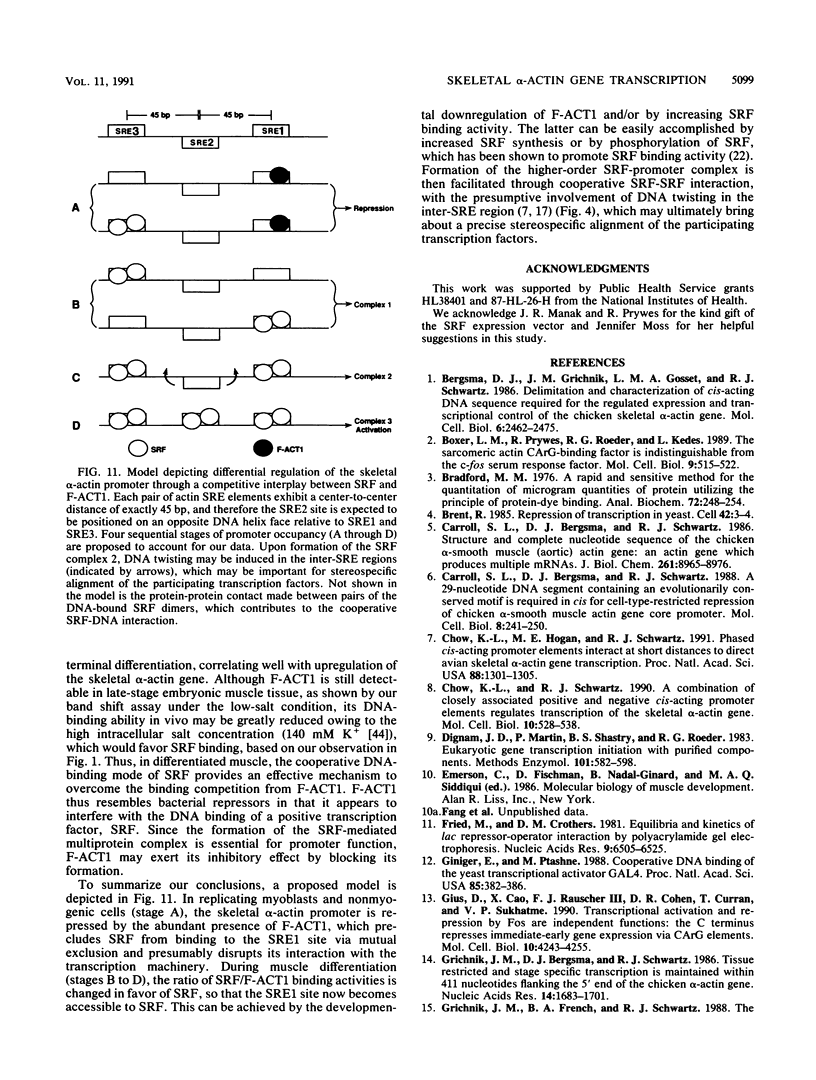
Abstract
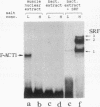
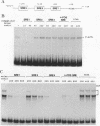
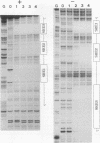
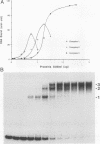
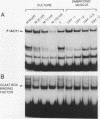
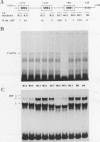
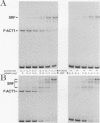
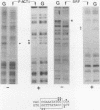
Three upstream CBAR cis-acting promoter elements, containing the inner core CC(A/T)6GG of the serum response element (SRE), are required for myogenic cell type-restricted expression of the avian skeletal alpha-actin gene (K.L. Chow and R.J. Schwartz, Mol. Cell. Biol. 10:528-538, 1990). These actin SRE elements display differential binding properties with two distinct nuclear proteins, serum response factor (SRF) and another factor described here as F-ACT1. SRF is able to bind to all actin SREs with various affinities. This multisite interaction is marked by cooperative binding events in that the two high-affinity proximal and distal SREs facilitate the weak central-site interaction with SRF, leading to the formation of a higher-order SRF-promoter complex. Functional analyses reveal that undisrupted multiple SRF-DNA interactions are absolutely essential for promoter activity in myogenic cells. F-ACT1, present at higher levels in nonmyogenic cells and replicating myoblasts than in myotubes, binds solely to the proximal SRE, and its binding is mutually exclusive with that of SRF owing to their overlapping base contacts. The cooperative promoter binding by SRF, however, can effectively displace prebound F-ACT1. In addition, an intact F-ACT1 binding site acts as a negative promoter element by restricting developmentally timed expression in myoblasts. F-ACT1 may therefore act as a repressor of skeletal alpha-actin gene transcription. This interplay between F-ACT1 and SRF may constitute a developmental as well as a physiologically regulated mechanism which modulates sarcomeric actin gene expression.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergsma D. J., Grichnik J. M., Gossett L. M., Schwartz R. J. Delimitation and characterization of cis-acting DNA sequences required for the regulated expression and transcriptional control of the chicken skeletal alpha-actin gene. Mol Cell Biol. 1986 Jul;6(7):2462–2475. doi: 10.1128/mcb.6.7.2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxer L. M., Prywes R., Roeder R. G., Kedes L. The sarcomeric actin CArG-binding factor is indistinguishable from the c-fos serum response factor. Mol Cell Biol. 1989 Feb;9(2):515–522. doi: 10.1128/mcb.9.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brent R. Repression of transcription in yeast. Cell. 1985 Aug;42(1):3–4. doi: 10.1016/s0092-8674(85)80091-x. [DOI] [PubMed] [Google Scholar]
- Carroll S. L., Bergsma D. J., Schwartz R. J. A 29-nucleotide DNA segment containing an evolutionarily conserved motif is required in cis for cell-type-restricted repression of the chicken alpha-smooth muscle actin gene core promoter. Mol Cell Biol. 1988 Jan;8(1):241–250. doi: 10.1128/mcb.8.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll S. L., Bergsma D. J., Schwartz R. J. Structure and complete nucleotide sequence of the chicken alpha-smooth muscle (aortic) actin gene. An actin gene which produces multiple messenger RNAs. J Biol Chem. 1986 Jul 5;261(19):8965–8976. [PubMed] [Google Scholar]
- Chow K. L., Hogan M. E., Schwartz R. J. Phased cis-acting promoter elements interact at short distances to direct avian skeletal alpha-actin gene transcription. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1301–1305. doi: 10.1073/pnas.88.4.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow K. L., Schwartz R. J. A combination of closely associated positive and negative cis-acting promoter elements regulates transcription of the skeletal alpha-actin gene. Mol Cell Biol. 1990 Feb;10(2):528–538. doi: 10.1128/mcb.10.2.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignam J. D., Martin P. L., Shastry B. S., Roeder R. G. Eukaryotic gene transcription with purified components. Methods Enzymol. 1983;101:582–598. doi: 10.1016/0076-6879(83)01039-3. [DOI] [PubMed] [Google Scholar]
- Fried M., Crothers D. M. Equilibria and kinetics of lac repressor-operator interactions by polyacrylamide gel electrophoresis. Nucleic Acids Res. 1981 Dec 11;9(23):6505–6525. doi: 10.1093/nar/9.23.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giniger E., Ptashne M. Cooperative DNA binding of the yeast transcriptional activator GAL4. Proc Natl Acad Sci U S A. 1988 Jan;85(2):382–386. doi: 10.1073/pnas.85.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gius D., Cao X. M., Rauscher F. J., 3rd, Cohen D. R., Curran T., Sukhatme V. P. Transcriptional activation and repression by Fos are independent functions: the C terminus represses immediate-early gene expression via CArG elements. Mol Cell Biol. 1990 Aug;10(8):4243–4255. doi: 10.1128/mcb.10.8.4243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grichnik J. M., Bergsma D. J., Schwartz R. J. Tissue restricted and stage specific transcription is maintained within 411 nucleotides flanking the 5' end of the chicken alpha-skeletal actin gene. Nucleic Acids Res. 1986 Feb 25;14(4):1683–1701. doi: 10.1093/nar/14.4.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson T. A., Kedes L. Identification of multiple proteins that interact with functional regions of the human cardiac alpha-actin promoter. Mol Cell Biol. 1989 Aug;9(8):3269–3283. doi: 10.1128/mcb.9.8.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson T. A., Taylor A., Kedes L. DNA bending is induced by a transcription factor that interacts with the human c-FOS and alpha-actin promoters. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2162–2166. doi: 10.1073/pnas.86.7.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward L. J., Schwartz R. J. Sequential expression of chicken actin genes during myogenesis. J Cell Biol. 1986 Apr;102(4):1485–1493. doi: 10.1083/jcb.102.4.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward L. J., Zhu Y. Y., Schwartz R. J. Cellular localization of muscle and nonmuscle actin mRNAs in chicken primary myogenic cultures: the induction of alpha-skeletal actin mRNA is regulated independently of alpha-cardiac actin gene expression. J Cell Biol. 1988 Jun;106(6):2077–2086. doi: 10.1083/jcb.106.6.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochschild A., Ptashne M. Cooperative binding of lambda repressors to sites separated by integral turns of the DNA helix. Cell. 1986 Mar 14;44(5):681–687. doi: 10.1016/0092-8674(86)90833-0. [DOI] [PubMed] [Google Scholar]
- Lassar A. B., Buskin J. N., Lockshon D., Davis R. L., Apone S., Hauschka S. D., Weintraub H. MyoD is a sequence-specific DNA binding protein requiring a region of myc homology to bind to the muscle creatine kinase enhancer. Cell. 1989 Sep 8;58(5):823–831. doi: 10.1016/0092-8674(89)90935-5. [DOI] [PubMed] [Google Scholar]
- Manak J. R., de Bisschop N., Kris R. M., Prywes R. Casein kinase II enhances the DNA binding activity of serum response factor. Genes Dev. 1990 Jun;4(6):955–967. doi: 10.1101/gad.4.6.955. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Medford R. M., Nguyen H. T., Nadal-Ginard B. Transcriptional and cell cycle-mediated regulation of myosin heavy chain gene expression during muscle cell differentiation. J Biol Chem. 1983 Sep 25;258(18):11063–11073. [PubMed] [Google Scholar]
- Minty A., Kedes L. Upstream regions of the human cardiac actin gene that modulate its transcription in muscle cells: presence of an evolutionarily conserved repeated motif. Mol Cell Biol. 1986 Jun;6(6):2125–2136. doi: 10.1128/mcb.6.6.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa T., Kedes L. Duplicated CArG box domains have positive and mutually dependent regulatory roles in expression of the human alpha-cardiac actin gene. Mol Cell Biol. 1987 Aug;7(8):2803–2813. doi: 10.1128/mcb.7.8.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohun T. J., Taylor M. V., Garrett N., Gurdon J. B. The CArG promoter sequence is necessary for muscle-specific transcription of the cardiac actin gene in Xenopus embryos. EMBO J. 1989 Apr;8(4):1153–1161. doi: 10.1002/j.1460-2075.1989.tb03486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscat G. E., Gustafson T. A., Kedes L. A common factor regulates skeletal and cardiac alpha-actin gene transcription in muscle. Mol Cell Biol. 1988 Oct;8(10):4120–4133. doi: 10.1128/mcb.8.10.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman C., Runswick M., Pollock R., Treisman R. Isolation and properties of cDNA clones encoding SRF, a transcription factor that binds to the c-fos serum response element. Cell. 1988 Dec 23;55(6):989–1003. doi: 10.1016/0092-8674(88)90244-9. [DOI] [PubMed] [Google Scholar]
- Petropoulos C. J., Rosenberg M. P., Jenkins N. A., Copeland N. G., Hughes S. H. The chicken skeletal muscle alpha-actin promoter is tissue specific in transgenic mice. Mol Cell Biol. 1989 Sep;9(9):3785–3792. doi: 10.1128/mcb.9.9.3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan-Dinh-Tuy F., Tuil D., Schweighoffer F., Pinset C., Kahn A., Minty A. The 'CC.Ar.GG' box. A protein-binding site common to transcription-regulatory regions of the cardiac actin, c-fos and interleukin-2 receptor genes. Eur J Biochem. 1988 May 2;173(3):507–515. doi: 10.1111/j.1432-1033.1988.tb14027.x. [DOI] [PubMed] [Google Scholar]
- Phillips S. E., Manfield I., Parsons I., Davidson B. E., Rafferty J. B., Somers W. S., Margarita D., Cohen G. N., Saint-Girons I., Stockley P. G. Cooperative tandem binding of met repressor of Escherichia coli. Nature. 1989 Oct 26;341(6244):711–715. doi: 10.1038/341711a0. [DOI] [PubMed] [Google Scholar]
- Prywes R., Dutta A., Cromlish J. A., Roeder R. G. Phosphorylation of serum response factor, a factor that binds to the serum response element of the c-FOS enhancer. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7206–7210. doi: 10.1073/pnas.85.19.7206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prywes R., Roeder R. G. Purification of the c-fos enhancer-binding protein. Mol Cell Biol. 1987 Oct;7(10):3482–3489. doi: 10.1128/mcb.7.10.3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quitschke W. W., DePonti-Zilli L., Lin Z. Y., Paterson B. M. Identification of two nuclear factor-binding domains on the chicken cardiac actin promoter: implications for regulation of the gene. Mol Cell Biol. 1989 Aug;9(8):3218–3230. doi: 10.1128/mcb.9.8.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznikoff W. S., Siegele D. A., Cowing D. W., Gross C. A. The regulation of transcription initiation in bacteria. Annu Rev Genet. 1985;19:355–387. doi: 10.1146/annurev.ge.19.120185.002035. [DOI] [PubMed] [Google Scholar]
- Riggs K. J., Merrell K. T., Wilson G., Calame K. Common factor 1 is a transcriptional activator which binds in the c-myc promoter, the skeletal alpha-actin promoter, and the immunoglobulin heavy-chain enhancer. Mol Cell Biol. 1991 Mar;11(3):1765–1769. doi: 10.1128/mcb.11.3.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan W. A., Jr, Franza B. R., Jr, Gilman M. Z. Two distinct cellular phosphoproteins bind to the c-fos serum response element. EMBO J. 1989 Jun;8(6):1785–1792. doi: 10.1002/j.1460-2075.1989.tb03572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröter H., Shaw P. E., Nordheim A. Purification of intercalator-released p67, a polypeptide that interacts specifically with the c-fos serum response element. Nucleic Acids Res. 1987 Dec 23;15(24):10145–10158. doi: 10.1093/nar/15.24.10145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz R. J., Rothblum K. N. Gene switching in myogenesis: differential expression of the chicken actin multigene family. Biochemistry. 1981 Jul 7;20(14):4122–4129. doi: 10.1021/bi00517a027. [DOI] [PubMed] [Google Scholar]
- Schwartz R. J., Stone E. M. Cloning of contractile protein genes. Cell Muscle Motil. 1983;3:195–257. doi: 10.1007/978-1-4615-9296-9_7. [DOI] [PubMed] [Google Scholar]
- Struhl K. Negative control at a distance mediates catabolite repression in yeast. 1985 Oct 31-Nov 6Nature. 317(6040):822–824. doi: 10.1038/317822a0. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Moffatt B. A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986 May 5;189(1):113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- Sweadner K. J., Goldin S. M. Active transport of sodium and potassium ions: mechanism, function, and regulation. N Engl J Med. 1980 Apr 3;302(14):777–783. doi: 10.1056/NEJM198004033021404. [DOI] [PubMed] [Google Scholar]
- Taylor M., Treisman R., Garrett N., Mohun T. Muscle-specific (CArG) and serum-responsive (SRE) promoter elements are functionally interchangeable in Xenopus embryos and mouse fibroblasts. Development. 1989 May;106(1):67–78. doi: 10.1242/dev.106.1.67. [DOI] [PubMed] [Google Scholar]
- Treisman R. Identification and purification of a polypeptide that binds to the c-fos serum response element. EMBO J. 1987 Sep;6(9):2711–2717. doi: 10.1002/j.1460-2075.1987.tb02564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman R. Identification of a protein-binding site that mediates transcriptional response of the c-fos gene to serum factors. Cell. 1986 Aug 15;46(4):567–574. doi: 10.1016/0092-8674(86)90882-2. [DOI] [PubMed] [Google Scholar]
- Treisman R. Transient accumulation of c-fos RNA following serum stimulation requires a conserved 5' element and c-fos 3' sequences. Cell. 1985 Oct;42(3):889–902. doi: 10.1016/0092-8674(85)90285-5. [DOI] [PubMed] [Google Scholar]
- Tuil D., Clergue N., Montarras D., Pinset C., Kahn A., Phan-Dinh-Tuy F. CC Ar GG boxes, cis-acting elements with a dual specificity. Muscle-specific transcriptional activation and serum responsiveness. J Mol Biol. 1990 Jun 20;213(4):677–686. doi: 10.1016/S0022-2836(05)80255-4. [DOI] [PubMed] [Google Scholar]
- Vergara J., Tsien R. Y., Delay M. Inositol 1,4,5-trisphosphate: a possible chemical link in excitation-contraction coupling in muscle. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6352–6356. doi: 10.1073/pnas.82.18.6352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh K. Cross-binding of factors to functionally different promoter elements in c-fos and skeletal actin genes. Mol Cell Biol. 1989 May;9(5):2191–2201. doi: 10.1128/mcb.9.5.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh K., Schimmel P. Two nuclear factors compete for the skeletal muscle actin promoter. J Biol Chem. 1987 Jul 15;262(20):9429–9432. [PubMed] [Google Scholar]