Abstract
Context:
Serum thyroglobulin (Tg) measurements are central to the management of patients treated for differentiated thyroid carcinoma. For decades, Tg measurements have relied on methods that are subject to interference by commonly found substances in human serum and plasma, such as Tg autoantibodies. As a result, many patients need additional imaging studies to rule out cancer persistence or recurrence that could be avoided with more sensitive and specific testing methods.
Objectives:
The aims of this review are to: 1) briefly review the interferences common to Tg immunoassays; 2) introduce readers to liquid chromatography-tandem mass spectrometry as a method for quantifying proteins in human serum/plasma; and 3) discuss the potential benefits and limitations of the method in the quantification of serum Tg.
Results:
Mass spectrometric methods have traditionally lacked the sensitivity, robustness, and throughput to be useful clinical assays. These methods failed to meet the necessary clinical benchmarks due to the nature of the mass spectrometry workflow and instrumentation. Over the past few years, there have been major advances in reagents, automation, and instrumentation for the quantification of proteins using mass spectrometry. More recently, methods using mass spectrometry to detect and quantify Tg have been developed and are of sufficient quality to be used in the management of patients.
Conclusions:
Novel serum Tg assays that use mass spectrometry may avoid the issue of autoantibody interference and other problems with currently available immunoassays for Tg. Prospective studies are needed to fully understand the potential benefits of novel Tg assays to patients and care providers.
Although differentiated thyroid cancers (DTCs) only account for approximately 1% of all malignancies in the United States, they are the most common endocrine malignancy (1). Because DTCs retain many characteristics of thyroid follicular cells, the thyroid-specific protein thyroglobulin (Tg) acts as a tumor marker for evidence of disease persistence or recurrence after initial treatment with surgery and radioiodine ablation (2). Even if patients do not undergo radioactive iodine ablation, thyroglobulin concentrations can be used as a postoperative marker for disease progression or recurrence (3). Interval assessment of serum Tg continues to serve as the most common method of follow-up surveillance for patients with DTC, but the presence of antithyroglobulin autoantibodies (TgAb) in 10–30% of patients can cause falsely low or high Tg results, depending on the assay configuration (4). Although a change in antibody titers can generally be used to help follow disease progression (5, 6), patients with DTC and positive TgAb continue to pose a significant challenge for clinicians due to the uncertainty that arises regarding their disease status. These patients typically undergo additional testing and imaging studies to identify possible foci of persistent or recurrent disease, although most do not have clinically significant disease (7–9). Due to the additional challenges posed in following long-term disease in patients with DTC and positive TgAb, the most recent American Thyroid Association guidelines identify a need for development of Tg assays that have limited interference by TgAb (1). This review will focus on the current limitations of the available Tg assays and summarize the research to date regarding new Tg assays with decreased interference by TgAb.
Serum Thyroglobulin Immunoassays Are Imperfect
In sandwich immunoassays, a capture antibody bound to a bead or some other solid surface recognizes and binds to an epitope on a target protein. The capture antibody is used to isolate the protein from the rest of the proteins in the mixture, and then another antibody, sometimes called the reporter antibody because it has a molecule or enzyme attached to it to generate a signal, is used to bind to a distinct epitope on the protein making a sandwich (composed of solid surface-antibody-target protein-reporter antibody). The solid surface holds onto the capture antibody and any sandwiches formed, whereas all other nontarget proteins are washed away. Sandwiches are then detected by measuring the signal from the reporter antibody.
The presence of the target protein in the sample is thus inferred from the association of the reporter molecule to the solid surface. Imperfections of immunoassays used on human serum and plasma samples directly stem from this indirect estimate of protein concentration (Table 1). For example, antibodies that bind to the reagent antibodies in the assay can bridge the capture and reporter antibodies without any target protein present. These interferences, sometimes called “heterophilic antibodies” (although “antireagent antibodies” is more accurate because the antibodies can recognize specific motifs or modifications of the reagent antibodies), can be present in a surprisingly large portion of the population (10, 11). This is well illustrated by the Beckman Access Tg assay, for which falsely elevated results attributable to antireagent antibodies are present in at least 0.5–3% of the population tested (12–14). Thus, in as many as 3% of patients without recurrence of carcinoma, a falsely elevated concentration of Tg will be detected and could be mistaken for residual disease or metastasis. Serum Tg is not the only protein assay affected by antireagent antibodies (15–17). Many lives have been fundamentally changed for the worse due to this all-too-common phenomenon (18).
Table 1.
Issues with Immunoassay Performance in Human Serum and Plasma
| Issue | Description | How LC-MS/MS Helps | Refs. |
|---|---|---|---|
| Autoantibodies | Epitopes normally recognized by reagent antibodies are masked by endogenous autoantibodies and prevent reagent antibody from binding the analyte. | All proteins are digested with protease | 6 |
| Antireagent interferences | Capture and reporter antibody are bound by the same interfering substance, bridging the gap that would normally be filled by analyte. | All proteins are digested with protease | 12–14, 18, 26, 27 |
| Hook effect | Capture and reporter antibody binding sites are completely saturated with analyte, which prevents capture and reporter antibody from binding the same analyte. | There is no sandwich formation | 28 |
| Poor interplatform concordance | Intellectual property concerns have forced each immunoassay to detect different epitopes leading to differences in calibration and in the relative results for some patients who have modifications to their epitopes in vivo. | Direct detection of peptide analytes rather than calibration of an indirect signal | 28 |
| Microclots | Plasma samples that are not inverted immediately after blood draw can result in the formation of small fibrin strands that can bridge capture and reporter antibody. | All proteins are digested with protease | 73–76 |
In other cases, endogenous autoantibodies recognize the target protein in the assay. Often, the epitopes recognized by these autoantibodies overlap or are near the epitopes recognized by the reagent antibodies. Immunoglobulins are large and can sterically interfere with the binding of reagent antibodies, which leads to falsely decreased or negative results. Most reports indicate that approximately 20% of thyroid cancer patients have detectable levels of antithyroglobulin autoantibody in their serum, with as many as 25% reported in 1 study (4). In our population, 20.9% are positive for Tg autoantibodies during follow-up (n = 1019 patients over 36 mo), and 75.6% of these have at least 1 Tg result < 1 ng/mL by sandwich immunoassay. In patients with TgAb and Tg concentrations below the actionable cutoff, it was previously common to perform a standard addition or recovery experiment in which a specific amount of Tg is added to the sample and then run in the Tg assay. Although 80% recovery of the spiked-in amount has been suggested to indicate that a low or negative result is accurate (19, 20), there are examples of TgAb-positive samples that test negative for Tg, which have > 80% recovery (and therefore what appears to be an accurate result) and actionable amounts of Tg results detectable with another assay (eg, by mass spectrometry) (4, 21). As a result, recovery experiments are not recommended by the most recent guidelines (1, 2).
Competitive immunoassays, such as RIAs, suffer from the same types of interferences as sandwich immunoassays, but the effect of the interferences may be different. For example, autoantibodies may lead to a false-positive result rather than a false-negative result when autoantibody competes with reagent antibody for labeled thyroglobulin protein (22). Although certain RIA reagents appear to be relatively resistant to interference with TgAb (23), it must be recognized that no immunoassay platform is free from interferences when using human serum and plasma as samples.
Autoantibodies to thyroglobulin have significant implications in the posttreatment monitoring of patients. Given that complete removal of all thyroid tissue and its antigenic components, whether normal or cancerous, results in the disappearance of antibodies to thyroid antigens, the presence of continued TgAb production suggests that persistent disease remains (5). However, the clinical significance of the persistent disease remains unknown, especially because the amount of autoantibodies produced can vary dramatically between patients; therefore, there is no specific titer that can be used as a clinical indicator of clinically significant disease (6). Patients with DTC and positive TgAb will often undergo subsequent testing with radioactive iodine scans and/or 18F-fluorodeoxyglucose-positron emission tomography/computed tomography to identify possible foci of persistent or recurrent disease (8, 24). In addition, for many patients with TgAb and DTC, no additional foci of disease are found, yet these patients undergo repeat imaging studies and may suffer from significant anxiety associated with the unknown clinical status of their disease.
Importantly, different TgAb assays are themselves variably sensitive to the presence of TgAb (25). The potential impact of this variability on patient monitoring, ie, using TgAb to identify potentially spurious Tg results or to identify potential recurrence, has been recently reviewed (6). From the perspective of the interpretation of serum Tg results, one quickly recognizes that there may be a small fraction of patients whose serum Tg immunoassay results are not questioned as falsely low or elevated, even when they should be.
There are many other issues with immunoassays that are beyond the scope of this review, but which have been reviewed elsewhere (16, 26–28). The presence of antibodies and other substances that cause falsely low or falsely elevated results in the indirect estimation of the concentration of a protein is a universal problem for immunoassays used in human serum and plasma. It is with these complications in mind that analytical and clinical chemists have sought to make a better thyroglobulin assay using mass spectrometry.
Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS) Methods to Measure Protein Concentrations
In mass spectrometric assays, the indirect measurement of proteins using antibodies is replaced by direct detection of the analyte itself as it strikes the detector within the instrument (29). The mass spectrometer is essentially a mass selector. It can sort molecules based on molecular weight and electric charge into very well-resolved bins, and any ions that reach the detector have the correct properties. In modern mass spectrometers, molecules of specific mass and charge can be isolated and fragmented, and then the molecular fragments can be measured. The combination of the mass and charge of the intact molecule and that of each fragment (MS/MS) is relatively specific for each molecule. This specificity is augmented with high-performance liquid chromatography (LC-MS/MS), which separates molecules based on, eg, hydrophobicity. Putting it all together, one can be confident that anything that 1) elutes at a specific time from the liquid chromatography column; 2) has a specific mass and charge in the intact state; and 3) has a specific fragment that hits the detector, is the molecule that one is interested in detecting.
The measurement of proteins by LC-MS/MS, particularly large proteins like Tg, requires 1 additional step: proteolytic digestion of the proteins into peptides, which are small enough to be detectable by the mass spectrometer. A standard protein LC-MS/MS assay therefore has the following steps: 1) digestion of all serum proteins into peptides; 2) separation of the peptides with liquid chromatography; and 3) specific detection of the molecule (intact and fragments) using mass spectrometry. This approach has been used to accurately quantify several different proteins directly in human serum (30, 31).
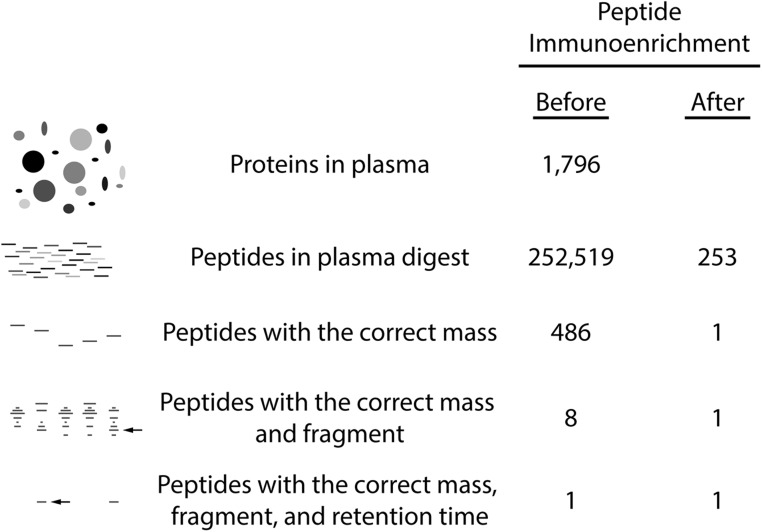
To predict how specific the method could be, one can calculate the likelihood of interference from peptides other than the peptide of interest (Figure 1). For example, to quantify Tg, one could proteolytically digest all of the proteins in plasma with trypsin and then run LC-MS/MS on the digest to quantify the peptide VIFDANAPVAVR, a peptide found only in human thyroglobulin and in no other protein encoded by the human genome. To calculate how many peptides would be present in the digest, one can start with the fact that almost 2000 proteins have been detected in human plasma (32) and the knowledge that trypsin is a specific protease that cleaves at lysine and arginine (amino acid symbols K and R, respectively). Using a computer to predict all of the peptides in a tryptic digest of serum, one can determine that there will be > 250 000 different peptides. Of those, approximately 500 will have the correct mass and charge to be selected for fragmentation, and 8 of those will have the correct fragment of interest (Dr Jimmy Eng, PhD, University of Washington, personal communication). In LC-MS/MS assays, liquid chromatography is used to separate peptides from one another before they enter the mass spectrometer. Liquid chromatography is not perfect in separating molecules from one another, but many peptides can be resolved from one another based on size and hydrophobicity. As a result, liquid chromatography can be used to separate the 1 peptide of interest from the other 7 peptides that have the same intact and fragment mass and result in a specific method for the detection of the 1 peptide of interest (Figure 1).
Figure 1.
Immunoaffinity enrichment and mass spectrometry are a foundation of analytical specificity. Tandem mass spectrometers transmit peptide ions of the correct mass and charge that also have the correct fragments. When mass spectrometry is combined with upstream peptide enrichment using antibodies and liquid chromatography, an analytically specific process can be used to quantify peptides. To predict the specificity of LC-MS/MS methods, serum proteins were digested with trypsin into peptides in silico (1796 proteins out of the 1929 proteins previously identified [32] were used in the experiment, and all precursor peptides with 2 missed-proteolytic cleavages, neutral mass 600–5000, and charge states of +2, +3, and +4 were included in the analysis]. The peptides that had the same mass per charge (m/z) as the Tg peptide VIFDANAPVAVR (m/z = 636.4 ± 0.35) and those that had the correct fragment (m/z = 1059.4 ± 0.35) were identified using spectral matching algorithms (Dr. Jimmy Eng, personal communication). The effects of immunoaffinity enrichment (1000-fold) are shown (compare before and after).
The Pitfalls of LC-MS/MS
Although quite specific, LC-MS/MS lacks sensitivity when compared with immunoassays. For example, direct measurement of proteins in serum is limited to abundant proteins above about 1 μg/mL (eg, albumin, apolipoprotein A-I, complement C3, ceruloplasmin). The reason the method lacks sensitivity is tied to the number of peptides and other molecules in the serum digest that elute from the liquid chromatography column at the same time as the peptide of interest. Mass spectrometers need molecules to be ionized in order to be detected, and when many molecules elute off of the liquid chromatography column at the same time, it is impossible to ionize all of them efficiently (this is called ion suppression) (33, 34). As a result, a low abundance peptide will be less efficiently charged with many other molecules around. As discussed in the next section, there is a way around this problem (ie, peptide immunoaffinity enrichment).
Besides sensitivity issues, mass spectrometric quantification of large proteins will have other problems that are not as easily solved. For example, nonsymptomatic germline mutations in the peptide of interest could lead to a different mass (making it nondetectable by mass spectrometry) and lead to an apparent concentration half of what one would expect given the actual protein concentration (or could even lead to a complete loss of the peptide in a homozygous patient). With the increasing number of publicly available genome sequences, it will be progressively easier to avoid the selection of polymorphic peptides as surrogates in LC-MS/MS assays (35). In addition, the monitoring of more than 1 peptide for each target protein helps identify single-point polymorphisms, which will help ensure that negative results are truly negative. As another example, posttranslational modifications of proteins, including proteolytic cleavages, are quite common. A cleavage or phosphorylation in the middle of the peptide of interest would lead to a loss of the peptide in an LC-MS/MS assay and a falsely low measurement of the protein concentration. For these reasons, LC-MS/MS assays will not be perfect. There will be certain patients for whom the assay will give an incorrect answer. However, when compared with the very large number of patients at risk of getting the wrong answer by immunoassay (up to ∼20%), it is likely that LC-MS/MS will make far fewer mistakes than current immunoassay technology.
One problem that both immunoassays and LC-MS/MS assays share is calibration within a lab and standardization between laboratories. Mass spectrometric methods use stable isotope-labeled molecules as internal standards, which are detected simultaneously with the endogenous analyte. It is the ratio of the number of molecules of the endogenous analyte to the internal standard that is compared against a calibration curve to generate a concentration. This is in contrast to immunoassays in which a change in the amount of light emitted or absorbed by a captured reporter antibody is compared to a calibration curve. The direct detection of analyte in mass spectrometers should make it easy to standardize measurements across laboratories, but the calibration of the mass spectrometric signal is only as good as the calibrators themselves. Indeed, large efforts are under way to standardize the quantification of vitamin D and T by LC-MS/MS (36, 37). Fortunately, there is a standard reference material available for thyroglobulin, BCR-457, which was purified from cadaveric thyroid tissue (38, 39). It will be important to demonstrate the commutability of this reference material between LC-MS/MS assays developed in different laboratories.
Another hurdle for the success of LC-MS/MS assays of large proteins is the variability of the trypsin digestion of serum (40, 41). In this respect, proper calibration materials will be vital for excellent functional sensitivity, which can be defined as the lowest concentration of an analyte in an assay that has between-assay variability less than 20% of the measured value over the typical interval of measurements. For Tg, this is 6–12 months (2). It is perhaps not surprising that the optimal matrix for spiked calibrators in LC-MS/MS protein assays seems to be human serum and that native human serum may provide the most reliable calibrator of all (42, 43).
How Could LC-MS/MS Work Better Than Immunoassays for the Measurement of Tg?
In contrast to albumin and apolipoprotein A-I, Tg is one of the least abundant proteins in the blood, especially in patients who have been treated with thyroidectomy and radioablation. To put the problem in perspective, the proteolytic digestion of serum will generate 1 peptide from Tg for every 40 000 000 peptides from albumin (when Tg is 1 ng/mL and albumin is 4 g/dL). As discussed in the preceding section, ion suppression makes it impossible to quantify Tg peptides in unmodified serum digests. Fortunately, antibodies are specific enough to purify peptides of interest from serum digests. By using peptide immunoaffinity enrichment, our laboratory has demonstrated the potential for using LC-MS/MS to quantify Tg in human samples (43). There are many other proteins for which sensitive assays have been developed using peptide immunoaffinity enrichment before LC-MS/MS (30, 44).
The benefit of the immunoaffinity enrichment of the peptides from the serum digests is illustrated in Figure 1. Although the mass spectrometer is a very specific detector and is theoretically capable of identifying and quantifying the 1 peptide of interest out of more than 250 000 peptides in the digest, the enrichment step is able to greatly simplify the number of peptides before LC-MS/MS, which reduces the amount of ion suppression and greatly improves the sensitivity of the method. Different studies have reported different amounts of peptide enrichment using antibodies, from 120-fold in early reports up to more than 18 000-fold in more recent reports (45–49). Using a conservative estimate of 1000-fold, there are only 253 peptides left out of the 252 519 peptides in the digest after enrichment. The LC-MS/MS platform can easily handle that many peptides to result in a very sensitive assay. Thus, although antibodies are never perfectly specific, the immunoaffinity enrichment step enables the sensitive detection of a single Tg peptide from a digest of human serum.
Current Status of Thyroglobulin LC-MS/MS Assays
In 2008, the first LC-MS/MS assay for thyroglobulin was described (43). The sensitivity of the assay was not sufficient for clinical use (3 ng/mL). Over the next few years, reagent (50) and instrument changes improved the analytical sensitivity of the assay (<1 ng/mL). When this new assay was compared with a standard thyroglobulin immunoassay, there was good agreement in patients without autoantibodies, which is to be expected. However, in patients with thyroglobulin autoantibodies, there are a number of samples that appear to have false-negative results by immunoassay and a few that could be false-positive results (Figure 2). Because LC-MS/MS has not been adopted as the “gold standard” for thyroglobulin measurement as it has for some small proteins (51–53), it is not possible to say that positive immunoassay results on samples with negative LC-MS/MS are false-positives. As mentioned previously, the absence of multiple peptides in an LC-MS/MS assay could increase confidence in a negative LC-MS/MS assay result, but more studies are needed, as discussed in Prospective Studies.
Figure 2.
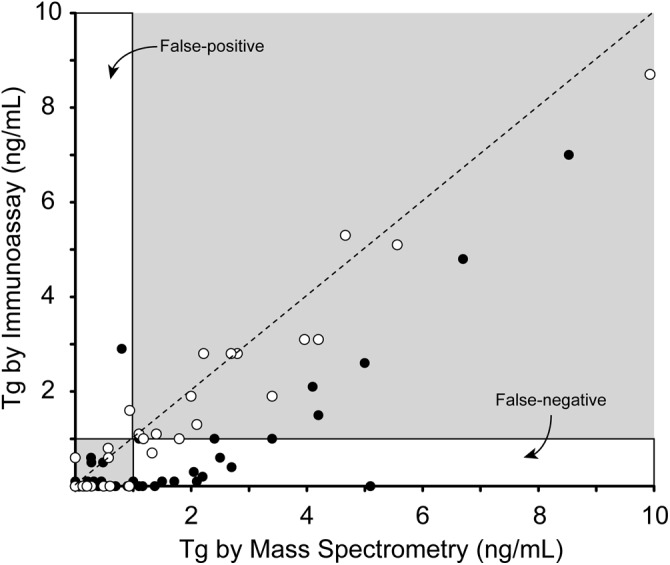
The analytical performance of an LC-MS/MS assay for Tg. Concentrations of Tg in serum samples measured by immunoassay (Beckman Access 2) and LC-MS/MS are plotted for samples without autoantibodies (open circles) and with (filled circles). Regions of the comparison plot that correspond to results that are potentially falsely high and low are illustrated with respect to a clinical cutoff of 1 ng/mL. For reference, the line of identity is shown (dashed).
Other groups have also made improvements to the immunoaffinity enrichment approach that we first described, and now 2 commercial laboratories offer serum thyroglobulin measurement: ARUP (Salt Lake City, Utah) and Quest Diagnostics (San Juan Capistrano, California). Recently, the details of these methods have been published (54, 55), and the analytical sensitivity of the assays (0.4–0.5 ng/mL) should be sufficient for clinical use in many cases. As the guidelines recommend (2), it will be important to verify that these assays have the needed functional sensitivity over at least 6 months of assay operation in TgAb-positive and -negative pools with 0.5–0.6 ng/mL Tg. Both assays had good agreement with a sandwich immunoassay in patients without detectable TgAb, strongly supporting the idea that it is possible to reliably calibrate a mass spectrometric signal into an accurate concentration. The most important question that can be asked of these assays is how well they perform in samples from patients that have interfering TgAb. None of the previously published papers have patient outcomes associated with their samples, which, as discussed in Prospective Studies, will be vital in understanding how well the assays serve clinicians and patients. However, both groups presented comparisons of their assays with sandwich immunoassays, and similar to what was shown in Figure 2, both LC-MS/MS assays had results in TgAb-positive samples that were on average higher than those seen by sandwich immunoassay. The group from Quest also compared their assay against a highly respected clinically used competitive immunoassay and saw on average lower results, which coincides with what would be expected. Perhaps most importantly, the group from ARUP compared their LC-MS/MS assay with the LC-MS/MS assay of another reference laboratory that used a different enrichment protocol, a different trypsin digestion protocol, a different calibration scheme (ie, 1 laboratory uses commercial calibrators and the other makes standards with BCR-457 spiked into TgAb-negative human serum), and a different LC-MS/MS platform. The results were concordant across a small population of patients with and without TbAg, which supports the proposal that standardization between laboratories will be possible. Thus, whereas there are no data available to assess how well these 3 methods perform in a clinical setting, LC-MS/MS as a moderately sensitive, highly specific platform for the measurement of Tg in the serum of DTC patients has been analytically validated.
Until the technology is greatly simplified and the reagents are disseminated more widely (50), LC-MS/MS methods will only be available in moderate to large reference laboratories. Although these laboratory-developed tests are not specifically cleared by the Food and Drug Administration, a thorough validation will be required before the assays are put into use in the laboratory. The validation must adhere to the guidelines established by the Clinical Laboratory Improvement Amendments (CLIA) of 1988, and it is strongly recommended that the protocols developed and described by the Clinical Laboratory Standards Institute be used as a framework for that validation. If all of this is done properly, it will be easy to understand the influence of lot changes of trypsin, of using different mass spectrometers in the same laboratory, and of other subtle variations of the procedure during the course of clinical care that could affect assay performance.
The newest immunoassays for thyroglobulin are reported to have functional sensitivities that are as low as 0.1 ng/mL or lower, and these improved assays can improve patient care (56–58). As such, the LC-MS/MS assays appear to be at a disadvantage when compared with traditional immunoassays. However, it must be recognized that at low Tg concentrations, many patients will have detectable or undetectable autoantibodies that interfere with the immunoassay, and that the assay will therefore have insufficient sensitivity for meaningful clinical use.
Relative Cost of the Lab Test
The exact cost of a laboratory test may refer to: 1) the amount of money spent by the laboratory performing the test; 2) the cost of the test billed to the patient/insurance company; or 3) the amount of money that the patient actually pays for the lab test before the deductible. Importantly, the amount of money that a laboratory spends to run an individual test is very difficult to calculate—just thinking of the indirect costs required to run the laboratory (keeping the lights on, the water running, the computers online, etc.) and the vastly varying reagent and labor costs around the world. However, in any given setting, one might be able to compare the relative amounts of money that a laboratory might spend to run a laboratory test.
To consider the direct costs of a laboratory assay, the reagents, instrument, and technologist time must all be taken into account (59). In the case of immunoassays on automated instruments, the reagents arrive as “plug-and-play” components and are generally less expensive than the many reagents that need to be made from scratch for a mass spectrometric assay. The instruments for an immunoassay are generally 3- to 6-fold less expensive than the LC-MS/MS equipment, which may also include an automated pipettor/sample-handling device. And the amount of technologist time required per sample is also less for immunoassays, generally 6- to 30-fold less. As a result, mass spectrometric assays for proteins are at least 6 times as expensive as their immunoassay counterparts. Importantly, mass spectrometry is a novel platform for the measurement of proteins in the clinical laboratory, and as more analytes are ported over to the novel platform, it will get less expensive.
Outside of the laboratory, a more sensitive and specific assay could impact many costs associated with the management of patients with DTC. The current guidelines from the American Thyroid Association recommend long-term follow-up of Tg-negative, TgAb-positive patients with interval imaging studies and monitoring of the TgAb titer (1). Follow-up imaging studies to look for persistent or recurrent disease often include increased utilization of neck ultrasound (60), radioiodine diagnostic whole-body scans (9), and fluorodeoxyglucose-positron emission tomography imaging (8), all of which increase the cost of long-term surveillance for these patients (ie, Medicare reimbursement rates for these imaging studies are 10–100 times that of a Tg assay). In addition, patients with thyroid cancer report decreased quality of life compared to age-matched peers (61), and it is possible that patients with TgAb have a worse quality of life compared to their peers without TgAb, but this has not been studied. Although the overall healthcare costs and quality of life indicators in thyroid cancer patients with and without TgAb have not been compared, a more sensitive and specific assay for Tg could decrease the overall cost of long-term treatment and surveillance in patients with DTC.
Prospective Studies
As mentioned above, the publications so far that describe thyroglobulin assays by LC-MS/MS do not take the necessary next step in identifying the clinical sensitivity and specificity of the assay (43, 54, 55). This could be performed in a retrospective manner in which stored clinical samples from patients that go on to have recurrent carcinoma and those that do not are all measured by the new assay. A retrospective comparison of the original immunoassay results with the LC-MS/MS results could also document the improved performance of the assay and be used to model the improvement in patient care. Unfortunately, because there is no gold standard for the measurement of thyroglobulin in serum, the interpretation of retrospective comparison studies will be complicated, but it will be a necessary first step.
Due to the increased expense of novel LC-MS/MS assays and the good performance of immunoassays in most patients without interfering autoantibodies, actual benefits to patients and the healthcare system need to be evaluated via prospective clinical studies. It is anticipated that superior thyroglobulin assays could benefit nearly all patients with autoantibodies (results via sandwich immunoassay are lower in these patients) by identifying patients with circulating thyroglobulin earlier than immunoassays. Given that TgAb assays are not 100% sensitive and that some patients with interfering TgAb will be missed as a result (25), it is expected that some portion of patients with undetectable interfering TgAb could also benefit from LC-MS/MS testing. For RIA, in which falsely elevated levels of Tg in TgAb-positive patients could go unnoticed, prospective studies may also demonstrate a benefit in patients with TgAb. On the other hand, because the analytical sensitivity of the LC-MS/MS assay is currently lower than available immunoassays, some recurrences, especially in lymph nodes, may not be detectable, which would lessen the clinical performance of the LC-MS/MS assay. If prospective studies demonstrate that the LC-MS/MS assay is more robust in actual clinical use, it is expected that the total number of imaging studies after ultrasound will be lower (1, 9, 60, 62–67). However, many centers may continue to rely on advanced imaging studies, even in patients with a low risk of recurrence (68–72). It will be interesting to see whether prospective studies actually demonstrate an improvement in patient outcomes or healthcare expenditures in low-risk patients with autoantibodies or with immunoassay results that do not match their clinical situation.
Meeting the Needs of Patients and Care Providers
Even after prospective studies are completed, the most economical reflexive testing strategy for serum thyroglobulin may still include an initial immunoassay screen for thyroglobulin and antithyroglobulin autoantibodies. At this time, it is possible to envision a reflexive strategy in which only samples with Tg levels below the clinical cutoff by a sandwich immunoassay and detectable amounts of autoantibodies would have Tg measured by LC-MS/MS. As the field eagerly awaits prospective studies, this may be the most appropriate approach to consider, recognizing that the assay may ultimately benefit all antibody-positive patients or even all DTC patients.
The development of sensitive LC-MS/MS assays for the quantification of serum thyroglobulin has been a great triumph for analytical and clinical chemistry. It represents a synergy of many different areas of research and innovation (50). The fact that thyroglobulin is the first peptide immunoaffinity enrichment-LC-MS/MS assay available for clinical use is not surprising because it is the single best-studied analyte in terms of interferences and how those issues affect patient management. It is only the first example of a potential paradigm shift in clinical chemistry wherein proteins are measured by mass spectrometry rather than often-frustrating immunoassays.
Acknowledgments
This work was partially supported by National Cancer Institute/National Institutes of Health (NIH) Grant U24CA160034 that funds the Clinical Proteomic Tumor Analysis Consortium program (to A.N.H.) and National Institute of Child Health and Human Development/NIH Grant K12 HD053984 (to M.Y.R.).
Disclosure Summary: M.Y.R. has nothing to disclose. A.N.H. is an inventor on US Patent 7,807,172.
Footnotes
- DTC
- differentiated thyroid cancer
- LC-MS/MS
- liquid chromatography-tandem mass spectrometry
- Tg
- thyroglobulin
- TgAb
- antithyroglobulin autoantibody.
References
- 1. American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer; Cooper DS, Doherty GM, Haugen BR, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1214 [DOI] [PubMed] [Google Scholar]
- 2. Demers LM, Spencer CA. Laboratory medicine practice guidelines: laboratory support for the diagnosis and monitoring of thyroid disease. Clin Endocrinol (Oxf). 2003;58:138–140 [DOI] [PubMed] [Google Scholar]
- 3. Durante C, Montesano T, Attard M, et al. Long-term surveillance of papillary thyroid cancer patients who do not undergo postoperative radioiodine remnant ablation: is there a role for serum thyroglobulin measurement? J Clin Endocrinol Metab. 2012;97:2748–2753 [DOI] [PubMed] [Google Scholar]
- 4. Spencer CA, Takeuchi M, Kazarosyan M, et al. Serum thyroglobulin autoantibodies: prevalence, influence on serum thyroglobulin measurement, and prognostic significance in patients with differentiated thyroid carcinoma. J Clin Endocrinol Metab. 1998;83:1121–1127 [DOI] [PubMed] [Google Scholar]
- 5. Chiovato L, Latrofa F, Braverman LE, et al. Disappearance of humoral thyroid autoimmunity after complete removal of thyroid antigens. Ann Intern Med. 2003;139:346–351 [DOI] [PubMed] [Google Scholar]
- 6. Spencer CA. Clinical review: clinical utility of thyroglobulin antibody (TgAb) measurements for patients with differentiated thyroid cancers (DTC). J Clin Endocrinol Metab. 2011;96:3615–3627 [DOI] [PubMed] [Google Scholar]
- 7. Fish SA, Langer JE, Mandel SJ. Sonographic imaging of thyroid nodules and cervical lymph nodes. Endocrinol Metab Clin North Am. 2008;37:401–417 [DOI] [PubMed] [Google Scholar]
- 8. Ozkan E, Soydal C, Araz M, Aras G, Ibis E. The additive clinical value of 18F-FDG PET/CT in defining the recurrence of disease in patients with differentiated thyroid cancer who have isolated increased antithyroglobulin antibody levels. Clin Nucl Med. 2012;37:755–758 [DOI] [PubMed] [Google Scholar]
- 9. Rosario PW, Mineiro Filho AF, Lacerda RX, dos Santos DA, Calsolari MR. The value of diagnostic whole-body scanning and serum thyroglobulin in the presence of elevated serum thyrotropin during follow-up of anti-thyroglobulin antibody-positive patients with differentiated thyroid carcinoma who appeared to be free of disease after total thyroidectomy and radioactive iodine ablation. Thyroid. 2012;22:113–116 [DOI] [PubMed] [Google Scholar]
- 10. Goto M, Kuribayashi K, Umemori Y, et al. High prevalence of human anti-mouse antibodies in the serum of colorectal cancer patients. Anticancer Res. 2010;30:4353–4356 [PubMed] [Google Scholar]
- 11. Koshida S, Asanuma K, Kuribayashi K, et al. Prevalence of human anti-mouse antibodies (HAMAs) in routine examinations. Clin Chim Acta. 2010;411:391–394 [DOI] [PubMed] [Google Scholar]
- 12. Preissner CM, O'Kane DJ, Singh RJ, Morris JC, Grebe SK. Phantoms in the assay tube: heterophile antibody interferences in serum thyroglobulin assays. J Clin Endocrinol Metab. 2003;88:3069–3074 [DOI] [PubMed] [Google Scholar]
- 13. Spencer C, Fatemi S, Singer P, Nicoloff J, Lopresti J. Serum basal thyroglobulin measured by a second-generation assay correlates with the recombinant human thyrotropin-stimulated thyroglobulin response in patients treated for differentiated thyroid cancer. Thyroid. 2010;20:587–595 [DOI] [PubMed] [Google Scholar]
- 14. Verburg FA, Waschle K, Reiners C, Giovanella L, Lentjes EG. Heterophile antibodies rarely influence the measurement of thyroglobulin and thyroglobulin antibodies in differentiated thyroid cancer patients. Horm Metab Res. 2010;42:736–739 [DOI] [PubMed] [Google Scholar]
- 15. Bolstad N, Warren DJ, Bjerner J, et al. Heterophilic antibody interference in commercial immunoassays; a screening study using paired native and pre-blocked sera. Clin Chem Lab Med. 2011;49:2001–2006 [DOI] [PubMed] [Google Scholar]
- 16. Hoofnagle AN, Wener MH. The fundamental flaws of immunoassays and potential solutions using tandem mass spectrometry. J Immunol Methods. 2009;347:3–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Todd DJ, Knowlton N, Amato M, et al. Erroneous augmentation of multiplex assay measurements in patients with rheumatoid arthritis due to heterophilic binding by serum rheumatoid factor. Arthritis Rheum. 2011;63:894–903 [DOI] [PubMed] [Google Scholar]
- 18. Rotmensch S, Cole LA. False diagnosis and needless therapy of presumed malignant disease in women with false-positive human chorionic gonadotropin concentrations. Lancet. 2000;355:712–715 [DOI] [PubMed] [Google Scholar]
- 19. Bachelot A, Cailleux AF, Klain M, et al. Relationship between tumor burden and serum thyroglobulin level in patients with papillary and follicular thyroid carcinoma. Thyroid. 2002;12:707–711 [DOI] [PubMed] [Google Scholar]
- 20. Ligabue A, Poggioli MC, Zacchini A. Interference of specific autoantibodies in the assessment of serum thyroglobulin. J Nucl Biol Med. 1993;37:273–279 [PubMed] [Google Scholar]
- 21. Spencer CA. Recoveries cannot be used to authenticate thyroglobulin (Tg) measurements when sera contain Tg autoantibodies: Clin Chem. 1996;42:661–663 [PubMed] [Google Scholar]
- 22. Schneider AB, Pervos R. Radioimmunoassay of human thyroglobulin: effect of antithyroglobulin autoantibodies. J Clin Endocrinol Metab. 1978;47:126–137 [DOI] [PubMed] [Google Scholar]
- 23. Black EG, Hoffenberg R. Should one measure serum thyroglobulin in the presence of anti-thyroglobulin antibodies? Clin Endocrinol (Oxf). 1983;19:597–601 [DOI] [PubMed] [Google Scholar]
- 24. Chung JK, Park YJ, Kim TY, et al. Clinical significance of elevated level of serum antithyroglobulin antibody in patients with differentiated thyroid cancer after thyroid ablation. Clin Endocrinol (Oxf). 2002;57:215–221 [DOI] [PubMed] [Google Scholar]
- 25. Spencer C, Petrovic I, Fatemi S. Current thyroglobulin autoantibody (TgAb) assays often fail to detect interfering TgAb that can result in the reporting of falsely low/undetectable serum Tg IMA values for patients with differentiated thyroid cancer. J Clin Endocrinol Metab. 2011;96:1283–1291 [DOI] [PubMed] [Google Scholar]
- 26. Hennig C, Rink L, Fagin U, Jabs WJ, Kirchner H. The influence of naturally occurring heterophilic anti-immunoglobulin antibodies on direct measurement of serum proteins using sandwich ELISAs. J Immunol Methods. 2000;235:71–80 [DOI] [PubMed] [Google Scholar]
- 27. Kricka LJ. Human anti-animal antibody interferences in immunological assays. Clin Chem. 1999;45:942–956 [PubMed] [Google Scholar]
- 28. Spencer CA, Takeuchi M, Kazarosyan M. Current status and performance goals for serum thyroglobulin assays. Clin Chem. 1996;42:164–173 [PubMed] [Google Scholar]
- 29. Strathmann FG, Hoofnagle AN. Current and future applications of mass spectrometry to the clinical laboratory. Am J Clin Pathol. 2011;136:609–616 [DOI] [PubMed] [Google Scholar]
- 30. Boja ES, Rodriguez H. Mass spectrometry-based targeted quantitative proteomics: achieving sensitive and reproducible detection of proteins. Proteomics. 2012;12:1093–1110 [DOI] [PubMed] [Google Scholar]
- 31. Lehmann S, Hoofnagle A, Hochstrasser D, et al. Quantitative clinical chemistry proteomics (qCCP) using mass spectrometry: general characteristics and application. Clin Chem Lab Med. 2012;23:1–16 [DOI] [PubMed] [Google Scholar]
- 32. Farrah T, Deutsch EW, Omenn GS, et al. A high-confidence human plasma proteome reference set with estimated concentrations in PeptideAtlas. Mol Cell Proteomics. 2011;10:M110.006353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bonfiglio R, King RC, Olah TV, Merkle K. The effects of sample preparation methods on the variability of the electrospray ionization response for model drug compounds. Rapid Commun Mass Spectrom. 1999;13:1175–1185 [DOI] [PubMed] [Google Scholar]
- 34. Matuszewski BK, Constanzer ML, Chavez-Eng CM. Matrix effect in quantitative LC/MS/MS analyses of biological fluids: a method for determination of finasteride in human plasma at picogram per milliliter concentrations. Anal Chem. 1998;70:882–889 [DOI] [PubMed] [Google Scholar]
- 35. Abecasis GR, Auton A, Brooks LD, et al. An integrated map of genetic variation from 1,092 human genomes. Nature. 2012;491:56–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sempos CT, Vesper HW, Phinney KW, Thienpont LM, Coates PM. Vitamin D status as an international issue: national surveys and the problem of standardization. Scand J Clin Lab Invest Suppl. 2012;243:32–40 [DOI] [PubMed] [Google Scholar]
- 37. Yun YM, Botelho JC, Chandler DW, et al. Performance criteria for testosterone measurements based on biological variation in adult males: recommendations from the Partnership for the Accurate Testing of Hormones. Clin Chem. 2012;58:1703–1710 [DOI] [PubMed] [Google Scholar]
- 38. Feldt-Rasmussen U, Profilis C, Colinet E, et al. Human thyroglobulin reference material (CRM 457). 1st Part: assessment of homogeneity, stability and immunoreactivity. Ann Biol Clin (Paris). 1996;54:337–342 [PubMed] [Google Scholar]
- 39. Feldt-Rasmussen U, Profilis C, Colinet E, et al. Human thyroglobulin reference material (CRM 457). 2nd Part: physicochemical characterization and certification. Ann Biol Clin (Paris). 1996;54:343–348 [PubMed] [Google Scholar]
- 40. Addona TA, Abbatiello SE, Schilling B, et al. Multi-site assessment of the precision and reproducibility of multiple reaction monitoring-based measurements of proteins in plasma. Nat Biotechnol. 2009;27:633–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hoofnagle AN. Quantitative clinical proteomics by liquid chromatography-tandem mass spectrometry: assessing the platform. Clin Chem. 2010;56:161–164 [DOI] [PubMed] [Google Scholar]
- 42. Agger SA, Marney LC, Hoofnagle AN. Simultaneous quantification of apolipoprotein A-I and apolipoprotein B by liquid-chromatography-multiple- reaction-monitoring mass spectrometry. Clin Chem. 2010;56:1804–1813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hoofnagle AN, Becker JO, Wener MH, Heinecke JW. Quantification of thyroglobulin, a low-abundance serum protein, by immunoaffinity peptide enrichment and tandem mass spectrometry. Clin Chem. 2008;54:1796–1804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Becker JO, Hoofnagle AN. Replacing immunoassays with tryptic digestion-peptide immunoaffinity enrichment and LC-MS/MS. Bioanalysis. 2012;4:281–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Anderson NL, Anderson NG, Haines LR, Hardie DB, Olafson RW, Pearson TW. Mass spectrometric quantitation of peptides and proteins using stable isotope standards and capture by anti-peptide antibodies (SISCAPA). J Proteome Res. 2004;3:235–244 [DOI] [PubMed] [Google Scholar]
- 46. Anderson NL, Jackson A, Smith D, Hardie D, Borchers C, Pearson TW. SISCAPA peptide enrichment on magnetic beads using an in-line bead trap device. Mol Cell Proteomics. 2009;8:995–1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kuhn E, Addona T, Keshishian H, et al. Developing multiplexed assays for troponin I and interleukin-33 in plasma by peptide immunoaffinity enrichment and targeted mass spectrometry. Clin Chem. 2009;55:1108–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Schoenherr RM, Zhao L, Whiteaker JR, et al. Automated screening of monoclonal antibodies for SISCAPA assays using a magnetic bead processor and liquid chromatography-selected reaction monitoring-mass spectrometry. J Immunol Methods. 2010;353:49–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Whiteaker JR, Zhao L, Anderson L, Paulovich AG. An automated and multiplexed method for high throughput peptide immunoaffinity enrichment and multiple reaction monitoring mass spectrometry-based quantification of protein biomarkers. Mol Cell Proteomics. 2010;9:184–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rodriguez H, Rivers R, Kinsinger C, et al. Reconstructing the pipeline by introducing multiplexed multiple reaction monitoring mass spectrometry for cancer biomarker verification: an NCI-CPTC initiative perspective. Proteomics Clin Appl. 2010;4:904–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Barr JR, Maggio VL, Patterson DG, Jr, et al. Isotope dilution—mass spectrometric quantification of specific proteins: model application with apolipoprotein A-I. Clin Chem. 1996;42:1676–1682 [PubMed] [Google Scholar]
- 52. Kaiser P, Akerboom T, Ohlendorf R, Reinauer H. Liquid chromatography-isotope dilution-mass spectrometry as a new basis for the reference measurement procedure for hemoglobin A1c determination. Clin Chem. 2010;56:750–754 [DOI] [PubMed] [Google Scholar]
- 53. Miller WG, Thienpont LM, Van Uytfanghe K, et al. Toward standardization of insulin immunoassays. Clin Chem. 2009;55:1011–1018 [DOI] [PubMed] [Google Scholar]
- 54. Clarke NJ, Zhang Y, Reitz RE. A novel mass spectrometry-based assay for the accurate measurement of thyroglobulin from patient samples containing antithyroglobulin autoantibodies. J Investig Med. 2012;60:1157–1163 [DOI] [PubMed] [Google Scholar]
- 55. Kushnir MM, Rockwood AL, Roberts WL, Abraham D, Hoofnagle AN, Meikle WA. Measurement of thyroglobulin by liquid chromatography/tandem mass spectrometry in serum and plasma in presence of anti-thyroglobulin autoantibodies [published online ahead of print February 8, 2013]. Clin Chem. doi:10.1373/clinchem.2012.195594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Iervasi A, Iervasi G, Ferdeghini M, et al. Clinical relevance of highly sensitive Tg assay in monitoring patients treated for differentiated thyroid cancer. Clin Endocrinol (Oxf). 2007;67:434–441 [DOI] [PubMed] [Google Scholar]
- 57. Schlumberger M, Hitzel A, Toubert ME, et al. Comparison of seven serum thyroglobulin assays in the follow-up of papillary and follicular thyroid cancer patients. J Clin Endocrinol Metab. 2007;92:2487–2495 [DOI] [PubMed] [Google Scholar]
- 58. Smallridge RC, Meek SE, Morgan MA, et al. Monitoring thyroglobulin in a sensitive immunoassay has comparable sensitivity to recombinant human TSH-stimulated thyroglobulin in follow-up of thyroid cancer patients. J Clin Endocrinol Metab. 2007;92:82–87 [DOI] [PubMed] [Google Scholar]
- 59. Travers EM. Cost analysis in the toxicology laboratory. Clin Lab Med. 1990;10:591–623 [PubMed] [Google Scholar]
- 60. Soyluk O, Boztepe H, Aral F, Alagol F, Ozbey NC. Papillary thyroid carcinoma patients assessed to be at low or intermediary risk after primary treatment are at greater risk of long term recurrence if they are thyroglobulin antibody positive or do not have distinctly low thyroglobulin at initial assessment. Thyroid. 2011;21:1301–1308 [DOI] [PubMed] [Google Scholar]
- 61. Husson O, Haak HR, Oranje WA, Mols F, Reemst PH, van de Poll-Franse LV. Health-related quality of life among thyroid cancer survivors: a systematic review. Clin Endocrinol (Oxf). 2011;75:544–554 [DOI] [PubMed] [Google Scholar]
- 62. Chindris AM, Diehl NN, Crook JE, Fatourechi V, Smallridge RC. Undetectable sensitive serum thyroglobulin (<0.1 ng/ml) in 163 patients with follicular cell-derived thyroid cancer: results of rhTSH stimulation and neck ultrasonography and long-term biochemical and clinical follow-up. J Clin Endocrinol Metab. 2012;97:2714–2723 [DOI] [PubMed] [Google Scholar]
- 63. Rosario PW, Furtado Mde S, Mineiro Filho AF, Lacerda RX, Calsolari MR. Value of diagnostic radioiodine whole-body scanning after initial therapy in patients with differentiated thyroid cancer at intermediate and high risk for recurrence. Thyroid. 2012;22:1165–1169 [DOI] [PubMed] [Google Scholar]
- 64. Rosario PW, Mineiro Filho AF, Prates BS, Silva LC, Calsolari MR. Postoperative stimulated thyroglobulin of less than 1 ng/mL as a criterion to spare low-risk patients with papillary thyroid cancer from radioactive iodine ablation. Thyroid. 2012;22:1140–1143 [DOI] [PubMed] [Google Scholar]
- 65. Rosario PW, Xavier AC, Calsolari MR. Value of postoperative thyroglobulin and ultrasonography for the indication of ablation and 131I activity in patients with thyroid cancer and low risk of recurrence. Thyroid. 2011;21:49–53 [DOI] [PubMed] [Google Scholar]
- 66. Vaisman A, Orlov S, Yip J, et al. Application of post-surgical stimulated thyroglobulin for radioiodine remnant ablation selection in low-risk papillary thyroid carcinoma. Head Neck. 2010;32:689–698 [DOI] [PubMed] [Google Scholar]
- 67. Webb RC, Howard RS, Stojadinovic A, et al. The utility of serum thyroglobulin measurement at the time of remnant ablation for predicting disease-free status in patients with differentiated thyroid cancer: a meta-analysis involving 3947 patients. J Clin Endocrinol Metab. 2012;97:2754–2763 [DOI] [PubMed] [Google Scholar]
- 68. Cherk MH, Francis P, Topliss DJ, Bailey M, Kalff V. Incidence and implications of negative serum thyroglobulin but positive I-131 whole-body scans in patients with well-differentiated thyroid cancer prepared with rhTSH or thyroid hormone withdrawal. Clin Endocrinol (Oxf). 2012;76:734–740 [DOI] [PubMed] [Google Scholar]
- 69. Giovanella L, Ceriani L, De Palma D, Suriano S, Castellani M, Verburg FA. Relationship between serum thyroglobulin and 18FDG-PET/CT in 131I-negative differentiated thyroid carcinomas. Head Neck. 2012;34:626–631 [DOI] [PubMed] [Google Scholar]
- 70. Park EK, Chung JK, Lim IH, et al. Recurrent/metastatic thyroid carcinomas false negative for serum thyroglobulin but positive by posttherapy I-131 whole body scans. Eur J Nucl Med Mol Imaging. 2009;36:172–179 [DOI] [PubMed] [Google Scholar]
- 71. Phan HT, Jager PL, van der Wal JE, et al. The follow-up of patients with differentiated thyroid cancer and undetectable thyroglobulin (Tg) and Tg antibodies during ablation. Eur J Endocrinol. 2008;158:77–83 [DOI] [PubMed] [Google Scholar]
- 72. Robenshtok E, Grewal R, Fish S, Sabra M, Tuttle RM. A low post-operative non-stimulated thyroglobulin does not exclude the presence of RAI avid metastatic foci in intermediate risk differentiated thyroid cancer patients. Thyroid. 2012;15:15. [DOI] [PubMed] [Google Scholar]
- 73. Beyne P, Vigier JP, Bourgoin P, Vidaud M. Comparison of single and repeat centrifugation of blood specimens collected in BD evacuated blood collection tubes containing a clot activator for cardiac troponin I assay on the ACCESS analyzer: Clin Chem. 2000;46:1869–1870 [PubMed] [Google Scholar]
- 74. Fleming SM, O'Byrne L, Finn J, Grimes H, Daly KM. False-positive cardiac troponin I in a routine clinical population. Am J Cardiol. 2002;89:1212–1215 [DOI] [PubMed] [Google Scholar]
- 75. Kazmierczak SC, Sekhon H, Richards C. False-positive troponin I measured with the Abbott AxSYM attributed to fibrin interference. Int J Cardiol. 2005;101:27–31 [DOI] [PubMed] [Google Scholar]
- 76. Strathmann FG, Ka MM, Rainey PM, Baird GS. Use of the BD vacutainer rapid serum tube reduces false-positive results for selected Beckman Coulter Unicel DxI immunoassays. Am J Clin Pathol. 2011;136:325–329 [DOI] [PubMed] [Google Scholar]