Abstract
Context:
It has been hypothesized that increased plasma bile acids (BAs) contribute to metabolic improvements after Roux-en-Y gastric bypass (RYGB) surgery by the G protein-coupled receptor TGR5-mediated effects on glucagon-like peptide-1 secretion and thyroid hormones.
Objective:
The objective of this study was to evaluate the importance of bariatric surgery-induced alterations in BA physiology on factors that regulate glucose homeostasis (insulin secretion and sensitivity) and energy metabolism (resting energy expenditure and thyroid hormone axis).
Design, Participants, Intervention, and Main Outcome Measure:
Eighteen extremely obese subjects were studied before and after 20% weight loss, induced by either laparoscopic adjustable gastric banding (LAGB) (n = 10) or RYGB surgery (n = 8).
Results:
Plasma BAs more than doubled after RYGB [fasting: 1.08 (0.26–1.42) to 2.28 (1.59–3.28) μmol/L, P = .03; postprandial: 2.46 ± 1.59 to 6.00 ± 2.75 μmol/L, P = .01] but were either lower or did not change after LAGB [fasting: 1.80 (1.49–2.19) to 0.92 (0.73–1.15) μmol/L, P = .02; postprandial: 3.71 ± 2.61 to 2.82 ± 1.75 μmol/L, P = .14]. Skeletal muscle expression of TGR5 targets, Kir6.2 and cyclooxygenase IV, increased after RYGB but not LAGB. Surgery-induced changes in BAs were associated with increased peak postprandial plasma glucagon-like peptide-1 (r2 = 0.509, P = .001) and decreased serum TSH (r2 = 0.562, P < .001) but did not correlate with the change in insulin response to a meal (r2 = 0.013, P = .658), insulin sensitivity (assessed as insulin stimulated glucose disposal during a hyperinsulinemic-euglycemic clamp procedure) (r2 = 0.001, P = .995), or resting energy expenditure (r2 = 0.004, P = .807).
Conclusions:
Compared with LAGB, RYGB increases circulating BAs and TGR5 signaling, but this increase in BAs is not a significant predictor of changes in glucose homeostasis or energy metabolism.
Roux-en Y gastric bypass (RYGB) surgery and laparoscopic adjustable gastric banding (LAGB) are the 2 most commonly performed bariatric surgical procedures in the world (1). Although both procedures improve metabolic function by causing weight loss, it has been suggested that RYGB has weight loss-independent effects on glycemic control and ameliorating type 2 diabetes (2, 3). The observation that plasma bile acid (BA) concentrations increase after upper intestinal bypass surgeries (4–7) has led to the notion that BAs contribute to weight-independent improvements in glucose homeostasis (8).
Bile acids could improve metabolic function through several potential mechanisms. Circulating BAs bind to TGR5, a plasma membrane-bound G protein-coupled receptor that is present in enteroendocrine cells, skeletal muscle, and brown adipose tissue (9). The activation of TGR5 increases glucagon-like peptide-1 (GLP-1) release (10), which can improve insulin secretion and insulin sensitivity (11). Activation of the TGR5 receptor in skeletal muscle and brown adipose tissue also mediates the conversion of T4 to T3 through iodothyronine deiodinase, which could facilitate weight loss by increasing resting energy expenditure (REE) (12). In addition, BAs can also increase insulin secretion through the activation of the farnesoid X receptor in pancreatic β-cells (13).
The purpose of this study was to evaluate the importance of bariatric surgery-induced alterations in BA physiology on metabolic function by testing the following hypotheses: 1) weight loss induced by RYGB surgery causes greater alterations in serum BA concentrations and BA-responsive TGR5 elements in skeletal muscle than the same weight loss induced by LAGB; 2) alterations in serum BA concentrations are associated with factors that regulate glucose homeostasis (postprandial plasma GLP-1 concentrations, insulin secretion in response to a mixed meal, and skeletal muscle insulin sensitivity); and 3) alterations in serum BA concentrations are associated with changes in factors involved in energy metabolism (plasma thyroid hormones and REE). Accordingly, we evaluated both fasting and postprandial serum BAs and BA-responsive TGR5 elements in skeletal muscle of obese subjects, in whom we had measured several other metabolic end points (insulin and GLP-1 response to a mixed meal, insulin sensitivity assessed by using the hyperinsulinemic-euglycemic clamp procedure, and REE) before and after 20% weight loss induced by either RYGB or LAGB surgery (14).
Materials and Methods
Study subjects
Eighteen consecutive, eligible patients who were scheduled to undergo RYGB (n = 8; 2 men, 6 women; 43 ± 7 years old) or LAGB (n = 10; 1 man, 9 women; 47 ± 14 years old) procedures at Barnes-Jewish Hospital (St Louis, Missouri) participated in this study, which was approved by the Washington University Institutional Review Board. All participants provided written informed consent. Potential participants who had diabetes were excluded to avoid the confounding effects of differences in baseline glycemic control, glucose toxicity, and subsequent postsurgical changes in diabetes medications on the outcome measures. Blood and muscle tissue samples analyzed for the present study were obtained from the subjects during their participation in a study that evaluated insulin sensitivity, β-cell function, and the metabolic response to a mixed meal. The primary outcomes from that study and details of the study procedures (briefly described below) were recently reported (14).
Experimental procedures
A hyperinsulinemic-euglycemic clamp procedure and a mixed-meal metabolic study were performed before surgery in the Clinical Research Unit at Washington University School of Medicine. Subjects were admitted to the Clinical Research Unit in the evening on 2 occasions, approximately 1 week apart, before each study and consumed a standard meal. Studies were conducted the following morning after a 12-hour overnight fast. Although an experienced weight management dietitian provided weekly dietary counseling in an attempt to match weight loss in both groups and adjusted the dietary intake to help maintain weight stability for at least 2 weeks before final metabolic testing, subjects continued to lose weight in the last 2 weeks before final testing (additional 1.26% weight loss in the RYGB group and 1.10% in the LAGB) (Supplemental Figure 1, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org).
Hyperinsulinemic-euglycemic clamp procedure
A primed-continuous infusion of [6,6-2H2]glucose was started and maintained for 7.5 hours. After 3.5 hours, insulin was infused at a rate of 50 mU/m2 body surface area per minute−1 for 4 hours. Euglycemia (plasma glucose ∼100 mg/dL) was maintained by infusing 20% dextrose enriched to 2.5% with [6,6-2H2]glucose. Blood samples were obtained before isotope tracer infusion and during the final 30 minutes of the basal period and insulin clamp to assess plasma substrate and hormone concentrations and glucose kinetics. Vastus lateralis muscle tissue was obtained by percutaneous biopsy 60 minutes after starting the glucose tracer infusion.
Mixed-meal metabolic study
Subjects ingested a liquid meal (containing 46 g of glucose, 9 g of fat, and 9 g of protein) provided in 7 equally divided aliquots every 5 minutes over 30 minutes. Blood samples were obtained to determine plasma insulin, C-peptide, and GLP-1 concentrations before and at 1 hour after starting the meal to analyze BA composition. REE was determined before meal ingestion by using indirect calorimetry.
Analyses of blood samples and calculations
Serum BA content was assayed by using electrospray ionization liquid chromatography mass spectrometry (sensitivity ≥ 0.01 μmol/L, coefficient of variation 1%–13%), as previously described (15, 16). The bile acids quantified included the following: primary (cholic acid and chenodeoxycholic acid), secondary (deoxycholic acid, lithocholic acid, and ursodeoxycholic acid), glycine-conjugated (glycocholic acid, glycodeoxycholic acid, glycochenodeoxycholic acid, glycolithocholic acid, and glycoursodeoxycholic acid), and taurine-conjugated (taurocholic acid, taurodeoxycholic acid, taurochenodeoxycholic acid, taurolithocholic acid, and tauroursodeoxycholic acid) BAs.
Plasma TSH concentration was determined by chemiluminescent assay (sensitivity ≥ 0.01 μIU/mL, coefficient of variation 2.94–3.45%) (Abbott Diagnostics, Ontario, Canada). Plasma active GLP-1 concentration was measured by using an ELISA (Millipore, Billerica, Massachusetts). The increase in the glucose disposal rate during insulin infusion was used as an index of skeletal muscle insulin sensitivity, and a minimal model analysis was used to calculate the insulin secretion rate by evaluating plasma glucose, C-peptide, and insulin concentrations obtained over 5 hours after subjects consumed a mixed meal (14, 17).
Quantitative PCR analyses
RNA was isolated from frozen muscle tissue by using TRIzol reagent (Life Technologies, Grand Island, New York). cDNA was synthesized (Superscript VILO kit; Life Technologies), and gene-specific amplification was performed by using SYBR Green chemistry on an ABI 7500 quantitative PCR cycler (Applied Biosystems, Foster City, California). Results were analyzed by comparing the threshold crossing of each sample after normalization to the housekeeping 36B4 gene. Changes in threshold crossing (DCt) were used to calculate the relative levels of each mRNA compared with the control gene by using the formula 2−DCt. The following primer sets were used: Kir6.2 forward, TCCTGATCCTCATCGTGCAGA, reverse, CCCACACGTAGCATGAAGCA; cyclooxygenase (COX) IV forward, CAGGGTATTTAGCCTAGTTGGC, reverse, GCCGATCCATATAAGCTGGGA; and 36B4 forward, GTGATGTGCAGCTGATCAAGACT, reverse, GATGACCAGCCCAAAGGAGA.
Statistical analyses
Data were examined for normality according to the Shapiro-Wilks criteria, and nonnormally distributed data sets were log transformed. A 2-way, repeated-measures ANOVA with post hoc paired and unpaired Student's t tests was used to compare the effects of surgery on the in vivo metabolic outcomes in the 2 groups. We also performed a repeated-measures analysis of covariance, adjusting for age, sex, race, change in weight, and change in fat mass. A Student's t test for paired samples was used to evaluate the effect of surgery-induced weight loss on gene expression of TGR5 downstream elements. Results are expressed as means ± SD for normally distributed variables and backtransformed mean and 95% confidence intervals for nonnormally distributed variables. Pearson's correlation and multiple linear regression analysis with independent variables of age, sex, race, change in body weight, and fat mass were used to examine associations between variables of interest. A P ≤ .05 was considered statistically significant. We estimated that 8 subjects in each group would allow us to detect a 50% difference in the change in plasma BA concentrations between surgical groups with a power of 0.9 and an alpha value of 0.05. Based on statistical power analysis (18), a total of 18 subjects would be needed to detect a significant correlation of moderate strength (r2 ≥ 0.30) between the change in plasma bile acids and change in metabolic outcomes (plasma GLP-1 and TSH, glucose disposal, insulin secretion rate, and REE), at a 95% level of significance (2 sided P < .05) and with 80% power (β = .20).
Results
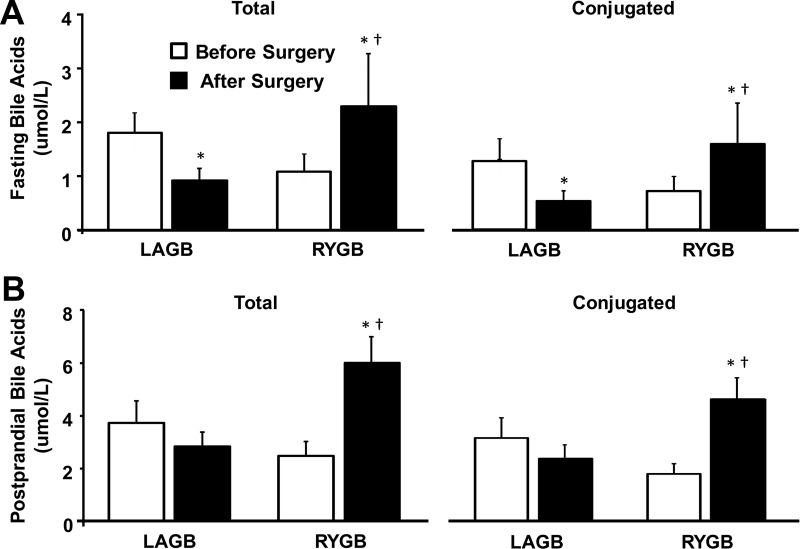
Total and conjugated serum BA concentrations, obtained 1 hour after subjects began to ingest a mixed meal, were 2- to 3-fold greater than fasting BA concentrations in both groups, both before and after surgery-induced weight loss (Figure 1). Compared with presurgery values, total fasting and postprandial BA concentrations were approximately 2.5-fold higher after RYGB surgery but were either lower or did not significantly change after LAGB (Figure 1). The increase in serum BAs after RYGB surgery was due to a proportionate increase in all BA species (primary, secondary, and conjugated BAs); glycine-conjugated BAs represented the major BA before and after surgery in both groups (Supplemental Figure 2).
Figure 1.
Total and conjugated bile acid concentrations during fasting (A) and postprandial (B) conditions before and after 20% weight loss induced by RYGB surgery or LAGB. *, Significantly different from Before Surgery value, P < .03; †, significantly different from LAGB, P < .05. Values are means ± SEM.
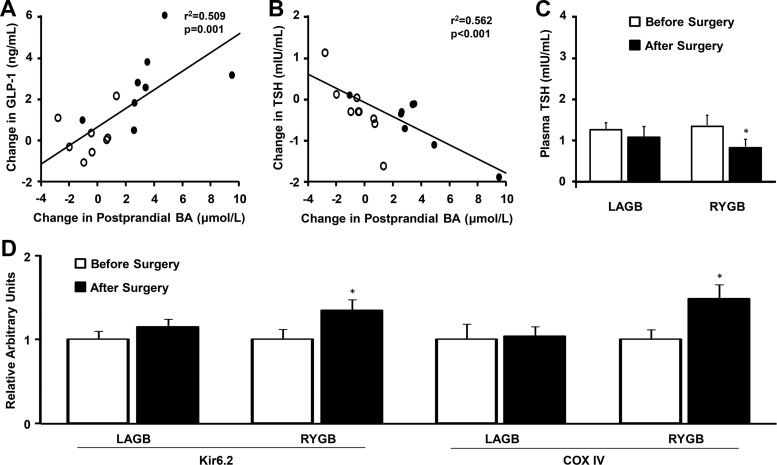
The change in postprandial BA concentrations after surgery was positively correlated with the change in peak postprandial GLP-1 concentrations (Figure 2A). However, no significant correlation was observed between the surgery-induced change in postprandial total serum BAs and the change in the insulin secretion rate in response to a mixed meal (r2 = 0.013, P = .658) or insulin-stimulated glucose disposal (r2 < 0.001, P = .995), even after adjustment for the independent variables of age, sex, race, change in body weight, and change in fat mass.
Figure 2.
Relationship between RYGB (closed circles) and LAGB (open circles)-induced changes in postprandial total BA concentrations and the change in plasma GLP-1 (A) and TSH (B). Plasma TSH concentration (C) and skeletal muscle gene expression of Kir6.2 and COX IV (D) before and after surgery-induced 20% weight loss. *, Significantly different from Before Surgery value, P < .05. Values are means ± SEM.
Plasma TSH decreased after weight loss induced by RYGB but not LAGB (Figure 2C). The change in plasma TSH after surgery was negatively correlated with the change in plasma BAs (Figure 2B). Plasma free T3 decreased after weight loss in both the LAGB and RYGB groups (0.38 ± 0.03 to 0.31 ± 0.02 ng/dL and 0.35 ± 0.02 to 0.31 ± 0.03 ng/dL, respectively), but there was no difference between groups (P = .440). REE, expressed per kilogram fat-free mass (FFM), decreased by approximately 15% after weight loss induced by either RYGB (31.4 ± 3.4 to 27.4 ± 3.6 kcal/kg FFM per day) (P < .001) or LAGB (32.5 ± 4.0 to 26.5 ± 3.0 kcal/kg FFM per day) (P < .001), but there was no difference between groups. Individual changes in REE after surgery were not associated with changes in either fasting or postprandial serum BA concentrations (r2 = 0.004, P = .807 and r2 = 0.003, P = .826, respectively). Weight loss induced by RYGB, but not LAGB, increased skeletal muscle expression of mitochondrial TGR5 downstream elements, COX IV and Kir6.2 (Figure 2D).
Discussion
The results from the present study demonstrate profound differences between the effects of weight loss induced by RYGB surgery and LAGB on BA physiology. We found that both fasting and postprandial total plasma BA concentrations more than doubled after RYGB but tended to decrease after LAGB. The observed effect of RYGB on serum BAs was independent of weight loss because both groups were studied after losing the same proportion (20%) of their initial body weight. The increase in serum BAs in the RYGB group was associated with cellular and systemic metabolic effects, manifested by increased skeletal muscle gene expression of TGR5 downstream targets (mitochondrial COX IV and Kir6.2), and by significant correlations between the changes in postprandial BAs and both meal-induced increases in plasma GLP-1 and changes in plasma TSH concentrations. However, we did not detect any relationship between changes in plasma BAs and the insulin response to a mixed meal, skeletal muscle insulin sensitivity, or REE. These findings suggest that increased BAs after RYGB surgery are associated with intestinal GLP-1 secretion and thyroid hormone function via TGR5-mediated mechanisms but do not predict changes in the major factors that regulate glucose homeostasis or overall resting energy metabolism.
The mechanism responsible for the increase in plasma BA concentrations after RYGB surgery is unclear. We speculate that RYGB increases the enterohepatic circulation of BAs by increasing the delivery of BAs to the ileum for reabsorption and delivery to the liver, rather than by altering microbial intestinal BA metabolism because all BA species increased in proportion to their original relative distribution. This possible explanation is supported by the results from a study conducted in a rodent ileal transposition (IT) model, which increases the proximal absorption of intestinal BAs by moving a segment of ileum to the upper jejunum (19). The increase in enterohepatic recycling may be enhanced by augmented gallbladder filling through TGR5 activation. Similar to our observations after RYGB in people, IT in rodents caused an increase in plasma BAs and GLP-1 concentrations. Moreover, the changes in IT animals were also independent of weight loss because control rodents that were pair fed to IT animals did not demonstrate any changes in serum BA concentrations.
In summary, our data show that active weight loss induced by RYGB surgery, but not LAGB, increases both fasting and postprandial plasma BA concentrations. Although the increase in BAs was associated with increased TGR5 signaling and selected TGR5 targets (GLP-1 secretion and TSH), it did not translate into important physiologically or clinically meaningful effects on meal-induced insulin secretion, insulin sensitivity, or energy expenditure.
Supplementary Material
Acknowledgments
We thank Dr Ken Schectman for assistance with statistical analyses, Courtney Tiemann for help with subject recruitment and dietary counseling, Terri Pietka for technical assistance, Janine Kampelman for assistance with skeletal muscle biopsies, the staff of the Clinical Research Unit for their help in performing the studies, and the study subjects for their participation. This study is registered with the Clinical Trial Registration (www.clinicaltrials.gov) NCT00981500.
This work and manuscript were supported by National Institutes of Health Grants DK 37948, DK 56341 (Nutrition Obesity Research Center), UL1 RR024992 (Clinical and Translational Science Award), DK084310, and U01 DK08505.
Disclosure Summary: S.K. and R.K. received a grant from Ethicon Endo-Surgery (Cincinnati, OH). S.K. has served on a Scientific Advisory Board for Ethicon Endo-Surgery and is a shareholder of Aspire Bariatrics. The other authors have no disclosures.
Footnotes
- BA
- bile acid
- COX
- cyclooxygenase
- FFM
- fat-free mass
- GLP-1
- glucagon-like peptide-1
- IT
- ileal transposition
- LAGB
- laparoscopic adjustable gastric banding
- RYGB
- Roux-en Y gastric bypass
- REE
- resting energy expenditure.
References
- 1. Dixon JB, Straznicky NE, Lambert EA, Schlaich MP, Lambert GW. Surgical approaches to the treatment of obesity. Nat Rev Gastroenterol Hepatol. 2011;8:429–437 [DOI] [PubMed] [Google Scholar]
- 2. Pories WJ, Dohm GL. Full and durable remission of type 2 diabetes? Through surgery? Surg Obes Relat Dis. 2009;5:285–288 [DOI] [PubMed] [Google Scholar]
- 3. Goldfine AB, Shoelson SE, Aguirre V. Expansion and contraction: treating diabetes with bariatric surgery. Nat Med. 2009;15:616–617 [DOI] [PubMed] [Google Scholar]
- 4. Patti ME, Houten SM, Bianco AC, et al. Serum bile acids are higher in humans with prior gastric bypass: potential contribution to improved glucose and lipid metabolism. Obesity (Silver Spring). 2009;17:1671–1677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pournaras DJ, Glicksman C, Vincent RP, et al. The role of bile after Roux-en-Y gastric bypass in promoting weight loss and improving glycaemic control. Endocrinology. 2012;153:3613–3619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nakatani H, Kasama K, Oshiro T, Watanabe M, Hirose H, Itoh H. Serum bile acid along with plasma incretins and serum high-molecular weight adiponectin levels are increased after bariatric surgery. Metabolism. 2009;58:1400–1407 [DOI] [PubMed] [Google Scholar]
- 7. Jansen PL, van Werven J, Aarts E, et al. Alterations of hormonally active fibroblast growth factors after Roux-en-Y gastric bypass surgery. Dig Dis. 2011;29:48–51 [DOI] [PubMed] [Google Scholar]
- 8. Laferrere B. Gut feelings about diabetes. Endocrinol Nutr. 2012;59:254–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen X, Lou G, Meng Z, Huang W. TGR5: a novel target for weight maintenance and glucose metabolism. Exp Diabetes Res. 2011;2011:853501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Thomas C, Gioiello A, Noriega L, et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009;10:167–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. D'Alessio DA, Kahn SE, Leusner CR, Ensinck JW. Glucagon-like peptide 1 enhances glucose tolerance both by stimulation of insulin release and by increasing insulin-independent glucose disposal. J Clin Invest. 1994;93:2263–2266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Watanabe M, Houten SM, Mataki C, et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439:484–489 [DOI] [PubMed] [Google Scholar]
- 13. Dufer M, Horth K, Wagner R, et al. Bile acids acutely stimulate insulin secretion of mouse β-cells via farnesoid X receptor activation and K(ATP) channel inhibition. Diabetes. 2012;61:1479–1489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bradley D, Conte C, Mittendorfer B, et al. Gastric bypass and banding equally improve insulin sensitivity and β cell function. J Clin Invest. 2012;122:4667–4674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Melgarejo T, Williams DA, O'Connell NC, Setchell KD. Serum unconjugated bile acids as a test for intestinal bacterial overgrowth in dogs. Dig Dis Sci. 2000;45:407–414 [DOI] [PubMed] [Google Scholar]
- 16. Hagio M, Matsumoto M, Fukushima M, Hara H, Ishizuka S. Improved analysis of bile acids in tissues and intestinal contents of rats using LC/ESI-MS. J Lipid Res. 2009;50:173–180 [DOI] [PubMed] [Google Scholar]
- 17. Breda E, Toffolo G, Polonsky KS, Cobelli C. Insulin release in impaired glucose tolerance: oral minimal model predicts normal sensitivity to glucose but defective response times. Diabetes. 2002;51(suppl 1):S227–S233 [DOI] [PubMed] [Google Scholar]
- 18. Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed Hillsdale, NJ: L. Erlbaum Associates; 1988 [Google Scholar]
- 19. Kohli R, Kirby M, Setchell KD, et al. Intestinal adaptation after ileal interposition surgery increases bile acid recycling and protects against obesity-related comorbidities. Am J Physiol Gastrointest Liver Physiol. 2010;299:G652–G660 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.