07-9261com. [DOI] [PubMed] [Google Scholar]
- Selman C, Tullet JM, Wieser D, Irvine E, Lingard SJ, Choudhury AI, Claret M, Al-Qassab H, Carmignac D, Ramadani F, Woods A, Robinson IC, Schuster E, Batterham RL, Kozma SC, Thomas G, et al. Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science. 2009;326:140–144. doi: 10.1126/science.1177221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapahi P, Chen D, Rogers AN, Katewa SD, Li PW, Thomas EL, Kockel L. With TOR, less is more: a key role for the conserved nutrient-sensing TOR pathway in aging. Cell Metab. 2010;11:453–465. doi: 10.1016/j.cmet.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Budanov AV, Park EJ, Birse R, Kim TE, Perkins GA, Ocorr K, Ellisman MH, Bodmer R, Bier E, Karin M. Sestrin as a feedback inhibitor of TOR that prevents age-related pathologies. Science. 2010;327:1223–1228. doi: 10.1126/science.1182228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katewa SD, Kapahi P. Role of TOR signaling in aging and related biological processes in Drosophila melanogaster. Exp Gerontol. 2011;46:382–390. doi: 10.1016/j.exger.2010.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzilai N, Huffman DM, Muzumdar RH, Bartke A. The critical role of metabolic pathways in aging. Diabetes. 2012;61:1315–1322. doi: 10.2337/db11-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Magalhaes JP. A role for Ras signaling in modulating mammalian aging by the GH/IGF1 axis. Aging (Albany NY) 2011;3:336–337. doi: 10.18632/aging.100309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge L, Alic N, Bjedov I, Piper MD. Ageing in Drosophila: the role of the insulin/Igf and TOR signalling network. Exp Gerontol. 2011;46:376–381. doi: 10.1016/j.exger.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demidenko ZN. Rapamycin for life: a step to immortality. Cell Cycle. 2011;10:4206. doi: 10.4161/cc.10.24.18562. [DOI] [PubMed] [Google Scholar]
- Passtoors WM, Beekman M, Deelen J, van der Breggen R, Maier AB, Guigas B, Derhovanessian E, van Heemst D, de Craen AJ, Gunn DA, Pawelec G, Slagboom PE. Gene expression analysis of mTOR pathway: association with human longevity. Aging Cell. 2012 doi: 10.1111/acel.12015. [DOI] [PubMed] [Google Scholar]
- Haselton A, Sharmin E, Schrader J, Sah M, Poon P, Fridell YW. Partial ablation of adult Drosophila insulin-producing neurons modulates glucose homeostasis and extends life span without insulin resistance. Cell Cycle. 2010;9:3063–3071. doi: 10.4161/cc.9.15.12458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagosklonny MV. Aging and immortality: quasi-programmed senescence and its pharmacologic inhibition. Cell Cycle. 2006;5:2087–2102. doi: 10.4161/cc.5.18.3288. [DOI] [PubMed] [Google Scholar]
- Tsang CK, Qi H, Liu LF, Zheng XFS. Targeting mammalian target of rapamycin (mTOR) for health and diseases. Drug Disc Today. 2007;12:112–124. doi: 10.1016/j.drudis.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Inoki K, Corradetti MN, Guan KL. Dysregulation of the TSC-mTOR pathway in human disease. Nat Genet. 2005;37:19–24. doi: 10.1038/ng1494. [DOI] [PubMed] [Google Scholar]
- Chen WQ, Zhong L, Zhang L, Ji XP, Zhang M, Zhao YX, Zhang C, Zhang Y. Oral rapamycin attenuates inflammation and enhances stability of atherosclerotic plaques in rabbits independent of serum lipid levels. Br J Pharmacol. 2009;156:941–951. doi: 10.1111/j.1476-5381.2008.00102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagosklonny MV. Validation of anti-aging drugs by treating age-related diseases. Aging (Albany NY) 2009;1:281–288. doi: 10.18632/aging.100034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagosklonny MV. Prospective treatment of age-related diseases by slowing down aging. Am J Pathol. 2012;181:1142–1146. doi: 10.1016/j.ajpath.2012.06.024. [DOI] [PubMed] [Google Scholar]
- Dazert E, Hall MN. mTOR signaling in disease. Curr Opin Cell Biol. 2011 doi: 10.1016/j.ceb.2011.09.003. [DOI] [PubMed] [Google Scholar]
- Zhao C, Vollrath D. mTOR pathway activation in age-related retinal disease. Aging (Albany NY) 2011;3:346–347. doi: 10.18632/aging.100303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha AK, Xu XJ, Balon TW, Brandon A, Kraegen EW, Ruderman NB. Insulin resistance due to nutrient excess: is it a consequence of AMPK downregulation? Cell Cycle. 2011;10:3447–3451. doi: 10.4161/cc.10.20.17886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu MK, Gong XG, Guan KL. mTOR in podocyte function: is rapamycin good for diabetic nephropathy? Cell Cycle. 2011;10:3415–3416. doi: 10.4161/cc.10.20.17686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson DL. Normalizing a hyperactive mTOR initiates muscle growth during obesity. Aging (Albany NY) 2011;3:83–84. doi: 10.18632/aging.100290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumder S, Caccamo A, Medina DX, Benavides AD, Javors MA, Kraig E, Strong R, Richardson A, Oddo S. Lifelong rapamycin administration ameliorates age-dependent cognitive deficits by reducing IL-1beta and enhancing NMDA signaling. Aging Cell. 2012;11:326–335. doi: 10.1111/j.1474-9726.2011.00791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagosklonny MV. Revisiting the antagonistic pleiotropy theory of aging: TOR-driven program and quasi-program. Cell Cycle. 2010;9:3151–3156. doi: 10.4161/cc.9.16.13120. [DOI] [PubMed] [Google Scholar]
- Blagosklonny MV, Hall MN. Growth and aging: a common molecular mechanism. Aging (Albany NY) 2009;1:357–362. doi: 10.18632/aging.100040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pani G. From growing to secreting: new roles for mTOR in aging cells. Cell Cycle. 2011;10:2450–2453. doi: 10.4161/cc.10.15.16886. [DOI] [PubMed] [Google Scholar]
- Coppe JP, Patil CK, Rodier F, Sun Y, Munoz DP, Goldstein J, Nelson PS, Desprez PY, Campisi J. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6:2853–2868. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund A, Patil CK, Campisi J. p38MAPK is a novel DNA damage response-independent regulator of the senescence-associated secretory phenotype. Embo J. 2011;30:1536–1548. doi: 10.1038/emboj.2011.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laberge RM, Zhou L, Sarantos MR, Rodier F, Freund A, de Keizer PL, Liu S, Demaria M, Cong YS, Kapahi P, Desprez PY, Hughes RE, Campisi J. Glucocorticoids suppress selected components of the senescence-associated secretory phenotype. Aging Cell. 2012;11:569–578. doi: 10.1111/j.1474-9726.2012.00818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilsen PM, Noll JE, Suetani RJ, Schulz RB, Al-Ejeh F, Evdokiou A, Lane DP, Callen DF. Mutant p53 uses p63 as a molecular chaperone to alter gene expression and induce a pro-invasive secretome. Oncotarget. 2011;2:1203–1217. doi: 10.18632/oncotarget.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Keizer PL, Laberge RM, Campisi J. p53: Pro-aging or pro-longevity? Aging (Albany NY) 2010;2:377–379. doi: 10.18632/aging.100178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagosklonny MV. Cell senescence and hypermitogenic arrest. EMBO Rep. 2003;4:358–362. doi: 10.1038/sj.embor.embor806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso C, Lio D, Cavallone L, Franceschi C. Aging, longevity, inflammation, and cancer. Ann N Y Acad Sci. 2004;1028:1–13. doi: 10.1196/annals.1322.001. [DOI] [PubMed] [Google Scholar]
- Salvioli S, Capri M, Valensin S, Tieri P, Monti D, Ottaviani E, Franceschi C. Inflamm-aging, cytokines and aging: state of the art, new hypotheses on the role of mitochondria and new perspectives from systems biology. Curr Pharm Des. 2006;12:161–3171. doi: 10.2174/138161206777947470. [DOI] [PubMed] [Google Scholar]
- Lisanti MP, Martinez-Outschoorn UE, Pavlides S, Whitaker-Menezes D, Pestell RG, Howell A, Sotgia F. Accelerated aging in the tumor microenvironment: connecting aging, inflammation and cancer metabolism with personalized medicine. Cell Cycle. 2011;10:2059–2063. doi: 10.4161/cc.10.13.16233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisanti MP, Martinez-Outschoorn UE, Lin Z, Pavlides S, Whitaker-Menezes D, Pestell RG, Howell A, Sotgia F. Hydrogen peroxide fuels aging, inflammation, cancer metabolism and metastasis: the seed and soil also needs “fertilizer”; Cell Cycle. 2011;10:2440–2449. doi: 10.4161/cc.10.15.16870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J, Keller JN. Regulation of energy metabolism by inflammation: a feedback response in obesity and calorie restriction. Aging (Albany NY) 2010;2:361–368. doi: 10.18632/aging.100155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss EP, Villareal DT, Fontana L, Han DH, Holloszy JO. Dehydroepiandrosterone (DHEA) replacement decreases insulin resistance and lowers inflammatory cytokines in aging humans. Aging (Albany NY) 2011;3:533–542. doi: 10.18632/aging.100327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasko I, Stampfer-Kountchev M, Robatscher P, Veerhuis R, Eikelenboom P, Grubeck-Loebenstein B. How chronic inflammation can affect the brain and support the development of Alzheimer's disease in old age: the role of microglia and astrocytes. Aging Cell. 2004;3:169–176. doi: 10.1111/j.1474-9728.2004.00101.x. [DOI] [PubMed] [Google Scholar]
- Wanner SP, Garami A, Pakai E, Oliveira DL, Gavva NR, Coimbra CC, Romanovsky AA. Aging reverses the role of the transient receptor potential vanilloid-1 channel in systemic inflammation from anti-inflammatory to proinflammatory. Cell Cycle. 2012;11:343–349. doi: 10.4161/cc.11.2.18772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samani NJ, Boultby R, Butler R, Thompson JR, Goodall AH. Telomere shortening in atherosclerosis. Lancet. 2001;358:472–473. doi: 10.1016/S0140-6736(01)05633-1. [DOI] [PubMed] [Google Scholar]
- Gadalla SM, Cawthon R, Giri N, Alter BP, Savage SA. Telomere length in blood, buccal cells, and fibroblasts from patients with inherited bone marrow failure syndromes. Aging (Albany NY) 2010;2:867–874. doi: 10.18632/aging.100235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njajou OT, Hsueh WC, Blackburn EH, Newman AB, Wu SH, Li R, Simonsick EM, Harris TM, Cummings SR, Cawthon RM. Association between telomere length, specific causes of death, and years of healthy life in health, aging, and body composition, a population-based cohort study. J Gerontol A Biol Sci Med Sci. 2009;64:860–864. doi: 10.1093/gerona/glp061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, Cawthon RM. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci U S A. 2004;101:17312–17315. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel ES, Merkin SS, Cawthon R, Blackburn EH, Adler NE, Pletcher MJ, Seeman TE. The rate of leukocyte telomere shortening predicts mortality from cardiovascular disease in elderly men. Aging (Albany NY) 2009;1:81–88. doi: 10.18632/aging.100007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramatges MM, Bertuch AA. Measuring relative telomere length: is tissue an issue? Aging (Albany NY) 2010;2:756–757. doi: 10.18632/aging.100236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemielity S, Kimura M, Parker KM, Parker JD, Cao X, Aviv A, Keller L. Short telomeres in short-lived males: what are the molecular and evolutionary causes? Aging Cell. 2007;6:225–233. doi: 10.1111/j.1474-9726.2007.00279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekaert S, De Meyer T, Rietzschel ER, De Buyzere ML, De Bacquer D, Langlois M, Segers P, Cooman L, Van Damme P, Cassiman P, Van Criekinge W, Verdonck P, De Backer GG, Gillebert TC, Van Oostveldt P. Telomere length and cardiovascular risk factors in a middle-aged population free of overt cardiovascular disease. Aging Cell. 2007;6:639–647. doi: 10.1111/j.1474-9726.2007.00321.x. [DOI] [PubMed] [Google Scholar]
- Kuhlow D, Florian S, von Figura G, Weimer S, Schulz N, Petzke KJ, Zarse K, Pfeiffer AF, Rudolph KL, Ristow M. Telomerase deficiency impairs glucose metabolism and insulin secretion. Aging (Albany NY) 2010;2:650–658. doi: 10.18632/aging.100200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panossian LA, Porter VR, Valenzuela HF, Zhu X, Reback E, Masterman D, Cummings JL, Effros RB. Telomere shortening in T cells correlates with Alzheimer's disease status. Neurobiol Aging. 2003;24:77–84. doi: 10.1016/s0197-4580(02)00043-x. [DOI] [PubMed] [Google Scholar]
- Ishikawa N, Nakamura K, Izumiyama-Shimomura N, Aida J, Ishii A, Goto M, Ishikawa Y, Asaka R, Matsuura M, Hatamochi A, Kuroiwa M, Takubo K. Accelerated in vivo epidermal telomere loss in Werner syndrome. Aging (Albany NY) 2011;3:417–429. doi: 10.18632/aging.100315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gems DH, de la Guardia YI. Alternative Perspectives on Aging in C. elegans: Reactive Oxygen Species or Hyperfunction? Antioxid Redox Signal. 2012 doi: 10.1089/ars.2012.4840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gems D, Partridge L. Genetics of Longevity in Model Organisms: Debates and Paradigm Shifts. Annu Rev Physiol. 2012 doi: 10.1146/annurev-physiol-030212-183712. [DOI] [PubMed] [Google Scholar]
- Blagosklonny MV. Rapamycin and quasi-programmed aging: Four years later. Cell Cycle. 2010;9:1859–1862. doi: 10.4161/cc.9.10.11872. [DOI] [PubMed] [Google Scholar]
- Blagosklonny MV. An anti-aging drug today: from senescence-promoting genes to anti-aging pill. Drug Disc Today. 2007;12:218–224. doi: 10.1016/j.drudis.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Pan KZ, Palter JE, Rogers AN, Olsen A, Chen D, Lithgow GJ, Kapahi P. Inhibition of mRNA translation extends lifespan in Caenorhabditis elegans. Aging Cell. 2007;6:111–119. doi: 10.1111/j.1474-9726.2006.00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen M, Taubert S, Crawford D, Libina N, Lee SJ, Kenyon C. Lifespan extension by conditions that inhibit translation in Caenorhabditis elegans. Aging Cell. 2007;6:95–110. doi: 10.1111/j.1474-9726.2006.00267.x. [DOI] [PubMed] [Google Scholar]
- Mehta R, Chandler-Brown D, Ramos FJ, Shamieh LS, Kaeberlein M. Regulation of mRNA translation as a conserved mechanism of longevity control. Adv Exp Med Biol. 2010;694:14–29. doi: 10.1007/978-1-4419-7002-2_2. [DOI] [PubMed] [Google Scholar]
- Blagosklonny MV. NCI's provocative questions on cancer: some answers to ignite discussion. Oncotarget. 2011;2:1352–1367. doi: 10.18632/oncotarget.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powe DG, Voss MJ, Zanker KS, Habashy HO, Green AR, Ellis IO, Entschladen F. Beta-blocker drug therapy reduces secondary cancer formation in breast cancer and improves cancer specific survival. Oncotarget. 2010;1:628–638. doi: 10.18632/oncotarget.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquier E, Ciccolini J, Carre M, Giacometti S, Fanciullino R, Pouchy C, Montero MP, Serdjebi C, Kavallaris M, Andre N. Propranolol potentiates the anti-angiogenic effects and anti-tumor efficacy of chemotherapy agents: implication in breast cancer treatment. Oncotarget. 2011;2:797–809. doi: 10.18632/oncotarget.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CC, Chan WL, Chen YC, Chen TJ, Lin SJ, Chen JW, Leu HB. Angiotensin II receptor blockers and risk of cancer in patients with systemic hypertension. Am J Cardiol. 2011;107:1028–1033. doi: 10.1016/j.amjcard.2010.11.026. [DOI] [PubMed] [Google Scholar]
- Shen XZ, Bernstein KE. The peptide network regulated by angiotensin converting enzyme (ACE) in hematopoiesis. Cell Cycle. 2011;10:1363–1369. doi: 10.4161/cc.10.9.15444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadoshima J, Izumo S. Rapamycin selectively inhibits angiotensin II-induced increase in protein synthesis in cardiac myocytes in vitro. Potential role of 70-kD S6 kinase in angiotensin II-induced cardiac hypertrophy. Circ Res. 1995;77:1040–1052. doi: 10.1161/01.res.77.6.1040. [DOI] [PubMed] [Google Scholar]
- Anisimov VN. Metformin for aging and cancer prevention. Aging (Albany NY) 2010;2:760–774. doi: 10.18632/aging.100230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demidenko ZN. Chronological lifespan in stationary culture: from yeast to human cells. Aging (Albany NY) 2011;3:1041–1042. doi: 10.18632/aging.100406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anisimov VN, Berstein LM, Popovich IG, Zabezhinski MA, Egormin PA, Piskunova TS, Semenchenko AV, Tyndyk ML, Yurova MN, Kovalenko IG, Poroshina TE. If started early in life, metformin treatment increases life span and postpones tumors in female SHR mice. Aging (Albany NY) 2011;3:148–157. doi: 10.18632/aging.100273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anisimov VN, Egormin PA, Piskunova TS, Popovich IG, Tyndyk ML, Yurova MN, Zabezhinski MA, Anikin IV, Karkach AS, Romanyukha AA. Metformin extends life span of HER-2/neu transgenic mice and in combination with melatonin inhibits growth of transplantable tumors in vivo. Cell Cycle. 2010;9:188–197. doi: 10.4161/cc.9.1.10407. [DOI] [PubMed] [Google Scholar]
- Gosmanova EO, Canada RB, Mangold TA, Rawls WN, Wall BM. Effect of metformin-containing antidiabetic regimens on all-cause mortality in veterans with type 2 diabetes mellitus. Am J Med Sci. 2008;336:241–247. doi: 10.1097/MAJ.0b013e31816250e6. [DOI] [PubMed] [Google Scholar]
- Menendez JA, Cufi S, Oliveras-Ferraros C, Vellon L, Joven J, Vazquez-Martin A. Gerosuppressant metformin: less is more. Aging (Albany NY) 2011;3:348–362. doi: 10.18632/aging.100316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandezr E, Miller RA. Rapamycin fed late in life extends lifespan in genetically heterogenous mice. Nature. 2009;460:392–396. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RA, Harrison DE, Astle CM, Baur JA, Boyd AR, de Cabo R, Fernandez E, Flurkey K, Javors MA, Nelson JF, Orihuela CJ, Pletcher S, Sharp ZD, Sinclair D, Starnes JW, Wilkinson JE, et al. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci. 2011;66:191–201. doi: 10.1093/gerona/glq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anisimov VN, Zabezhinski MA, Popovich IG, Piskunova TS, Semenchenko AV, Tyndyk ML, Yurova MN, Rosenfeld SV, Blagosklonny MV. Rapamycin increases lifespan and inhibits spontaneous tumorigenesis in inbred female mice. Cell Cycle. 2011;10:4230–4236. doi: 10.4161/cc.10.24.18486. [DOI] [PubMed] [Google Scholar]
- Anisimov VN, Zabezhinski MA, Popovich IG, Piskunova TS, Semenchenko AV, Tyndyk ML, Yurova MN, Antoch MP, Blagosklonny MV. Rapamycin extends maximal lifespan in cancer-prone mice. Am J Pathol. 2010;176:2092–2097. doi: 10.2353/ajpath.2010.091050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson JE, Burmeister L, Brooks SV, Chan CC, Friedline S, Harrison DE, Hejtmancik JF, Nadon N, Strong R, Wood LK, Woodward MA, Miller RA. Rapamycin slows aging in mice. Aging Cell. 2012;11:675–682. doi: 10.1111/j.1474-9726.2012.00832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna A, Kapahi P. Rapamycin: killing two birds with one stone. Aging (Albany NY) 2011;3:1043–1044. doi: 10.18632/aging.100405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo VD, Fontana L. Intermittent supplementation with rapamycin as a dietary restriction mimetic. Aging (Albany NY) 2011;3:1039–1040. doi: 10.18632/aging.100401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagosklonny MV. Increasing healthy lifespan by suppressing aging in our lifetime: Preliminary proposal. Cell Cycle. 2010;9:4788–4794. doi: 10.4161/cc.9.24.14360. [DOI] [PubMed] [Google Scholar]
- Blagosklonny MV. How to save Medicare: the anti-aging remedy. Aging (Albany NY) 2012;4:547–552. doi: 10.18632/aging.100479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagosklonny MV. Molecular damage in cancer: an argument for mTOR-driven aging. Aging (Albany NY) 2011;3:1130–1141. doi: 10.18632/aging.100422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pospelova TV, Demidenko ZN, Bukreeva EI, Pospelov VA, Gudkov AV, Blagosklonny MV. Pseudo-DNA damage response in senescent cells. Cell Cycle. 2009;8:4112–4118. doi: 10.4161/cc.8.24.10215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leontieva OV, Lenzo F, Demidenko ZN, Blagosklonny MV. Hyper-mitogenic drive coexists with mitotic incompetence in senescent cells. Cell Cycle. 2012;11:4642–4649. doi: 10.4161/cc.22937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodier F, Munoz DP, Teachenor R, Chu V, Le O, Bhaumik D, Coppe JP, Campeau E, Beausejour CM, Kim SH, Davalos AR, Campisi J. DNA-SCARS: distinct nuclear structures that sustain damage-induced senescence growth arrest and inflammatory cytokine secretion. J Cell Sci. 2011;124:68–81. doi: 10.1242/jcs.071340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodier F, Coppe JP, Patil CK, Hoeijmakers WA, Munoz DP, Raza SR, Freund A, Campeau E, Davalos AR, Campisi J. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol. 2009;11:973–979. doi: 10.1038/ncb1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagosklonny MV. mTOR-driven aging: speeding car without brakes. Cell Cycle. 2009;8:4055–4059. doi: 10.4161/cc.8.24.10310. [DOI] [PubMed] [Google Scholar]
- Blagosklonny MV. Why men age faster but reproduce longer than women: mTOR and evolutionary perspectives. Aging (Albany NY) 2010;2:265–273. doi: 10.18632/aging.100149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagosklonny MV. Rapamycin-induced glucose intolerance: Hunger or starvation diabetes. Cell Cycle. 2011;10:4217–4224. doi: 10.4161/cc.10.24.18595. [DOI] [PubMed] [Google Scholar]
- Blandino-Rosano M, Chen AY, Scheys JO, Alejandro EU, Gould AP, Taranukha T, Elghazi L, Cras-Meneur C, Bernal-Mizrachi E. mTORC1 signaling and regulation of pancreatic beta-cell mass. Cell Cycle. 2012;11 doi: 10.4161/cc.20036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge L, Gems D. Mechanisms of ageing: public or private? Nat Rev Genet. 2002;3:165–175. doi: 10.1038/nrg753. [DOI] [PubMed] [Google Scholar]
- Bishop NA, Guarente L. Two neurons mediate diet-restriction-induced longevity in C. elegans. Nature. 2007;447:545–549. doi: 10.1038/nature05904. [DOI] [PubMed] [Google Scholar]
- Krakauer T, Buckley M, Issaq HJ, Fox SD. Rapamycin Protects Mice from Staphylococcal Enterotoxin B-Induced Toxic Shock and Blocks Cytokine Release In vitro and In Vivo. Antimicrob Agents Chemother. 2010 doi: 10.1128/AAC.01015-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss G, Skurnick JH, Goldsmith LT, Santoro NF, Park SJ. Menopause and hypothalamic-pituitary sensitivity to estrogen. JAMA. 2004;292:2991–2996. doi: 10.1001/jama.292.24.2991. [DOI] [PubMed] [Google Scholar]
- Prior JC. Ovarian aging and the perimenopausal transition: the paradox of endogenous ovarian hyperstimulation. Endocrine. 2005;26:297–300. doi: 10.1385/ENDO:26:3:297. [DOI] [PubMed] [Google Scholar]
- Horiuchi S. Postmenopausal acceleration of age-related mortality increase. J Gerontol A Biol Sci Med Sci. 1997;52:B78–92. doi: 10.1093/gerona/52a.1.b78. [DOI] [PubMed] [Google Scholar]
- Heys KR, Cram SL, Truscott RJ. Massive increase in the stiffness of the human lens nucleus with age: the basis for presbyopia? Mol Vis. 2004;10:956–963. [PubMed] [Google Scholar]
- Truscott RJ. Presbyopia. Emerging from a blur towards an understanding of the molecular basis for this most common eye condition. Exp Eye Res. 2009;88:241–247. doi: 10.1016/j.exer.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Wendt M, Croft MA, McDonald J, Kaufman PL, Glasser A. Lens diameter and thickness as a function of age and pharmacologically stimulated accommodation in rhesus monkeys. Exp Eye Res. 2008;86:746–752. doi: 10.1016/j.exer.2008.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen AM, Denham DB, Fernandez V, Borja D, Ho A, Manns F, Parel JM, Augusteyn RC. In vitro dimensions and curvatures of human lenses. Vision Res. 2006;46:1002–1009. doi: 10.1016/j.visres.2005.10.019. [DOI] [PubMed] [Google Scholar]
- McGinty SJ, Truscott RJ. Presbyopia: the first stage of nuclear cataract? Ophthalmic Res. 2006;38:137–148. doi: 10.1159/000090645. [DOI] [PubMed] [Google Scholar]
- Blagosklonny MV. Program-like aging and mitochondria: instead of random damage by free radicals. J Cell Biochem. 2007;102:1389–1399. doi: 10.1002/jcb.21602. [DOI] [PubMed] [Google Scholar]
- Michno K, van de Hoef D, Wu H, Boulianne GL. Modeling age-related diseases in Drosophila: can this fly? Curr Top Dev Biol. 2005;71:199–223. doi: 10.1016/S0070-2153(05)71006-1. [DOI] [PubMed] [Google Scholar]
- Nelson B, Nishimura S, Kanuka H, Kuranaga E, Inoue M, Hori G, Nakahara H, Miura M. Isolation of gene sets affected specifically by polyglutamine expression: implication of the TOR signaling pathway in neurodegeneration. Cell Death Differ. 2005;12:1115–1123. doi: 10.1038/sj.cdd.4401635. [DOI] [PubMed] [Google Scholar]
- Khurana V, Lu Y, Steinhilb ML, Oldham S, Shulman JM, Feany MB. TOR-mediated cell-cycle activation causes neurodegeneration in a Drosophila tauopathy model. Curr Biol. 2006;16:230–241. doi: 10.1016/j.cub.2005.12.042. [DOI] [PubMed] [Google Scholar]
- Chaudhuri A, Bowling K, Funderburk C, Lawal H, Inamdar A, Wang Z, O'Donnell JM. Interaction of genetic and environmental factors in a Drosophila parkinsonism model. J Neurosci. 2007;27:2457–2467. doi: 10.1523/JNEUROSCI.4239-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birse RT, Choi J, Reardon K, Rodriguez J, Graham S, Diop S, Ocorr K, Bodmer R, Oldham S. High-fat-diet-induced obesity and heart dysfunction are regulated by the TOR pathway in Drosophila. Cell Metab. 2010;12:533–544. doi: 10.1016/j.cmet.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessells R, Fitzgerald E, Piazza N, Ocorr K, Morley S, Davies C, Lim HY, Elmen L, Hayes M, Oldham S, Bodmer R. d4eBP acts downstream of both dTOR and dFoxo to modulate cardiac functional aging in Drosophila. Aging Cell. 2009;8:542–552. doi: 10.1111/j.1474-9726.2009.00504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teleman AA, Ratzenbock I, Oldham S. Drosophila: a model for understanding obesity and diabetic complications. Exp Clin Endocrinol Diabetes. 2012;120:184–185. doi: 10.1055/s-0032-1304566. [DOI] [PubMed] [Google Scholar]
- Luong N, Davies CR, Wessells RJ, Graham SM, King MT, Veech R, Bodmer R, Oldham SM. Activated FOXO-mediated insulin resistance is blocked by reduction of TOR activity. Cell Metab. 2006;4:133–142. doi: 10.1016/j.cmet.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Broughton SJ, Piper MD, Ikeya T, Bass TM, Jacobson J, Driege Y, Martinez P, Hafen E, Withers DJ, Leevers SJ, Partridge L. Longer lifespan, altered metabolism, and stress resistance in Drosophila from ablation of cells making insulin-like ligands. Proc Natl Acad Sci U S A. 2005;102:3105–3110. doi: 10.1073/pnas.0405775102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldham S. Obesity and nutrient sensing TOR pathway in flies and vertebrates: Functional conservation of genetic mechanisms. Trends Endocrinol Metab. 2011;22:45–52. doi: 10.1016/j.tem.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diop SB, Bodmer R. Drosophila as a model to study the genetic mechanisms of obesity-associated heart dysfunction. J Cell Mol Med. 2012;16:966–971. doi: 10.1111/j.1582-4934.2012.01522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris SN, Coogan C, Chamseddin K, Fernandez-Kim SO, Kolli S, Keller JN, Bauer JH. Development of diet-induced insulin resistance in adult Drosophila melanogaster. Biochim Biophys Acta. 2012;1822:1230–1237. doi: 10.1016/j.bbadis.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haselton AT, Fridell YW. Adult Drosophila melanogaster as a model for the study of glucose homeostasis. Aging (Albany NY) 2010;2:523–526. doi: 10.18632/aging.100185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musselman LP, Fink JL, Narzinski K, Ramachandran PV, Hathiramani SS, Cagan RL, Baranski TJ. A high-sugar diet produces obesity and insulin resistance in wild-type Drosophila. Dis Model Mech. 2011;4:842–849. doi: 10.1242/dmm.007948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luong N, Davies CR, Wessells RJ, Graham SM, King MT, Veech R, Bodmer R, Oldham SM. Activated FOXO-mediated insulin resistance is blocked by reduction of TOR activity. Cell Metab. 2006;4:133–142. doi: 10.1016/j.cmet.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Biteau B, Karpac J, Hwangbo D, Jasper H. Regulation of Drosophila lifespan by JNK signaling. Exp Gerontol. 2011;46:349–354. doi: 10.1016/j.exger.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasper H, Kennedy BK. Niche science: the aging stem cell. Cell Cycle. 2012;11:2959–2960. doi: 10.4161/cc.21558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biteau B, Karpac J, Supoyo S, Degennaro M, Lehmann R, Jasper H. Lifespan extension by preserving proliferative homeostasis in Drosophila. PLoS Genet. 2010;6:e1001159. doi: 10.1371/journal.pgen.1001159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biteau B, Hochmuth CE, Jasper H. JNK activity in somatic stem cells causes loss of tissue homeostasis in the aging Drosophila gut. Cell Stem Cell. 2008;3:442–455. doi: 10.1016/j.stem.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskalev A, Shaposhnikov M. Pharmacological inhibition of NF-kappaB prolongs lifespan of Drosophila melanogaster. Aging (Albany NY) 2011;3:391–394. doi: 10.18632/aging.100314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brignull HR, Morley JF, Garcia SM, Morimoto RI. Modeling polyglutamine pathogenesis in C. elegans. Methods Enzymol. 2006;412:256–282. doi: 10.1016/S0076-6879(06)12016-9. [DOI] [PubMed] [Google Scholar]
- Vosseller K. O-GlcNAc and aging: C. elegans as a genetic model to test O-GlcNAc roles in type II diabetic insulin resistance. Aging (Albany NY) 2010;2:749–751. doi: 10.18632/aging.100225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman MM, Stuchlick O, El-Karim EG, Stuart R, Kipreos ET, Wells L. Intracellular protein glycosylation modulates insulin mediated lifespan in C.elegans. Aging (Albany NY) 2010;2:678–690. doi: 10.18632/aging.100208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams TW, Dumas KJ, Hu PJ. EAK proteins: novel conserved regulators of C. elegans lifespan. Aging (Albany NY) 2010;2:742–747. doi: 10.18632/aging.100214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou KI, Pincus Z, Slack FJ. Longevity and stress in Caenorhabditis elegans. Aging (Albany NY) 2011;3:733–753. doi: 10.18632/aging.100367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solari F. CeKlotho opens a new road for investigation in worm aging. Aging (Albany NY) 2010;2:539–540. doi: 10.18632/aging.100198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmookler Reis RJ, Xu L, Lee H, Chae M, Thaden JJ, Bharill P, Tazearslan C, Siegel E, Alla R, Zimniak P, Ayyadevara S. Modulation of lipid biosynthesis contributes to stress resistance and longevity of C. elegans mutants. Aging (Albany NY) 2011;3:125–147. doi: 10.18632/aging.100275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulbert AJ. Longevity, lipids and C. elegans. Aging (Albany NY) 2011;3:81–82. doi: 10.18632/aging.100288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chateau MT, Araiz C, Descamps S, Galas S. Klotho interferes with a novel FGF-signalling pathway and insulin/Igf-like signalling to improve longevity and stress resistance in Caenorhabditis elegans. Aging (Albany NY) 2010;2:567–581. doi: 10.18632/aging.100195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rongo C. Epidermal growth factor and aging: a signaling molecule reveals a new eye opening function. Aging (Albany NY) 2011;3:896–905. doi: 10.18632/aging.100384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alavez S, Lithgow GJ. Pharmacological maintenance of protein homeostasis could postpone age-related disease. Aging Cell. 2012;11:187–191. doi: 10.1111/j.1474-9726.2012.00789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lithgow GJ. Does anti-aging equal anti-microbial? Sci Aging Knowledge Environ. 2003;2003:PE16. doi: 10.1126/sageke.2003.25.pe16. [DOI] [PubMed] [Google Scholar]
- Briaud I, Dickson LM, Lingohr MK, McCuaig JF, Lawrence JC, Rhodes CJ. Insulin receptor substrate-2 proteasomal degradation mediated by a mammalian target of rapamycin (mTOR)-induced negative feedback down-regulates protein kinase B-mediated signaling pathway in beta-cells. J Biol Chem. 2005;280:2282–2293. doi: 10.1074/jbc.M412179200. [DOI] [PubMed] [Google Scholar]
- Elghazi L, Balcazar N, Blandino-Rosano M, Cras-Meneur C, Fatrai S, Gould AP, Chi MM, Moley KH, Bernal-Mizrachi E. Decreased IRS signaling impairs beta-cell cycle progression and survival in transgenic mice overexpressing S6K in beta-cells. Diabetes. 2010;59:2390–2399. doi: 10.2337/db09-0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachdi L, Balcazar N, Osorio-Duque F, Elghazi L, Weiss A, Gould A, Chang-Chen KJ, Gambello MJ, Bernal-Mizrachi E. Disruption of Tsc2 in pancreatic beta cells induces beta cell mass expansion and improved glucose tolerance in a TORC1-dependent manner. Proc Natl Acad Sci U S A. 2008;105:9250–9255. doi: 10.1073/pnas.0803047105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigeyama Y, Kobayashi T, Kido Y, Hashimoto N, Asahara S, Matsuda T, Takeda A, Inoue T, Shibutani Y, Koyanagi M, Uchida T, Inoue M, Hino O, Kasuga M, Noda T. Biphasic response of pancreatic beta-cell mass to ablation of tuberous sclerosis complex 2 in mice. Mol Cell Biol. 2008;28:2971–2979. doi: 10.1128/MCB.01695-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonda DJ, Evans TA, Santocanale C, Llosa JC, Vina J, Bajic VP, Castellani RJ, Siedlak SL, Perry G, Smith MA, Lee HG. Evidence for the progression through S-phase in the ectopic cell cycle re-entry of neurons in Alzheimer disease. Aging (Albany NY) 2009;1:382–388. doi: 10.18632/aging.100044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frade JM. Unscheduled re-entry into the cell cycle induced by NGF precedes cell death in nascent retinal neurones. J Cell Sci. 2000;113(Pt 7):1139–1148. doi: 10.1242/jcs.113.7.1139. [DOI] [PubMed] [Google Scholar]
- Song B, Davis K, Liu XS, Lee HG, Smith M, Liu X. Inhibition of Polo-like kinase 1 reduces beta-amyloid-induced neuronal cell death in Alzheimer's disease. Aging (Albany NY) 2011;3:846–851. doi: 10.18632/aging.100382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frade JM, Lopez-Sanchez N. A novel hypothesis for Alzheimer disease based on neuronal tetraploidy induced by p75 (NTR) Cell Cycle. 2010;9:1934–1941. doi: 10.4161/cc.9.10.11582. [DOI] [PubMed] [Google Scholar]
- Futatsugi A, Utreras E, Rudrabhatla P, Jaffe H, Pant HC, Kulkarni AB. Cyclin-dependent kinase 5 regulates E2F transcription factor through phosphorylation of Rb protein in neurons. Cell Cycle. 2012;11:1603–1610. doi: 10.4161/cc.20009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanga S, Lanni C, Govoni S, Uberti D, D'Orazi G, Racchi M. Unfolded p53 in the pathogenesis of Alzheimer's disease: is HIPK2 the link? Aging (Albany NY) 2010;2:545–554. doi: 10.18632/aging.100205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan B, DePinho RA. mTORC1 signaling governs hematopoietic stem cell quiescence. Cell Cycle. 2009;8:1003–1006. doi: 10.4161/cc.8.7.8045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castilho RM, Squarize CH, Chodosh LA, Williams BO, Gutkind JS. mTOR mediates Wnt-induced epidermal stem cell exhaustion and aging. Cell Stem Cell. 2009;5:279–289. doi: 10.1016/j.stem.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratajczak MZ, Shin DM, Ratajczak J, Kucia M, Bartke A. A novel insight into aging: are there pluripotent very small embryonic-like stem cells (VSELs) in adult tissues overtime depleted in an Igf-1-dependent manner? Aging (Albany NY) 2010;2:875–883. doi: 10.18632/aging.100231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy P, Liu L, Adhikari D, Jagarlamudi K, Rajareddy S, Shen Y, Du C, Tang W, HŠmŠlŠinen T, Peng SL, Lan ZJ, Cooney AJ, Huhtaniemi I, Liu K. Oocyte-specific deletion of Pten causes premature activation of the primordial follicle pool. Science. 2008;319:611–613. doi: 10.1126/science.1152257. [DOI] [PubMed] [Google Scholar]
- Reddy P, Adhikari D, Zheng W, Liang S, Hamalainen T, Tohonen V, Ogawa W, Noda T, Volarevic S, Huhtaniemi I, Liu K. PDK1 signaling in oocytes controls reproductive aging and lifespan by manipulating the survival of primordial follicles. Hum Mol Genet. 2009;18:2813–2824. doi: 10.1093/hmg/ddp217. [DOI] [PubMed] [Google Scholar]
- Adhikari D, Liu K. mTOR signaling in the control of activation of primordial follicles. Cell Cycle. 2010;9:1673–1674. doi: 10.4161/cc.9.9.11626. [DOI] [PubMed] [Google Scholar]
- Adhikari D, Flohr G, Gorre N, Shen Y, Yang H, Lundin E, Lan Z, Gambello MJ, Liu K. Disruption of Tsc2 in oocytes leads to overactivation of the entire pool of primordial follicles. Mol Hum Reprod. 2009;15:765–770. doi: 10.1093/molehr/gap092. [DOI] [PubMed] [Google Scholar]
- Blagosklonny MV. Aging, stem cells, and mammalian target of rapamycin: a prospect of pharmacologic rejuvenation of aging stem cells. Rejuvenation Res. 2008;11:801–808. doi: 10.1089/rej.2008.0722. [DOI] [PubMed] [Google Scholar]
- Leontieva OV, Blagosklonny MV. Yeast-like chronological senescence in mammalian cells: phenomenon, mechanism and pharmacological suppression. Aging (Albany NY) 2011;3:1078–1091. doi: 10.18632/aging.100402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabrizio P, Wei M. Conserved role of medium acidification in chronological senescence of yeast and mammalian cells. Aging (Albany NY) 2011;3:1127–1129. doi: 10.18632/aging.100412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger Z, Ravikumar B, Menzies FM, Oroz LG, Underwood BR, Pangalos MN, Schmitt I, Wullner U, Evert BO, O'Kane CJ, Rubinsztein DC. Rapamycin alleviates toxicity of different aggregate-prone proteins. Hum Mol Genet. 2006;15:433–442. doi: 10.1093/hmg/ddi458. [DOI] [PubMed] [Google Scholar]
- Williams A, Jahreiss L, Sarkar S, Saiki S, Menzies FM, Ravikumar B, Rubinsztein DC. Aggregate-prone proteins are cleared from the cytosol by autophagy: therapeutic implications. Curr Top Dev Biol. 2006;76:89–101. doi: 10.1016/S0070-2153(06)76003-3. [DOI] [PubMed] [Google Scholar]
- Sarkar S, Rubinsztein DC. Huntington's disease: degradation of mutant huntingtin by autophagy. Febs J. 2008;275:4263–4270. doi: 10.1111/j.1742-4658.2008.06562.x. [DOI] [PubMed] [Google Scholar]
- Sarkar S, Ravikumar B, Floto RA, Rubinsztein DC. Rapamycin and mTOR-independent autophagy inducers ameliorate toxicity of polyglutamine-expanded huntingtin and related protein-pathies. Cell Death Differ. 2009;16:46–56. doi: 10.1038/cdd.2008.110. [DOI] [PubMed] [Google Scholar]
- Menzies FM, Rubinsztein DC. Broadening the therapeutic scope for rapamycin treatment. Autophagy. 2010;6:286–287. doi: 10.4161/auto.6.2.11078. [DOI] [PubMed] [Google Scholar]
- Cao K, Graziotto JJ, Blair CD, Mazzulli JR, Erdos MR, Krainc D, Collins FS. Rapamycin reverses cellular phenotypes and enhances mutant protein clearance in Hutchinson-Gilford progeria syndrome cells. Sci Transl Med. 2011;3:89ra58. doi: 10.1126/scitranslmed.3002346. [DOI] [PubMed] [Google Scholar]
- Kharas MG, Okabe R, Ganis JJ, Gozo M, Khandan T, Paktinat M, Gilliland DG, Gritsman K. Constitutively active AKT depletes hematopoietic stem cells and induces leukemia in mice. Blood. 2010;115:1406–1415. doi: 10.1182/blood-2009-06-229443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay N. Interplay between FOXO, TOR, and Akt. Biochim Biophys Acta. 2011;1813:1965–1970. doi: 10.1016/j.bbamcr.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh HS, McBurney M, Robbins PD. SIRT1 negatively regulates the mammalian target of rapamycin. PLoS One. 2010;5:e9199. doi: 10.1371/journal.pone.0009199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagosklonny MV. Linking calorie restriction to longevity through sirtuins and autophagy: any role for TOR. Cell Death Dis. 2010;1:e12. doi: 10.1038/cddis.2009.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheaffer KL, Updike DL, Mango SE. The Target of Rapamycin pathway antagonizes pha-4/FoxA to control development and aging. Curr Biol. 2008;18:1355–1364. doi: 10.1016/j.cub.2008.07.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CC, Jeon SM, Bhaskar PT, Nogueira V, Sundararajan D, Tonic I, Park Y, Hay N. FoxOs inhibit mTORC1 and activate Akt by inducing the expression of Sestrin3 and Rictor. Dev Cell. 2010;18:592–604. doi: 10.1016/j.devcel.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh SW, Mukhopadhyay A, Svrzikapa N, Jiang F, Davis RJ, Tissenbaum HA. JNK regulates lifespan in Caenorhabditis elegans by modulating nuclear translocation of forkhead transcription factor/DAF-16. Proc Natl Acad Sci U S A. 2005;102:4494–4499. doi: 10.1073/pnas.0500749102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian P, Longo VD. Linking Klotho, Nrf2, MAP kinases and aging. Aging (Albany NY) 2010;2:632–633. doi: 10.18632/aging.100219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belgardt BF, Mauer J, Bruning JC. Novel roles for JNK1 in metabolism. Aging (Albany NY) 2010;2:621–626. doi: 10.18632/aging.100192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer NF, Poynter ME, Im SY, Daynes RA. Constitutive activation of NF-kappa B in an animal model of aging. Int Immunol. 1997;9:1581–1588. doi: 10.1093/intimm/9.10.1581. [DOI] [PubMed] [Google Scholar]
- Vaughan S, Jat PS. Deciphering the role of nuclear factor-kappaB in cellular senescence. Aging (Albany NY) 2011;3:913–919. doi: 10.18632/aging.100390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilstra JS, Robinson AR, Wang J, Gregg SQ, Clauson CL, Reay DP, Nasto LA, St Croix CM, Usas A, Vo N, Huard J, Clemens PR, Stolz DB, Guttridge DC, Watkins SC, Garinis GA, et al. NF-kappaB inhibition delays DNA damage-induced senescence and aging in mice. J Clin Invest. 122:2601–2612. doi: 10.1172/JCI45785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreesen O, Stewart CL. Accelerated aging syndromes, are they relevant to normal human aging? Aging (Albany NY) 2011;3:889–895. doi: 10.18632/aging.100383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagosklonny MV. Hormesis does not make sense except in the light of TOR-driven aging. Aging (Albany NY) 2011;3:1051–1062. doi: 10.18632/aging.100411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Kittler O, Zhu J, Yang J, Liu G, Hendricks W, Lengauer C, Gabelli SB, Kinzler KW, Vogelstein B, Huso DL, Zhou S. PI3Kalpha inhibitors that inhibit metastasis. Oncotarget. 2010;1:339–348. doi: 10.18632/oncotarget.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber GL, Parat MO, Binder ZA, Gallia GL, Riggins GJ. Abrogation of PIK3CA or PIK3R1 reduces proliferation, migration, and invasion in glioblastoma multiforme cells. Oncotarget. 2011;2:833–849. doi: 10.18632/oncotarget.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441:424–430. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12:9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Gruppuso PA, Boylan JM, Sanders JA. The physiology and pathophysiology of rapamycin resistance: implications for cancer. Cell Cycle. 2011;10:1050–1058. doi: 10.4161/cc.10.7.15230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia B, Nahle Z, Kenney AM. Double trouble: when sonic hedgehog signaling meets TSC inactivation. Cell Cycle. 2010;9:456–459. doi: 10.4161/cc.9.3.10532. [DOI] [PubMed] [Google Scholar]
- Adams JR, Schachter NF, Liu JC, Zacksenhaus E, Egan SE. Elevated PI3K signaling drives multiple breast cancer subtypes. Oncotarget. 2011;2:435–447. doi: 10.18632/oncotarget.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett JT, Chakrabarty A, Arteaga CL. Will PI3K pathway inhibitors be effective as single agents in patients with cancer? Oncotarget. 2011;2:1314–1321. doi: 10.18632/oncotarget.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolosky ML, Stadelman KM, Chappell WH, Abrams SL, Martelli AM, Stivala F, Libra M, Nicoletti F, Drobot LB, Franklin RA, Steelman LS, McCubrey JA. Involvement of Akt-1 and mTOR in sensitivity of breast cancer to targeted therapy. Oncotarget. 2011;2:538–550. doi: 10.18632/oncotarget.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green AS, Chapuis N, Lacombe C, Mayeux P, Bouscary D, Tamburini J. LKB1/AMPK/mTOR signaling pathway in hematological malignancies: from metabolism to cancer cell biology. Cell Cycle. 2011;10:2115–2120. doi: 10.4161/cc.10.13.16244. [DOI] [PubMed] [Google Scholar]
- Hart JR, Vogt PK. Phosphorylation of AKT: a mutational analysis. Oncotarget. 2011;2:467–476. doi: 10.18632/oncotarget.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell WH, Steelman LS, Long JM, Kempf RC, Abrams SL, Franklin RA, Basecke J, Stivala F, Donia M, Fagone P, Malaponte G, Mazzarino MC, Nicoletti F, Libra M, Maksimovic-Ivanic D, Mijatovic S, et al. Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR inhibitors: rationale and importance to inhibiting these pathways in human health. Oncotarget. 2011;2:135–164. doi: 10.18632/oncotarget.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin D, Colombi M, Moroni C, Hall MN. Rapamycin passes the torch: a new generation of mTOR inhibitors. Nat Rev Drug Discov. 2011;10:868–880. doi: 10.1038/nrd3531. [DOI] [PubMed] [Google Scholar]