Abstract
Context:
Colonization of the gastrointestinal tract with methanogenic archaea (methanogens) significantly affects host metabolism and weight gain in animal models, and breath methane is associated with a greater body mass index (BMI) among obese human subjects.
Objective:
The objective of the study was to characterize the relationship between methane and hydrogen on breath test (as a surrogate for colonization with the hydrogen requiring methanogen, Methanobrevibacter smithii), body weight, and percent body fat in a general population cohort.
Design and Subjects:
This was a prospective study (n = 792) of consecutive subjects presenting for breath testing.
Setting:
The study was conducted at a tertiary care center.
Outcome Measurements:
BMI and percent body fat were measured.
Results:
Subjects were classified into 4 groups based on breath testing: normal (N) (methane <3 ppm and hydrogen <20 ppm at or before 90 minutes); hydrogen positive only (H+) [methane <3 ppm and hydrogen ≥20 ppm); methane positive only (M+) (methane ≥3 ppm and hydrogen <20 ppm), or methane and hydrogen positive (M+/H+) (methane ≥3 ppm and hydrogen ≥20 ppm]. There were significant differences in age but not in gender across the groups. After controlling for age as a confounding variable, M+/H+ subjects had significantly higher BMI than other groups (N: 24.1 ± 5.2 kg/m2; H+: 24.2 ± 4.5 kg/m2; M+: 24.0 ± 3.75 kg/m2; M+/H+: 26.5 ± 7.1 kg/m2, P < .02) and also had significantly higher percent body fat (N: 28.3 ± 10.0%; H+: 27.5 ± 9.0%; M+: 28.0 ± 8.9%; M+/H+; 34.1 ± 10.9%, P < .001).
Conclusions:
The presence of both methane and hydrogen on breath testing is associated with increased BMI and percent body fat in humans. We hypothesize that this is due to colonization with the hydrogen-requiring M smithii, which affects nutrient availability for the host and may contribute to weight gain.
Obesity constitutes a significant and rapidly increasing public health challenge and is associated with increased risks for coronary artery disease, hypertension, stroke, type 2 diabetes, certain cancers, and premature death (1, 2). Elucidating mechanisms contributing to the development of obesity is central to defining preventive approaches. Research has begun to define the relationship between gut flora and metabolism (3–5). Alterations in the relative abundance of Bacteroidetes and Firmicutes have been linked to changes in metabolism and weight increases both in mice (6) and humans (4). Furthermore, cocolonization with the methanogenic archaea, Methanobrevibacter smithii, results in a greater weight gain in germ-free animals than infection with B thetaiotaomicron alone (7).
A current hypothesis is that the role of M smithii in weight gain in animals is facilitative and involves syntrophic relationships with other microbes. M smithii produces methane as a byproduct of its hydrogen-requiring anaerobic metabolism. By scavenging hydrogen, M smithii allows for the increased productivity and metabolism of these syntropes, facilitating short-chain fatty acid (SCFA) production and enhancing the availability of calories to the host (8).
M smithii is the predominant methanogen in the human gastrointestinal (GI) tract (9), and we have shown that methane on breath testing is associated with higher levels of M smithii in stool (10). Furthermore, we recently showed that methane-positive obese subjects have an average 6.7 kg/m2 greater body mass index (BMI) than methane-negative obese controls (11). However, the relationship between methane, BMI, and body fat has not been evaluated in a general population cohort. Herein we present the first large-scale prospective human study to characterize the association with methane on breath test (as a surrogate of intestinal M smithii colonization) across a spectrum of ages, body weights, and BMIs.
Materials and Methods
Study population
Consecutive subjects presenting for lactulose breath testing were eligible for participation. Exclusion criteria were based on the ability to safely perform bioimpedance anthropometric measurements, and pregnant women and those with cardiac pacing/defibrillation devices were excluded. All subjects provided informed consent prior to participating in the study. The study was approved by Institutional Review Board at Cedars-Sinai Medical Center (Los Angeles, California).
Questionnaire
Subjects completed a demographic and medical questionnaire and a bowel symptom questionnaire (12) rating their last 7 days of intestinal complaints (bloating, diarrhea, constipation, and abdominal pain) on a visual analog scale from 0 to 100 mm, 100 being the most severe.
Lactulose breath test
Subjects presented to the medical center, having fasted for 12 hours as described previously (13). Breath samples were collected in a dual-bag system (Quintron Instrument Co, Milwaukee, Wisconsin). After an initial breath collection, subjects ingested 10 g of lactulose syrup and then 250 mL of water. Breath samples were collected every 15 minutes for 2 hours and analyzed using the Breathtracker gas chromatograph (Quintron Instrument Co). Outputs included hydrogen, methane, and carbon dioxide. Hydrogen and methane were corrected for carbon dioxide to standardize to alveolar gas levels and reported in parts per million (ppm). Subjects with methane 3 ppm or greater were considered methane positive, as described previously (13). Subjects with hydrogen greater than 20 ppm at or before 90 minutes during the test were considered hydrogen positive.
Anthropometrics
Bioimpedance testing was performed using the InBody scale (Biospace Co, Ltd, Seoul, Korea), which has been validated in other studies (12). BMI and percent body fat were determined based on height (measured via stadiometer) and electrical conductance.
Outcome measures
Subjects were divided into 4 groups: normal (N) (<3 ppm methane and < 20 ppm hydrogen at or before 90 minutes); hydrogen positive only (H+) (<3 ppm methane and hydrogen ≥ 20 ppm at or before 90 minutes); methane positive only (M+) (methane ≥ 3 ppm and hydrogen < 20 ppm at or before 90 minutes); and methane and hydrogen positive (M+/H+) (methane ≥ 3 ppm and hydrogen ≥ 20 ppm at or before 90 minutes). Primary outcome measures were BMI and percent body fat, and primary analyses compared these measures across the 4 groups.
Data and statistical analysis
Age was compared across the groups by ANOVA and then Dunnett's post hoc test and gender by the Fisher exact test. Visual analog scale scores were compared across the groups by the Kruskal-Wallis test because of nonnormality. BMI and percent body fat were analyzed by analysis of covariance (ANCOVA) models. The initial ANCOVA models were 2-way factorial models (sex at 2 levels and group at 4 levels) with age as a covariate. Because the gender-by-group interaction was not significant (P = .28 for BMI and P = .37 for percent body fat), the interaction term was dropped in the ANCOVA model for each outcome. Age was significant and was retained in each model. Least squares (adjusted) means were used to compare the H+/M+ group to each of the other 3 groups. A 2-sided significance level of P = .05 was used throughout. SAS version 9.2 (SAS Institute, Cary, North Carolina) was used for statistical calculations.
Results
Demographics
A total of 792 subjects participated in the study. Subject demographics were noted and somewhat different between groups (Table 1). Subjects in the methane-positive only (M+) and methane- and hydrogen-positive (H+/M+) groups were older than those in the normal (N) and hydrogen-positive only (H+) groups. The percentage of females was lower in the H+ and M+ groups. Baseline GI complaints were not different between groups, although H+/M+ and M+ subjects tended to have a greater degree of constipation than other groups (Table 1).
Table 1.
Demographic Comparison of the Study Cohort
| Baseline | Variable | Total (n = 792) | N (n = 343) | H+ (n = 320) | M+ (n = 101) | H+/M+ (n = 28) | P Valuea |
|---|---|---|---|---|---|---|---|
| Demographic | Age, y | 47.3 ± 16.3 | 46.7 ± 16.2 | 45.7 ± 15.9 | 53.2 ± 15.5 | 50.1 ± 19.7 | <.001 |
| Gender, % female | 70.8 | 75.51 | 67.5 | 65.35 | 71.43 | .073 | |
| Intestinal symptoms (mean VAS) | Bloating | 61.3 ± 28.4 | 61.3 ± 29.4 | 61.1 ± 27.8 | 61.7 ± 27.6 | 60.8 ± 26.2 | .97 |
| Abd pain | 47.9 ± 31.7 | 49.8 ± 31.4 | 46.4 ± 31.6 | 49.1 ± 32.6 | 39.0 ± 31.4 | .30 | |
| Constipation | 42.3 ± 35.1 | 40.4 ± 34.8 | 41.8 ± 35.5 | 47.6 ± 34.8 | 52.8 ± 33.8 | .16 | |
| Diarrhea | 35.4 ± 33.7 | 36.2 ± 33.9 | 36.0 ± 34.0 | 31.2 ± 32.9 | 34.2 ± 30.2 | .53 |
Data are expressed as mean ± SD.
P value is for comparison of differences among the 4 groups.
Body composition
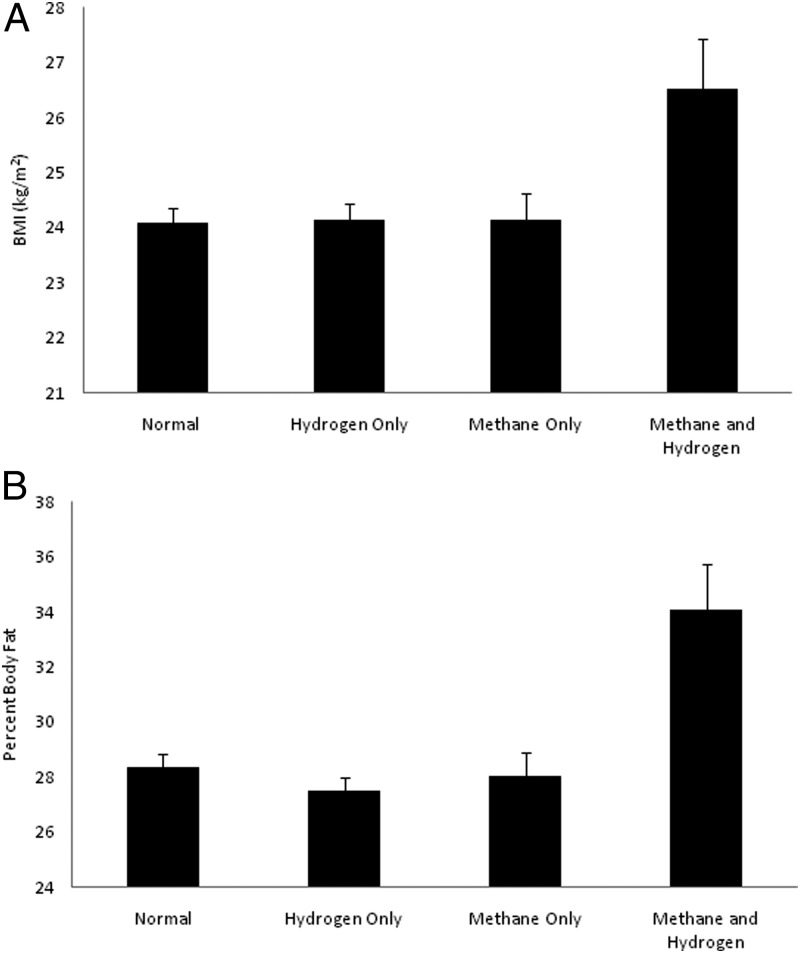
H+/M+ subjects had a greater BMI than any of the other 3 groups (Figure 1A). Similarly, percent body fat was greatest in the H+/M+ group (Figure 1B). Gender was not significantly different between groups. ANOVA indicated that age differed across the groups. Dunnett's post hoc test indicated that the M+ group was the only group that differed significantly from the N group for age. Adjusting for age, BMI was still significantly higher in the H+/M+ group than the other 3 groups (N: 24.1 ± 5.2 kg/m2; H+: 24.2 ± 4.5 kg/m2; M+: 24.0 ± 3.75 kg/m2; H+/M+: 26.5 ± 7.1 kg/m2, P < .02 for each comparison). Using a similar analysis, the H+/M+ group had a higher percent body fat than the other groups (N: 28.3 ± 10.0%; H+: 27.5 ± 9.0%; M+: 28.0 ± 8.9%; H+/M+: 34.1 ± 10.9%) (P ≤ .001 for each comparison).
Figure 1.
Body composition and production of methane and hydrogen on a breath test. A, BMI by group. A significance level of P < .02 between the methane-and-hydrogen group and each of the other groups is shown. Error bars denote SEM. B, Percent body fat by group. A significance level of P ≤ .001 between the methane-and-hydrogen group and each of the other groups is shown.
Discussion
In this study, we demonstrate clear associations between the presence of both methane and hydrogen on breath testing and increased BMI as well as increased percent body fat in an analysis of nearly 800 subjects. This study is the first of its kind to identify the production of methane and hydrogen as an indicator of higher BMI and fat content in human subjects.
Obesity is a public health problem and is undoubtedly multifactorial. Dysregulations are seen in multiple areas of energy intake, expenditure, and storage. There is growing interest in the potential role of gut flora in the pathogenesis of obesity. Research by Gordon, Bäckhed, and others (3–7) have shown an intriguing relationship between microbial flora and weight gain in mouse models, including an association between alterations in the relative abundance of Firmicutes vs Bacteroidetes in the gut and potentially enhanced nutritional harvest (3). Intestinal flora have been implicated in many mechanisms that may contribute to weight gain, including enhanced lipopolysaccharide production leading to insulin resistance (5), suppression of fasting-induced adipose factor (14), suppression of AMP-activated protein kinase-driven fatty acid oxidation in the liver (15), incretin regulation (16), and increased SCFA production and absorption, thereby providing increased lipogenic substrates to the host (17). Increased methanogens have also been observed in the cecal flora of Ob/Ob mice (3). We believe that this large-scale human study may support a role for methanogens, and specifically M smithii, in human obesity.
The human GI tract is colonized by up to 1012 microbial species, including bacteria and archaea, of which M smithii is the most abundant methane-producing organism (9). We have shown that methane-positive individuals have M smithii in the GI tract, that increased methane on breath testing is associated with higher levels of M smithii in stool in subjects with irritable bowel syndrome (IBS) (10), and that methane-positive obese subjects have an average 6.7 kg/m2 greater BMI than methane-negative obese controls (11). Although M smithii was originally thought to inhabit only the large bowel, weakening the likelihood that it could play a significant role in caloric harvest and weight gain, we recently showed using a rat model that M smithii colonization in fact occurs throughout the small intestine (18). Importantly, the number of bowel segments colonized with M smithii was directly related to the degree of weight gain in this rat model and was further enhanced in the presence of a high-fat diet (18).
A current hypothesis is that the role of M smithii in weight gain in animals is facilitative and involves a syntrophic relationship with other microbes, whereby M smithii scavenges hydrogen produced by syntrophic organisms for its hydrogen-requiring anaerobic metabolism, producing methane as a byproduct. This scavenging of hydrogen allows the syntrophic organisms to be more productive, increasing SCFA production and availability of calories for the host (8). Our results may support this hypothesis as the presence of both hydrogen and methane on breath test, but not either methane or hydrogen alone, is associated with higher BMI and percent body fat, perhaps because these subjects have an abundance of hydrogen to fuel methane production.
In addition, methane itself (in gaseous form as generated by intestinal methanogens) could also contribute to enhanced energy harvest. We previously noted an association between breath methane and constipation (constipation-predominant irritable bowel syndrome) in human subjects (13) and, using an in vivo animal model, demonstrated that methane gas directly slows transit in the gut by 59% (19). We hypothesize that the slowing of transit could result in greater time to harvest nutrients and absorb calories, representing another potential mechanism for weight gain.
Although the mean age of the methane producers was higher than that of the controls, the results retained significance, even when controlling for age as a confounding variable. Furthermore, there is currently no evidence to suggest that methane production increases with age but rather plateaus in adulthood (20), making it unlikely that age could affect the study findings. Diet may affect overall intestinal flora and M smithii levels in animal models (18). Our study does not account for dietary differences among subjects. However, given the large sample size, these individual variations may be mitigated between groups. Similarly, the study population consisted of individuals presenting for lactulose breath testing and may differ from the general population. In addition, differences in race and ethnicity were not addressed in this study. These factors may play a role in host/microbial relationships and should be explored in future studies.
In summary, this study demonstrates for the first time that individuals with both methane and hydrogen on breath test have higher BMIs and percent body fat. We hypothesize that this is due to excessive colonization with the hydrogen-requiring methanogen M smithii, which enhances energy harvest and delivery of nutrients to the host organism through syntrophic relationships with other microbes. Further work is needed to define the effects of GI flora on human metabolism and to further elucidate the roles of M smithii and methane in the growing obesity epidemic.
Acknowledgments
We thank the Beatrice and Samuel A. Seaver Foundation for their support for this project. We are thankful to the Clinical and Translational Science Institute staff for their assistance.
This study was supported by a grant from the Beatrice and Samuel A. Seaver Foundation and by the National Center for Research Resources Grant UL1RR033176 and is now at the National Center for Advancing Translational Sciences, Grant UL1TR000124.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ANCOVA
- analysis of covariance
- BMI
- body mass index
- GI
- gastrointestinal
- SCFA
- short-chain fatty acid.
References
- 1. Malnick SD, Knobler H. The medical complications of obesity. Q J M. 2006;99:565–579 [DOI] [PubMed] [Google Scholar]
- 2. Flegal KM, Graubard BI, Williamson DF, Gail MH. Cause-specific excess deaths associated with underweight, overweight, and obesity. JAMA. 2007;298:2028–2037 [DOI] [PubMed] [Google Scholar]
- 3. Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031 [DOI] [PubMed] [Google Scholar]
- 4. Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–1023 [DOI] [PubMed] [Google Scholar]
- 5. Cani PD, Bibiloni R, Knauf C, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:1470–1481 [DOI] [PubMed] [Google Scholar]
- 6. Ley RE, Backhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci USA. 2005;102:11070–11075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Samuel BS, Gordon JI. A humanized gnotobiotic mouse model of host-archaeal-bacterial mutualism. Proc Natl Acad Sci USA. 2006;103:10011–10016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schink B. Energetics of syntrophic cooperation in methanogenic degradation. Microbiol Mol Biol Rev. 1997;61:262–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Flourie B, Etanchaud F, Florent C, Pellier P, Bouhnik Y, Rambaud JC. Comparative study of hydrogen and methane production in the human colon using caecal and faecal homogenates. Gut. 1990;31:684–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kim G, Deepinder F, Morales W, et al. Methanobrevibacter smithii is the predominant methanogen in patients with constipation-predominant IBS and methane on breath. Digest Dis Sci. 2012;57(12):3213–3218 [DOI] [PubMed] [Google Scholar]
- 11. Basseri RJ, Basseri B, Pimentel M, et al. Intestinal methane production in obese individuals is associated with a higher body mass index. Gastroenterol Hepatol. 2012;8:22–28 [PMC free article] [PubMed] [Google Scholar]
- 12. Gibson AL, Holmes JC, Desautels RL, Edmonds LB, Nuudi L. Ability of new octapolar bioimpedance spectroscopy analyzers to predict 4-component-model percentage body fat in Hispanic, black, and white adults. Am J Clin Nutr. 2008;87:332–338 [DOI] [PubMed] [Google Scholar]
- 13. Pimentel M, Mayer AG, Park S, Chow EJ, Hasan A, Kong Y. Methane production during lactulose breath test is associated with gastrointestinal disease presentation. Digest Dis Sci. 2003;48:86–92 [DOI] [PubMed] [Google Scholar]
- 14. Backhed F, Ding H, Wang T, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA. 2004;101:15718–15723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Backhed F, Manchester JK, Semenkovich CF, Gordon JI. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc Natl Acad Sci USA. 2007;104:979–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cani PD, Lecourt E, Dewulf EM, et al. Gut microbiota fermentation of prebiotics increases satietogenic and incretin gut peptide production with consequences for appetite sensation and glucose response after a meal. Am J Clin Nutr. 2009;90:1236–1243 [DOI] [PubMed] [Google Scholar]
- 17. Samuel BS, Shaito A, Motoike T, et al. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc Natl Acad Sci USA. 2008;105:16767–16772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mathur R, Kim G, Morales W, et al. Intestinal Methanobrevibacter smithii but not total bacteria is related to diet-induced weight gain in rats [published online June 7, 2012]. Obesity (Silver Spring). doi:10.1038/oby.2012.141 [DOI] [PubMed] [Google Scholar]
- 19. Pimentel M, Lin HC, Enayati P, et al. Methane, a gas produced by enteric bacteria, slows intestinal transit and augments small intestinal contractile activity. Am J Physiol. 2006;290:G1089–G1095 [DOI] [PubMed] [Google Scholar]
- 20. Peled Y, Gilat T, Liberman E, Bujanover Y. The development of methane production in childhood and adolescence. J Pediatr Gastroenterol Nutr. 1985;4:575–579 [DOI] [PubMed] [Google Scholar]